Abstract
The barbel chub (Squaliobarbus curriculus) exhibits remarkable resistance to grass carp reovirus (GCRV), a devastating pathogen in aquaculture. To reveal the molecular basis of this resistance, we investigated complement factor D (DF)—a rate-limiting serine protease governing alternative complement pathway activation. Molecular cloning revealed that the barbel chub DF (ScDF) gene encodes a 1251-bp cDNA sequence translating into a 250-amino acid protein. Crucially, bioinformatic characterization identified a unique N-glycosylation site at Asn139 in ScDF, representing a structural divergence absent in grass carp (Ctenopharyngodon idella) DF (CiDF). While retaining a conserved Tryp_SPc domain harboring the catalytic triad (His61, Asp109, and Ser204) and substrate-binding residues (Asp198, Ser219, and Gly221), sequence and phylogenetic analyses confirmed ScDF’s evolutionary conservation, displaying 94.4% amino acid identity with CiDF and clustering within the Cyprinidae. Expression profiling revealed constitutive ScDF dominance in the liver, and secondary prominence was observed in the heart. Upon GCRV challenge in S. curriculus kidney (SCK) cells, ScDF transcription surged to a 438-fold increase versus uninfected controls at 6 h post-infection (hpi; p < 0.001)—significantly preceding the 168-hpi response peak documented for CiDF in grass carp. Functional validation showed that ScDF overexpression suppressed key viral capsid genes (VP2, VP5, and VP7) and upregulated the interferon regulator IRF9. Moreover, recombinant ScDF protein incubation induced interferon pathway genes and complement C3 expression. Collectively, ScDF’s rapid early induction (peaking at 6 hpi) and multi-pathway coordination may contribute to barbel chub’s GCRV resistance. These findings may provide molecular insights into the barbel chub’s high GCRV resistance compared to grass carp and novel perspectives for anti-GCRV breeding strategies in fish.
Key Contribution:
This study reveals that ScDF harbors a unique Asn139 glycosylation site absent in the grass carp. Furthermore, ScDF demonstrates earlier responsiveness to GCRV infection compared to its ortholog in grass carp. Functional analyses confirm that ScDF overexpression significantly suppresses GCRV replication while activating the interferon regulatory factor IRF9. Recombinant ScDF protein further induces the synergistic upregulation of interferon and complement C3 expression, suggesting its potential as a novel anti-GCRV target for breeding in fish.
1. Introduction
Grass carp reovirus (GCRV), a double-stranded RNA virus of the genus Aquareovirus, causes hemorrhagic disease in grass carp (Ctenopharyngodon idella), posing a severe threat to aquaculture [1]. Characterized by high infectivity and lethality, this disease causes substantial annual economic losses. Notably, the barbel chub (Squaliobarbus curriculus), a cyprinid fish within the same subfamily (Leuciscinae) as grass carp, exhibits remarkable GCRV resistance: artificial GCRV infection trials revealed significantly higher survival rates in barbel chub than grass carp, with GCRV outbreaks rarely occurring in natural or cultured barbel chub populations [2]. This species-specific divergence makes the barbel chub an ideal model for identifying key immune regulators against GCRV.
Evidence suggests that GCRV resistance correlates with unique immune defense mechanisms: barbel chub demonstrates superior serum complement activity, lysozyme concentrations, and phagocytic capacity compared to grass carp [2,3]. Critically, KEGG enrichment analysis of serum proteomes from our previous work revealed that the coagulation–complement cascade pathway exhibits as the most prominent differential expression between barbel chub and grass carp [3]. This finding also implies that early complement regulation may be central to the barbel chub’s defense against GCRV.
The complement system bridges innate and adaptive immunity in vertebrates, executing multifaceted roles in pathogen defense, including opsonization (C3b-mediated), inflammation (C3a/C5a-driven chemotaxis), and direct pathogen lysis via the membrane attack complex (MAC) [4,5,6,7]. In teleost fish, where adaptive immunity is less developed, the complement system serves as a primary defense barrier against viruses [8,9,10]. Fish complement activation occurs through three pathways: the classical pathway (CP), the lectin pathway (LP), and the alternative pathway (AP) [11,12,13]. Complement factor D (DF), a rate-limiting serine protease of the AP, initiates cascade amplification through cleavage of factor B (Bf) to form C3 convertase (C3bBb), following spontaneous C3 hydrolysis [4,7,14,15].
