Identification of Suitable Habitats for Threatened Elasmobranch Species in the OSPAR Maritime Area
Abstract
1. Introduction
2. Materials and Methods
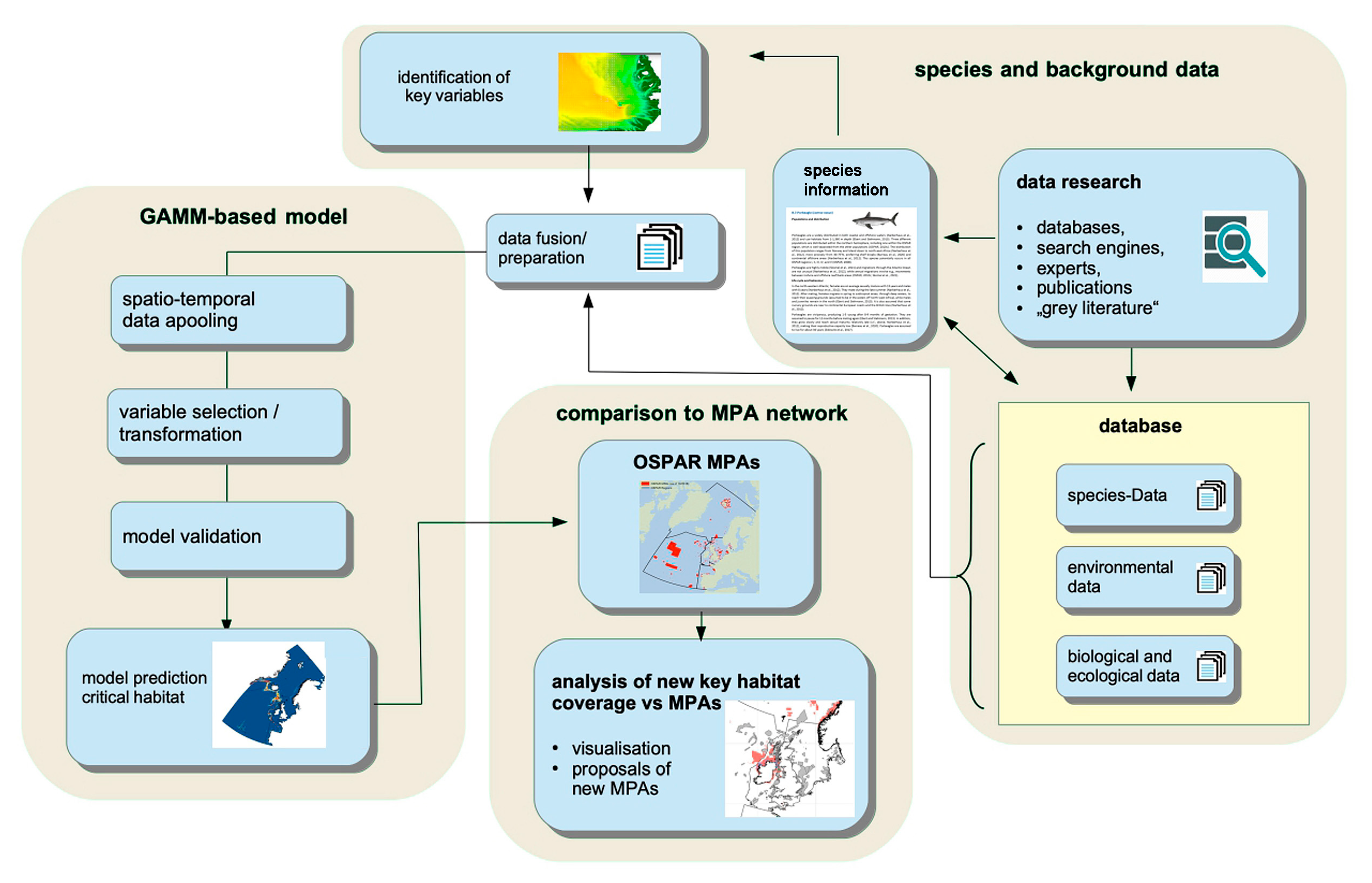
2.1. General Approach and Definitions
2.2. Species Data: Sources, Preparation, and Suitability for Modelling
2.3. Definition and Validation of Suitable Habitats
2.4. Predictors: Sources and Preparation
2.5. Statistical Methods
3. Results
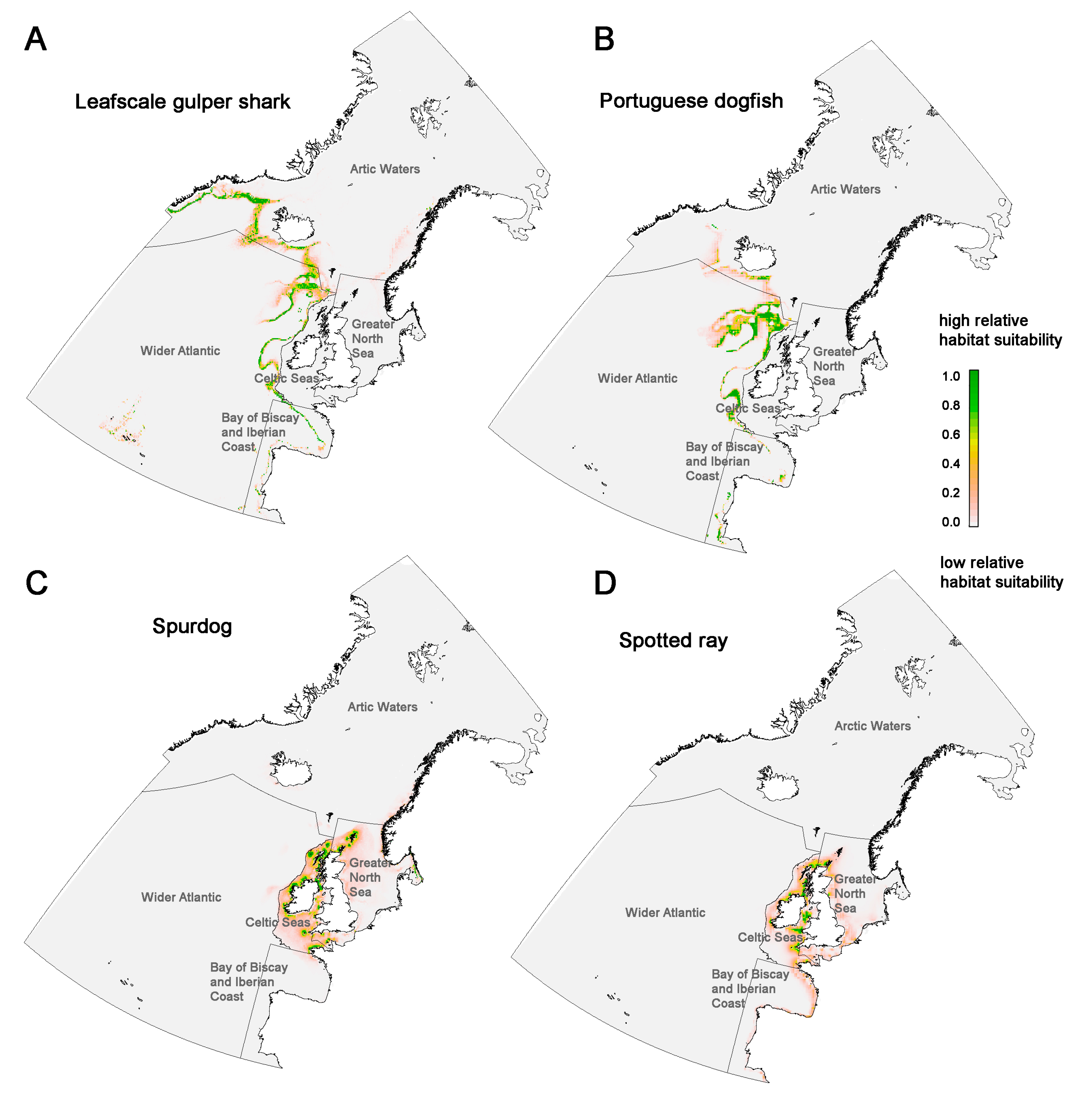
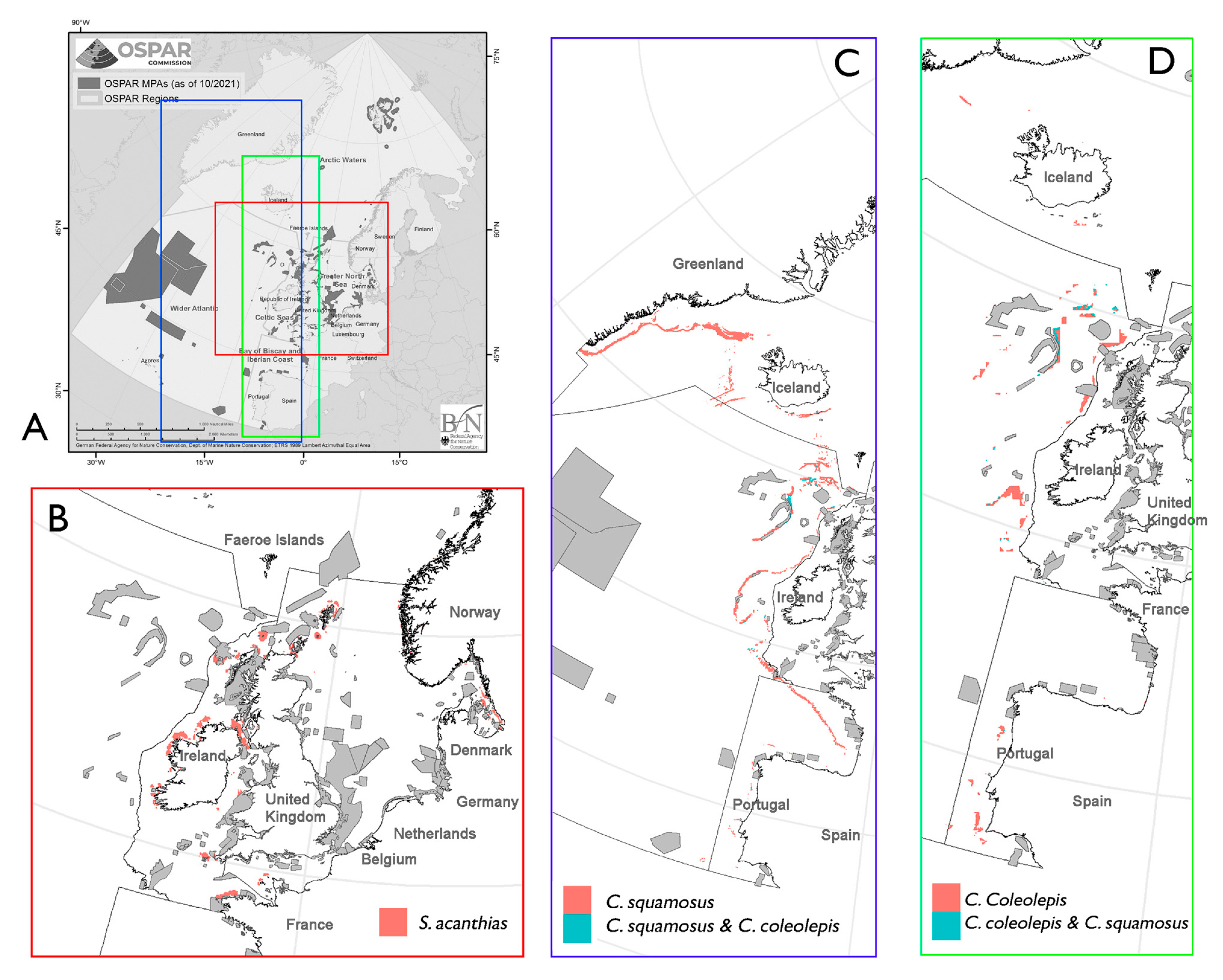
