The Effects of Interactions Between Key Environmental Factors on Non-Specific Indicators in Carassius auratus
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Husbandry of Experimental Fish
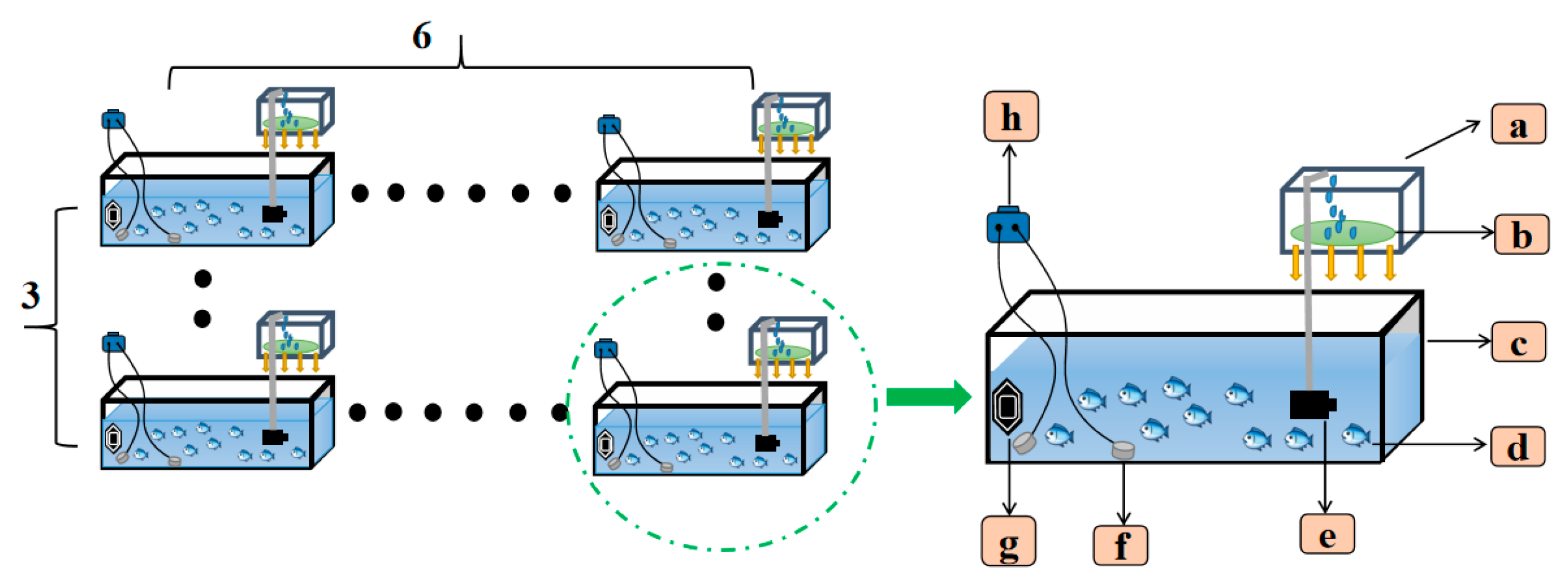
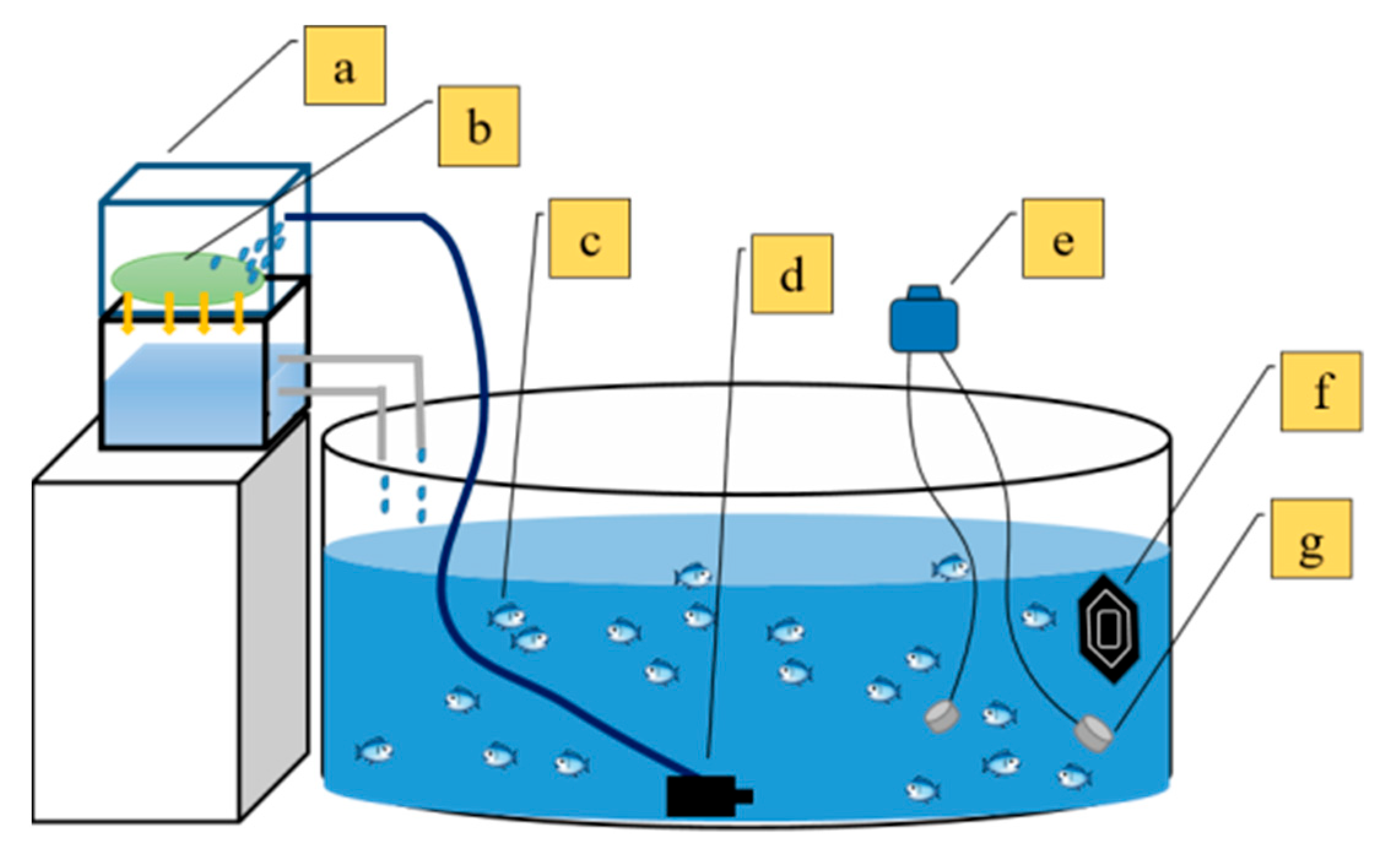
2.2. Experimental Design
2.2.1. Temperature and Oxygen Control Methodology
- Temperature control module: A closed-loop temperature regulation system based on a PID algorithm was established, integrating immersion heaters with digital temperature sensors. The compensation mechanism was automatically activated when the detected temperature deviated by >0.5 °C from the setpoint. The power of the heater was 100 W (Sensen Group Co., Ltd., Zhoushan, China). The temperature control range was 15–34 °C.
- Dissolved oxygen regulation module: A micro-aeration device coupled with optical DO probes was utilized, achieving dynamic DO balance through fuzzy-PID control. Specifically, the hypoxic group was maintained through synergistic regulation of biological oxygen consumption and micro-oxygen supplementation, while normoxic and hyperoxic groups were stabilized using gradient aeration strategies.
2.2.2. Experiment I: Effects of Thermo-Oxygen Stress on Physiological Parameters and Gene Expression in Crucian Carp
2.2.3. Experiment II: Effects of Warm Oxygen Stress on Stress Behavior and Cortisol in Crucian Carp
2.2.4. Experiment III: Effects of Thermo-Oxygen Stress on Muscle Texture Properties in Crucian Carp
2.3. Determination of Stress Parameters
2.4. Observation of Stress-Related Behavioral Characteristics
2.4.1. Hypoxic Stress-Induced Behavioral Alterations
- Sustained surface stress response: Persistent surfacing behavior or surface-proximal swimming for aerial oxygen uptake;
- Transient stress compensation: Temporary surfacing episodes, followed by resumption of normal swimming activity;
- Immobility-mediated stress adaptation: Reduced locomotor activity to minimize metabolic demand;
- Active stress avoidance: Vertical exploration of the water column in search of oxygen-enriched zones (Figure 3).
