Trace Metal Contamination in Commercial Fish from the Ecuadorian Amazon: Preliminary Health Risk Assessment in a Local Market
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Element Analysis
2.3. Health Risk Assessment
| Metal | Standard 1 | Standard 2 | ||
|---|---|---|---|---|
| Oral Reference Dose mg/kg/Day | References | Oral Reference Dose mg/kg/Day | References | |
| Aluminum (Al) | 0.29 | [42] | 1 | [47] |
| Arsenic (As) | 0.002 | [10] | 0.00006 | [48] |
| Cadmium (Cd) | 0.0008 | [10] | 0.001 | [49] |
| Chromium (Cr) | 0.05 | [43] | 0.0009 | [50] |
| Copper (Cu) | 10 | [45] | 0.04 | [51] |
| Iron (Fe) | 100 | [43] | 0.7 | [52] |
| Manganese (Mn) | 1 | [10] | 0.14 | [53] |
| Nickel (Ni) | 70 | [43] | 0.02 | [54] |
| Lead (Pb) | 0.3 | [44] | 0.00 | [55] |
| Zinc (Zn) | 100 | [45] | 0.3 | [56] |
| Mercury (Hg) | 0.0012 | [43] | 0.0003 | [57] |
2.4. Statistical Analysis
3. Results
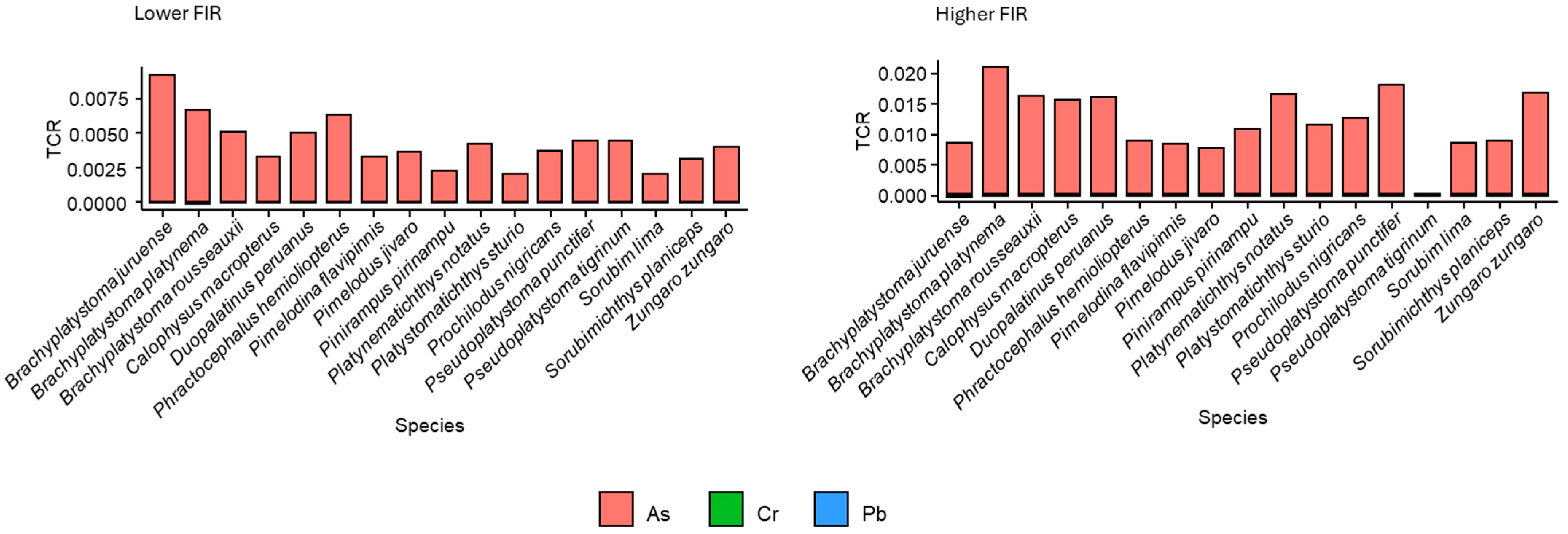
3.1. Health Risk Assessment Results
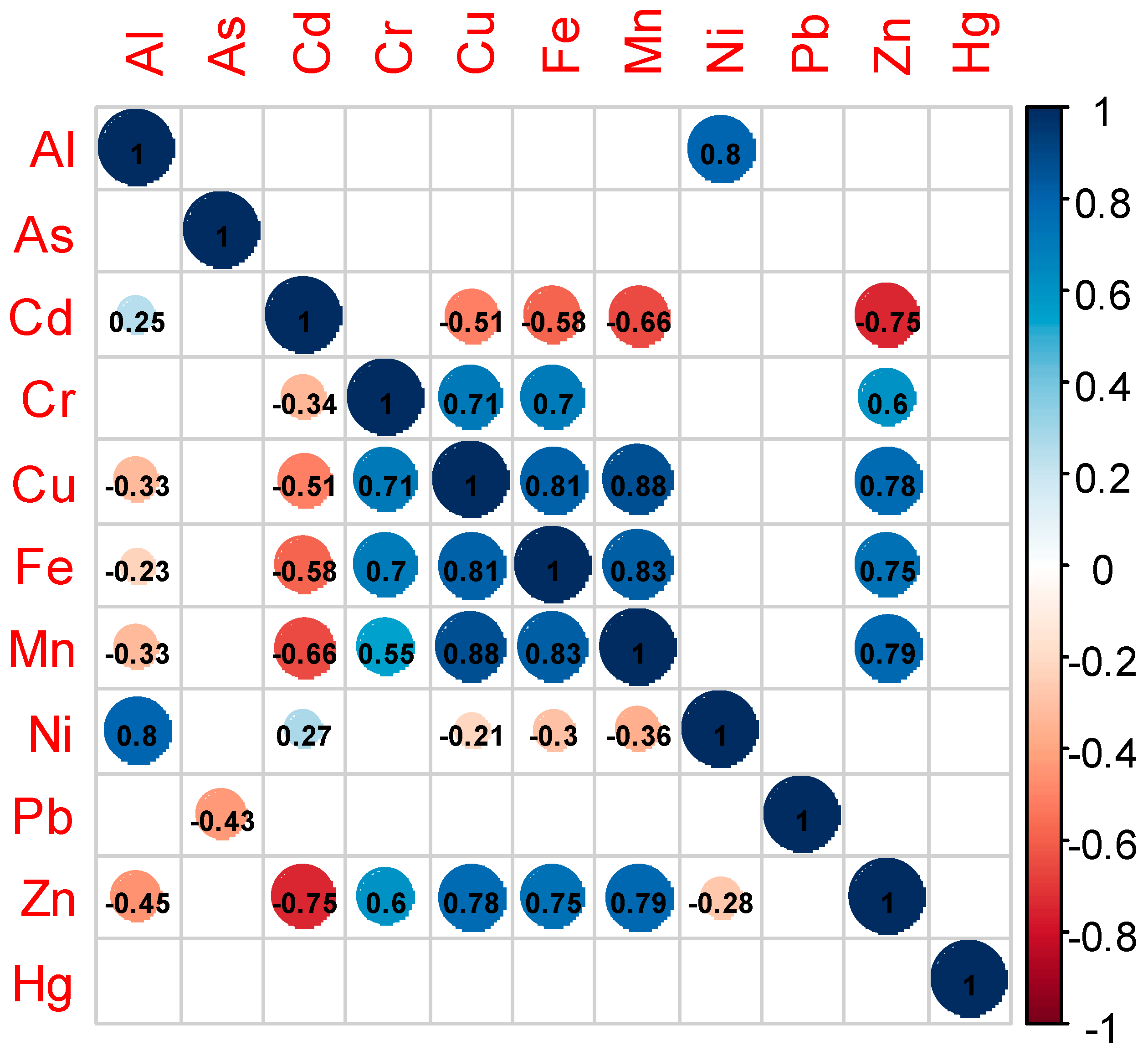
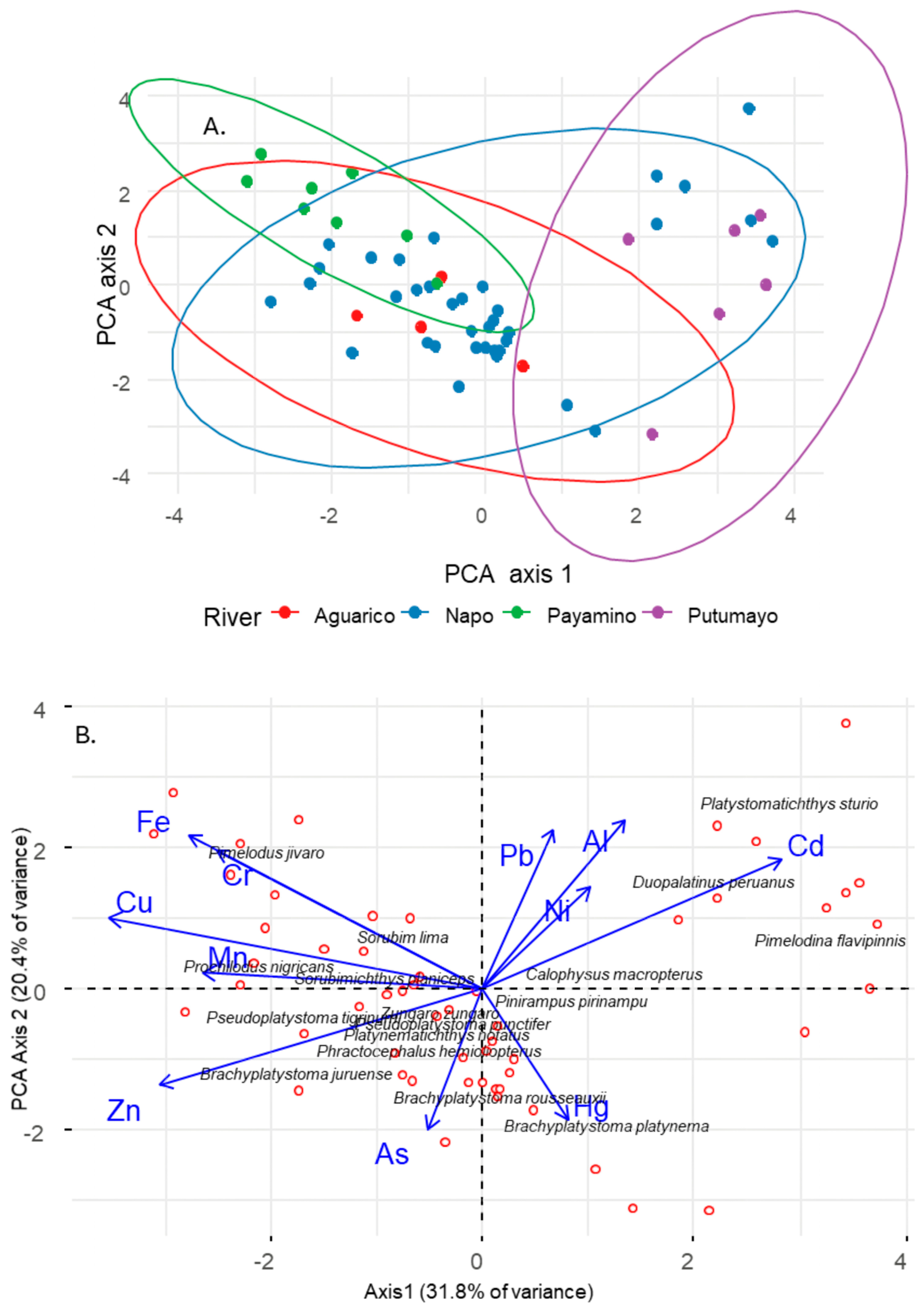
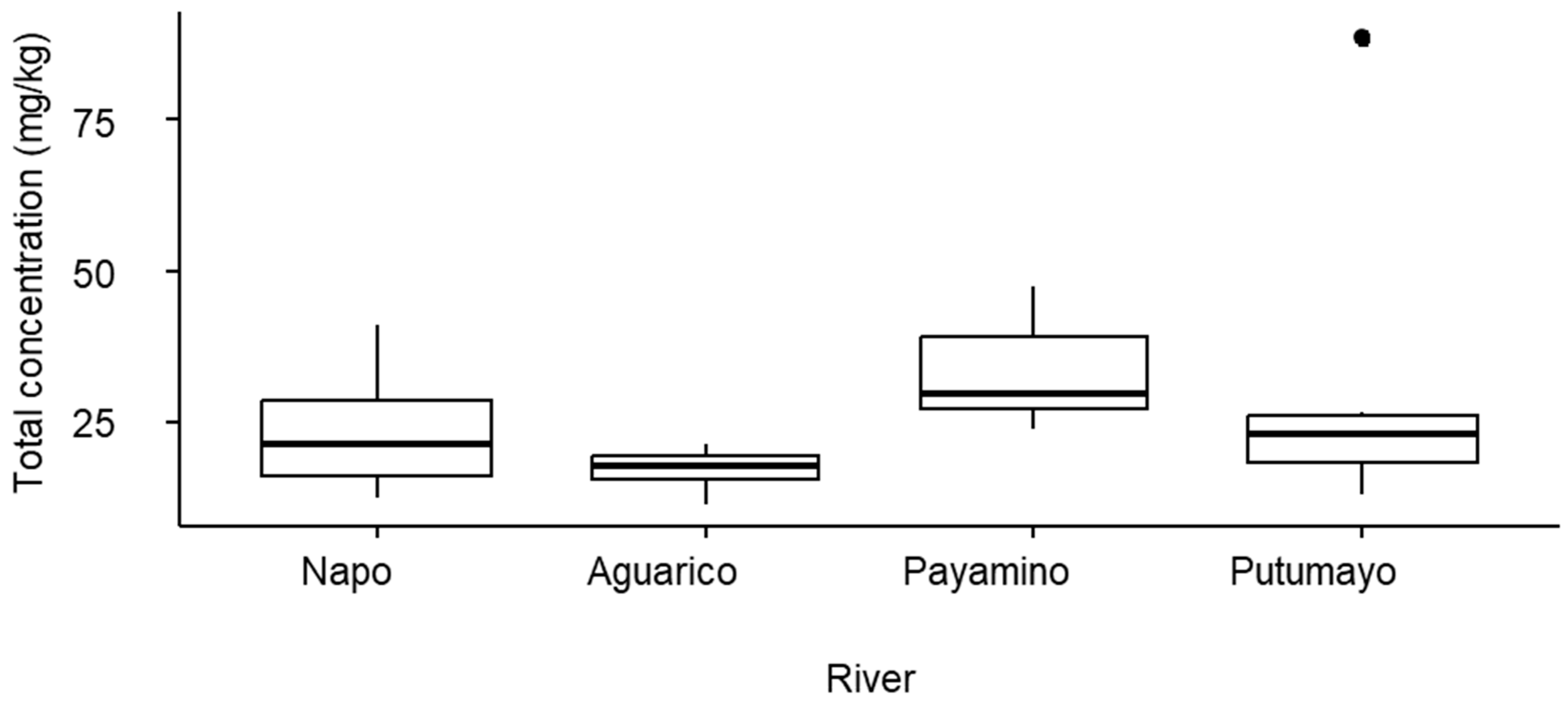
3.2. Factors Driving Trace Metal Concentrations
4. Discussion
4.1. Health Risk Assessment
4.2. Drivers of Trace Metal Concentrations in Fish
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Wei, S.; Ali, A.; Khan, I.; Sun, Q.; Xia, Q.; Wang, Z.; Han, Z.; Liu, Y.; Liu, S. Research Progress on Nutritional Value, Preservation and Processing of Fish—A Review. Foods 2022, 11, 3669. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The Contribution of Fisheries and Aquaculture to the Global Protein Supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. 4 The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ali, H. Trophic Transfer, Bioaccumulation, and Biomagnification of Non-Essential Hazardous Heavy Metals and Metalloids in Food Chains/Webs—Concepts and Implications for Wildlife and Human Health Webs—Concepts and Implications for Wildlife and Human Health. Hum. Ecol. Risk Assess. 2018, 25, 1353–1376. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The Toxicity of Cadmium and Resulting Hazards for Human Health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead Exposure and Cardiovascular Disease—A Systematic Review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of Drinking-Water by Arsenic in Bangladesh: A Public Health Emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar] [PubMed]
- FAO; OMS Codex Alimentarius. Norma General Para los Contaminantes y las Toxinas Presentes en los Alimentos y Piensos. CXS 193-1995. 1995. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193s.pdf (accessed on 1 May 2025).
