Abstract
The mountainous rivers of Central Asia host diverse ichthyofauna threatened by increasing anthropogenic pressures, particularly water pollution, abstraction, and hydropower development. This study provides valuable morphometric and ecological data for Schizothorax eurycephalus (snow trout) and Triplophysa ferganaensis (stone loach) in the Shakhimardan River basin, Uzbekistan. S. eurycephalus exhibited positive allometric growth, while T. ferganaensis showed negative near-isometric growth. The mean Fulton’s Condition Factor was 1.0 for S. eurycephalus and 0.7 for T. ferganaensis, with site-specific variations. Strong correlations among morphometric parameters, particularly length–height relationships, support non-invasive monitoring techniques. Dietary analysis revealed S. eurycephalus was predominantly herbivorous, with around 70% algae consumption. Early sexual maturity was observed in S. eurycephalus males, whereas T. ferganaensis showed no clear maturity signs, but swollen bellies suggested ongoing or recent reproductive activity. These baseline morphometric and ecological data establish a solid foundation for future ecological assessments, conservation strategies, and the design and monitoring of mitigation measures to address anthropogenic impacts in this vulnerable region.
Keywords:
ichthyology; snowtrout; snow barbel; Schizothoracinae; Shohimardon; Margilansay River; Syr Darya River Key Contribution:
This study provides valuable morphometric and ecological baseline data for S. eurycephalus and T. ferganaensis, including condition factor, length–height relationships, and data on sexual maturity.
1. Introduction
The mountains of Central Asia, particularly the Tien Shan mountains, feature unique riverine ecosystems and a diverse native fish fauna adapted to cold-water, steep-sloped high-altitude ecosystems [1]. Snow trout (Schizothorax Heckel 1838) and loach (Triplophysa Rendahl 1933) are widespread in the region. Schizothorax are valuable species for touristic (recreational) fishing and constitute a food source for local communities. Both genera fulfill important ecological roles [2,3]. However, Schizothorax and Triplophysa populations across the region are increasingly exposed to anthropogenic pressures, notably water abstraction for irrigational use and hydropower development, leading to deterioration and fragmentation of habitats [4,5,6,7,8]. Despite the ecological significance of many Central Asian fish species and their use as bioindicator for freshwater ecosystem health, detailed, species-specific morphometric, and ecological information remains limited (but see [7,9,10]).
Morphometric data, including relationships between fish length, weight, width, and height, are valuable for understanding fish condition and fundamental aspects of their ecology [11,12]. Incorporating multiple body dimensions can yield more robust assessments of fish condition than traditional length–weight-only metrics [11,12]. Alongside morphometrics, initial observations on ecological parameters, such as diet composition [13] and indicators of sexual maturity [14], including the identification of potential spawning or nursery areas, can provide critical insights into resource utilization, reproductive strategies, and habitat requirements. Collectively, such morphometric and ecological datasets underpin effective fisheries management and conservation.
The Aral Sea basin is home to three species of Schizothorax, each associated with a distinct sub-catchment—the Amu Darya, Syr Darya, and Zeravshan Rivers [3,15,16]. This study, conducted in a tributary system of the Syr Darya River, focuses on Schizothorax eurycephalus (Berg, 1932) and Triplophysa ferganaensis (Sheraliev and Peng, 2021) [7,17]. The objectives of this research are to (1) quantify the length, weight, width, and height relationships of the target species, and to (2) present ecological observations on diet composition and sexual maturity. Therefore, this work provides a crucial empirical basis for further applied studies and the development of ecologically sound conservation measures.
2. Materials and Methods
2.1. Study Area and Field Sampling
This study was conducted in the Shakhimardan River basin, located south of the Fergana Valley and flowing northward into the Syr Darya River. Specifically, sampling was conducted in the tributary network of the Shakhimardan exclave, Uzbekistan, including the Koksu and Aksu Rivers, as well as the Shakhimardan River (Figure 1). These rivers are characterized by fast-flowing, cold waters and rocky substrates, typical habitats of snow trout, S. eurycephalus, and stone loach, T. ferganaensis. These two species are the sole inhabitants of the study area [2,8].
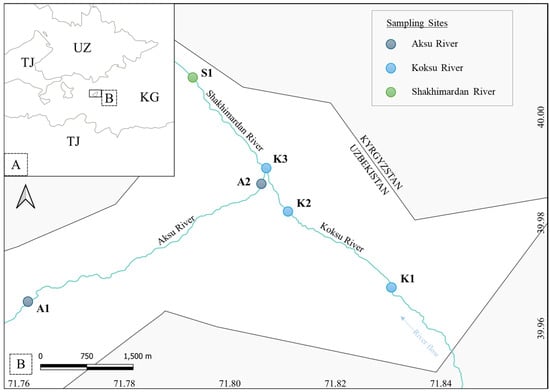
Figure 1.
