Impact of Novel Diets on the Distribution of Mucosal Immune Cells in the Digestive System of High-Growth Genetically Selected Gilthead Seabream (Sparus aurata) in a Long-Term Feeding Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Sampling
2.3. In Situ Hybridization
2.4. Immunohistochemistry and Histochemistry
2.5. Image Acquisition and mRNA+ Cell Counting
2.6. Statistical Analyses
2.7. Ethical Statement
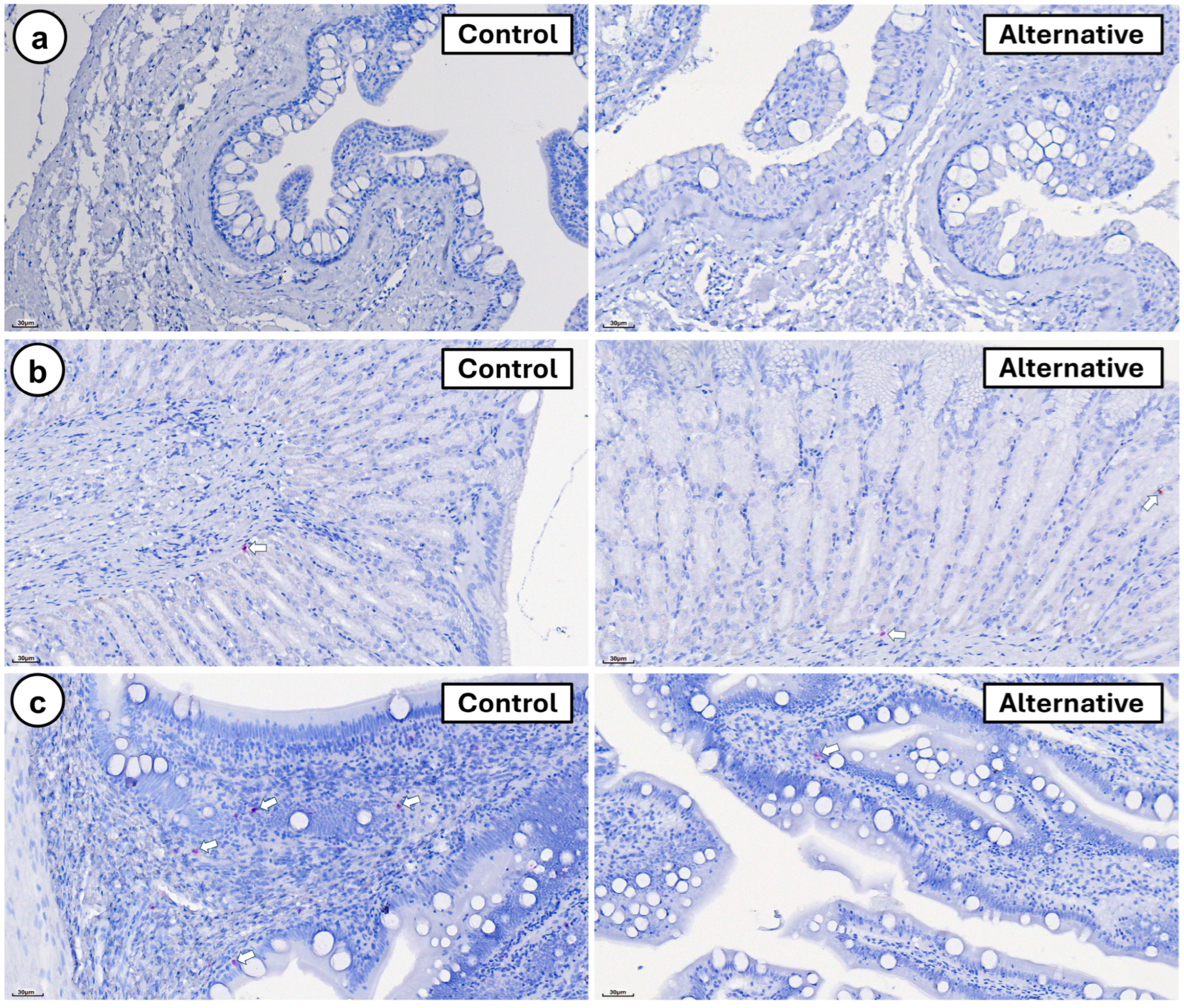
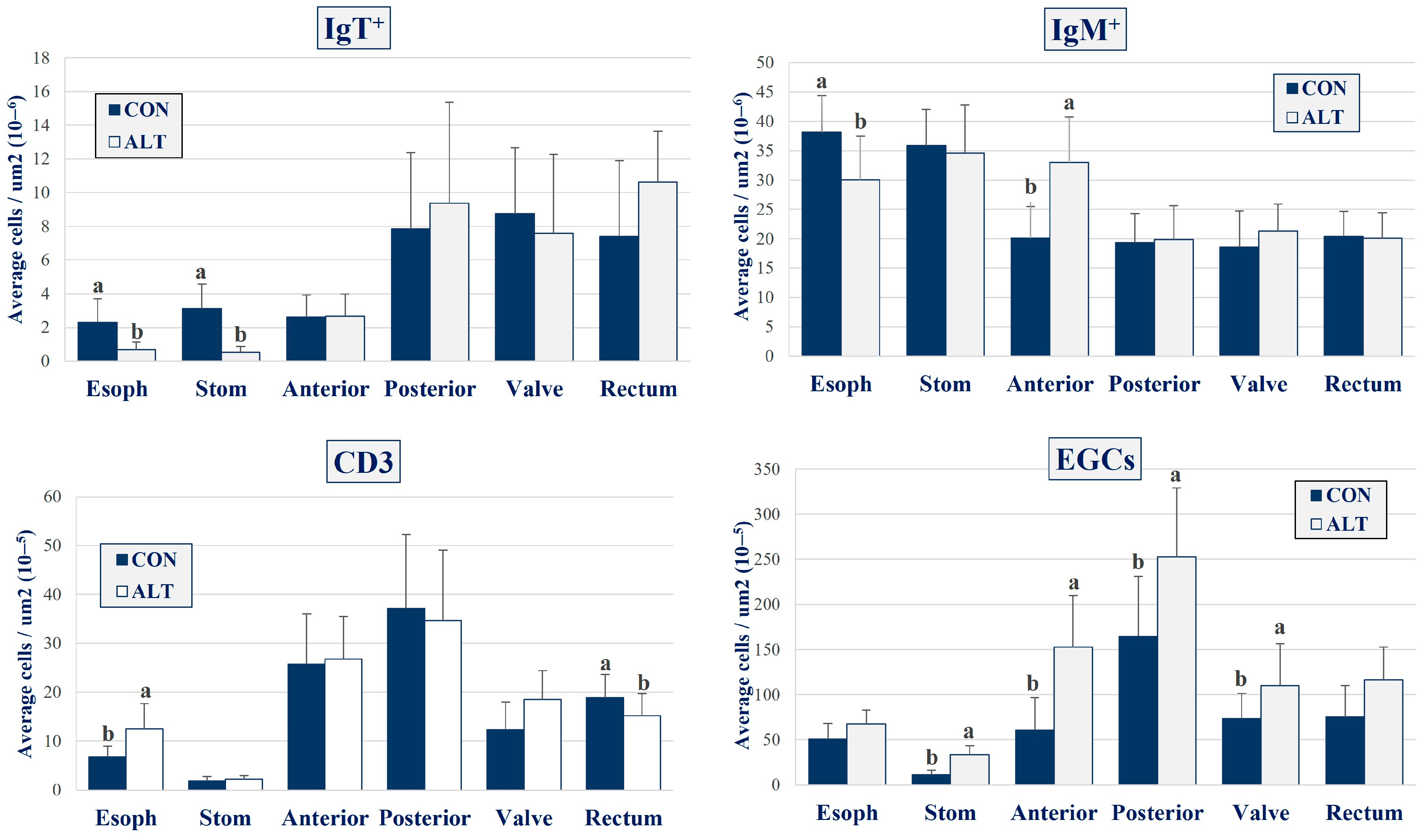
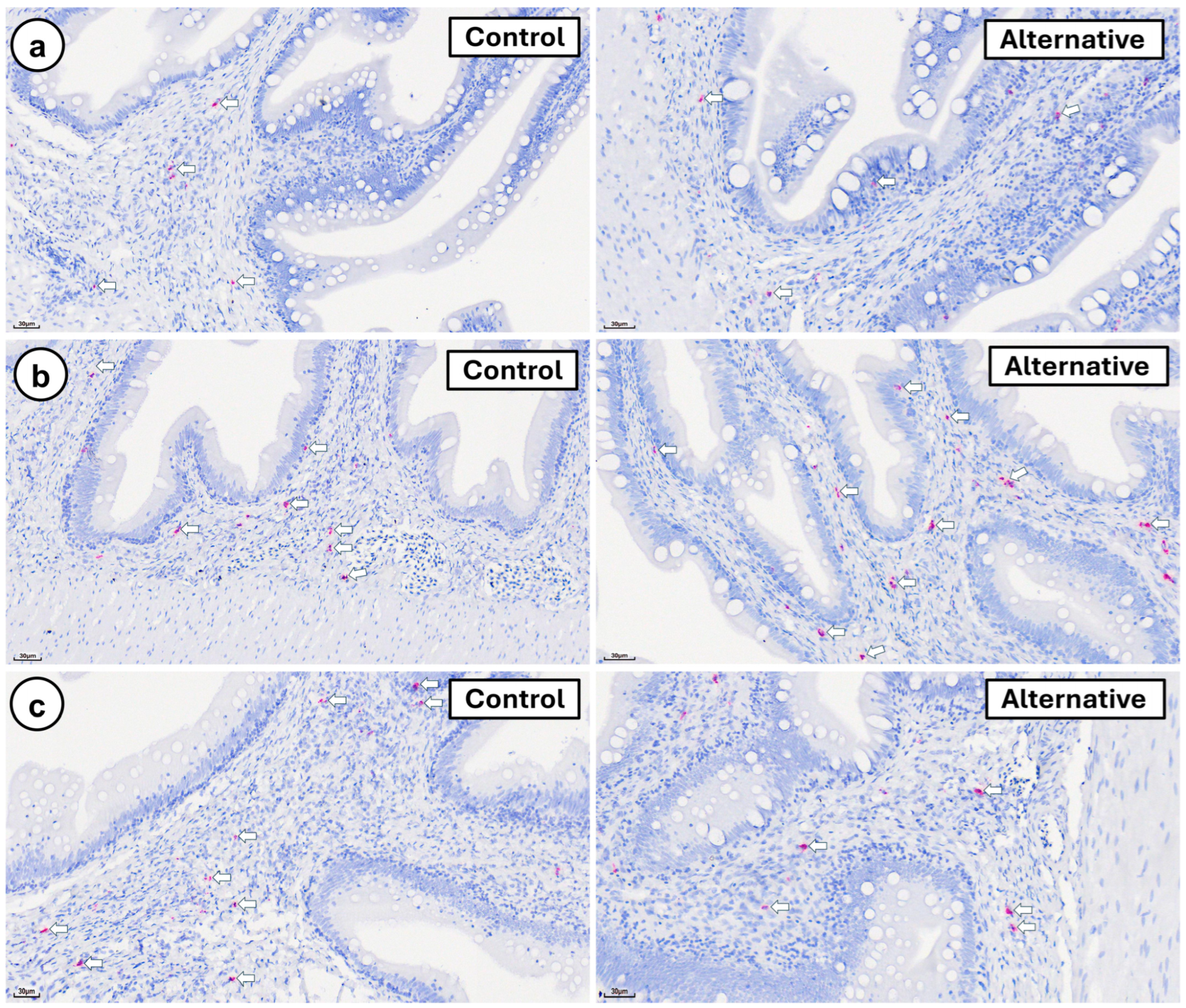
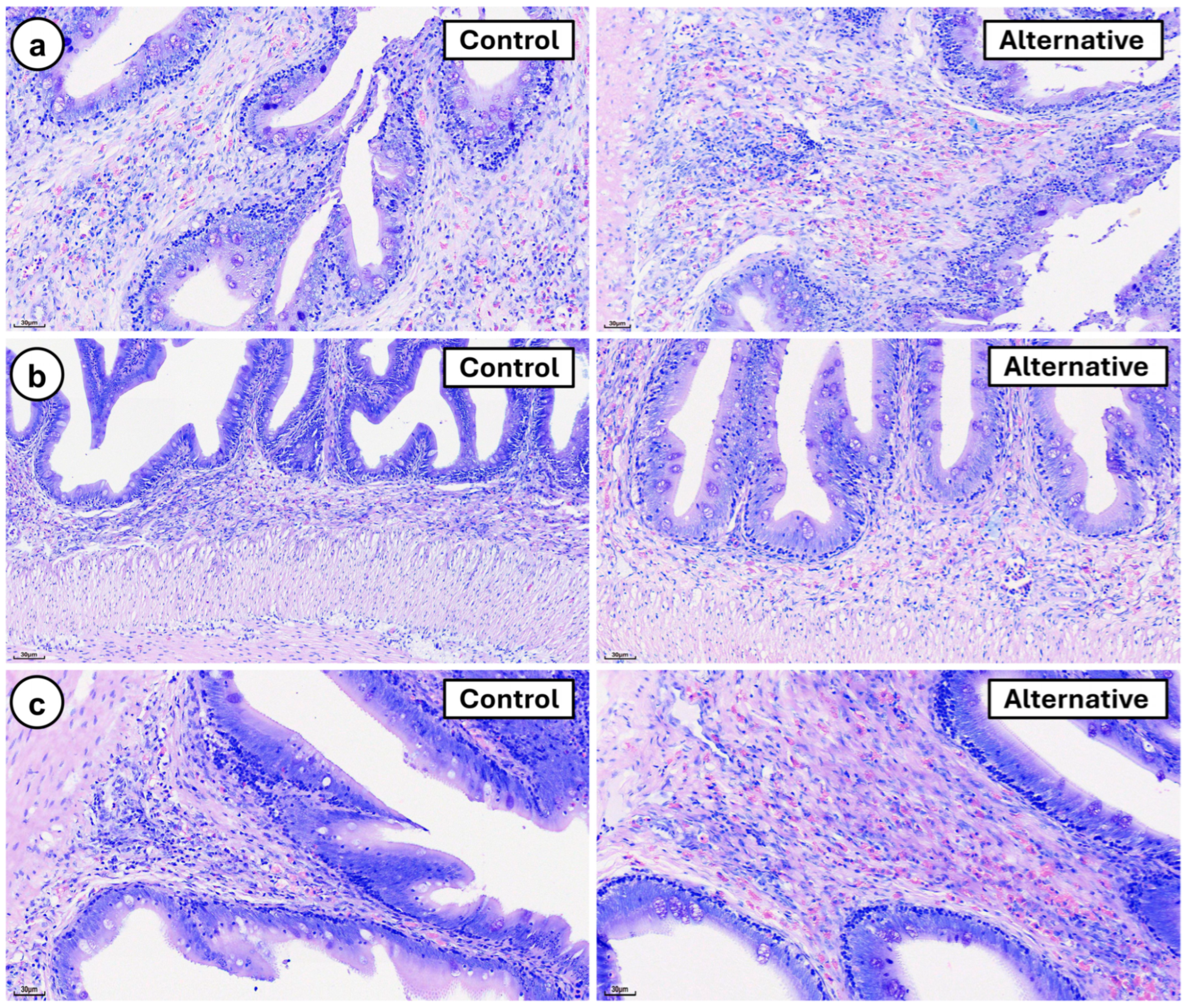
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; Valenti, W.C. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Qiu, H.; Dai, M.; Chen, N.; Li, S. Apparent digestibility of ten protein ingredients for largemouth bass (Micropterus salmoides). Aquac. Res. 2022, 53, 6846–6854. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Ferrer-Llagostera, P.; Kallas, Z.; Reig, L.; De Gea, D.A. The use of insect meal as a sustainable feeding alternative in aquaculture: Current situation, Spanish consumers’ perceptions and willingness to pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards sustainable aquafeeds: Complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile Nile tilapia (Oreochromis niloticus). PLoS ONE 2016, 11, e0156684. [Google Scholar] [CrossRef]
- Carvalho, M.; Montero, D.; Rosenlund, G.; Fontanillas, R.; Ginés, R.; Izquierdo, M. Effective complete replacement of fish oil by combining poultry and microalgae oils in practical diets for gilthead sea bream (Sparus aurata) fingerlings. Aquaculture 2020, 529, 735696. [Google Scholar] [CrossRef]