DF’s indispensable role is evident in mammals: human DF deficiency increases susceptibility to Neisseria meningitidis, while DF-knockout mice develop bacterial colitis [16,17]. DF genes have been cloned and characterized in a variety of fish, and data suggest that fish DF plays an important role in the host immune defense [18,19,20]. Notably, several teleost DF genes have been cloned and functionally characterized, with accumulating evidence demonstrating their inducible expression profiles upon diverse pathogen challenges. Following Edwardsiella ictaluri infection, channel catfish (Ictalurus punctatus) exhibits significant DF transcriptional upregulation in the gill, liver, spleen, and kidney tissues [19]. Similarly, Aeromonas hydrophila exposure triggers a 3.7-fold and 16-fold DF elevation in hepatic and renal tissues of blunt snout bream (Megalobrama amblycephala), respectively [18]. During Streptococcus iniae and rock bream iridovirus (RBIV) co-infection, splenic DF transcripts in rock bream (Oplegnathus fasciatus) peak distinctly at 6 h and 12 h post-infection (hpi) [20]. In olive flounder (Paralichthys olivaceus) infected with viral hemorrhagic septicemia virus (VHSV), the kidney, liver, and spleen display transient DF upregulation within 24 h, with the maximal induction (6 hpi) observed specifically in renal tissue [21]. In striking contrast, grass carp exhibits delayed DF activation during late-phase GCRV infection (144–168 hpi), where sustained high expression persists in liver, spleen, and intestinal tissues [22]. Critically, despite the established roles in initiating the alternative complement pathway and confirmed pathogen-responsive induction across teleosts, precise antiviral mechanisms of DF—particularly against dsRNA viruses such as GCRV—remain poorly characterized, including its molecular interplay with interferon signaling, the kinetics of immune complex formation, and species-specific functional divergence.
Significantly, our prior comparative transcriptomics results revealed pronounced upregulation of complement factors (including DF) in GCRV-infected barbel chub versus grass carp [2,22]. In grass carp, DF (CiDF) expression dynamically responds to GCRV infection, suggesting its involvement in antiviral responses [22,23]. The previous results suggest that the rapid and potent activation of ScDF may be the key node for the antiviral advantage of barbel chub, and we hypothesize that the rapid and/or potent response of ScDF is the key to its antiviral advantage. We further raise critical questions: does DF from the highly resistant barbel chub (ScDF) possess unique structural or functional attributes? How does ScDF confer superior antiviral capacity? In order to address these questions, we cloned the full-length cDNA of ScDF, analyzed its gene and protein structure distinctions from CiDF, and investigated its tissue-specific expression patterns, temporal dynamics in SCK cells post-GCRV challenge, and functional roles via overexpression and recombinant protein assays. This study may elucidate ScDF’s molecular characteristics and antiviral mechanisms, provide insights into the divergent anti-GCRV strategies between barbel chub and grass carp, and offer novel perspectives for anti-GCRV breeding strategies in fish.
2. Materials and Methods
2.1. Experimental Fish, Cell Line, and Virus
Healthy barbel chub (Squaliobarbus curriculus) (body length: 10–15 cm) were provided from Hunan Haibo Laboratory (Kaifu District, Changsha, China). The fish were acclimated in a recirculating aquaculture system (28 °C) and fed a commercial feed twice a day before processing (feeding amount: 3% of fish body weight daily). Aquaculture water quality was maintained within optimal survival ranges for fish: pH 7.0–8.5, dissolved oxygen (DO) > 5 mg/L, and total ammonia nitrogen (TAN) < 0.2 mg/L. The S. curriculus kidney (SCK) cell line, maintained in our laboratory, was cultured in M199 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) at 28 °C. The grass carp reovirus (GCRV) strain JX-0901 was propagated in SCK cells, with a viral titer of 106.5/mL determined using the TCID50 method. Aliquots were stored at −80 °C.
2.2. RNA Extraction, cDNA Synthesis, and Quantitative PCR
Total RNA was extracted from tissues and cells using the TransZol Up Plus RNA Kit (TransGen Biotech, Beijing, China), treated with DNase I, and reverse-transcribed into cDNA using the RevertAid™ cDNA Synthesis Kit (Thermo Fisher, Waltham, MA, USA). Quantitative PCR (qPCR) was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) on a Bio-Rad CFX96 system under the following conditions: 95 °C for 30 s; 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The β-actin gene served as the internal control (primers shown in Table 1), and the relative expression of target genes was quantified using the 2−ΔΔCt method [2]. For analyzing the tissue mRNA quantity of ScDF, the 2−ΔCt normalization was employed according to previous descriptions [22].

Table 1.
Primers used for full-length cDNA cloning and qPCR (all qPCR amplicons ranged from 200 to 250 bp in length).