Location of the study area (A) in Central Asia (UZ = Uzbekistan, KG = Kyrgyzstan, TJ = Tajikistan); (B) detailed map showing the sampling sites in the Aksu, Koksu, and Shakhimardan Rivers.
The morphological state of the river systems is near natural with only local bank protection measures. The Aksu River is a glacier-fed stream. The Koksu River’s flow is fed by underground water sources downstream of two lakes formed by natural earthen dams [18]. Apart from water abstractions for small-scale irrigation, the flow regimes are still largely intact. However, one diversion hydropower plant has recently been commissioned at the Koksu River [19]; another one at the Shakhimardan River downstream of the Aksu and Koksu River confluence is currently under construction.
We conducted semi-quantitative, single-pass electrofishing fish surveys between 25 March and 1 April 2025 using a backpack generator (EFKO FEG 1500, EFKO Elektrofischfanggeräte GmbH, Leutkirch im Allgäu, Germany) and a stationary device (Electracatch WFC7 0–250 V/0–10 Amps DC control box, Electracatch International Ltd., Peterborough, United Kingdom) in combination with a Honda 2000i 2kVA generator. In total, we sampled 3.5 km, consisting of six sites along a longitudinal gradient. Site length ranged from 291 to 900 m, with a mean of 592 m. Two sites are located in Aksu River, three in Koksu River, and one in Shakhimardan River (Table 1). Fish were stunned with electric fishing gear, caught with dip nets, and transferred into holding tanks. After capture, each specimen was measured to the nearest mm for total length (TL; from the tip of the snout to the end of the caudal fin), body height (H; maximum vertical body height), and body width (W; maximum horizontal body width) (Figure 2), and to the nearest 0.1 g for weight. Fish stocks were calculated as abundance per 100 m based on the sampled river length. Specimens were also sexed by putting gentle pressure on the abdomen to check for milt with the males and eggs with females. Body width and height measurements were obtained only for a subset of the specimens. After measurements, all fish were released back into the river at the site of capture.

Table 1.
Sampling sites and site-specific catch rates for Schizothorax eurycephalus and Triplophysa ferganaensis.
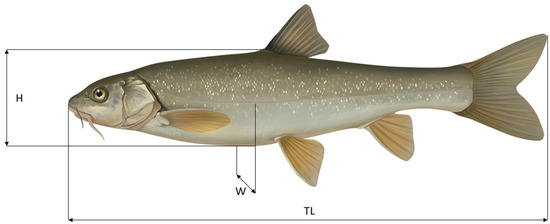
Figure 2.
Morphometric fish measurements, exemplified by Schizothorax eurycephalus. TL = total length, H = maximum body height, W = maximum body width. Fish illustration © Jennifer Clausen 2023, https://www.jacdraws.com/ (accessed 20 June 2025).
For gut content analysis, three specimens caught from Koksu River in September 2021 were euthanized using clove oil as an anesthetic, and their gastrointestinal tracts were carefully dissected. Subsequently, the gut contents were separated into different categories (algae, macroinvertebrates, and miscellaneous) and weighed to determine the percentage of each category relative to the total gut content.
For each species, two specimens were preserved in ethanol and deposited in the Fish Collection of the Natural History Museum Vienna, Austria: S. eurycephalus (NMW-101620) and T. ferganaensis (NMW-101621). All sampling was performed in accordance with ethical and legal guidelines (State Ministry of Ecology, Environmental Protection and Climate Change of the Republic of Uzbekistan, Approval Code: 02-02/6-1741).
2.2. Data Analysis
We first conducted descriptive statistics for standardized fish abundance [ind./100 m] and morphometric parameters, i.e., total length [mm], weight [g], body width [mm], and body height [mm] for the entire dataset and each sampling site, respectively. To assess the linear relationships among morphometric traits, we performed a Pearson correlation analysis. A correlation matrix was visualized displaying pairwise scatterplots, Pearson correlation coefficients, histograms of individual variables with overlaid density curves, and 95% confidence ellipses for bivariate relationships.
Fulton’s condition factor (K), also known as the coefficient of condition, was estimated as
where ‘W’ denotes the weight of the fish and ‘L’ its TL. While Fulton’s condition factor is typically used when fish have isometric growth, it remains valuable even when allometric growth is considered more appropriate [20].
The length–weight relationships (LWRs) and length–height relationships (LHRs) were calculated based on the formula
where ‘W’ represents the weight of fish in grams (or, in the case of LHR: height in centimeters), ‘L’ the TL in centimeters, ‘a’ is the scaling constant, and ‘b’ is the allometric coefficient (slope). The values of ‘a’ and ‘b’ were estimated by logarithm-based linear regression, represented as
following methodologies outlined by Froese [20] and Le Cren [21]. We calculated the 95% confidence limits for ‘a’ and ‘b’, along with the coefficient of determination (R2), using the equations from Sparre and Venema [22].