- Dourson, M.L. Let the IRIS Bloom:Regrowing the Integrated Risk Information System (IRIS) of the U.S. Environmental Protection Agency. Regul. Toxicol. Pharmacol. 2018, 97, A4–A5. [Google Scholar] [CrossRef] [PubMed]
- Oluyemi, E.A.; Olabanji, I.O. Heavy Metals Determination in Some Species of Frozen Fish Sold at Ile-Ife Main Market, South West Nigeria. Ife J. Sci. 2011, 13, 355–362. [Google Scholar]
- Şirin, M.; Bayrak, E.Y.; Baltaş, H. Human Health Risk Assessment of Heavy Metals Accumulation in Different Genders and Tissues of Whiting Fish (Merlangius Merlangus Euxinus Nordmann, 1840) from Rize, Turkey. J. Food Compos. Anal. 2024, 127, 105971. [Google Scholar] [CrossRef]
- Jarosz-Krzemińska, E.; Mikołajczyk, N.; Adamiec, E. Content of Toxic Metals and As in Marine and Freshwater Fish Species Available for Sale in EU Supermarkets and Health Risk Associated with Its Consumption. J. Sci. Food Agric. 2021, 101, 2818–2827. [Google Scholar] [CrossRef]
- Hossain, M.K.; Parvin, A.; Parvin, A.; Islam, F.; Saha, B.; Kabir, M.A.; Shahjadee, U.F.; Hossain, A.; Moniruzzaman, M.; Suchi, P.D. Health Hazardous Index Based Trace Metals and Essential Acids Analysis of Size-Dependent Market Available Hilsa Fish, Bangladesh: Experimental and Chemometric Approaches. Mar. Pollut. Bull. 2024, 208, 116975. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Heavy Metals in Commercial Fish in New Jersey. Environ. Res. 2005, 99, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hajrić, D.; Smajlović, M.; Antunović, B.; Smajlović, A.; Alagić, D.; Tahirović, D.; Brenjo, D.; Članjak-Kudra, E.; Djedjibegović, J.; Porobić, A. Risk Assessment of Heavy Metal Exposure via Consumption of Fish and Fish Products from the Retail Market in Bosnia and Herzegovina. Food Control 2022, 133, 108631. [Google Scholar] [CrossRef]
- Valiente-Diaz, C.; Alonso-Llamazares, C.; Machado-Schiaffino, G.; Soto-López, V.; Garcia-Vazquez, E. A Study Investigating Heavy Metals in Salmonids Products Marketed in Spain. Food Control 2025, 168, 110891. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Luo, Y.; Guo, C.; Ma, X.; Fan, J.; Chen, J.; Geng, N. Occurrence, Accumulation, and Health Risks of Heavy Metals in Chinese Market Baskets. Sci. Total Environ. 2022, 829, 154597. [Google Scholar] [CrossRef]
- Reis, R.E.; Albert, J.S.; Di Dario, F.; Mincarone, M.M.M.; Petry, P.L.; Rocha, L.R. Fish Biodiversity and Conservation in South America. J. Fish Biol. 2016, 89, 12–47. [Google Scholar] [CrossRef]
- Azevedo-Santos, V.M.; Garcia-Ayala, J.R.; Fearnside, P.M.; Esteves, F.A.; Pelicice, F.M.; Laurance, W.F.; Benine, R.C. Amazon Aquatic Biodiversity Imperiled by Oil Spills. Biodivers. Conserv. 2016, 25, 2831–2834. [Google Scholar] [CrossRef]
- Moulatlet, G.M.; Yacelga, N.; Rico, A.; Mora, A.; Hauser-Davis, R.A.; Cabrera, M.; Capparelli, M.V. A Systematic Review on Metal Contamination Due to Mining Activities in the Amazon Basin and Associated Environmental Hazards. Chemosphere 2023, 339, 139700. [Google Scholar] [CrossRef]
- Swenson, J.J.; Carter, C.E.; Domec, J.-C.; Delgado, C.I. Gold Mining in the Peruvian Amazon: Global Prices, Deforestation, and Mercury Imports. PLoS ONE 2011, 6, e18875. [Google Scholar] [CrossRef] [PubMed]
- Yusta-García, R.; Orta-Martínez, M.; Mayor, P.; González-Crespo, C.; Rosell-Melé, A. Water Contamination from Oil Extraction Activities in Northern Peruvian Amazonian Rivers. Environ. Pollut. 2017, 225, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E. Bioaccumulation of Non-Essential Hazardous Heavy Metals and Metalloids in Freshwater Fish. Risk to Human Health. Environ. Chem. Lett. 2018, 16, 903–917. [Google Scholar] [CrossRef]
- Alcalá-Orozco, M.; Caballero-Gallardo, K.; Olivero-Verbel, J. Biomonitoring of Mercury, Cadmium and Selenium in Fish and the Population of Puerto Nariño, at the Southern Corner of the Colombian Amazon. Arch. Environ. Contam. Toxicol. 2020, 79, 354–370. [Google Scholar] [CrossRef]
- Echevarría, G.; Lujan, N.K.; Montoya, J.; Granda-Albuja, M.G.; Valdiviezo-Rivera, J.; Sánchez, F.; Cuesta, F.; Ríos-Touma, B. Abiotic and Biotic Factors Influencing Heavy Metals Pollution in Fisheries of the Western Amazon. Sci. Total Environ. 2024, 908, 168506. [Google Scholar] [CrossRef]
- Anticona, C.; Coe, A.; Bergdahl, I.A.; San Sebastian, M. Easier Said than Done: Challenges of Applying the Ecohealth Approach to the Study on Heavy Metals Exposure among Indigenous Communities of the Peruvian Amazon. BMC Public Health 2013, 13, 437. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Carranza-Lopez, L.; Caballero-Gallardo, K.; Ripoll-Arboleda, A.; Muñoz-Souza, D. Human Exposure and Risk Assessment Associated with Mercury Pollution in the Caqueta River, Colombian Amazon. Environ. Sci. Pollut. Res. 2016, 23, 20761–20771. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Valentini, J.; Fillion, M.; Souza Passos, C.J.; García, S.C.; Mergler, D.; Barbosa, F., Jr. Mercury Exposure and Oxidative Stress in Communities of the Brazilian Amazon. Sci. Total Environ. 2010, 408, 806–811. [Google Scholar] [CrossRef]
- Arantes, F.P.; Savassi, L.A.; Santos, H.B.; Gomes, M.V.T.; Bazzoli, N. Bioaccumulation of Mercury, Cadmium, Zinc, Chromium, and Lead in Muscle, Liver, and Spleen Tissues of a Large Commercially Valuable Cat Fi Sh Species from Brazil. Acad. Bras. Cienc. 2016, 88, 137–147. [Google Scholar] [CrossRef]