2.3. Cloning and Bioinformatics Analysis of ScDF
Total RNA was extracted from barbel chub liver tissue, and the full-length cDNA sequence of ScDF was amplified using PCR (primers ScDF-F/R shown in Table 1). The PCR product was purified using the FastPure Gel DNA Extraction Mini Kit (Vazyme) and cloned into the pMD19-T (Takara, Dalian, China) vector for sequencing. The obtained ScDF sequence was subjected to bioinformatics analysis: the physicochemical properties of the protein were predicted using ExPASy Compute pI/Mw (https://web.expasy.org/compute_pi/, accessed on 5 August 2024); the signal peptide was analyzed using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/, accessed on 5 August 2024); the N-glycosylation sites were predicted based on NetNGlyc 1.0 (http://www.cbs.dtu.dk/services/NetNGlyc/, accessed on 5 August 2024); the protein domains were identified using SMART (http://smart.embl-heidelberg.de/, accessed on 5 August 2024); the tertiary structure was predicted via homology modeling using SWISS-MODEL (https://swissmodel.expasy.org/, accessed on 6 August 2024) and visualized with PyMOL 3.0 software (https://www.pymol.org/, accessed on 6 August 2024); the phylogenetic tree was constructed using MEGA 11 [24] (neighbor-joining method, bootstrap = 1000) and edited online using the iTOL tool (http://itol.embl.de, accessed on 8 August 2024), according to previous descriptions [2]. The amino acid sequences of DFs and their accession numbers used for phylogenetic tree construction, and their corresponding species information are presented in Table 2. Species were chosen based on the following: (i) phylogenetic representativeness, (ii) availability of high-quality DF sequences, and (iii) relevance to teleost complement evolution, with emphasis on the Cyprinidae.

Table 2.
Species and accession numbers of DF sequences used for phylogenetic analysis.
2.4. Plasmid Construction
For overexpression assays, the ScDF coding sequence was cloned into the pEGFP-N1-Flag (Vazyme, Nanjing, China) using Xho I/BamH I restriction sites, generating the recombinant plasmid pEGFP-N1-Flag-ScDF. For recombinant protein expression, the signal peptide-truncated ScDF (Ile21~Gln250) sequence was ligated into pCold-TF-His (Takara, Dalian, China) using Nde I/Xba I restriction sites, yielding the recombinant plasmid pCold-TF-His-ScDFΔSP. Signal peptide truncation was applied only for prokaryotic expression due to Escherichia coli’s inability to process eukaryotic signal peptides. For eukaryotic expression assays, full-length ScDF was used to ensure proper secretion. All constructs were verified by sequencing.
2.5. Overexpression and Viral Challenge Assay
SCK cells (5 × 105 cells/well) were transfected with pEGFP-N1-Flag-ScDF or the empty vector (control) using PEI MAX (Polysciences, Niles, IL, USA). After 24 h, the cells were infected with GCRV (multiplicity of infection (MOI) = 1). Cells were harvested at 6, 12, 24, and 48 hpi for qPCR analysis of expression of viral genes (VP2, VP5, and VP7) and immune genes, including interferon regulatory factor 9 (IRF9), nuclear factor kappa B (NF-κB), tumor necrosis factor alpha (TNF-α), and interleukin 8 (IL-8).
2.6. Recombinant Protein Incubation Assay
For prokaryotic expression, the recombinant plasmid pCold-TF-His-ScDFΔSP was transformed into E. coli BL21(DE3) competent cells for overnight culture. Selected transformants were isolated and grown in LB medium supplemented with 100 μg/mL ampicillin (Beyotime, Shanghai, China) at 37 °C under 220 rpm shaking for 4 h. At the mid-log phase of bacteria (OD600 reached 0.4–0.6), isopropyl-β-d-thiogalactopyranoside (IPTG; Beyotime) was added to a final concentration of 1 mM, followed via induction at 16 °C with 140 rpm shaking for 24 h. Bacterial cultures were then subjected to ultrasonication and centrifugation. The resulting supernatant was purified using a His-Tag Protein Purification Kit with NTA-Ni Magnetic Agarose Beads (Beyotime). The purified recombinant ScDF proteins were resolved using 12% SDS-PAGE (GenScript, Nanjing, China) and stained with Coomassie Brilliant Blue R-250 (Bio-Rad, Hercules, CA, USA) for visualization. Protein concentration was determined using the BCA Protein Assay Kit (Beyotime) and adjusted to 20 μg/mL. For the recombinant protein incubation assay, SCK cells were incubated with ScDF or BSA (control), followed by GCRV infection (MOI = 1). At 1 and 24 hpi, SCK cells were harvested, and the mRNA expression of interferon 1 (IFN-1), IFN-2, interferon-stimulated gene 15 (ISG15), TNF-α, IRF9, and C3 were quantified via qPCR.