The growth pattern was classified based on the value of ‘b’: b = 3 constitutes isometric growth, which is when weight and length increase proportionally and small fish have the same shape as large specimens; b > 3 represents positive allometric growth, which is when larger fish have increased in weight more than in length; b < 3 is negative allometric growth, which is when larger fish have increased in length more than weight [20].
All statistical analyses and graphical visualizations were performed using Microsoft Excel 2021 and R version 4.3.0.
3. Results
A total of 174 S. eurycephalus and 57 T. ferganaensis were captured across the six sampling sites (Table 1). The highest numbers of each species were recorded at the most upstream site in Aksu River (site A1).
S. eurycephalus was present at all surveyed locations. In contrast, T. ferganaensis was not detected at the two most upstream sites in the Koksu River (K1–K2). In the Aksu River, both species were encountered at the upstream (A1) and downstream (A2) sites, with S. eurycephalus exhibiting greater abundance than T. ferganaensis at both sites. Only in the Koksu River at the downstream end (K3), S. eurycephalus and T. ferganaensis were caught in similar abundances. At the Shakhimardan site (S1), both species were present, with twelve S. eurycephalus and two T. ferganaensis captured (Table 1).
Standardized fish density for S. eurycephalus ranged from 1.5 ind./100 m at S1 to 10.2 ind./100 m at A1 (Table 2). For T. ferganaensis, where present, catch rates varied from 0.5 ind./100 m at S1 to 4.1 ind./100 m at A1 (Table 2).

Table 2.
Standardized fish density, morphometrics, and condition factor for Schizothorax eurycephalus and Triplophysa ferganaensis per sampling site.
3.1. Morphometrics
Fish body metrics (length, weight, width, height) were all positively correlated with R ≥ 0.91 for all comparisons in S. eurycephalus (Figure 3a) and R ≥ 0.81 in T. ferganaensis. For the latter, correlations between fish length, weight and width were highest (R > 0.90). Correlations with R < 0.90 were largely found to be related to fish height (Figure 3b). Considering the high correlations, we focus on selected parameters in the section below (see Table 2 and Table 3).
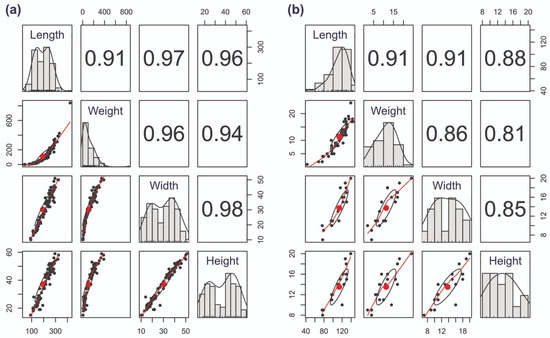
Figure 3.
Pairwise correlation matrix for morphometric measurements of (a) Schizothorax eurycephalus (n = 174 for length and weight, n = 88 for width and height) and (b) Triplophysa ferganaensis (n = 57 for length and weight, n = 18 for width and height). Scatterplots below the diagonal display the bivariate relationships between variables, with Pearson correlation coefficients shown above the diagonal. Histograms on the diagonal depict the distribution of each variable, overlaid with density curves. Ellipses represent 95% confidence intervals for the correlation in each bivariate plot. The red line is a smoothed density curve; the red dot represents the mean of the bivariate distribution for each pair of variables. All variables are measured in millimeters, except for weight measured in grams.

Table 3.
Length–weight relationship of Schizothorax eurycephalus and Triplophysa ferganaensis.
S. eurycephalus specimens from S1 exhibited the largest mean length (257.3 mm) and weight (213 g), while those from the mid-section of Koksu River (K2) were the smallest (mean TL = 182.1 mm) and lightest fish (mean weight = 67.8 g). The broadest range of observed lengths (39–422 mm) and weights (1–839 g) for S. eurycephalus was recorded at A2. Overall, the data show that the weight of S. eurycephalus generally increases from upstream to downstream, with the exception of K1, where two fish ready to spawn were caught (Figure 4a).