- Basta, P.C.; de Vasconcellos, A.C.S.; Hallwass, G.; Yokota, D.; Pinto, D.D.O.D.E.R.; de Aguiar, D.S.; de Souza, C.C.; Oliveira-da-Costa, M. Risk Assessment of Mercury-Contaminated Fish Consumption in the Brazilian Amazon: An Ecological Study. Toxics 2023, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Arsel, M. Oil and Conflict in the Ecuadorian Amazon. Eur. Rev. Lat. Am. Caribb. Stud. Rev. Eur. Estud. Latinoam. Caribe 2018, 106, 209–218. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Cuenca-Cumbicus, J.; D’Orio, G.; Flores-Toala, J.; Segovia-Cáceres, S.; Bonilla-Bonilla, A.; Straface, S. Gold Mining in the Amazon Region of Ecuador: History and a Review of Its Socio-Environmental Impacts. Land 2022, 11, 221. [Google Scholar] [CrossRef]
- Coronel Vargas, G.; Au, W.W.; Izzotti, A. Public Health Issues from Crude-Oil Production in the Ecuadorian Amazon Territories. Sci. Total Environ. 2020, 719, 134647. [Google Scholar] [CrossRef]
- Echevarría, G.; Nugra, F.; Zuña, L.; Jiménez-Prado, P. Las Pesquerías Continentales En La Amazonía Ecuatoriana: El Caso de Las Cuencas de Los Ríos Napo y Marañón. In Pesquería en la Amazonía Ecuatoriana. Estado Actual y Perspectivas de Manejo Sustentable; Jiménez-Prado, P., Carrillo-Moreno, C., Robles, M., Eds.; The Nature Conservancy: Quito, Ecuador, 2025; pp. 19–34. [Google Scholar]
- Mascia, C.; Capone, W.; Valenti, D. Determination of Heavy Metals in Animal Tissues. Farmaco 1990, 45 (Suppl. S6), 777–781. [Google Scholar] [CrossRef]
- Maier, E.A.; Demesmay, C.; Olle, M.; Lamotte, A.; Lagarde, F.; Heimburger, R.; Leroy, M.J.; Asfari, Z.; Muntau, H. European Comission. In The Certification of the Contents (Mass Fractions) of Arsenobetaine in Solution (CRM 626) and of Total Arsenic, Arsenobetaine Dimethylarsinic Acid in Tuna Fish Tissue (CRM 627); Publications Office of the European Union: Luxembourg, 1997; p. 65. [Google Scholar]
- Snell, J.; Embteborg, H.; Schimmel, H. Certification Report. In The Certification of the Mass Fractions of Elements in Fish Muscle; Certified Reference Material ERM®-BB422 2012; Publications Office of the European Union: Luxembourg, 2012; p. 68. [Google Scholar]
- Sirén, A. El Consumo de Pescado y Fauna Acuática Silvestre en la Amazonía Ecuatoriana; Organización de las Naciones Unidas Para la Alimentacion y la Agricu: Rome, Italy, 2011; ISBN 9789253069545. [Google Scholar]
- Isaac, V.J.; Almeida, M.; Giarrizzo, T.; Deus, C.; Vale, R.; Klein, G.; Begossi, A. Food Consumption as an Indicator of the Conservation of Natural Resources in Riverine Communities of the Brazilian Amazon. Agrar. Sci. 2015, 87, 2229–2242. [Google Scholar] [CrossRef]
- FAO/WHO. Evaluation of Certain Food Additives and Contaminants. Seventy-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives; Technical Report Series 966; World Health Organization: Rome, Italy, 2011; ISBN 9789241209663. [Google Scholar]
- National Research Council. Recommended Dietary Allowances, 10th ed; National Academies Press (US): Washington, DC, USA, 1989. [Google Scholar]
- USFDA. Food and Drug Administration, Guidance Document for Nickel in Shell Fish; DHHS/PHS/FDA/CFSAN/Office of Seafood: Washington, DC, USA, 1993.
- INEN. Norma Técnica Ecuatoriana NTE INEN 184. Atún y Bonito en Conserva. Requisitos. 2013. Available online: https://www.normalizacion.gob.ec/buzon/reglamentos/RTE-184.pdf (accessed on 1 May 2025).
- USEPA. Integrated Risk Information System (IRIS). United States Environmental Protection. Available online: https://www.epa.gov/iris (accessed on 1 May 2025.).
- USEPA. Provisional Peer Reviewed Toxicity Values for Aluminum (CASRN 7429-90-5); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2006.
- USEPA. Arsenic, Inorganic CASRN 7440-38-2|DTXSID4023886. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D278 (accessed on 1 May 2025).
- USEPA. Cadmium CASRN 7440-43-9|DTXSID1023940. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D141 (accessed on 1 May 2025).
- USEPA. Chromium(VI) CASRN 18540-29-9|DTXSID7023982. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D144 (accessed on 1 May 2025).
- USEPA. Copper CASRN 7440-50-8|DTXSID2023985. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr=368 (accessed on 1 May 2025).
- USEPA. Provisional Peer Reviewed Toxicity Values for Iron and Compounds (CASRN 7439-89-6); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2006.
- USEPA. Manganese CASRN 7439-96-5|DTXSID2024169. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D373 (accessed on 1 May 2025).
- USEPA. Nickel, Soluble Salts CASRN Various|DTXSID5024215. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D271 (accessed on 1 May 2025).
- USEPA. Lead and Compounds (Inorganic) CASRN 7439-92-1|DTXSID2024161. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D277 (accessed on 1 May 2025).
- USEPA. Zinc and Compounds CASRN 7440-66-6|DTXSID7035012. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D426 (accessed on 1 May 2025).
- USEPA. Mercury, Elemental CASRN 7439-97-6|DTXSID1024172. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr=370 (accessed on 1 May 2025).
- Freire, W.B.; Ramírez-Luzuriaga, M.J.; Belmont, P.; Mendieta, M.J.; Silva-Jaramillo, K.; Romero, N.; Sáenz, K.; Piñeiros, P.; Gómez, L.F.; Monge, R. Tomo I: Encuesta Nacional de Salud y Nutrición de la Población Ecuatoriana de cero a 59 Años. ENSANUT-ECU 2012. 2014. Available online: https://biblio.flacsoandes.edu.ec/libros/digital/55040.pdf (accessed on 1 May 2025).
- Ungureanu, E.L.; Mocanu, A.L.; Stroe, C.A.; Duță, D.E.; Mustățea, G. Assessing Health Risks Associated with Heavy Metals in Food: A Bibliometric Analysis. Foods 2023, 12, 3974. [Google Scholar] [CrossRef]
- USEPA Risk Assessment Guidance for Superfund: Volume I Human Health Evaluation Manual; USEPA: Washington, DC, USA, 1989.