- Carvalho, M.; Marotta, B.; Xu, H.; Geraert, P.A.; Kaushik, S.; Montero, D.; Izquierdo, M. Complete replacement of fish oil by three microalgal products rich in n-3 long-chain polyunsaturated fatty acids in early weaning microdiets for gilthead sea bream (Sparus aurata). Aquaculture 2022, 558, 738354. [Google Scholar] [CrossRef]
- Pulido-Rodriguez, L.F.; Cardinaletti, G.; Secci, G.; Randazzo, B.; Bruni, L.; Cerri, R.; Olivotto, I.; Tibaldi, E.; Parisi, G. Appetite regulation, growth performances and fish quality are modulated by alternative dietary protein ingredients in gilthead sea bream (Sparus aurata) culture. Animals 2021, 11, 1919. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, B.; Zarantoniello, M.; Cardinaletti, G.; Cerri, R.; Giorgini, E.; Belloni, A.; Contò, M.; Tibaldi, E.; Olivotto, I. Hermetia illucens and poultry by-product meals as alternatives to plant protein sources in gilthead seabream (Sparus aurata) diet: A multidisciplinary study on fish gut status. Animals 2021, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Perera, E.; Simó-Mirabet, P.; Shin, H.S.; Rosell-Moll, E.; Naya-Catalá, F.; Heras, V.d.L.; Martos-Sitcha, J.A.; Karalazos, V.; Armero, E.; Arizcun, M.; et al. Selection for growth is associated in gilthead sea bream (Sparus aurata) with diet flexibility, changes in growth patterns and higher intestine plasticity. Aquaculture 2019, 507, 349–360. [Google Scholar] [CrossRef]
- Carvalho, M.; Ginés, R.; Martín, I.; Zamorano, M.J.; Acosta, F.; Fontanillas, R.; Montero, D. Genetic selection for high growth improves the efficiency of gilthead sea bream (Sparus aurata) in using novel diets with insect meal, single-cell protein and a DHA rich-microalgal oil. Aquaculture 2024, 578, 740034. [Google Scholar] [CrossRef]