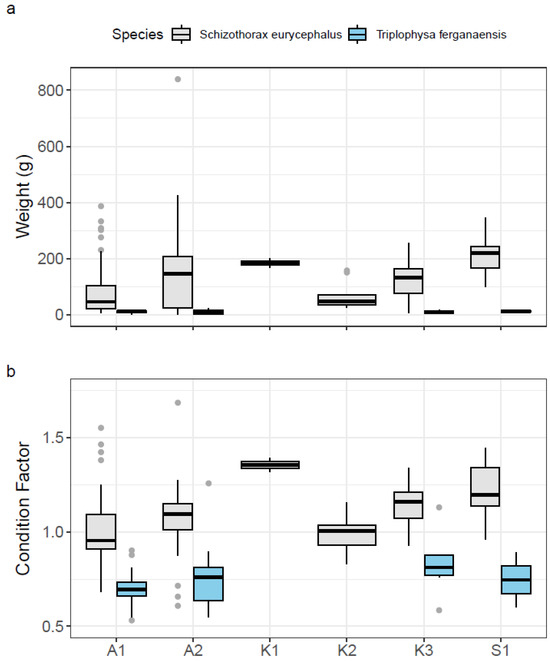
Figure 4.
(a) Fish weight and (b) Fulton’s condition factor by sampling site and species. Grey dots represent statistical outliers, i.e., values that fall outside 1.5 times the interquartile range.
Fulton’s condition factor (K) for S. eurycephalus was highest at K1 (mean = 1.4) and S1 (mean = 1.2), and lowest at K2 (Table 2). Site A2 exhibited the highest variability, ranging from 0.6 to 1.7 (Table 2; Figure 4b). The overall mean value was 1.0.
Mean total lengths for T. ferganaensis ranged from 104.3 mm at site A2 to 117.8 mm at A1, with mean weight ranging from 10.2 g (K3) and 12.5 g (S1) (Table 2; Figure 4a). Even though the mean fish weight was the lowest at site K3, this location including A2 featured the highest condition factor of K = 0.8. The lowest condition factor of T. ferganaensis was at sites A1 and S1 with K = 0.7 (Table 2; Figure 4b). The overall mean value was 0.7.
Table 3 presents the statistics related to the length–weight relationship estimates, including length and weight ranges and confidence intervals for estimated parameters. Table 4 contains the statistics related to the length–height relationship estimates.

Table 4.
Length–height relationship of Schizothorax eurycephalus and Triplophysa ferganaensis.
3.2. Insights into Sexual Maturity and Diet
Of the 174 S. eurycephalus individuals examined, 23% (n = 40) exhibited milt upon gentle abdominal pressure, indicating male sexual maturity. No females releasing eggs were observed during this procedure. The smallest male S. eurycephalus found to be sexually mature measured 77 mm TL and weighed 3 g, although the majority of mature males were >100 mm TL. Of the 57 T. ferganaensis individuals examined, only three (5%) exhibited milt, with the smallest mature male measuring 122 mm TL and weighing 13 g. Except for these three individuals, we did not observe clear signs of sexual maturity in T. ferganaensis during our sampling campaign. However, we noted that their bellies appeared noticeably more swollen compared to other seasons, suggesting that maturation was underway or that the reproductive season had already started for this species.
Preliminary gut content analysis was conducted on three S. eurycephalus specimens caught in Koksu River during September 2021 sampling. This examination revealed a diet primarily composed of algae, which accounted for approximately 70% of the gut contents by volume. Macroinvertebrate remains constituted a secondary dietary component, comprising about 28% by volume. The remaining 2% consisted of miscellaneous, undefined material.
4. Discussion
Foundational morphometric data for S. eurycephalus and T. ferganaensis, two ecologically important species in the mountainous river systems of the Fergana Valley, Central Asia, remain scarce or undocumented, despite the urgent need for species-specific information to inform conservation and monitoring in this rapidly changing region [23,24]. This study addresses this gap by quantifying relationships of fish length, weight, height, and width and providing ecological observations on diet composition and sexual maturity for these fishes. These findings provide valuable data for future applied research and the development of ecologically sound conservation strategies.
S. eurycephalus occurs in the entire Shakhimardan River basin. T. ferganaensis, however, is absent from the mid and upper sections of Koksu River [23]. Standardized fish density of S. eurycephalus was highest in the two Aksu River sites, as well as in the downstream reach of Koksu River. At both Aksu River sites, we detected aggregations of S. eurycephalus, which have gathered for upstream spawning migration. Interestingly, the two specimens caught in the upstream section of Koksu River, exhibited the highest condition factor; their bellies were thick, suggesting egg development, although the sex could not be determined. Moreover, both specimens were caught in a river section where no fish could be documented in previous surveys due to an artificial waterfall blocking upstream movements [23]. The partial removal of the waterfall in early 2025 seemed to have allowed the first upstream migration of S. eurycephalus since many decades. Fish specimens with the highest condition factors, similar to those in the Koksu, were also recorded in the Aksu River, further suggesting egg development in these well-conditioned individuals. The absence of T. ferganaensis from the Koksu River’s upstream and mid-sections suggests specific habitat preferences or limiting factors for this species in those reaches [2,8].