2.7. Statistical Analysis
Data are presented as the mean ± SEM (n = 3). Significance was determined using one-way or two-way ANOVA with Tukey’s multiple comparison test using the Statistical Package for Social Sciences Version 18.0 (SPSS Inc., Chicago, IL, USA). Differences were considered significant at p < 0.05.
3. Results
3.1. Molecular Characterization of ScDF Reveals a Unique Glycosylation Site
Gene structure analysis using barbel chub genomic annotation files and whole-genome sequencing data identified the ScDF gene as comprising four introns and five exons, along with a 433-bp 5′ untranslated region (UTR) and a 65-bp 3′ UTR (Figure 1A). To investigate structural differences between barbel chub and grass carp DF, a comparative analysis revealed that grass carp CiDF (GenBank: XM_051917986.1, chromosome 13) shares an identical exon-intron architecture (four introns and five exons) with ScDF but exhibits a shorter 5′ UTR (38 bp) and a longer 3′ UTR (131 bp) (Figure 1A). These findings suggest evolutionary conservation of DF gene organization between fish species, while highlighting the expanded 5′ UTR in ScDF as a potential regulatory divergence compared to CiDF (Figure 1A).
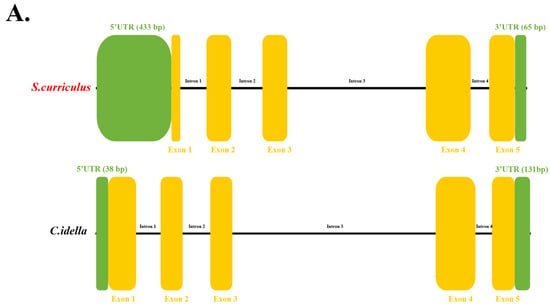
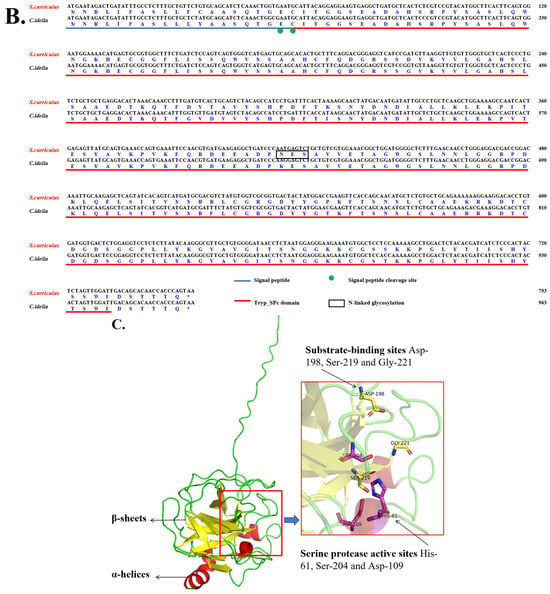
Figure 1.
Structural features of ScDF. (A) Gene structure schematic of ScDF and CiDF. Yellow blocks represent exons, green blocks represent UTRs, and black lines between yellow blocks represent introns. (B) Amino acid sequence alignment of ScDF and CiDF revealing a unique N-glycosylation site at Asn139 in ScDF. (C) Tertiary structure of ScDF predicted using SWISS-MODEL (https://swissmodel.expasy.org/, accessed on 6 August 2024). Substrate-binding sites and serine protease active sites of ScDF are displayed and highlighted.
Subsequent cloning yielded the full-length ScDF cDNA of 1251 bp, containing a 753-bp open reading frame (ORF) encoding a 250-amino acid protein. Bioinformatics analysis predicted a molecular weight of 27.5 kDa and an isoelectric point (pI) of 6.30 for ScDF. Crucially, sequence alignment identified a unique N-glycosylation site at Asn139 in ScDF, which is absent in CiDF (Figure 1B).
Tertiary structure modeling demonstrated that ScDF folds into three α-helices and 13 β-sheets (Figure 1C), retaining the conserved catalytic triad (His61, Asp109, and Ser204) and substrate-binding residues (Asp198, Ser219, and Gly221) (Figure 1C). This structural configuration confirms a functional serine protease conformation consistent with CiDF, suggesting that Asn139 glycosylation may contribute to functional specialization despite overall structural homology [22].