- Moroni, F.; Carvalho, M.; Di Rosa, A.R.; Torrecillas, S.; Fontanillas, R.; Haffray, P.; Montero, D. Genetic selection and novel feeds containing single cell protein as a substitute for fishmeal in european sea bass: Effects on growth, fatty acid profile and E-sensing analysis of fillets. Aquac. Rep. 2024, 35, 102021. [Google Scholar] [CrossRef]
- Yamamoto, T.; Murashita, K.; Matsunari, H.; Oku, H.; Furuita, H.; Okamoto, H.; Amano, S.; Suzuki, N. Selectively bred juvenile F2 amago salmon Oncorhynchus masou ishikawae fed a low fishmeal diet exhibit growth comparable to unselected juveniles fed a fishmeal-based diet. Fish. Sci. 2015, 81, 83–93. [Google Scholar] [CrossRef]
- Geay, F.; Ferraresso, S.; Zambonino-Infante, J.L.; Bargelloni, L.; Quentel, C.; Vandeputte, M.; Mazurais, D. Effects of the total replacement of fish-based diet with plant-based diet on the hepatic transcriptome of two European sea bass (Dicentrarchus labrax) half-subfamilies showing different growth rates with the plant-based diet. BMC Genom. 2011, 12, 522. [Google Scholar] [CrossRef]
- Montero, D.; Moyano, F.J.; Carvalho, M.; Sarih, S.; Fontanillas, R.; Zamorano, M.J.; Torrecillas, S. Nutritional innovations in superior gilthead seabream (Sparus aurata) genotypes: Implications in the utilization of emerging new ingredients through the study of the patterns of secretion of digestive enzymes. Aquaculture 2023, 577, 739958. [Google Scholar] [CrossRef]
- Cao, J.; Xu, H.; Yu, Y.; Xu, Z. Regulatory roles of cytokines in T and B lymphocytes-mediated immunity in teleost fish. Dev. Comp. Immunol. 2023, 140, 104621. [Google Scholar] [CrossRef]