The length–weight relationship for S. eurycephalus indicates positive allometric growth, meaning that individuals become proportionally heavier for their length as they grow [2]. While positive allometry is common in many fishes, the genus Schizothorax exhibits considerable plasticity in growth patterns, with isometric or negative allometric growth reported for other species or populations under differing environmental conditions [2,25,26]. This variability highlights the importance of establishing population-specific baselines, as growth trajectories can be influenced by local factors such as food availability, water temperature regimes, and flow velocity [2,27]. For T. ferganaensis, the length–weight relationship indicates negative allometric growth, meaning that as individuals mature, their length increases more than their weight [20]. However, the confidence interval overlaps with isometric growth, indicating that the pattern is negative near-isometric. The coefficient of determination was low for the length–height relationship of T. ferganaensis, likely attributable to the limited number of specimens measured for these parameters. This suggests a need for further research with a larger sample size to establish a more robust length–height relationship for this species.
The mean condition factor of 1.0 for S. eurycephalus suggests a generally good condition of the Shakhimardan population. This condition, vital for resilience, could be further monitored as a bioindicator, especially given that stressors like water pollution and habitat degradation have been shown to impact the health and condition of other Schizothorax species [28,29]. Our gut analysis indicate a predominantly herbivorous diet for S. eurycephalus—with algae constituting around 70% of the gut content—which is consistent with feeding strategies of many congeners [1,30]. Alterations to periphyton availability due to flow regulation or water quality changes could, therefore, significantly impact this population. For T. ferganaensis, the mean condition factor (K = 0.7) was considerably lower than that of S. eurycephalus. While direct K value comparisons between species require caution due to inherent differences in body morphology [11], this value provides a species-specific baseline for T. ferganaensis in these rivers. The observed differences in condition factor between sites for both species likely reflect localized variations in habitat quality, food availability, or other environmental stressors.
The strong correlations observed among morphometric parameters for S. eurycephalus and T. ferganaensis underscore the integrated nature of these metrics, with established length–height relationships proving particularly valuable for non-invasive monitoring techniques. For instance, using infrared scanners [31] or other camera setups [32], fish height can be measured to estimate the total length via species-specific length–height ratios, facilitating assessments of migrating fish populations [31]. The baseline morphometric data presented here will also provide essential input for designing fish passage facilities, intake screens, and trash racks as the dimensions of these structures must be tailored to the target species’ size and swimming capabilities to ensure effectiveness [33,34].
The observation of sexually mature male S. eurycephalus at small body sizes (3 g) and the presence of juveniles (5–30 mm) in shallow, vegetated irrigational tributaries suggest early reproductive maturity and localized spawning habitats. The co-occurrence of these small mature males with juveniles (5–30 mm) in shallow, vegetated tributaries near site A2 strongly identifies these areas as critical spawning and nursery habitats. Such microhabitats have been documented as essential for other Schizothorax species [35]. These findings highlight the ecological importance of preserving low-velocity, fine-substrate areas as critical spawning and nursery grounds. The increasing and largely unchecked deterioration and fragmentation of river habitats due to hydropower development [24] and water abstraction without adequate mitigation measures poses significant risks to population sustainability [36].
Limitations of this study include the spring-only sampling period, which may not capture seasonal variability in condition factors, a phenomenon documented for S. labiatus in India [37]. Future research should aim to expand the temporal scope of sampling to assess seasonal variations in fish’ condition, diet and habitat use, particularly focusing on life-history patterns. Detailed habitat and migration assessments, genetic studies to delineate population structures [38,39], and investigations into swimming performance and environmental tolerances [40,41] will be crucial for developing robust, evidence-based conservation and management strategies for the increasingly threatened fish fauna of Central Asia.
5. Conclusions
This study provides important morphometric and ecological data for S. eurycephalus and T. ferganaensis, in a mountainous tributary of the Syr Darya River in Central Asia. The assessments include the relationships of fish length, weight, height, and width, as well as Fulton’s condition factor. Regarding fish ecology, this article provides observations on sexual maturity and diet composition.
S. eurycephalus exhibited positive allometric growth and a mean condition factor indicative of good health. T. ferganaensis showed negative near-isometric growth close to isometric and a comparatively lower condition factor. The strong correlations among morphometric parameters for the target species constitute valuable data, enabling non-invasive monitoring, e.g., through length–height relationships, and provide critical baseline information for designing effective fish passage and protection facilities tailored to these species’ size.