3.2. ScDF Is an Extracellular Protein with Conserved Domain Architecture
Domain prediction analysis demonstrated that ScDF mainly contains a Tryp_SPc domain and a signal peptide composed of 20 amino acids at its N-terminus, which means that ScDF is most likely an extracellular protein. To further explore the conservation of ScDF protein sequence features and functional sites, we further aligned the ScDF protein sequence with various vertebrate DF protein sequences. The statistical results of the similarity and identity of the comparison showed that ScDF shared higher similarity and identity with the DFs from other cyprinid fish (Figure 2A). Among them, ScDF and CiDF showed the highest similarity (96.4%) and identity (94.4%) (Figure 2A). At the same time, protein sequence analysis and alignment showed that ScDF protein was highly conserved with a typical Tryp_SPc domain (amino acid positions 20~244), three serine protease active sites, three substrate binding sites and three cysteine residues possibly involved in disulfide bond pairing, which were also conserved in DFs from other teleosts (Figure 2B).
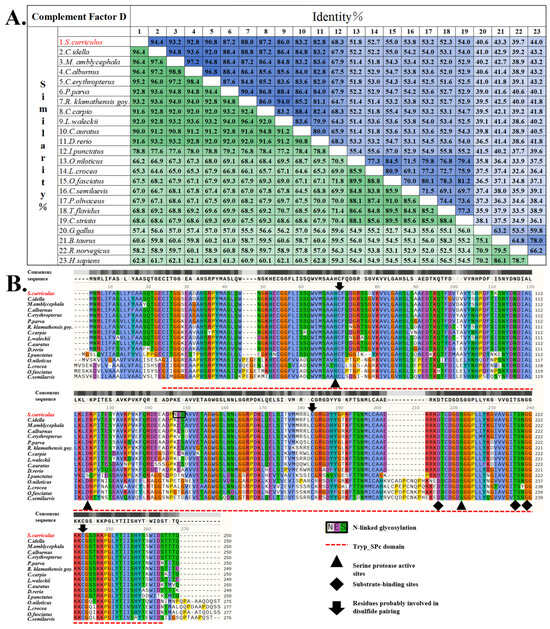
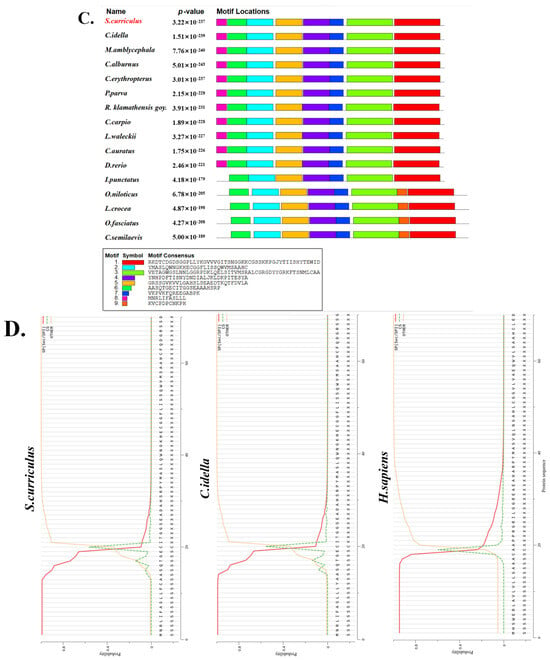
Figure 2.
ScDF is an extracellular protein with conserved domain architecture. (A) Amino acid sequence similarity and identity heatmap of 23 vertebrate DFs. The depth of the color block indicates the level of the similarity/identity value. (B) Multiple sequence alignment of 16 teleost DFs revealing the conserved Tryp_SPc domain with the catalytic triad and substrate-binding residues in ScDF. The top gray bar indicates sequence conservation levels through shading intensity, while colored highlights denote properties of conserved residues based on multiple sequence alignment. (C) Conserved motif distribution across 16 teleost DFs. The top 11 cyprinid DFs shared eight identical structural modules. (D) Signal peptide conservation in ScDF, CiDF, and HsDF predicted using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/, accessed on 5 August 2024). Accession numbers for DF sequences are listed in Table 2.
Subsequent motif comparison across various teleost DFs confirmed eight identical structural modules among all analyzed cyprinid DF proteins (Figure 2C). Notably, the signal peptide conservation in ScDF, CiDF, and human DF (HsDF) (Figure 2D) was observed, which underscored the indispensable role of signal peptide in DFs’ secretory trafficking and extracellular activation, consistent with the established mechanism of complement factor function.
3.3. Evolutionary Conservation of ScDF Within Vertebrate Complement Systems
Phylogenetic reconstruction using DF amino acid sequences from 23 vertebrates demonstrated that ScDF clusters within the Cyprinidae clade alongside CiDF (Figure 3). The topology exhibited high bootstrap support (>90%) for monophyletic groupings corresponding to major vertebrate lineages (Figure 3); teleosts formed a distinct branch separated from tetrapods (mammals/birds), with divergence timelines aligning with key evolutionary events. The initial cladogenesis occurred approximately 400 million years ago (Mya), during the divergence between teleost and tetrapod ancestors, followed by a secondary radiation of 300 Mya when amniotes split into mammalian and sauropsid (including avian) lineages. All DFs represented orthologs derived from a common vertebrate ancestor, with intra-clade sequence conservation (e.g., cyprinids) contrasting sharply with reduced homology across divergent lineages (e.g., mammals vs. teleosts), correlating positively with phylogenetic distance (Figure 2A and Figure 3).