- Jenberie, S.; van der Wal, Y.A.; Jensen, I.; Jørgensen, J.B. There and back again? A B cell’s tale on responses and spatial distribution in teleosts. Fish Shellfish Immunol. 2024, 148, 109479. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef]
- Castro, P.L.; Barac, F.; Hansen, T.J.; Fjelldal, P.G.; Hordvik, I.; Bjørgen, H.; Koppang, E.O. The distribution of IgT mRNA+ cells in the gut of the atlantic salmon (Salmo salar L.). Animals 2023, 13, 3191. [Google Scholar] [CrossRef]
- Parra, D.; Takizawa, F.; Sunyer, J.O. Evolution of B cell immunity. Annu. Rev. Anim. Biosci. 2013, 1, 65–97. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Galindo-Villegas, J.; Pereiro, P.; Estensoro, I.; Calduch-Giner, J.A.; Gómez-Casado, E.; Novoa, B.; Mulero, V.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Differential modulation of IgT and IgM upon parasitic, bacterial, viral, and dietary challenges in a perciform fish. Front. Immunol. 2016, 7, 637. [Google Scholar] [CrossRef]
- Bakke-McKellep, A.M.; Press, C.M.; Baeverfjord, G.; Krogdahl, Å.; Landsverk, T. Changes in immune and enzyme histochemical phenotypes of cells in the intestinal mucosa of Atlantic salmon, Salmo salar L., with soybean meal-induced enteritis. J. Fish Dis. 2008, 23, 115–127. [Google Scholar] [CrossRef]
- Rombout, J.H.W.M.; Abelli, L.; Picchietti, S.; Scapigliati, G.; Kiron, V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011, 31, 616–626. [Google Scholar] [CrossRef]