Among S. eurycephalus, one quarter of sampled individuals showed sexual maturity with milt expression, primarily in males > 100 mm TL, while no females released eggs; T. ferganaensis showed no clear maturity signs but had swollen bellies, suggesting ongoing or recent reproductive activity. Initial dietary observations for S. eurycephalus suggest an herbivorous diet consisting of around two thirds of algae.
These findings provide a baseline for future ecological assessments, conservation strategies, and the design and monitoring of mitigation measures aimed to sustain fish populations in this increasingly regulated region.
Author Contributions
Conceptualization, E.K., M.S. and D.S.H.; methodology, E.K., O.O., P.V., M.S. and D.S.H.; validation, E.K., O.O., P.V., M.S. and D.S.H.; formal analysis, E.K. and D.S.H.; investigation, E.K., O.O., P.V., B.K.K., M.S. and D.S.H.; resources, B.K.K. and D.S.H.; data curation, O.O., P.V., B.K.K., M.S. and D.S.H.; writing—original draft preparation, E.K., O.O., P.V., M.S. and D.S.H.; writing—review and editing, E.K., O.O., P.V., B.K.K., M.S. and D.S.H.; visualization, E.K., O.O. and D.S.H.; supervision, M.S. and D.S.H.; project administration, B.K.K. and D.S.H.; funding acquisition, E.K., B.K.K. and D.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 101022905. E.K. received support (Ernst Mach Grant) from the Austrian Federal Ministry of Education, Science and Research, OeAD-GmbH Agency for International Mobility and Cooperation in Education, Science and Research.
Institutional Review Board Statement
This study was approved by the State Ministry of Ecology, Environmental Protection and Climate Change of the Republic of Uzbekistan, Approval Code: 02-02/6-1741, Approval Date: 20 February 2025.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in Zenodo at https://doi.org/10.5281/zenodo.15672703 (accessed on 25 July 2025).
Acknowledgments
Thanks to Johan Coeck, Bernhard Zeiringer, and Falko Wagner for help during field work. Thanks to Jörg Freyhof and Akbarjon Rozimov for fruitful discussion on snow trout taxonomy, and to two anonymous reviewers for comments to improve the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Berg, L.S. Ryby presnykh vod SSSR i sopredel’nykh stran. In Fishes of Fresh Waters of the USSR and Adjacent Countries; Akad. Nauk SSSR: Moscow, Russia, 1948; Volume 2. [Google Scholar]
- Karimov, E.; Zeiringer, B.; Coeck, J.; Verhelst, P.; Karimov, B.; Omonov, O.; Schletterer, M.; Hayes, D.S. Length-Weight-Age Relationship of Schizothorax eurystomus Kessler, 1872 and Comparison to Other Snow Trout Species in Central Asia. Fishes 2024, 9, 94. [Google Scholar] [CrossRef]
- Rozimov, A.; Wang, Y.; Wang, M.; Zou, M.; Sobirov, J.; Karimov, E.; Kholmatov, B.; Freyhof, J.; Namozov, S.; Wang, C.; et al. Mitochondrial Genome Insights into the Phylogenetics and Biogeographic Evolution of Snow Trout (Cyprinidae, Schizothorax) in the Tien Shan Mountains. Zoosystematics Evol. 2025, 101, 91–102. [Google Scholar] [CrossRef]
- Amirbekova, F.; Isbekov, K.B.; Assylbekova, S.Z.; Sharipova, O.A.; Adyrbekova, K.; Bulavina, N. Biological Characteristics of a Rare and Vulnerable Species (SCHIZOTHORAX ARGENTATUS (Kessler, 1874)) of TOKYRAUYN RIVER and Approbation of Its Artificial Reproduction. Agriculture 2022, 12, 1121. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2025-1. 2025. Available online: https://www.iucnredlist.org (accessed on 5 May 2025).
- Yan, T.; Hu, J.; Cai, Y.; Xiong, S.; Yang, S.; Wang, X.; He, Z. Otolith Development in Larval and Juvenile Schizothorax davidi: Ontogeny and Growth Increment Characteristics. Chin. J. Oceanol. Limnol. 2017, 35, 1197–1204. [Google Scholar] [CrossRef]
- Sheraliev, B.; Peng, Z. Triplophysa ferganaensis, a New Loach Species from Fergana Valley in Central Asia (Teleostei: Nemacheilidae). J. Fish. Biol. 2021, 99, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Sheraliev, B.; Nazirov, B.; Khalimov, S. Notes on Distribution of Triplophysa ferganaensis Sheraliev & Peng, 2021 (Teleostei: Nemacheilidae). Ecol. Montenegrina 2025, 86, 188–196. [Google Scholar] [CrossRef]
- Rozimov, A.D. Morphometric Features of the Chinese Hook Snout Opsariichthys bidens Günther 1873 (Teleostei: Xenocyprididae) from the Chirchik River. Bull. Khorezm Mamun Acad. 2023, 5, 73–76. [Google Scholar]
- Rozimov, A.; Sheraliev, B. Length-Weight Relationship of Carassius gibelio from the Lower Reaches of the Amu Darya River. Sci. Innov. Int. Conf. Tashkent 2020, 26, 194–197. [Google Scholar]
- Jones, R.E.; Petrell, R.J.; Pauly, D. Using Modified Length–Weight Relationships to Assess the Condition of Fish. Aquac. Eng. 1999, 20, 261–276. [Google Scholar] [CrossRef]
- Nafizi, F.; Naim, M.; Kamel, N.; Pajar, S.; Abdullah, M. Prediction of Fish Weight Based on Length, Height, and Width, [Kuala Lumpur]: University of Kuala Lumpur; 2023. Available online: https://www.researchgate.net/publication/375831066_Prediction_of_Fish_Weight_based_on_Length_Height_and_Width (accessed on 22 July 2025).