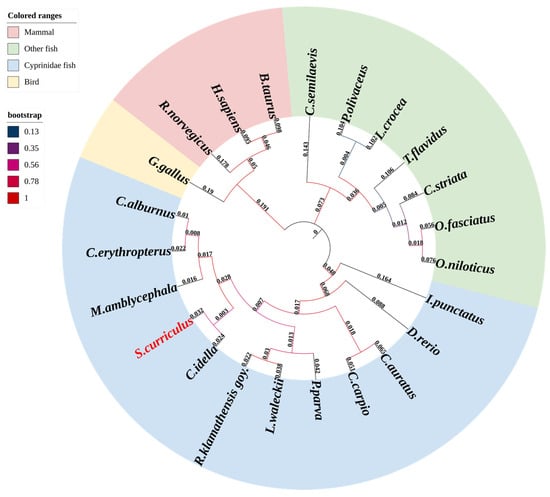
Figure 3.
Phylogenetic analysis of ScDF evolutionary conservation. A neighbor-joining phylogenetic tree was constructed with MEGA 11 (bootstrap = 1000 replicates), using DF amino acid sequences from 23 vertebrate species (accession numbers listed in Table 2).
3.4. Hepatic Dominance of ScDF and Its Early Response to GCRV Outpace CiDF
Tissue-specific profiling identified constitutive ScDF expression across nine tissues, including liver, heart, spleen, kidney, head kidney, intestines, muscle, gill, skin, and fin, with the liver exhibiting the highest abundance, followed by the heart (Figure 4A), consistent with the liver’s conserved role as the primary site of complement synthesis. During GCRV infection in SCK cells, ScDF transcription peaked dramatically at 6 h post-infection (hpi) (438-fold induction vs. 0 hpi; p < 0.001), coinciding with a sharp upregulation of the viral capsid gene VP7 expression (p < 0.001) (Figure 4B). Transcript levels of ScDF rapidly declined thereafter (12 hpi: 11-fold; 24 hpi: 3.5-fold; 48 hpi: 10-fold), demonstrating its tight synchronization with viral replication kinetics (Figure 4B). In stark contrast, grass carp CiDF exhibited a delayed response peak at 168 hpi [22]. This immediate early induction profile positions ScDF as a rapid-response effector in barbel chub antiviral defense, triggering immune activation prior to viral establishment.
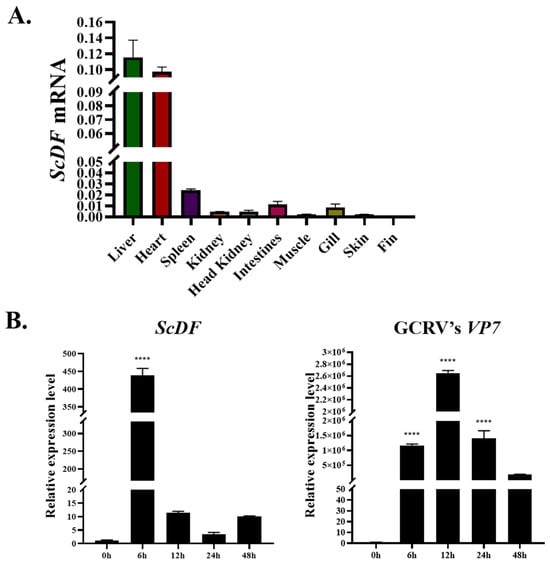
Figure 4.
Hepatic dominance and early antiviral response of ScDF. (A) Tissue-specific distribution of constitutive ScDF mRNA in healthy barbel chub. Relative expression was quantified using the 2−ΔCt method to reflect absolute transcript abundance of ScDF in various tissues. (B) Time-course analysis of ScDF and viral VP7 expression in GCRV-infected SCK cells. The 2−ΔΔCt calculation method was employed to reveal fold-changes of ScDF and viral VP7 expression. Data represent mean ± SEM (n = 3; **** p < 0.0001; one-way ANOVA with Tukey’s test).