- Bakke-McKellep, A.M.; Frøystad, M.K.; Lilleeng, E.; Dapra, F.; Refstie, S.; Krogdahl, Å.; Landsverk, T. Response to soy: T-cell-like reactivity in the intestine of Atlantic salmon, Salmo salar L. J. Fish Dis. 2007, 30, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Couto, A.; Kortner, T.M.; Penn, M.; Bakke, A.M.; Krogdahl, Å.; Oliva-Teles, A. Effects of dietary soy saponins and phytosterols on gilthead sea bream (Sparus aurata) during the on-growing period. Anim. Feed Sci. Technol. 2014, 198, 203–214. [Google Scholar] [CrossRef]
- Picchietti, S.; Miccoli, A.; Fausto, A.M. Gut immunity in european sea bass (Dicentrarchus labrax): A review. Fish Shellfish Immunol. 2021, 108, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.A.; Oliveira, F.P.S.; Pedrosa, V.F. Mast cells and eosinophilic granule cells in Oncorhynchus mykiss: Are they similar or different? Fish Shellfish Immunol. Rep. 2021, 2, 100029. [Google Scholar] [CrossRef]
- APROMAR. La Acuicultura en España; Asociación Empresarial de Acuicultura de España (APROMAR): Chiclana de la Frontera, Spain, 2023; Available online: www.apromar.es/wp-content/uploads/2023/09/LaacuiculturaEspana2023APROMAR_v2.pdf (accessed on 20 January 2024).
- Mhalhel, K.; Levanti, M.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Aragona, M.; Porcino, C.; Briglia, M.; Germanà, A.; Montalbano, G. Review on gilthead seabream (Sparus aurata) aquaculture: Life cycle, growth, aquaculture practices and challenges. J. Mar. Sci. Eng. 2023, 11, 2008. [Google Scholar] [CrossRef]
- AENOR. Pisciculture. In Guide to Good Practice for Sacrifice; AENOR: Madrid, Spain, 2016; Available online: www.mapa.gob.es/en/pesca/temas/calidad-seguridad-alimentaria/pisciculturaguiadepracticascorrectasparaelsacrificio_tcm38-291479.pdf (accessed on 20 January 2024).