3.1. Leafscale Gulper Shark (Centrophorus squamosus)
3.2. Portuguese Dogfish (Centroscymnus coelolepis)
3.3. Spurdog/Spiny Dogfish (Squalus acanthias)
3.4. Spotted Ray (Raja montagui)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geelhoed, S.C.V.; Authier, M.; Pigeault, R.; Gilles, A.; Carlström, J.; Evans, P.; Haelters, J.; Hammond, P.; Louzao, M.; Rogan, E.; et al. Abundance and Distribution of Cetaceans; OSPAR Commission: London, UK, 2023. [Google Scholar]
- Hooker, S.K.; Canadas, A.M.; Hyrenbach, K.D.; Corrigan, C.; Polovina, J.J.; Reeves, R.R. Making Protected Area Networks Effective for Marine Top Predators. Endanger. Species Res. 2011, 13, 203–218. [Google Scholar] [CrossRef]
- Blasco, G.D.; Ferraro, D.M.; Cottrell, R.S.; Halpern, B.S.; Froehlich, H.E. Substantial Gaps in the Current Fisheries Data Landscape. Front. Mar. Sci. 2020, 7, 612831. [Google Scholar] [CrossRef]
- OSPAR Commission North-East Atlantic Environment Strategy 2030. 2021. Available online: https://www.ospar.org/convention/strategy (accessed on 22 July 2025).