2.4.2. Thermal Stress Behavior
2.5. Isolation of Tissue RNA and Quantitative Real-Time PCR Analysis
2.5.1. Isolation of Liver Tissue RNA
2.5.2. Reverse Transcription of mRNA
2.5.3. Quantitative Real-Time PCR Analysis
2.6. Determination of Muscle Moisture Loss in Crucian Carp
- Drip loss
- Cooking loss
- Centrifugal loss
2.7. Determination of Crucian Carp Muscle Morphology and Texture Characteristics
2.8. Data Analysis
3. Results
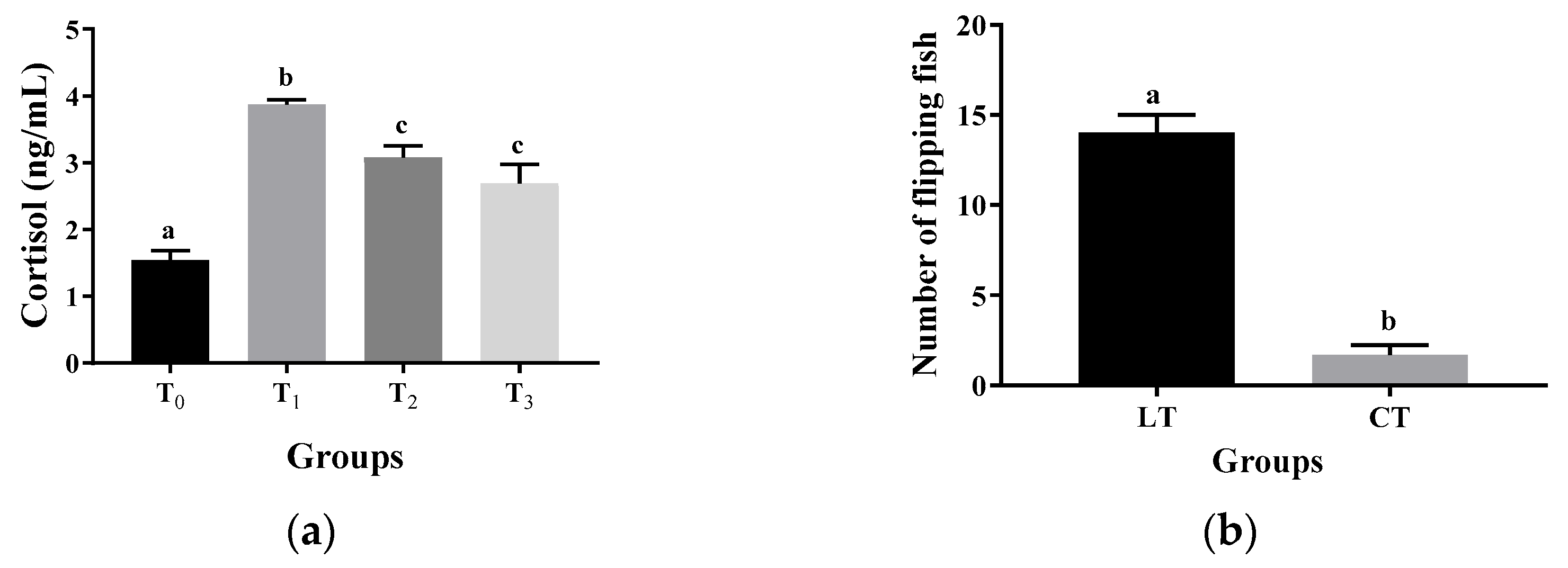
3.1. Effects of Thermal and Hypoxic Stress on Cortisol and Blood Glucose Levels in Crucian Carp
3.2. Behavioral Response Characteristics of Crucian Carp to Thermal and Hypoxic Stress
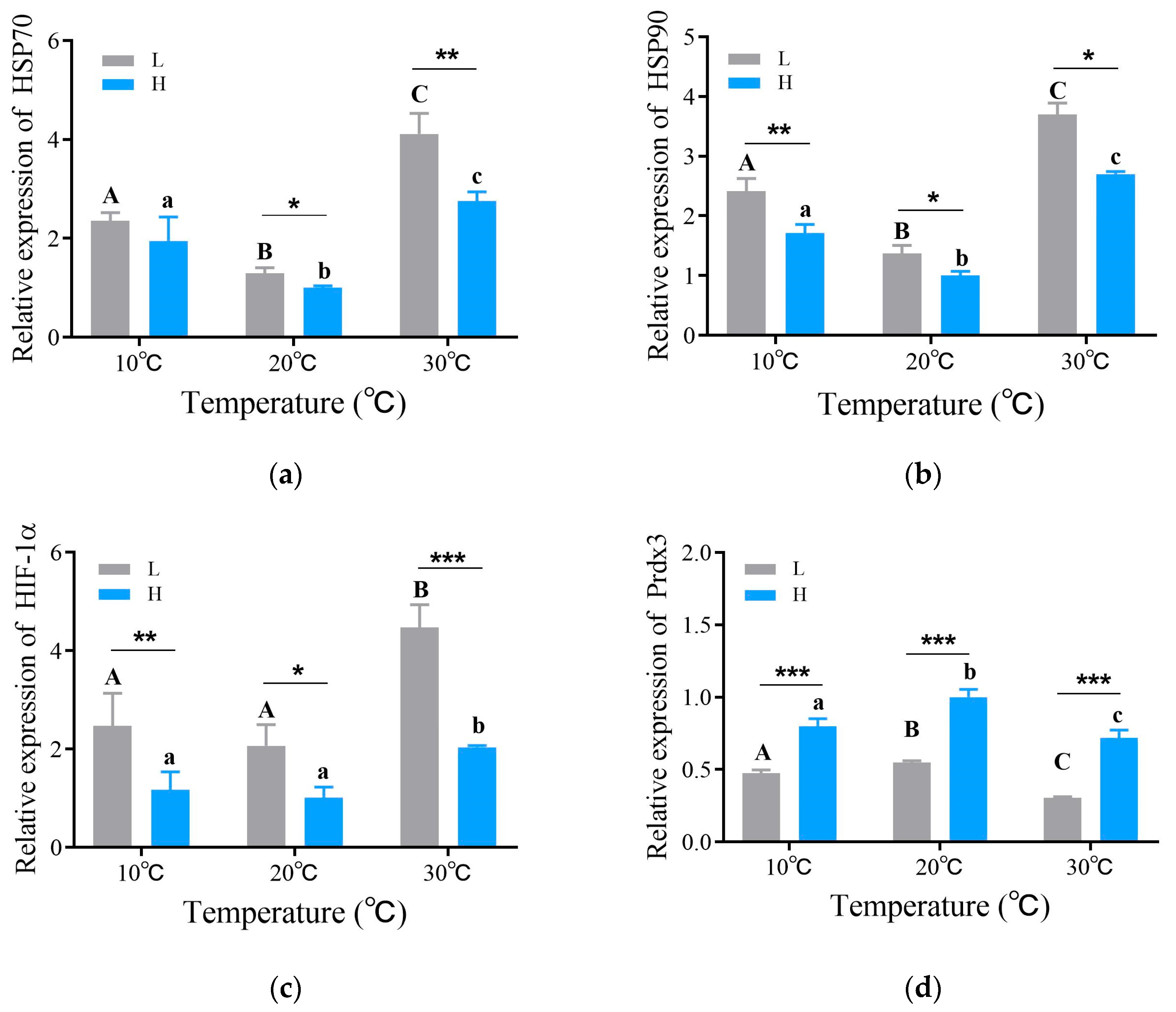
3.3. Gene Expression Response Patterns of Crucian Carp to Thermal and Hypoxic Stress
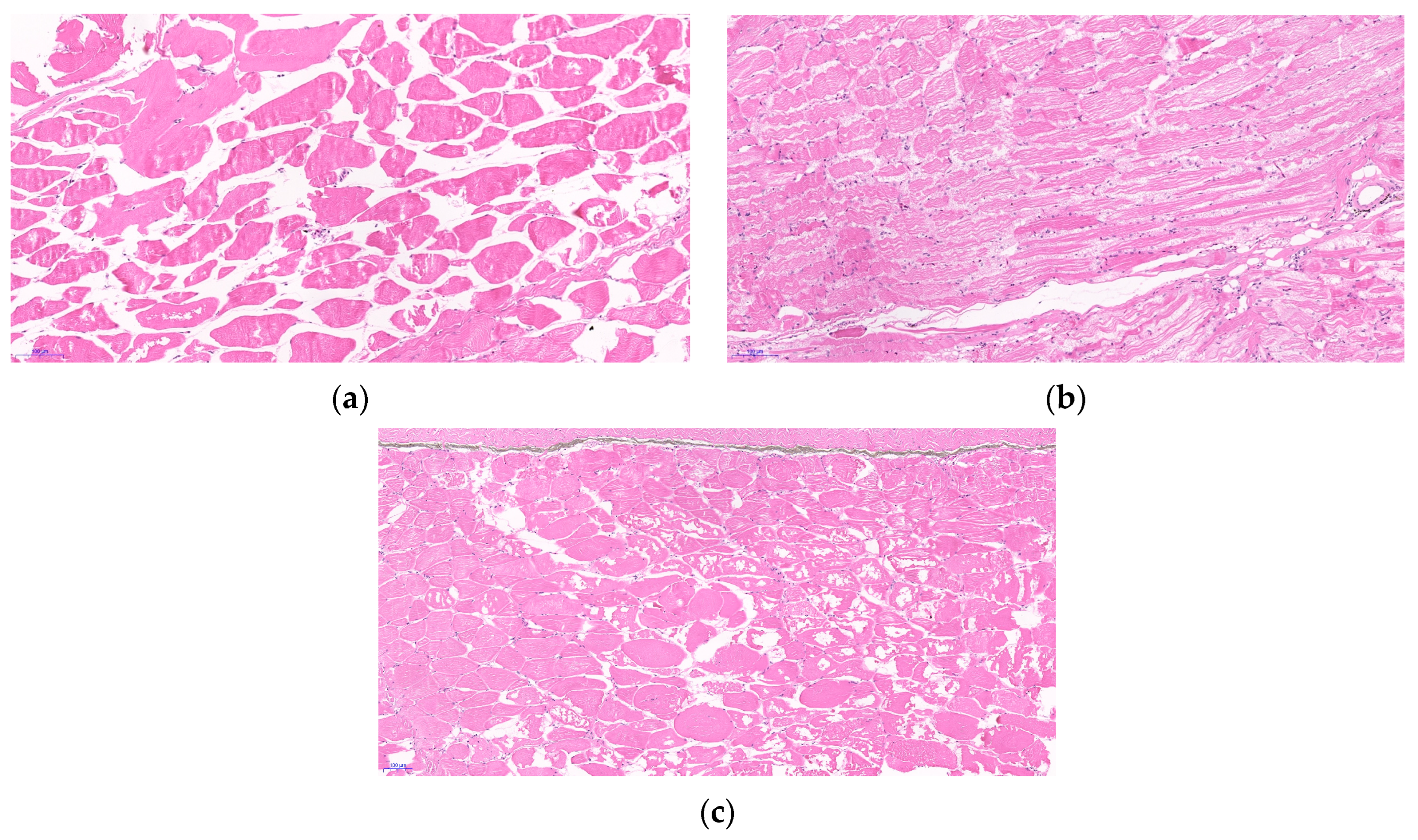
3.4. Variation Patterns in Crucian Carp Muscle Texture Parameters Under Stress Conditions
4. Discussion
4.1. Response Patterns of Crucian Carp Stress-Induced Parameters to Thermal and Hypoxic Stress
4.2. Response Patterns in Crucian Carp Stress-Induced Behaviors to Thermal and Hypoxic Stress
4.3. Response Patterns in Crucian Carp Gene Expression to Thermal and Hypoxic Stress
4.4. Effects of Thermal and Hypoxic Stress on Muscle Texture Parameters in Crucian Carp
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiloso, E.I.; Romli, M.; Nugraha, B.A.; Wiloso, A.R.; Setiawan, A.A.R.; Henriksson, P.J.G. Life cycle assessment of Indonesian canned crab (Portunus pelagicus). J. Ind. Ecol. 2022, 26, 1947–1960. [Google Scholar] [CrossRef]
- Zhuang, P.; Bi, B.; Zhao, F.; Zhang, T.; Peng, J.; Zhang, L.; Kong, L.; Yang, G. Perspective on the development strategy of freshwater fishery seed industry in Yunnan Province. J. China Agric. Resour. Reg. Plan. 2023, 44, 1–7. [Google Scholar]
- Wang, B.; Mao, H.P.; Zhao, J.; Liu, Y.; Wang, Y.F.; Du, X.X. Influences of oxygen and temperature interaction on the antibacterial activity, antioxidant activity, serum biochemical indices, blood indices and growth performance of crucian carp. PeerJ 2023, 10, e14530. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wang, Y.; Zhang, Z. Long-read RNA sequencing of Pacific abalone Haliotis discus hannai reveals innate immune system responses to environmental stress. Fish Shellfish. Immunol. 2022, 122, 131–145. [Google Scholar] [CrossRef]
- Wang, B.; Mao, H.; Zhao, J.; Liu, Y.; Wang, Y.; Du, X. Designing a Multi-Parameter Method to Assess the Adaptation Period of Crucian Carp under Stress Conditions of the Bionic Robot Fish. Fishes 2022, 7, 198. [Google Scholar] [CrossRef]
- Yao, W.; Li, X.; Zhang, C.; Wang, J.; Cai, Y.; Leng, X. Effects of dietary synbiotics supplementation methods on growth, intestinal health, non-specific immunity and disease resistance of Pacific white shrimp, Litopenaeus vannamei*. Fish Shellfish. Immunol. 2021, 112, 46–55. [Google Scholar] [CrossRef]