- Sonone, S.D.; Jorvekar, S.B.; Naik, D.D.; Saharia, N.; Borkar, R.M. Assessment of Heavy Metal Contamination Risk in Dry Fish from India: A Comprehensive Study. Food Control 2025, 167, 110804. [Google Scholar] [CrossRef]
- Luo, X.-S.; Ding, J.; Xu, B.; Wang, Y.-J.; Li, H.-B.; Yu, S. Incorporating Bioaccessibility into Human Health Risk Assessments of Heavy Metals in Urban Park Soils. Sci. Total Environ. 2012, 424, 88–96. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 1 May 2025).
- Pouillot, R.; Delignette-Muller, M.L.; Kelly, D.L.; Denis, J.B. The Mc2d Package. 2016. Available online: https://cran.r-project.org/web/packages/mc2d/index.html (accessed on 1 May 2025).
- Nugra, F.; Echevarría, G.; Amaya, J.; Zuña, L.; Jiménez-Prado, P. Caracterización Socioeconómica de Las Pesquerías Continentales En La Amazonía Ecuatoriana. In Pesquería en la Amazonía Ecuatoriana. Estado Actual y Perspectivas de Manejo Sustentable; Jiménez-Prado, P., Carrillo-Moreno, C., Robles, M., Eds.; The Nature Conservancy: Quito, Ecuador, 2025; pp. 161–188. [Google Scholar]
- Martin, S.; Griswold, W. Human Health Effects of Heavy Metals. Environ. Sci. Technol. Briefs Citiz. 2009, 15, 1–6. [Google Scholar]
- Houck, K.; Sorensen, M.V.; Lu, F.; Alban, D.; Alvarez, K.; Hidobro, D.; Doljanin, C.; Ona, A.I. The Effects of Market Integration on Childhood Growth and Nutritional Status: The Dual Burden of Under-and Over-nutrition in the Northern Ecuadorian Amazon. Am. J. Hum. Biol. 2013, 25, 524–533. [Google Scholar] [CrossRef]
- Sirisangarunroj, P.; Monboonpitak, N.; Karnpanit, W.; Sridonpai, P.; Singhato, A.; Laitip, N.; Ornthai, N.; Yafa, C.; Judprasong, K. Toxic Heavy Metals and Their Risk Assessment of Exposure in Selected Freshwater and Marine Fish in Thailand. Foods 2023, 12, 3967. [Google Scholar] [CrossRef]
- Meschede, M.S.C.; Zagui, G.S.; Celere, B.S.; Machado, G.P.; Gomes-Silva, G.; Santos, D.V.; Sierra, J.; Nadal, M.; Domingo, J.L.; Segura-Muñoz, S.I. Human Exposure to Elements through Consumption of Raw and Cooked Fish in an Urban Region of the Central Brazilian Amazon Biome: Health Risks. Environ. Pollut. 2024, 347, 123728. [Google Scholar] [CrossRef]
- Gonzalez, D.J.X.; Arain, A.; Fernandez, L.E. Mercury Exposure, Risk Factors, and Perceptions among Women of Childbearing Age in an Artisanal Gold Mining Region of the Peruvian Amazon. Environ. Res. 2019, 179, 108786. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, F.M.; Caldas, E.D. Arsenic, Lead, Mercury and Cadmium: Toxicity, Levels in Breast Milk and the Risks for Breastfed Infants. Environ. Res. 2016, 151, 671–688. [Google Scholar] [CrossRef]
- Crisponi, G.; Fanni, D.; Gerosa, C.; Nemolato, S.; Nurchi, V.M.; Crespo-Alonso, M.; Lachowicz, J.I.; Faa, G. The Meaning of Aluminium Exposure on Human Health and Aluminium-Related Diseases. Biomol. Concepts 2013, 4, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-D.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344. [Google Scholar] [CrossRef]
- Risher, J.F.; Murray, H.E.; Prince, G.R. Organic Mercury Compounds: Human Exposure and Its Relevance to Public Health. Toxicol. Ind. Health 2002, 18, 109–160. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 245. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.; Uzairu, A.; Ekwumemgbo, P.A.; Okunola, O.J. Evaluation of Heavy Metals Concentration in Imported Frozen Fish Trachurus Murphyi Species Sold in Zaria Market, Nigeria. Am. J. Chem. 2014, 4, 137–154. [Google Scholar]
- de Queiroz, T.K.L.; Naka, K.S.; Mendes, L.D.C.D.S.; Costa, B.N.S.; Jesus, I.M.D.; Câmara, V.D.M.; Lima, M.D.O. Human Blood Lead Levels and the First Evidence of Environmental Exposure to Industrial Pollutants in the Amazon. Int. J. Environ. Res. Public Health 2019, 16, 3047. [Google Scholar] [CrossRef]
- Bonotto, D.M.; Wijersiri, B.; Vergotti, M.; da Silveira, E.G.; Goonetilleke, A. Assessing Mercury Pollution in Amazon River Tributaries Using a Bayesian Network Approach. Ecotoxicol. Environ. Saf. 2018, 166, 354–358. [Google Scholar] [CrossRef]
- Nyholt, K.; Jardine, T.D.; Villamarín, F.; Jacobi, C.M.; Hawes, J.E.; Campos-silva, J.V.; Srayko, S.; Magnusson, W.E. High Rates of Mercury Biomagni Fi Cation in Fi Sh from Amazonian Fl Oodplain-Lake Food Webs. Sci. Total Environ. 2022, 833, 155161. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; McCord, S.A.; Driscoll, C.T.; Todorova, S.; Wu, S.; Araújo, J.F.; Vega, C.M.; Fernandez, L.E. Mercury Contamination in Riverine Sediments and Fish Associated with Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. Int. J. Environ. Res. Public Health 2018, 15, 1584. [Google Scholar] [CrossRef] [PubMed]
- Waichman, A.V.; de Souza Nunes, G.S.; de Oliveira, R.; López-Heras, I.; Rico, A. Human Health Risks Associated to Trace Elements and Metals in Commercial Fish from the Brazilian Amazon. J. Environ. Sci. 2025, 148, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Sirén, A.; Valbo-Jørgensen, J. Quantifying Fish Catches and Fish Consumption in the Amazon Basin. Aquat. Ecosyst. Health Manag. 2022, 25, 59–71. [Google Scholar] [CrossRef]
- Rivera-parra, J.L.; Beate, B.; Diaz, X. Artisanal and Small Gold Mining and Petroleum Production as Potential Sources of Heavy Metal Contamination in Ecuador: A Call to Action. Int. J. Environ. Res. Public Health 2021, 18, 2794. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Yuki Takeda, P.; de Matos Macchi, B.; do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What Can We Learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef]
- Adler, R.M.; Davée, J.R.; Schudel, G.; Ghosh, S.; Godoy, J.M.; Silbergeld, E.K.; Lees, P.S.J.; Bergquist, B.A. Mercury Pollution in Amapá, Brazil: Mercury Amalgamation in Artisanal and Small-Scale Gold Mining or Land-Cover and Land-Use Changes? Earth Space Chem. 2018, 2, 441–450. [Google Scholar] [CrossRef]
- Tsai, T.-L.; Kuo, C.-C.; Pan, W.-H.; Chung, Y.-T.; Chen, C.-Y.; Wu, T.-N.; Wang, S.-L. The Decline in Kidney Function with Chromium Exposure Is Exacerbated with Co-Exposure to Lead and Cadmium. Kidney Int. 2017, 92, 710–720. [Google Scholar] [CrossRef]
- Fundación EcoCiencia. Amazon Conservation MAAP #221: Minería Ilegal en Áreas Naturales Protegidas de la Amazonía Ecuatoriana; Fundación EcoCiencia: Quito, Ecuador, 2024; Available online: https://www.maapprogram.org/es/mineria-areas-protegidas-ecuador/ (accessed on 1 May 2025).