- Mercker, M.; Markones, N.; Borkenhagen, K.; Schwemmer, H.; Wahl, J.; Garthe, S. An Integrated Framework to Estimate Seabird Population Numbers and Trends. Jour. Wild. Mgmt. 2021, 85, 751–771. [Google Scholar] [CrossRef]
- Mercker, M.; Müller, M.; Werner, T.; Hennicke, J. Identification of Key Habitats of Bowhead and Blue Whales in the OSPAR Area of the North-East Atlantic—A Modelling Approach towards Effective Conservation. J. Mar. Sci. Eng. 2024, 12, 1445. [Google Scholar] [CrossRef]
- Mendel, B.; Schwemmer, P.; Peschko, V.; Müller, S.; Schwemmer, H.; Mercker, M.; Garthe, S. Operational Offshore Wind Farms and Associated Ship Traffic Cause Profound Changes in Distribution Patterns of Loons (Gavia spp.). J. Environ. Manag. 2019, 231, 429–438. [Google Scholar] [CrossRef]
- Lindén, A.; Maentyniemi, S. Using the Negative Binomial Distribution to Model Overdispersion in Ecological Count Data. Ecology 2011, 92, 1414–1421. [Google Scholar] [CrossRef]
- Wood, S. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman & Hall/CRC: New York, USA, 2017. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized Linear Mixed Models: A Practical Guide for Ecology and Evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; McPherson, J.M.; Araujo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Daniel Kissling, W.; et al. Methods to Account for Spatial Autocorrelation in the Analysis of Species Distributional Data: A Review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Saveliev, A.A. Spatial, Temporal and Spatial-Temporal Ecological Data Analysis with R-INLA; Highland Statistics Ltd.: Newburgh, UK, 2017; Volume I–II. [Google Scholar]
- IUCN The IUCN Red List of Threatened Species, Version 2024-1; IUCN: Gland, Switzerland, 2024.
- Coulon, N.; Elliott, S.; Teichert, N.; Auber, A.; McLean, M.; Barreau, T.; Feunteun, E.; Carpentier, A. Northeast Atlantic Elasmobranch Community on the Move: Functional Reorganization in Response to Climate Change. Glob. Chang. Biol. 2024, 30, e17157. [Google Scholar] [CrossRef]
- Elliott, S.A.M.; Carpentier, A.; Feunteun, E.; Trancart, T. Distribution and Life History Trait Models Indicate Vulnerability of Skates. Prog. Oceanogr. 2020, 181, 102256. [Google Scholar] [CrossRef]
- Ebert, D.A.; Stehmann, M.F.W. Sharks, Batoids and Chimaeras of the North Atlantic; FAO: Roma, Italy, 2013. [Google Scholar]
- Figueiredo, I.; Moura, T.; Neves, A.; Gordo, L.S. Reproductive Strategy of Leafscale Gulper Shark Centrophorus squamosus and the Portuguese Dogfish Centroscymnus coelolepis on the Portuguese Continental Slope. J. Fish Biol. 2008, 73, 206–225. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, C.; Sánchez, F. Is Centrophorus squamosus a Highly Migratory Deep-Water Shark? Deep Sea Res. Part I: Oceanogr. Res. Pap. 2014, 92, 1–10. [Google Scholar] [CrossRef]
- Moura, T.; Jones, E.; Clarke, M.W.; Cotton, C.F.; Crozier, P.; Daley, R.K.; Diez, G.; Dobby, H.; Dyb, J.E.; Fossen, I.; et al. Large-Scale Distribution of Three Deep-Water Squaloid Sharks: Integrating Data on Sex, Maturity and Environment. Fish. Res. 2014, 157, 47–61. [Google Scholar] [CrossRef]
- Veríssimo, A.; McDowell, J.R.; Graves, J.E. Genetic Population Structure and Connectivity in a Commercially Exploited and Wide-Ranging Deepwater Shark, the Leafscale Gulper (Centrophorus squamosus). Mar. Freshw. Res. 2012, 63, 505–512. [Google Scholar] [CrossRef]