- Read, T.; Combes, S.; Gidenne, T.; Destombes, N.; Bébin, K.; Balmisse, E.; Fortun-Lamothe, L. Influence of feeding strategy and diet for reproductive rabbit does on intake, performances, and health of young and females before and after weaning. J. Anim. Sci. 2016, 94, 4848–4859. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, S.; Huang, Y.; Chang, K.; Zhao, X. Addition of Chlorella sorokiniana meal in the diet of juvenile rainbow trout (Oncorhynchus mykiss): Influence on fish growth, gut histology, oxidative stress, immune response, and disease resistance against Aeromonas salmonicida. Fish Shellfish. Immunol. 2022, 129, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Boegner, M.; Schwenke, C.; Guertzgen, T.; Boegner, D.; Slater, M.J. Effect of ambient light intensity on growth performance and diurnal stress response of juvenile starry flounder (Platichthys stellatus) in recirculating aquaculture systems (RAS). Aquac. Eng. 2018, 83, 20–26. [Google Scholar] [CrossRef]
- Diaz-Puente, B.; Guinez, R.; Pita, A.; Minambres, M.; Presa, P. Genotype by environment interaction for shell length in Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 2020, 522, 151252. [Google Scholar] [CrossRef]
- Liang, Q.; Ou, M.; Li, Z.; Ren, Y.; Wei, W.; Qiao, X.; Hu, R.; Wu, X.; Liu, Y.; Wang, W. Functional analysis target of rapamycin (TOR) on the Penaeus vannamei in response to acute low temperature stress. Fish Shellfish. Immunol. 2020, 96, 53–61. [Google Scholar] [CrossRef]
- Wang, Y.B.; Chen, R.; Wang, Q.; Yue, Y.F.; Gao, Q.X.; Wang, C.H.; Zheng, H.F.; Peng, S.M. Transcriptomic Analysis of Large Yellow Croaker (Larimichthys crocea) during Early Development under Hypoxia and Acidification Stress. Vet. Sci. 2022, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, M.; Ghomeshi, M.; Ahadiyan, J.; Mohammadian, T.; Katopodis, C. Do fishways stress fish? Assessment of physiological and hydraulic parameters of rainbow trout navigating a novel W-weir fishway. Ecol. Eng. 2021, 169, 106330. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; An, D.; Wei, Y. Identification methodology of special behaviors for fish school based on spatial behavior characteristics. Comput. Electron. Agric. 2021, 185, 106169. [Google Scholar] [CrossRef]
- Rao, K.; Tang, L.; Zhang, X.; Xiang, H.; Tang, L.; Liu, Y.; Wang, W.; Jiang, J.; Ma, M.; Xu, Y.; et al. Fish forewarning of comprehensive toxicity in water environment based on Bayesian sequential method. J. Environ. Sci. 2021, 110, 150–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Shen, F.; Zhang, Z.; Guo, H.; Zhang, X. Barren environment damages cognitive abilities in fish: Behavioral and transcriptome mechanisms. Sci. Total Environ. 2021, 794, 148805. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Li, X.; Sun, S.; Liu, J.; Wan, M.; Huang, L.; Yang, D.; Huang, B.; Zhong, Z.; et al. Exposure to pyrazosulfuron-ethyl induces immunotoxicity and behavioral abnormalities in zebrafish embryos. Fish Shellfish. Immunol. 