- Dall’Agnol, R.; Sahoo, P.K.; Salomão, G.N.; de Araújo, A.D.M.; da Silva, M.S.; Powell, M.A.; Junior, J.F.; Ramos, S.J.; Martins, G.C.; da Costa, M.F. Soil-Sediment Linkage and Trace Element Contamination in Forested/Deforested Areas of the Itacaiúnas River Watershed, Brazil: To What Extent Land-Use Change Plays a Role? Sci. Total Environ. 2022, 828, 154327. [Google Scholar] [CrossRef]
- Sun, H.; Brocato, J.; Costa, M. Oral Chromium Exposure and Toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef]
- Bjørklund, G.; Chartrand, M.S.; Aaseth, J. Manganese Exposure and Neurotoxic Effects in Children. Environ. Res. 2017, 155, 380–384. [Google Scholar] [CrossRef]

| Species | River | N | Al | As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | Hg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brachyplatystoma juruense | Napo | 1 | Value | 5.44 | 0.14 | 0.01 | 0.04 | 0.60 | 4.79 | 0.09 | 0.00 | 0.03 | 6.88 | 1.49 |
| Brachyplatystoma platynema | Napo | 1 | Value | 19.94 | 0.04 | 0.01 | 0.02 | 0.35 | 4.76 | 0.12 | 0.00 | 0.01 | 4.52 | 3.44 |
| Brachyplatystoma platynema | Putumayo | 1 | Value | 12.32 | 0.17 | 0.03 | 0.01 | 0.09 | 0.02 | 0.01 | 0.01 | 0.07 | 0.21 | 0.20 |
| Brachyplatystoma rousseauxii | Napo | 4 | Mean | 8.54 | 0.08 | 0.01 | 0.02 | 0.35 | 2.49 | 0.08 | 0.00 | 0.04 | 5.78 | 2.08 |
| SD | 5.67 | 0.04 | 0.00 | 0.01 | 0.08 | 1.22 | 0.01 | 0.00 | 0.02 | 1.08 | 1.63 | |||
| Calophysus macropterus | Napo | 3 | Mean | 11.09 | 0.06 | 0.01 | 0.02 | 0.55 | 3.91 | 0.09 | 0.00 | 0.03 | 5.13 | 1.36 |
| SD | 11.86 | 0.01 | 0.00 | 0.01 | 0.15 | 2.80 | 0.04 | 0.00 | 0.03 | 1.16 | 0.57 | |||
| Calophysus macropterus | Putumayo | 3 | Mean | 22.96 | 0.04 | 0.03 | 0.01 | 0.14 | 0.01 | 0.01 | 0.01 | 0.09 | 0.15 | 0.62 |
| SD | 2.10 | 0.01 | 0.01 | 0.01 | 0.03 | 0.00 | 0.00 | 0.01 | 0.02 | 0.06 | 0.35 | |||
| Duopalatinus peruanus | Napo | 1 | Value | 21.44 | 0.08 | 0.04 | 0.03 | 0.15 | NA | 0.01 | 0.02 | 0.11 | 0.24 | NA |
| Phractocephalus hemioliopterus | Napo | 2 | Mean | 7.46 | 0.10 | 0.01 | 0.02 | 0.45 | 3.47 | 0.09 | 0.00 | 0.07 | 6.67 | 1.10 |
| SD | 2.14 | 0.04 | 0.01 | 0.00 | 0.01 | 0.51 | 0.02 | 0.00 | 0.07 | 0.82 | 0.34 | |||
| Pimelodina flavipinnis | Putumayo | 2 | Mean | 51.40 | 0.05 | 0.02 | 0.01 | 0.15 | 0.14 | 0.02 | 0.03 | 0.06 | 0.22 | 0.89 |
| SD | 50.16 | 0.02 | 0.00 | 0.01 | 0.01 | 0.09 | 0.00 | 0.02 | 0.00 | 0.04 | 0.42 | |||
| Pimelodus jivaro | Payamino | 8 | Mean | 20.44 | 0.05 | 0.01 | 0.04 | 0.77 | 6.81 | 0.18 | 0.01 | 0.08 | 4.56 | 0.06 |
| SD | 6.93 | 0.03 | 0.00 | 0.01 | 0.18 | 1.86 | 0.03 | 0.01 | 0.05 | 0.59 | 0.04 | |||
| Pinirampus pirinampu | Napo | 3 | Mean | 12.93 | 0.03 | 0.02 | 0.02 | 0.41 | 2.06 | 0.08 | 0.02 | 0.04 | 4.79 | 1.58 |
| SD | 8.73 | 0.02 | 0.03 | 0.01 | 0.31 | 2.46 | 0.06 | 0.01 | 0.04 | 4.47 | 2.23 | |||
| Platynematichthys notatus | Aguarico | 1 | Value | 7.90 | 0.05 | 0.01 | 0.02 | 0.68 | 2.49 | 0.17 | 0.00 | 0.04 | 6.04 | 1.03 |
| Platynematichthys notatus | Napo | 1 | Value | 6.94 | 0.08 | 0.01 | 0.02 | 0.49 | 3.01 | 0.09 | 0.00 | 0.04 | 3.98 | 0.61 |
| Platystomatichthys sturio | Napo | 4 | Mean | 27.26 | 0.03 | 0.04 | 0.02 | 0.23 | NA | 0.02 | 0.17 | 0.16 | 0.16 | NA |