- Narberhaus, I.; Krause, J.; Bernitt, U. Bedrohte Biodiversität in Der Deutschen Nord- Und Ostsee: Empfindlichkeiten Gegenüber Anthropogenen Nutzungen Und Den Effekten Des Klimawandels; Bundesamt für Naturschutz: Bonn, Germany, 2012. [Google Scholar]
- Thorburn, J.; Neat, F.; Bailey, D.M.; Noble, L.R.; Jones, C.S. Winter Residency and Site Association in the Critically Endangered North East Atlantic Spurdog Squalus acanthias. Mar. Ecol. Prog. Ser. 2015, 526, 113–124. [Google Scholar] [CrossRef]
- Zidowitz, H.; Kaschner, C.; Magath, V.; Thiel, R.; Weigmann, S.; Thiel, R. Gefährdung Und Schutz Der Haie Und Rochen in Den Deutschen Meeresgebieten Der Nord- Und Ostsee: BfN-Skripten 450; BfN: Bonn, Germany, 2017. [Google Scholar]
- Ellis, J.R.; Milligan, S.P.; Readdy, L.; Taylor, N.; Brown, M.J. Spawning and Nursery Grounds of Selected Fish Species in UK Waters; CEFAS: Lowestoft, UK, 2012. [Google Scholar]
- ICES. Report of the Working Group on Elasmobranch Fishes (WGEF). In ICES Scientific Reports; ICES: Copenhagen, Denmark, 2023; Volume 5, p. 837. [Google Scholar] [CrossRef]
- EU Council Regulation. Council Regulation (EU) 2022/109 of 27 January 2022 Fixing for 2022 the Fishing Opportunities for Certain Fish Stocks and Groups of Fish Stocks, Applicable in Union Waters and for Union Fishing Vessels in Certain Non-Union Waters; Council of the European Union: Brussels, Belgium, 2022; Document 32022R0109. [Google Scholar]
- OSPAR. OSPAR List of Threatened and/or Declining Species and Habitats; Agreement 2008–6, updated 2021; OSPAR: London, UK, 2021. [Google Scholar]
- ICES. ICES Roadmap for Bycatch of Endangered, Threatened, and Protected (ETP) Species; ICES Convention, policies, and strategy; ICES: Copenhagen, Denmark, 2022; 48p. [Google Scholar] [CrossRef]
- ICES/DATRAS International Council for the Exploration of the Sea (ICES) DATRAS: Database of Trawl Surveys with Access to Standard Data Products. Available online: https://www.ices.dk/data/data-portals/Pages/DATRAS.aspx (accessed on 22 July 2025).
- NMDC. Norway Institute of Marine Research (IMR) Bottom Trawl Survey Data (Norwegian Marine Data Centre—NMDC). Available online: http://metadata.nmdc.no/metadata-api/landingpage/15ce748250a85dda02e6e4362552f0b1 (accessed on 22 July 2025).
- Cornou, A.-S.; Dimeet, J.; Tetard, A.; Gaudou, O.; Quinio-Scavinner, M.; Fauconnet, L.; Dube, B.; Rochet, M.-J. Observations à Bord Des Navires de Pêche Professionnelle. Bilan de l’échantillonnage 2013. Ifremer 2015. [Google Scholar] [CrossRef]
- Solmundsson, J. MFRI Survey Data from Icelandic Waters—Marine and Freshwater Research Institute (MFRI) Database; MFRI: Hafnarfjörður, Iceland, 2022. [Google Scholar]
- ICES. OSPAR Request for Scientific Knowledge on Selected Elasmobranch Species to Update the OSPAR List Assessments; ICES Special Request Advice; ICES: Copenhagen, Denmark, 2020. [Google Scholar]
- Guillera-Arroita, G. Modelling of Species Distributions, Range Dynamics and Communities under Imperfect Detection: Advances, Challenges and Opportunities. Ecography 2017, 40, 281–295. [Google Scholar] [CrossRef]
- Elliott, S.A.M.; Milligan, R.J.; Heath, M.R.; Turrell, W.R.; Bailey, D.M. Disentangling Habitat Concepts for Demersal Marine Fish Management. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-36859-7. [Google Scholar]