2022, 131, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Ahmad, T.; Sharma, J.; Chakrabarti, R. Effect of temperature on food consumption, immune system, antioxidant enzymes, and heat shock protein 70 of Channa punctata (Bloch, 1793). Fish Physiol. Biochem. 2021, 47, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Gutierrez, J.; Peregrino-Uriarte, A.B.; Gomez-Jimenez, S.; Mata-Haro, V.; Yepiz-Plascencia, G. HIF-1 is involved in the regulation of expression of metallothionein and apoptosis incidence in different oxygen conditions in the white shrimp Litopenaeus vannamei. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2021, 262, 111072. [Google Scholar] [CrossRef]
- Antonio Martos-Sitcha, J.; Simo-Mirabet, P.; de Las Heras, V.; Alvar Calduch-Giner, J.; Perez-Sanchez, J. Tissue-Specific Orchestration of Gilthead Sea Bream Resilience to Hypoxia and High Stocking Density. Front. Physiol. 2019, 10, 840. [Google Scholar] [CrossRef]
- Agusti, C.; Carbajal, A.; Olvera-Maneu, S.; Domingo, M.; Lopez-Bejar, M. Blubber and serum cortisol concentrations as indicators of the stress response and overall health status in striped dolphins. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2022, 272, 111268. [Google Scholar] [CrossRef]
- Du, X.D.; Zhang, W.W.; He, J.; Zhao, M.J.; Wang, J.Q.; Dong, X.J.; Fu, Y.Y.; Xie, X.D.; Miao, S.Y. The Impact of Rearing Salinity on Flesh Texture, Taste, and Fatty Acid Composition in Largemouth Bass Micropterus salmoides. Foods 2022, 11, 3261. [Google Scholar] [CrossRef]
- Lou, F.; Liu, M.; Han, Z.; Gao, T. Comparative transcriptome reveals the thermal stress response differences between Heilongjiang population and Xinjiang population of Lota lota. Comp. Biochem. Physiol. D-Genom. Proteom. 2022, 42, 100960. [Google Scholar] [CrossRef] [PubMed]
- Blikra, M.J.; Jessen, F.; Feyissa, A.H.; Vaka, M.R.; Skipnes, D. Low-concentration salting of cod loins: The effect on biochemical properties and predicted water retention during heating. Lwt-food Sci. Technol. 2020, 118, 108702. [Google Scholar] [CrossRef]
- Lv, H.-B.; Ma, Y.-y.; Hu, C.-T.; Lin, Q.-Y.; Yue, J.-j.-y.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y.; Qiao, F. The individual and combined effects of hypoxia and high-fat diet feeding on nutrient composition and flesh quality in Nile tilapia (Oreochromis niloticus). Food Chem. 2021, 343, 128479. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Dong, X.; He, F.; Shi, Y.; Chai, T. Water holding capacity and microstructure of sturgeon (Acipenser gueldenstaedti) fillets as affected by low temperature vacuum heating. Int. J. Food Prop. 2021, 24, 1061–1073. [Google Scholar] [CrossRef]