- Martoja, R.; Martoja-Pierson, M. Técnicas de Histología Animal; Toray-Masson: Barcelona, Spain, 1970; p. 350. [Google Scholar]
- Ballesteros, N.A.; Castro, R.; Abos, B.; Rodríguez Saint-Jean, S.S.; Pérez-Prieto, S.I.; Tafalla, C. The pyloric caeca area is a major site for IgM+ and IgT+ B cell recruitment in response to oral vaccination in rainbow trout. PLoS ONE 2013, 8, e66118. [Google Scholar] [CrossRef]
- Løkka, G.; Austbø, L.; Falk, K.; Bromage, E.; Fjelldal, P.G.; Hansen, T.; Hordvic, I.; Koppang, E.O. Immune parameters in the intestine of wild and reared unvaccinated and vaccinated Atlantic salmon (Salmo salar L.). Dev. Comp. Immunol. 2014, 47, 6–16. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; La Patra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Estensoro, I.; Calduch-Giner, J.A.; Kaushik, S.; Pérez-Sánchez, J.; Sitjà-Bobadilla, A. Modulation of the IgM gene expression and IgM immunoreactive cell distribution by the nutritional background in gilthead sea bream (Sparus aurata) challenged with Enteromyxum leei (Myxozoa). Fish Shellfish Immunol. 2012, 33, 401–410. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Du, F.; Wang, H.; Ma, L. Concordance of RT-qPCR with immunohistochemistry and its beneficial role in breast cancer subtyping. Medicine 2023, 102, e35272. [Google Scholar] [CrossRef] [PubMed]
- Naya-Català, F.; Wiggers, G.A.; Piazzon, M.C.; López-Martínez, M.I.; Estensoro, I.; Calduch-Giner, J.A.; Martínez-Cuesta, M.C.; Requena, T.; Sitjà-Bobadilla, A.; Miguel, M.; et al. Modulation of gilthead sea bream gut microbiota by a bioactive egg white hydrolysate: Interactions between bacteria and host lipid metabolism. Front. Mar. Sci. 2021, 8, 698484. [Google Scholar] [CrossRef]
- Fuglem, B.; Jirillo, E.; Bjerkås, I.; Kiyono, H.; Nochi, T.; Yuki, Y.; Raida, M.; Fischer, U.; Koppang, E.O. Antigen-sampling cells in the salmonid intestinal epithelium. Dev. Comp. Immunol. 2010, 34, 768–774. [Google Scholar] [CrossRef]
- Bjørgen, H.; Koppang, E.O.; Gunnes, G.; Hordvik, I.; Moldal, T.; Kaldhusdal, M.; Dale, O.B. Ectopic epithelial cell clusters in salmonid intestines are associated with inflammation. J. Fish Dis. 2018, 41, 1031–1040. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Li, Y.; Xu, Z. The distribution and function of teleost IgT. Fish Shellfish Immunol. 2024, 144, 109281. [Google Scholar] [CrossRef]
- Buonocore, F.; Stocchi, V.; Nunez-Ortiz, N.; Randelli, E.; Gerdol, M.; Pallavicini, A.; Picchietti, S. Immunoglobulin T from sea bass (Dicentrarchus labrax L.): Molecular characterization, tissue localization and expression after nodavirus infection. BMC Mol. Biol. 2017, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Koppang, E.O.; Kvellestad, A.; Fischer, U. Fish mucosal immunity: Gill. In Mucosal Health in Aquaculture; Academic Press: Cambridge, MA, USA, 2015; pp. 93–133. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Yan, W.; Chang, M.; Yao, W.; Xu, Q.; Wang, X.; Gao, Q.; Nie, P. Ig heavy chain genes and their locus in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2010, 29, 594–599. [Google Scholar] [CrossRef]
- Torrecillas, S.; Terova, G.; Makol, A.; Serradell, A.; Valdenegro, V.; Gini, E.; Izquierdo, M.; Acosta, F.; Montero, D.; Wilson, B.A. Dietary phytogenics and galactomannan oligosaccharides in low fish meal and fish oil-based diets for European sea bass (Dicentrarchus labrax) juveniles: Effects on gut health and implications on in vivo gut bacterial translocation. PLoS ONE 2019, 14, e0222063. [Google Scholar] [CrossRef]
- Simó-Mirabet, P.; Piazzon, M.C.; Calduch-Giner, J.A.; Ortiz, Á.; Puyalto, M.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Sodium salt medium-chain fatty acids and Bacillus-based probiotic strategies to improve growth and intestinal health of gilthead sea bream (Sparus aurata). PeerJ 2017, 5, e4001. [Google Scholar] [CrossRef]
- Bjørgen, H.; Oaland, Ø.; Midtllyng, P.; Tafalla, C.; Krogdahl, Å.; Koppang, E.O. IgD-transcript positive cells suggest hypersensitivity in feed-related intestinal inflammation in the Atlantic salmon. Fish Shellfish Immunol. 2023, 132, 108469. [Google Scholar] [CrossRef]
- Seibel, H.; Krassilnikova, K.; Fichtner-Grabowski, F.T.; Rebl, A.; Schulz, C.; Hornburg, S.C. Interactions of plant-based feeding and handling stress on the expression of selected immune markers in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2022, 53, 4304–4315. [Google Scholar] [CrossRef]
- Habte-Tsion, H.M.; Hawkyard, M.; Sealey, W.M.; Bradshaw, D.; Meesala, K.M.; Bouchard, D.A. Effects of fishmeal substitution with mealworm meals (Tenebrio molitor and Alphitobius diaperinus) on the growth, physiobiochemical response, digesta microbiome, and immune genes expression of Atlantic salmon (Salmo salar). Aquac. Nutr. 2024, 1, 6618117. [Google Scholar] [CrossRef] [PubMed]