- Elith, J.; Philipps, S.; Hastie, T.; Dudik, M.; Chee, Y.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Osborne, J. Notes on the Use of Data Transformations. Pract. Assess. Res. Eval. 2002, 8, 6. [Google Scholar]
- Schneider, D.C. Seabirds and Fronts: A Brief Overview. Polar Res. 1990, 8, 17–21. [Google Scholar] [CrossRef]
- Arkhipkin, A.; Brickle, P.; Laptikhovsky, V. Links between Marine Fauna and Oceanic Fronts on the Patagonian Shelf and Slope. Arquipélago. Life Mar. Sci. 2013, 30, 19–37. [Google Scholar]
- Bost, C.A.; Cotté, C.; Bailleul, F.; Cherel, Y.; Charrassin, J.B.; Guinet, C.; Ainley, D.G.; Weimerskirch, H. The Importance of Oceanographic Fronts to Marine Birds and Mammals of the Southern Oceans. J. Mar. Syst. 2009, 78, 363–376. [Google Scholar] [CrossRef]
- Zuur, A.F.; Saveliev, A.A.; Ieno, E.N. Zero Inflated Models and Gerneralized Linear Mixed Models Withh R; Highland Statistics Ltd.: Newburgh, UK, 2012. [Google Scholar]
- Waggitt, J.J.; Evans, P.G.H.; Andrade, J.; Banks, A.N.; Boisseau, O.; Bolton, M.; Bradbury, G.; Brereton, T.; Camphuysen, C.J.; Durinck, J.; et al. Distribution Maps of Cetacean and Seabird Populations in the North-East Atlantic. J. Appl. Ecol. 2020, 57, 253–269. [Google Scholar] [CrossRef]
- Korner-Nievergelt, F.; Roth, T.; von Felten, S.; Guelat, J.; Almasi, B.; Korner-Nievergelt, P. Bayesian Data Analysis in Ecology Using Linear Models with R, BUGS, and Stan; Elsevier: London, UK, 2015. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Tibshirani, R. The Lasso Method for Variable Selection in the Cox Model. Stat. Med. 1997, 16, 385–395. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Wood, S. Package ‘Mgcv.’ Package ‘mgcv’ version 1.7–29. 2015, Volume 1, p. 729. Available online: https://cran.r-project.org/package=mgcv (accessed on 22 July 2025).
- ICES. NEAFC/OSPAR Joint Request on the Status and Distribution of Deep-Water Elasmobranchs; ICES Special Request Advice 2020, Sr.2020.09; ICES: Copenhagen, Denmark, 2020. [Google Scholar]
- Compagno, L.J.V.; Dando, D.; Fowler, S. Field Guide to Sharks of the World; HarperCollins: London, UK, 2005. [Google Scholar]
- OSPAR. Background Document for Leafscale Gulper Shark: Centrophorus Squamosus; OSPAR Commission: London, UK, 2010. [Google Scholar]
- Heessen, H.J.; Daan, N.; Ellis, J.R. Fish Atlas of the Celtic Sea, North Sea, and Baltic Sea; KNNV Publishing, Wageningen Academic Publishers: Wageningen, The Netherlands, 2015. [Google Scholar]
- Werner, T.; Hennicke, J. A Proposed Method to Evaulate the Extent to Which Critical Habitats of OSPAR T&D Species Are Covered by the OSPAR MPA Network: Annex 1. In Proceedings of the OSPAR Convention for the Protection of the Marine Environment of the North-East Atlantic—ICG-POSH Meeting, Edinburgh, UK, 21–23 November 2017. [Google Scholar]
- Bisch, A. Improving Knowledge on Chondrichthyans Using Fisheries Dependent Data. Master‘s Thesis, Station Marine de Dinard (CRESCO), Muséum National d’Histoire Naturelle, Paris, France, 2020. [Google Scholar]
- OSPAR. Case Reports for the OSPAR List of Threatened and/or Declining Species and Habitats; OSPAR Commission: London, UK, 2008. [Google Scholar]
- Sguotti, C.; Lynam, C.P.; García-Carreras, B.; Ellis, J.R.; Engelhard, G.H. Distribution of Skates and Sharks in the North Sea: 112 Years of Change. Glob. Change Biol. 2016, 22, 2729–2743. [Google Scholar] [CrossRef]