- Neyrao, I.M.; Biller, J.D.; Takahashi, L.S.; Urbinati, E.C. Modulation of immunity and hepatic antioxidant defense by corticosteroids in pacu (Piaractus mesopotamicus). Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2021, 260, 111025. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, S.; Liu, R.; Huang, M.; Dong, K.; Ge, J.; Gao, Q.; Zhou, Y. Effects of temperature, dissolved oxygen, and their interaction on the growth performance and condition of rainbow trout (Oncorhynchus mykiss). J. Therm. Biol. 2021, 98, 102928. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Wu, H.Y.; Murata, M.; Matsumoto, H.; Ohnuki, H.; Endo, H. Development of a remote monitoring system for stress response in fish from a physiological and behavioral perspective. Fish Physiol. Biochem. 2025, 51, 1–10. [Google Scholar] [CrossRef]
- Varghese, T.; Mishal, P.; Gupta, G.; Kumar, M.; Pal, A.K.; Dasgupta, S. Temporal changes in behavioural responses and serum metabolites of Cirrhinus mrigala exposed to acute hypoxia. J. Environ. Biol. 2019, 40, 641–647. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Zhang, X.; Shi, Y.; Li, W. Locomotor posture and swimming-intensity quantification in starvation-stress behavior detection of individual fish. Comput. Electron. Agric. 2022, 202, 107399. [Google Scholar] [CrossRef]
- Yalsuyi, A.M.; Hajimoradloo, A.; Ghorbani, R.; Jafari, V.-a.; Prokic, M.D.; Faggio, C. Behavior evaluation of rainbow trout (Oncorhynchus mykiss) following temperature and ammonia alterations. Environ. Toxicol. Pharmacol. 2021, 86, 103648. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, S.-J.; Fu, C. Behavioral adjustments to prior predation experience and food deprivation of a common cyprinid fish species vary between singletons and a group. PeerJ 2019, 7, 7236. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.A.; Aboagye, D.L.; Ramee, S.W.; Allen, P.J. Effects of acute thermal stress on acid-base regulation, haematology, ion-osmoregulation and aerobic metabolism in Channel Catfish (Ictalurus punctatus). Aquac. Res. 2019, 50, 2133–2141. [Google Scholar] [CrossRef]
- Zarei, S.; Ghafoori, H.; Vahdatiraad, L.; Sohrabi, T.; Heidari, B. Effects of HSP inducers on the gene expression of Heat Shock Proteins (HSPs) in cells extracted from sterlet sturgeon under temperature stress with antioxidant and immunity responses. Fish Physiol. Biochem. 2024, 50, 1409–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Qian, Z.; Ji, J.; Wang, T.; Yin, S.W.; Zhang, K. Characterization of HSP70 and HSP90 Gene Family in Takifugu fasciatus and Their Expression Profiles on Biotic and Abiotic Stresses Response. Genes 2024, 15, 1445. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Y.; Meng, W.; Zhong, X.; Shan, Y.; Gao, T. Comparative transcriptomic analysis brings new insights into the response to acute temperature acclimation in burbot (Lota lota lota). Aquac. Rep. 2021, 20, 100657. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ergun, S.; Celik, E.S.; Banni, M.; Ahmadifar, E.; Dawood, M.A.O. The impact of acute cold water stress on blood parameters, mortality rate and stress-related genes in Oreochromis niloticus, Oreochromis mossambicus and their hybrids. J. Therm. Biol. 2021, 100, 103049. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, T.; Ma, A.; Huang, Z.; Liu, Z.; Cui, W.; Zhang, J.; Zhu, C.; Guo, X.; Yuan, C. Metabolic responses in Scophthalmus maximus kidney subjected to thermal stress. Fish Shellfish. Immunol. 2020, 103, 37–46. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Lin, T.; Feng, G.; Wang, X.; Zhang, D. Muscle fiber plasticity, stress physiology, and muscle transcriptome determine the inter-individual difference of swimming performance in the large yellow croaker (Larimichthys crocea). Aquaculture 2023, 567, 739247. [Google Scholar] [CrossRef]
- Jia, Y.; Gao, Y.; Wan, J.; Gao, Y.; Li, J.; Guan, C. Altered physiological response and gill histology in black rockfish, Sebastes schlegelii, during progressive hypoxia and reoxygenation. Fish Physiol. Biochem. 2021, 47, 1133–1147. [Google Scholar] [CrossRef]
- Mandic, M.; Flear, K.; Qiu, P.; Pan, Y.K.; Perry, S.F.; Gilmour, K.M. Aquatic surface respiration improves survival during hypoxia in zebrafish (Danio rerio) lacking hypoxia-inducible factor 1-α. Proc. R. Soc. B-Biol. Sci. 2022, 289, 1863. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Yu, Y.-Y.; Bao, X.-X.; Zhou, J.-H.; Zeng, W.-W.; Peng, Z.-Q.; Yang, y.; Duan, N. miRNA and mRNA expression analysis reveals the effects of continuous heat stress on antibacterial responses to Aeromonas hydrophila lipopolysaccharide (LPS) in grass carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2022, 130, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Vera, L.M.; Montoya, A.; Pujante, I.M.; Perez-Sanchez, J.; Calduch-Giner, J.A.; Mancera, J.M.; Moliner, J.; Sanchez-Vazquez, F.J. Acute stress response in gilthead sea bream (Sparus aurata L.) is time-of-day dependent: Physiological and oxidative stress indicators. Chronobiol. Int. 2014, 31, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Olivetti de Mattos, B.; Fernando Lopez-Olmeda, J.; Guerra-Santos, B.; Espinosa Ruiz, C.; Maria Garcia-Beltran, J.; Angeles-Esteban, M.; Javier Sanchez-Vazquez, F.; Fortes-Silva, R. Coping with exposure to hypoxia: Modifications in stress parameters in gilthead seabream (Sparus aurata) fed spirulina (Arthrospira platensis) and brewer’s yeast (Saccharomyces cerevisiae). Fish Physiol. Biochem. 2019, 45, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Salahinejad, A.; Attaran, A.; Naderi, M.; Meuthen, D.; Niyogi, S.; Chivers, D.P. Chronic exposure to bisphenol S induces oxidative stress, abnormal anxiety, and fear responses in adult zebrafish (Danio rerio). Sci. Total Environ. 2021, 750, 141633. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Ma, Y.B.; Jin, X.W.; Ren, H.M.; Feng, L. Histidine improves flesh quality: An assessment of grass carp (Ctenopharyngodon idella) muscle in terms of texture, nutritional value and flavor. Food Chem. 2025, 474, 143214. [Google Scholar] [CrossRef]