- Sayramoğlu, H.; Öztürk, R.; Ustaoglu, D.; Terzi, Y.; Yandi, I.; Kayis, S.; Capkin, E.; Altinok, I. Effects of black soldier fly meal feeding on rainbow trout gut microbiota, immune-related gene expression, and Lactococcus petauri resistance. J. Insects Food Feed. 2023, 10, 141–157. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Naya-Català, F.; Perera, E.; Palenzuela, O.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Genetic selection for growth drives differences in intestinal microbiota composition and parasite disease resistance in gilthead sea bream. Microbiome 2020, 8, 168. [Google Scholar] [CrossRef]
- Zwollo, P.; Ray, J.C.; Sestito, M.; Kiernan, E.; Wiens, G.D.; Kaattari, S.; StJacques, B.; Epp, L. B cell signatures of BCWD-resistant and susceptible lines of rainbow trout: A shift towards more EBF-expressing progenitors and fewer mature B cells in resistant animals. Dev. Comp. Immunol. 2015, 48, 1–12. [Google Scholar] [CrossRef]
- Zwollo, P.; Hennessey, E.; Moore, C.; Marancik, D.P.; Wiens, G.D.; Epp, L. A BCWD-resistant line of rainbow trout exhibits higher abundance of IgT+ B cells and heavy chain tau transcripts compared to a susceptible line following challenge with Flavobacterium psychrophilum. Dev. Comp. Immunol. 2017, 74, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, S.; Montero, D.; Torrecillas, S.; Serradell, A.; Acosta, F.; Haffray, P.; Hostins, B.; Fontanillas, R.; Allal, F.; Bajek, A.; et al. Genetically superior European sea bass (Dicentrarchus labrax) and nutritional innovations: Effects of functional feeds on fish immune response, disease resistance, and gut microbiota. Aquac. Rep. 2023, 33, 101747. [Google Scholar] [CrossRef]
- Liu, Y.; Moore, L.; Koppang, E.O.; Hordvik, I. Characterization of the CD3ζ, CD3γδ and CD3ε subunits of the T cell receptor complex in Atlantic salmon. Dev. Comp. Immunol. 2008, 32, 26–35. [Google Scholar] [CrossRef]
- Koppang, E.O.; Bjerkås, E.; Bjerkås, I.; Sveier, H.; Hordvik, I. Vaccination induces major histocompatibility complex class II expression in the Atlantic salmon eye. Scand. J. Immunol. 2003, 58, 9–14. [Google Scholar] [CrossRef]
- Koppang, E.O.; Fischer, U.; Moore, L.; Tranulis, M.A.; Dijkstra, J.M.; Köllner, B.; Aune, L.; Jirillo, E.; Hordvik, I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J. Anat. 2010, 217, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Picchietti, S.; Buonocore, F.; Guerra, L.; Belardinelli, M.C.; De Wolf, T.; Couto, A.; Fausto, A.M.; Saraceni, P.R.; Miccoli, A.; Scapigliati, G. Molecular and cellular characterization of European sea bass CD3ε+ T lymphocytes and their modulation by microalgal feed supplementation. Cell Tissue Res. 2021, 384, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Munang’andu, H.M.; Lock, E.J.; Krogdahl, Å. Gut health and vaccination response in pre-smolt Atlantic salmon (Salmo salar) fed black soldier fly (Hermetia illucens) larvae meal. Fish Shellfish Immunol. 2019, 86, 1106–1113. [Google Scholar] [CrossRef]
- Castro, C.; Couto, A.; Diógenes, A.F.; Corraze, G.; Panserat, S.; Serra, C.R.; Oliva-Teles, A. Vegetable oil and carbohydrate-rich diets marginally affected intestine histomorphology, digestive enzymes activities, and gut microbiota of gilthead sea bream juveniles. Fish Physiol. Biochem. 2019, 45, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Naya-Català, F.; Torrecillas, S.; Piazzon, M.C.; Sarih, S.; Calduch-Giner, J.; Fontanillas, R.; Hostins, B.; Sitjà-Bobadilla, A.; Acosta, F.; Pérez-Sánchez, J.; et al. Can the genetic background modulate the effects of feed additives? Answers from gut microbiome and transcriptome interactions in farmed gilthead sea bream (Sparus aurata) fed with a mix of phytogenics, organic acids or probiotics. Aquaculture 2024, 586, 740770. [Google Scholar] [CrossRef]
- Brown, T.D.; Hori, T.S.; Xue, X.; Ye, C.L.; Anderson, D.M.; Rise, M.L. Functional genomic analysis of the impact of camelina (Camelina sativa) meal on Atlantic salmon (Salmo salar) distal intestine gene expression and physiology. Mar. Biotechnol. 2016, 18, 418–435. [Google Scholar] [CrossRef]
- Powell, M.D.; Wright, G.M.; Burka, J.F. Morphological and distributional changes in the eosinophilic granule cell (EGC) population of the rainbow trout (Oncorhynchus mykiss walbaum) intestine following systemic administration of capsaicin and substance P. J. Exp. Zool. 1993, 266, 19–30. [Google Scholar] [CrossRef]
- Öztop, M. Density, Distribution and staining properties of eosinophilic granular cells in oscar fish (Astronotus ocellatus) intestine. J. Hellenic Vet. Med. Soc. 2022, 73, 4957–4964. [Google Scholar] [CrossRef]