| SD | 9.38 | 0.03 | 0.01 | 0.01 | 0.08 | NA | 0.00 | 0.28 | 0.09 | 0.03 | NA | |||
| Prochilodus nigricans | Napo | 3 | Mean | 11.46 | 0.06 | 0.01 | 0.02 | 0.63 | 5.50 | 0.41 | 0.01 | 0.04 | 6.09 | NA |
| SD | 2.37 | 0.02 | 0.00 | 0.01 | 0.05 | 1.67 | 0.31 | 0.00 | 0.03 | 1.30 | NA | |||
| Pseudoplatystoma punctifer | Napo | 4 | Mean | 10.01 | 0.07 | 0.01 | 0.01 | 0.43 | 3.07 | 0.11 | 0.00 | 0.08 | 6.77 | 0.38 |
| SD | 7.32 | 0.03 | 0.00 | 0.00 | 0.08 | 0.93 | 0.04 | 0.00 | 0.05 | 2.09 | 0.22 | |||
| Pseudoplatystoma tigrinum | Aguarico | 1 | Value | 4.83 | 0.07 | 0.01 | 0.02 | 0.70 | 5.27 | 0.11 | NA | NA | 9.56 | 0.84 |
| Sorubim lima | Napo | 2 | Mean | 13.80 | 0.03 | 0.01 | 0.02 | 0.62 | 4.53 | 0.14 | 0.00 | 0.07 | 4.84 | 0.26 |
| SD | 5.21 | 0.03 | 0.00 | 0.00 | 0.02 | 1.20 | 0.00 | 0.00 | 0.00 | 0.16 | 0.05 | |||
| Sorubimichthys planiceps | Napo | 1 | Value | 24.48 | 0.05 | 0.01 | 0.02 | 0.39 | 4.95 | 0.12 | 0.01 | NA | 10.8 | 0.42 |
| Zungaro zungaro | Aguarico | 2 | Mean | 5.24 | 0.04 | 0.01 | 0.02 | 0.47 | 3.03 | 0.08 | 0.00 | NA | 4.64 | 0.75 |
| SD | 2.99 | 0.00 | 0.00 | 0.01 | 0.18 | 1.64 | 0.02 | 0.00 | 0.04 | 0.33 | 0.59 | |||
| Zungaro zungaro | Napo | 6 | Mean | 10.73 | 0.07 | 0.01 | 0.02 | 0.49 | 3.73 | 0.11 | 0.01 | 0.04 | 5.03 | 0.67 |
| SD | 6.57 | 0.01 | 0.00 | 0.01 | 0.22 | 1.47 | 0.03 | 0.00 | 0.03 | 0.81 | 0.32 |
| Trace Element | DII | CDI | ||
|---|---|---|---|---|
| Min | Max | Min | Max | |
| Al | 0.0130 | 0.3482 | 0.00980 | 0.34824 |
| As | 0.0001 | 0.0010 | 0.00006 | 0.00096 |
| Cd | 0.00001 | 0.0003 | 0.00001 | 0.00028 |
| Cr | 0.00002 | 0.0003 | 0.00002 | 0.00027 |
| Cu | 0.00039 | 0.0051 | 0.00030 | 0.0052 |
| Fe | 0.00037 | 0.0461 | 0.00028 | 0.04617 |
| Mn | 0.000032 | 0.0027 | 0.00002 | 0.00279 |
| Ni | 0.0000013 | 0.0011 | 0.000001 | 0.00112 |
| Pb | 0.000085 | 0.00105 | 0.00006 | 0.00105 |
| Zn | 0.00044 | 0.07339 | 0.00033 | 0.07339 |
| Hg | 0.00016 | 0.01411 | 0.00012 | 0.01411 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, G.E.E.; Orellana, F.R.S.; Vega, R.E.Y.; Valdiviezo-Rivera, J.S.; Ríos-Touma, B.P. Trace Metal Contamination in Commercial Fish from the Ecuadorian Amazon: Preliminary Health Risk Assessment in a Local Market. Fishes 2025, 10, 392. https://doi.org/10.3390/fishes10080392
Díaz GEE, Orellana FRS, Vega REY, Valdiviezo-Rivera JS, Ríos-Touma BP. Trace Metal Contamination in Commercial Fish from the Ecuadorian Amazon: Preliminary Health Risk Assessment in a Local Market. Fishes. 2025; 10(8):392. https://doi.org/10.3390/fishes10080392
Chicago/Turabian StyleDíaz, Gabriela Elena Echevarría, Fernando Rafael Sánchez Orellana, Rafael Enrique Yunda Vega, Jonathan Santiago Valdiviezo-Rivera, and Blanca Patricia Ríos-Touma. 2025. "Trace Metal Contamination in Commercial Fish from the Ecuadorian Amazon: Preliminary Health Risk Assessment in a Local Market" Fishes 10, no. 8: 392. https://doi.org/10.3390/fishes10080392
APA StyleDíaz, G. E. E., Orellana, F. R. S., Vega, R. E. Y., Valdiviezo-Rivera, J. S., & Ríos-Touma, B. P. (2025). Trace Metal Contamination in Commercial Fish from the Ecuadorian Amazon: Preliminary Health Risk Assessment in a Local Market. Fishes, 10(8), 392. https://doi.org/10.3390/fishes10080392