- Ullah, A.; Khan, W. Feeding Habits and Diet Composition of Schizothorax plagiostomus in Panjkora River, Malakand Region, Pakistan. Arx. Miscel·Lània Zoològica 2024, 22, 53–65. [Google Scholar] [CrossRef]
- Wang, C.; Yao, N.; Xia, L.; Wang, X.; Song, Y.; Serekbol, G.; Zi, F.; Lin, X.; Yan, J.; Chen, S. Age, Growth and Reproduction of Schizothorax pseudaksaiensis of the Turks River. Water 2023, 15, 4044. [Google Scholar] [CrossRef]
- Rozimov, A.; Li, X.; Karimov, E.; Sheraliev, B.; Wang, C.; Guo, B.; Freyhof, J. Names of Snow Barbels of the Genus Schizothorax in the Aral Sea Basin (Teleostei, Cyprinidae). in preparation.
- Sheraliev, B.; Peng, Z. Molecular Diversity of Uzbekistan’s Fishes Assessed with DNA Barcoding. Sci. Rep. 2021, 11, 16894. [Google Scholar] [CrossRef]
- Sheraliev, B.; Azamov, O.; Rozimov, A.; Kayumova, Y. DNA barcoding reveals broader distribution of Triplophysa ferganaensis Sheraliev & Peng, 2021 (Teleostei: Nemacheilidae) in the Fergana Valley. Acta NUUz 2024, 3/1/1, 172–174. [Google Scholar]
- Strom, A.; Abdrakhmatov, K. Tien Shan. In Rockslides and Rock Avalanches of Central Asia; Elsevier: Amsterdam, The Netherlands, 2018; pp. 117–258. ISBN 978-0-12-803204-6. [Google Scholar]
- Alapfy, B.; Hayes, D.S.; De Keyser, J.; Jorde, K.; Karimov, B.; Kopecki, I.; Verhelst, P. Progress in the Implementation of Sustainable Small Hydro in Central Asia. Int. J. Hydropower Dams. 2025, 32, 35–40. [Google Scholar]
- Froese, R. Cube Law, Condition Factor and Weight-Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Cren, E.D.L. The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201. [Google Scholar] [CrossRef]
- Sparre, P.; Venema, S.C. Manual. In Introduction to Tropical Fish Stock Assessment, 2nd ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; ISBN 978-92-5-103996-0. [Google Scholar]
- Karimov, E.; Karimov, B.; Schletterer, M.; Hayes, D. Ichthyological Research in the Koksu River (Uzbekistan) to Identify Key Fish Species in the Context of Small Hydropower Development. Exp. Biol. 2023, 95, 133–141. [Google Scholar] [CrossRef]
- De Keyser, J.; Fuentes, P.O.; Hayes, D.S.; Habersack, H. A Review of Hydropower in Central Asia: Past, Present, and Future. Renew. Sustain. Energy Rev. in review.