- Cataldi, E.; Cataudella, S.; Monaco, G.; Rossi, A.; Tancioni, L. A study of the histology and morphology of the digestive tract of the sea-bream, Sparus aurata. J. Fish Biol. 1987, 30, 135–145. [Google Scholar] [CrossRef]
- El-Araby, D.A.; Amer, S.A.; Attia, G.A.; Osman, A.; Fahmy, E.M.; Altohamy, D.E.; Tolba, S.A. Dietary spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture 2022, 546, 737413. [Google Scholar] [CrossRef]
- Dawood, M.A. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Islam, S.M.; Siddik, M.A.; Sørensen, M.; Brinchmann, M.F.; Thompson, K.D.; Francis, D.S.; Vatsos, I.N. Insect meal in aquafeeds: A sustainable path to enhanced mucosal immunity in fish. Fish Shellfish Immunol. 2024, 150, 109625. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bruni, L.; Jaramillo-Torres, A.; Gajardo, K.; Kortner, T.M.; Krogdahl, Å. Differential response of digesta-and mucosa-associated intestinal microbiota to dietary insect meal during the seawater phase of Atlantic salmon. Anim. Microbiom. 2021, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Solé-Jiménez, P.; Naya-Català, F.; Piazzon, M.C.; Estensoro, I.; Calduch-Giner, J.À.; Sitjà-Bobadilla, A.; Van Mullem, D.; Pérez-Sánchez, J. Reshaping of gut microbiota in gilthead sea bream fed microbial and processed animal proteins as the main dietary protein source. Front. Mar. Sci. 2021, 8, 705041. [Google Scholar] [CrossRef]
- Zhang, J.; Geng, M.; Xiao, J.; Chen, L.; Cao, Y.; Li, K.; Yang, J.; Wei, X. Comparative analysis of T-cell immunity between Streptococcus agalactiae susceptible and resistant tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2024, 154, 109967. [Google Scholar] [CrossRef]







| Diets | ||
|---|---|---|
| Ingredients (%) | Control | Alternative |
| Fishmeal Super Prime | 15.00 | 7.50 |
| Feather meal hydrolysate | 7.50 | |
| Porcine blood meal | 5.00 | |
| Poultry meal | 10.00 | |
| Worm meal (Tenebrio molitor) | 7.50 | |
| Aminopro NT70–C. glutamicum | 7.50 | |
| Soy protein concentrate | 16.00 | |
| Wheat gluten | 13.60 | 3.00 |
| Corn gluten meal | 7.00 | 3.00 |
| Soybean meal 48 | 6.00 | 5.80 |
| Wheat meal | 14.23 | 15.63 |
| Faba beans (low tannins) | 8.00 | 8.00 |
| Fish oil | 7.00 | |
| Salmon oil | 4.00 | |
| Algae oil (Veramaris®) 1 | 3.30 | |
| Rapeseed oil | 7.70 | 3.90 |
| Poultry fat | 2.85 | |
| Vitamin and mineral premix 2 | 1.00 | 1.00 |
| Proximate composition (% feed) | Control | Alternative |
| Crude protein | 43.0 | 46.0 |
| Crude fat | 18.0 | 18.0 |
| Fiber | 1.8 | 1.2 |
| Starch | 15.6 | 14.4 |
| Ash | 5.7 | 6.0 |
| Fatty acids (%fat) | Control | Alternative |
| Myristic acid (C14:0) | 0.5 | 0.3 |
| Palmitic acid (C16:0) | 1.9 | 2.4 |
| Stearic acid (C18:0) | 0.4 | 0.5 |
| Oleic acid (C18:1n-9) | 6.5 | 6.3 |
| Linoleic acid (LNA, C18:2n-6) | 2.1 | 2.6 |
| α-Linolenic acid (ALA, C18:3n-3) | 0.8 | 0.8 |
| Arachidonic acid (ARA, C20:4n-6) | 0.1 | 0.1 |
| Eicosapentaenoic acid (EPA, C20:5n-3) | 1.4 | 0.7 |
| Docosahexaenoic acid (DHA, 22:6n-3) | 0.8 | 1.5 |
| Probe | Accession No. | Target Region (bp) | Catalog No. | |
|---|---|---|---|---|
| Target | IgT | KX599200.1 | 681–1608 | 1270141 |
| IgM | JQ811851.4 | 751–1424 | 1573471 | |
| Control | DapB (negative) | EF191515 | 414–862 | 310043 |
| PPIB (positive) | NM_001140870 | 20–934 | 494421 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdeljaouad, S.; Sarmiento, P.; Ginés, R.; Duque, G.; Castro, P.L. Impact of Novel Diets on the Distribution of Mucosal Immune Cells in the Digestive System of High-Growth Genetically Selected Gilthead Seabream (Sparus aurata) in a Long-Term Feeding Trial. Fishes 2025, 10, 396. https://doi.org/10.3390/fishes10080396
Abdeljaouad S, Sarmiento P, Ginés R, Duque G, Castro PL. Impact of Novel Diets on the Distribution of Mucosal Immune Cells in the Digestive System of High-Growth Genetically Selected Gilthead Seabream (Sparus aurata) in a Long-Term Feeding Trial. Fishes. 2025; 10(8):396. https://doi.org/10.3390/fishes10080396
Chicago/Turabian StyleAbdeljaouad, Sirine, Paula Sarmiento, Rafael Ginés, Gabriela Duque, and Pedro L. Castro. 2025. "Impact of Novel Diets on the Distribution of Mucosal Immune Cells in the Digestive System of High-Growth Genetically Selected Gilthead Seabream (Sparus aurata) in a Long-Term Feeding Trial" Fishes 10, no. 8: 396. https://doi.org/10.3390/fishes10080396
APA StyleAbdeljaouad, S., Sarmiento, P., Ginés, R., Duque, G., & Castro, P. L. (2025). Impact of Novel Diets on the Distribution of Mucosal Immune Cells in the Digestive System of High-Growth Genetically Selected Gilthead Seabream (Sparus aurata) in a Long-Term Feeding Trial. Fishes, 10(8), 396. https://doi.org/10.3390/fishes10080396