3.5. ScDF Overexpression Suppresses GCRV Replication
Confocal microscopy and qPCR verification showed that ScDF could be significantly overexpressed in SCK cells (Figure 5A,B). Transient overexpression of Flag-tagged ScDF (via transfection with pEGFP-N1-Flag-ScDF) in SCK cells significantly inhibited viral replication under GCRV challenge: the relative mRNA expression levels of viral capsid genes VP2, VP5, and VP7 were reduced at 6–24 hpi (Figure 5C). Concurrently, immune gene expression analysis revealed marked upregulation of the interferon pathway regulator IRF9 (10.52-fold induction at 6 hpi, p < 0.001) and pro-inflammatory factors (TNF-α, NF-κB, and IL-8) after ScDF overexpression under GCRV challenge (Figure 5D). These data indicate that ScDF orchestrates early antiviral responses by coordinately activating interferon signaling and inflammatory cascades to suppress viral proliferation.
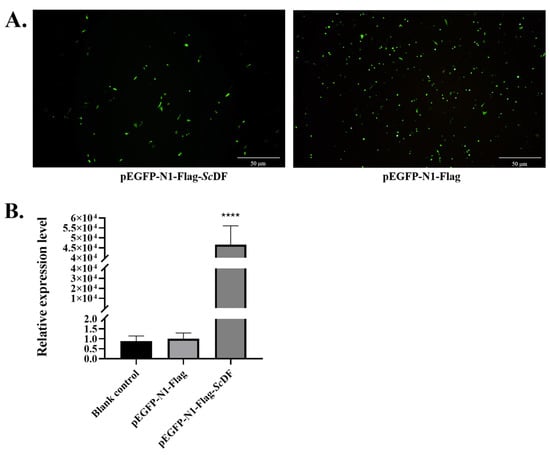
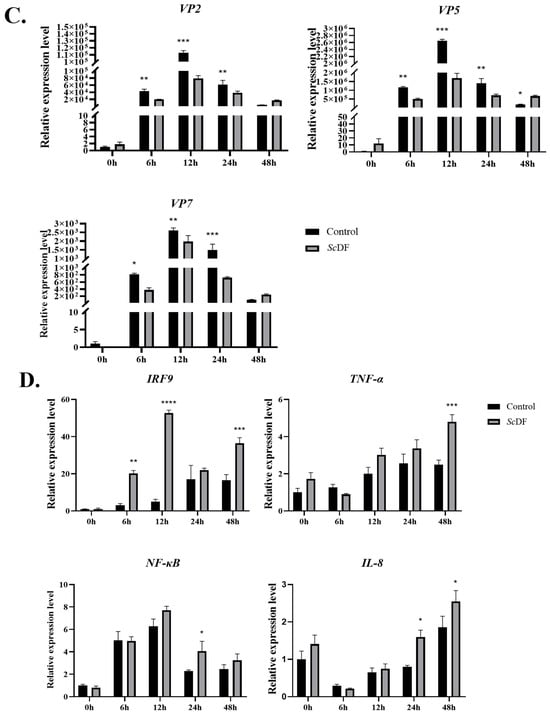
Figure 5.
Antiviral function of ScDF overexpression. (A) Transfections of pEGFP-N1-Flag-ScDF and pEGFP-N1-Flag (control) in SCK cells under confocal microscopy. (B) Confirmation of overexpression of ScDF at the mRNA level. Data represent mean ± SEM (n = 3; **** p < 0.0001 vs. blank control and pEGFP-N1-Flag groups; one-way ANOVA with Tukey’s test). (C) Suppression of viral capsid genes (VP2, VP5, and VP7) expression after ScDF overexpression during GCRV challenge at 0, 6, 12, 24, and 48 h. The *, **, and *** represent p < 0.05, p < 0.01, and p < 0.001, respectively. (D) Induction of immune regulator genes (IRF9, NF-κB, TNF-α, and IL-8) after ScDF overexpression under GCRV challenge at 0, 6, 12, 24, and 48 h. The 2−ΔΔCt calculation method was employed to reveal fold-changes of targeted gene expression. Data represent mean ± SEM (n = 3; * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 vs. vector control; two-way ANOVA with Tukey’s test).
3.6. Recombinant ScDF Protein Synergistically Activates Interferon and Complement Pathways
Recombinant His-tagged ScDF (with the signal peptide-truncated) was successfully obtained via prokaryotic expression. In the case of GCRV infection for 24 h, exogenous ScDF incubation (20 μg/mL) induced significant upregulation of interferon pathway genes (IFN-1: 3.21-fold, IFN-2: 2.97-fold, and ISG15: 3.75-fold; p < 0.01), complement component C3 (3.21-fold) and TNF-α (3.02-fold) compared to the control group (Figure 6). The results suggest that ScDF may directly stimulate cells to synergistically enhance interferon signaling and complement-inflammation networks, thereby amplifying the host antiviral state.