- Yousuf, T.; Bakhtiyar, Y.; Andrabi, S.; Wani, G.B. Length-Weight Relationship and Condition Factor of Seven Fish Species in Manasbal Lake, Kashmir, India. Croat. J. Fish. 2023, 81, 13–22. [Google Scholar] [CrossRef]
- Yousuf, T.; Andrabi, S.; Bakhtiyar, Y. Water Quality and Stock Assessment of Schizothorax niger (Alghad Snowtrout) in Manasbal Lake, Kashmir Himalaya. Environ. Monit. Assess. 2024, 197, 54. [Google Scholar] [CrossRef]
- Liu, M.; Xu, W.; Zhu, F.; Duan, X.; Liu, S.; Chen, D. Length–Weight Relationship and Spatiotemporal Distribution Pattern of Three Schizothoracinae Fishes Along the Nujiang River in the Qinghai–Tibetan Plateau, China. Fishes 2024, 9, 465. [Google Scholar] [CrossRef]
- Zargar, U.R.; Khanday, S.A.; Rather, M.I.; Dar, S.A.; Zargar, N.H.; Mir, A.H. Accelerated Eutrophication Alters Fish and Aquatic Health: A Quantitative Assessment by Using Integrative Multimarker, Hydrochemical, and GIS Modelling Method in an Urban Lake. Environ. Monit. Assess. 2024, 196, 40. [Google Scholar] [CrossRef]
- Khan, K.; Zeb, M.; Younas, M.; Sharif, H.M.A.; Yaseen, M.; Al-Sehemi, A.G.; Kavil, Y.N.; Shah, N.S.; Cao, X.; Maryam, A.; et al. Heavy Metals in Five Commonly Consumed Fish Species from River Swat, Pakistan, and Their Implications for Human Health Using Multiple Risk Assessment Approaches. Mar. Pollut. Bull. 2023, 195, 115460. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, C.; Malhotra, Y.R.; Dutta, S. Food and Feeding Habits of Schizothorax richardsonii (Gray and Hard) Inhabiting Neeru Nullah, Bhaderwah, Jammu. J. Indian. Inst. Sci. 2013, 73, 247–251. [Google Scholar]
- Haas, C.; Thumser, P.K.; Hellmair, M.; Pilger, T.J.; Schletterer, M. Monitoring of Fish Migration in Fishways and Rivers—The Infrared Fish Counter “Riverwatcher” as a Suitable Tool for Long-Term Monitoring. Water 2024, 16, 477. [Google Scholar] [CrossRef]
- Al-Abri, S.; Keshvari, S.; Al-Rashdi, K.; Al-Hmouz, R.; Bourdoucen, H. Computer Vision Based Approaches for Fish Monitoring Systems: A Comprehensive Study. Artif. Intell. Rev. 2025, 58, 185. [Google Scholar] [CrossRef]
- Deutscher Verband für Wasserwirtschaft und Kulturbau (DVWK) (Ed.) Fish Passes: Design, Dimensions and Monitoring; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; ISBN 978-92-5-104894-8. [Google Scholar]
- Leitfaden Zum Bau Von Fischaufstiegshilfen. Available online: https://www.bmluk.gv.at/themen/wasser/gewaesserbewirtschaftung/leitfaeden/leitfaden-zum-bau-von-fischaufstiegshilfen-2021.html (accessed on 25 May 2025).
- Chandra, S.; Ganie, P.A. Fisheries and Aquaculture of Snow Trouts in the Trans-Himalayan Region. In Fisheries and Aquaculture of the Temperate Himalayas; Pandey, P.K., Pandey, N., Akhtar, M.S., Eds.; Springer Nature: Singapore, 2023; pp. 131–150. ISBN 978-981-19-8302-3. [Google Scholar]
- Schmutz, S.; Friedrich, T.; Greimel, F.; Hayes, D.S.; Jungwirth, M.; Muhar, S.; Pinter, K.; Seliger, C.; Unfer, G.; Zeiringer, B. Beitrag einer nachhaltigen Wasserkraft zum Schutz der Fische. WasserWirtschaft 2025, 115, 18–23. [Google Scholar] [CrossRef]
- Jan, K.; Ahmed, I.; Dar, N.A. The Role of Sex, Season and Reproduction Status on Blood Parameters in Snow Trout (Schizothorax labiatus) from River Jhelum, Kashmir, India. Environ. Monit. Assess. 2022, 194, 674. [Google Scholar] [CrossRef]
- Sharma, A.; Dubey, V.K.; Johnson, J.A.; Rawal, Y.K.; Sivakumar, K. Introduced, Invaded and Forgotten: Allopatric and Sympatric Native Snow Trout Life-Histories Indicate Brown Trout Invasion Effects in the Himalayan Hinterlands. Biol. Invasions 2021, 23, 1497–1515. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, R.; Zhao, W.; Zhao, L.; Zhou, M.; Jing, T.; Luo, H. SNP Marker Development and Polymorphism Detection of Schizothorax waltoni. Conserv. Genet. Resour. 2020, 12, 199–203. [Google Scholar] [CrossRef]
- Senfan, K.; Zhijun, J.; Zhimin, L.; Qingsong, L.; Yongmeng, W.; Xiaotao, S.; Zhiying, T. Swimming Ability of Fifteen Target Fish from Eight Hydropower Stations in China. J. Lake Sci. 2022, 34, 1608–1619. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; Liu, K.; Pu, X.; Wang, Y. Swimming Ability of Schizothoracinae Fishes in Yarlung Zangbo River of China. J. Fish. Biol. 2024, 105, 95–109. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).