- Bañón, R.; Piñeiro, C.; Casas, M. Biological Aspects of Deep-Water Sharks Centroscymnus Coelolepis and Centrophorus Squamosus in Galician Waters (North-Western Spain). J. Mar. Biol. Assoc. United Kingd. 2006, 86, 843–846. [Google Scholar] [CrossRef]
- Barreau, T.; Rodriguez-Cabello, C.; Batsleer, J.; Johnston, G. Workshop to Review and Update OSPAR Status Assessments for Stocks of Listed Sharks, Skates and Rays in Suppot of OSPAR (WKSTATUS): Volume Issue 71: ICES Scientific Reports; ICES CIEM: Copenhagen, Denmark, 2020. [Google Scholar]
- Girard, M.; Buit, M.-H.D. Reproductive Biology of Two Deep-Water Sharks from the British Isles, Centroscymnus Coelolepis and Centrophorus Squamosus (Chondrichthyes: Squalidae). J. Mar. Biol. Assoc. United Kingd. 1999, 79, 923–931. [Google Scholar] [CrossRef]
- Clarke, M.W.; Connolly, P.L.; Bracken, J.J. Aspects of Reproduction of the Deep Water Sharks Centroscymnus Coelolepis and Centrophorus Squamosus from West of Ireland and Scotland. J. Mar. Biol. Assoc. United Kingd. 2001, 81, 1019–1029. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, C.; González-Pola, C.; Sánchez, F. Migration and Diving Behavior of Centrophorus Squamosus in the NE Atlantic. Combining Electronic Tagging and Argo Hydrography to Infer Deep Ocean Trajectories. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 115, 48–62. [Google Scholar] [CrossRef]
- Rodríguez-Cabello, C.; Sánchez, F. Catch and Post-Release Mortalities of Deep-Water Sharks Caught by Bottom Longlines in the Cantabrian Sea (NE Atlantic). J. Sea Res. 2017, 130, 248–255. [Google Scholar] [CrossRef]
- Shark Trust (2009) Chapter 1: The British Isles. Part 1: Skates and Rays. An Illustrated Compendium of Sharks, Skates, Rays and Chimaera. Available online: http://www.sharktrust.org/fact-files (accessed on 22 July 2025).
- Mas, F.; Forselledo, R.; Domingo, A.; Pin, O.; Troncoso, P.; Errico, E.; Marquez, A.; Tanaka, S.; Weigmann, S. New Records and Range Extension of the Portuguese Dogfish Centroscymnus Coelolepis in the South-Western Atlantic Ocean, with Comments on Its Morphology. J. Fish Biol. 2020, 96, 601–616. [Google Scholar] [CrossRef]
- Stehlik, L.L.; NOAA. Spiny Dogfish, Squalus Acanthias, Life History and Habitat Characteristics: NOAA Technical Memorandum NMFS-NE-203: Essential Fish Habitat Source Document: Second Edition; U.S. Department of Commerce: Woods Hole, MA, USA, 2007. [Google Scholar]
- Dell’apa, A.; Pennino, M.G.; Bonzek, C.F. Modeling the Habitat Distribution of Spiny Dogfish (Squalus Acanthias), By Sex, in Coastal Waters of the Northeastern United States. Fish. Bull. 2017, 115, 89–100. [Google Scholar] [CrossRef]
- OSPAR commission Background Document for Spotted Ray: Raja Montagui; OSPAR Commission: London, UK, 2010.
- Dutkiewicz, A.; Müller, R.D.; O’Callaghan, S.; Jónasson, H. Census of Seafloor Sediments in the World’s Ocean. Geology 2015, 43, 795–798. [Google Scholar] [CrossRef]
 “N_total” (blue dots) represents the total number of hauls (both zero and positive), and
“N_total” (blue dots) represents the total number of hauls (both zero and positive), and  “N_obs” (red dots) refers to the number of positive hauls exclusively.
“N_obs” (red dots) refers to the number of positive hauls exclusively.
 “N_total” (blue dots) represents the total number of hauls (both zero and positive), and
“N_total” (blue dots) represents the total number of hauls (both zero and positive), and  “N_obs” (red dots) refers to the number of positive hauls exclusively.