- Lu, Y.; Amenyogbe, E.; Yang, Y.; Wang, Z.L.; Jin, J.H.; Xie, R.T.; Droepenu, E.K.; Huang, J.S. Effects of hypoxia on the heart of the juvenile four-finger threadfin (Eleutheronema tetradactylum) based on physiological indicators and transcriptome analysis. Front. Mar. Sci. 2025, 12, 1530224. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, Y.; Duan, C.Y.; Yang, L.; Zhou, D.Y.; Zhang, Z.X.; Chen, H.P.; Li, G.L.; Zhu, C.H.; Tian, C.X. Effects of chronic high-temperature stress on muscle tissue integrity and metabolism-related genes in Clarias fuscus. Comp. Biochem. Physiol. D-Genom. Proteom. 2025, 55, 101497. [Google Scholar] [CrossRef]




| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| HSP70 | TGC CAT CCT CTC TGG TGA TAA GT | ACC AGC CGT TTC AAT TCC AA |
| HSP90 | CTC CCC AAC GTT CAC GAA | CGG CTT TGG TCA TCC CAA T |
| HIF-1α | TAA CCT CCC ACC TGG ACA AAG CCT C | TCG TTC TTG TCC GCT TCA TCA G |
| Prdx3 | ATC AAC ACC CCA CGC AAG ACT G | ACC GTT TGG ATC AAT GAG GAA CAG ACC |
| β-actin | ATG GTG GGG ATG GGA CAG A | CTG TGA GCA GGA CGG GGT G |
| Treatment | Cortisol’s p-Value | Blood Glucose’s p-Value |
|---|---|---|
| Temperature | <0.001 | <0.001 |
| Oxygen | <0.001 | <0.001 |
| Temperature × Oxygen | 0.003 | 0.020 |
| Treatment | HSP70 | HSP90 | HIF-1α | Prdx3 |
|---|---|---|---|---|
| Temperature | <0.001 | <0.001 | <0.001 | <0.001 |
| Oxygen | <0.001 | <0.001 | <0.001 | <0.001 |
| Temperature × Oxygen | 0.014 | 0.010 | 0.029 | 0.040 |
| Index (%) | S0 | S1 | S2 |
|---|---|---|---|
| Drip loss | 3.70 ± 0.24 a | 5.60 ± 0.41 b | 5.47 ± 0.37 b |
| Cooking loss | 18.74 ± 0.25 a | 22.31 ± 1.58 b | 21.29 ± 0.74 b |
| Centrifugal loss | 15.10 ± 0.14 a | 17.87 ± 0.45 b | 18.40 ± 0.54 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yang, H.; Mao, H.; Shi, Q. The Effects of Interactions Between Key Environmental Factors on Non-Specific Indicators in Carassius auratus. Fishes 2025, 10, 372. https://doi.org/10.3390/fishes10080372
Wang B, Yang H, Mao H, Shi Q. The Effects of Interactions Between Key Environmental Factors on Non-Specific Indicators in Carassius auratus. Fishes. 2025; 10(8):372. https://doi.org/10.3390/fishes10080372
Chicago/Turabian StyleWang, Bin, Hang Yang, Hanping Mao, and Qiang Shi. 2025. "The Effects of Interactions Between Key Environmental Factors on Non-Specific Indicators in Carassius auratus" Fishes 10, no. 8: 372. https://doi.org/10.3390/fishes10080372
APA StyleWang, B., Yang, H., Mao, H., & Shi, Q. (2025). The Effects of Interactions Between Key Environmental Factors on Non-Specific Indicators in Carassius auratus. Fishes, 10(8), 372. https://doi.org/10.3390/fishes10080372