“N_obs” (red dots) refers to the number of positive hauls exclusively.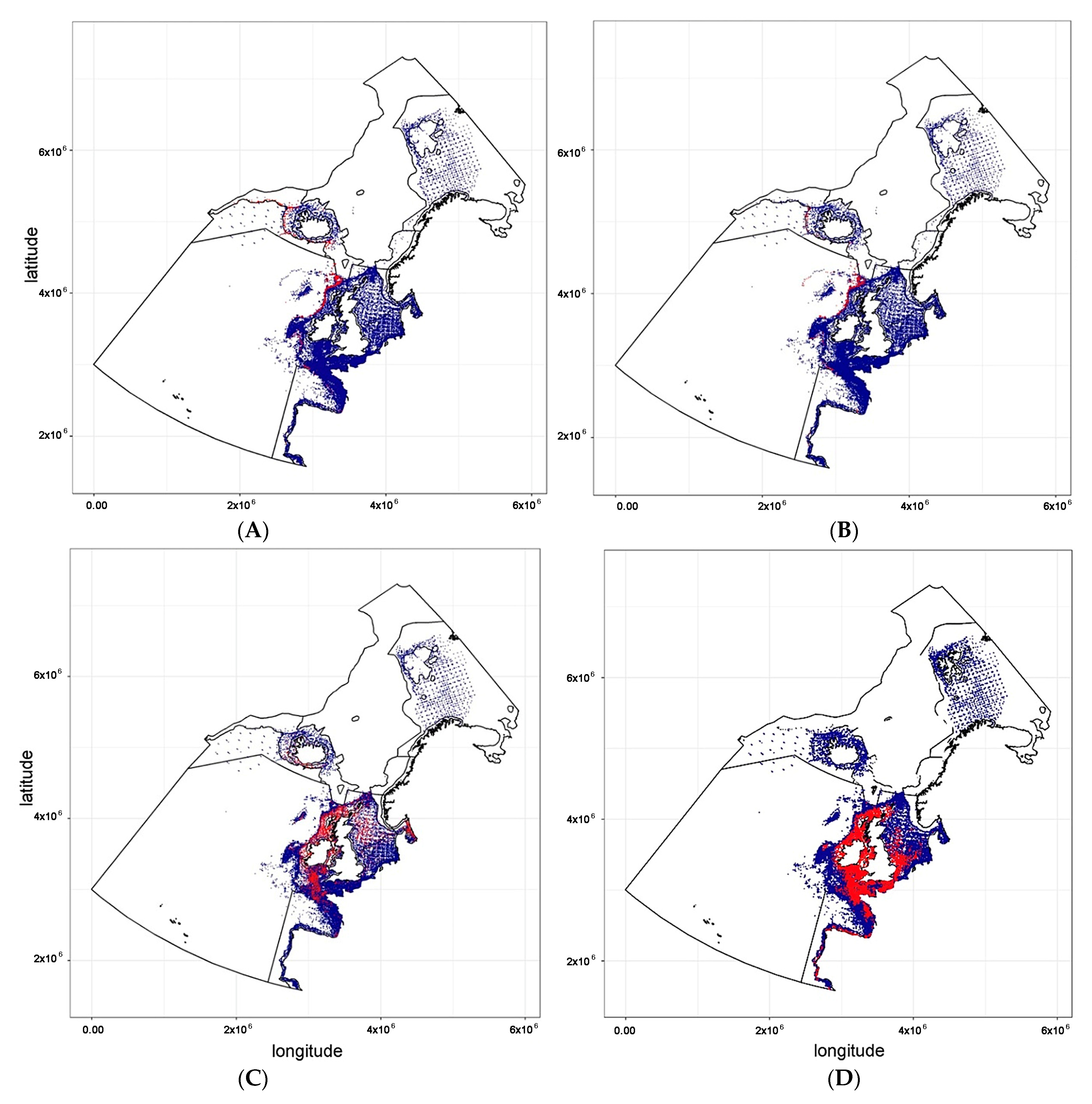
| Species | Total Hauls | Positive Hauls | Absences | %Zeros |
|---|---|---|---|---|
| Leafscale gulper shark | 329,664 | 11,451 | 318,213 | 96.5% |
| Portuguese dogfish | 329,311 | 6903 | 322,408 | 97.9% |
| Spurdog | 207,045 | 26,801 | 180,244 | 87.0% |
| Spotted ray | 213,973 | 23,140 | 190,833 | 89.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercker, M.; Müller, M.; Werner, T.; Hennicke, J. Identification of Suitable Habitats for Threatened Elasmobranch Species in the OSPAR Maritime Area. Fishes 2025, 10, 393. https://doi.org/10.3390/fishes10080393
Mercker M, Müller M, Werner T, Hennicke J. Identification of Suitable Habitats for Threatened Elasmobranch Species in the OSPAR Maritime Area. Fishes. 2025; 10(8):393. https://doi.org/10.3390/fishes10080393
Chicago/Turabian StyleMercker, Moritz, Miriam Müller, Thorsten Werner, and Janos Hennicke. 2025. "Identification of Suitable Habitats for Threatened Elasmobranch Species in the OSPAR Maritime Area" Fishes 10, no. 8: 393. https://doi.org/10.3390/fishes10080393
APA StyleMercker, M., Müller, M., Werner, T., & Hennicke, J. (2025). Identification of Suitable Habitats for Threatened Elasmobranch Species in the OSPAR Maritime Area. Fishes, 10(8), 393. https://doi.org/10.3390/fishes10080393