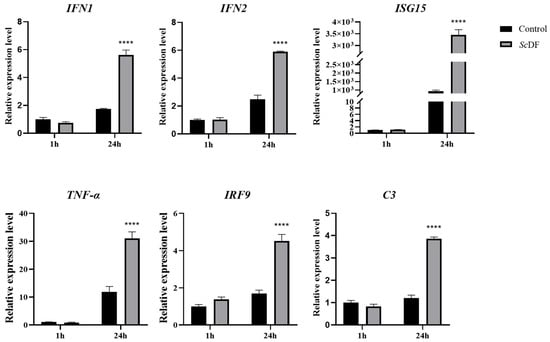
Figure 6.
Immunomodulatory effects of recombinant ScDF. Recombinant ScDF induced immune gene expression in GCRV-infected SCK cells. Cells were pre-incubated with 20 μg/mL recombinant ScDF (or BSA control) for 6 h, followed by GCRV infection (MOI = 1) for 24 h. Expression levels of interferon pathway genes (IFN-1, IFN-2, and ISG15), complement component C3, and inflammatory mediators (TNF-α and IRF9) were quantified using qPCR at 1 hpi and 24 hpi. The 2−ΔΔCt calculation method was employed to reveal fold-changes of targeted gene expression. Data represent mean ± SEM (n = 3; **** p < 0.0001; two-way ANOVA with Tukey’s test).
4. Discussion
As the first line of host immune defense, the complement system exhibits multiple biological functions, including lysis of pathogens, mediation of cellular inflammatory response, and regulation of phagocytosis [5]. A large number of experimental data has proven that complement system members play important roles in the struggle against pathogens in fish [13,25]. Similarly, complement components are also involved in the immune defense response of grass carp to GCRV infection, which involves multiple links such as signal transduction, cytokine regulation, and pathogen recognition [26,27]. In this study, we focused on functional complement molecules closely related to GCRV resistance from the perspective of immune mechanism differences between two species of fish (barbel chub and grass carp) under natural conditions and artificial infection.
This study systematically elucidates the potential role of barbel chub complement factor D (ScDF) in countering GCRV infection. By integrating molecular structural analyses, expression dynamics, and functional validation data, we demonstrate that ScDF orchestrates an efficient antiviral defense network via coordinating interferon, complement, and inflammatory pathways through its unique structural attributes and rapid early-response mechanism.
Bioinformatic analysis revealed a distinct N-glycosylation site at Asn139 in ScDF, which is notably absent in CiDF (Figure 1B). Glycosylation is recognized to enhance protein stability and optimize immune function [28,29,30,31], as evidenced by N-glycosylation-mediated regulation of C3 complement cascade activity in mammals [32]. Remarkably, ScDF and CiDF share 96.4% amino acid sequence similarity and 94.4% identity, and both retain conserved structural features, including the intact catalytic triad (His61, Asp109, and Ser204), identical substrate-binding residues (Asp198, Ser219, and Gly221), and equivalent tertiary architecture (3 α-helices and 13 β-sheets). However, the presence of the Asn139 glycosylation site likely constitutes a key structural basis for functional divergence between ScDF and CiDF. This observation aligns with established evolutionary patterns in teleost complement systems, where functional heterogeneity arises through structural diversification (e.g., thioester bonds and C345C domains) despite shared ancestry of components like C3, C4, and C5 [33]. Nevertheless, direct evidence linking glycosylation to ScDF enzymatic activity or stability remains elusive, and future studies should employ site-directed mutagenesis (e.g., Asn139) combined with mass spectrometry to verify glycosylation status and assess its impact on ScDF stability and enzymatic activity. This would clarify whether this structural divergence underlies functional differences between ScDF and CiDF.
The early-response kinetics of ScDF may represent a defining characteristic of barbel chub’s antiviral superiority. Tissue-specific profiling indicated that constitutive ScDF expression was highest in the liver, followed by the heart, with moderate levels in the spleen, which is consistent with the conserved hepatosynthesis of complement factors [9]. Crucially, during GCRV infection in SCK cells, ScDF transcription peaked sharply at 6 h post-infection (hpi), synchronizing with the replication peak of viral capsid gene VP7. This rapid activation starkly contrasts with the delayed response observed in grass carp, where CiDF peaks at 168 hpi [22]. Such temporal disparity may stem from dual mechanisms: firstly, ScDF possesses an extended 5′ UTR (433 bp versus 38 bp in CiDF) (Figure 1A), potentially harboring additional cis-regulatory elements that facilitate accelerated transcriptional activation; secondly, the elevated baseline complement activity in barbel chub provides a pre-emptive immunological resource pool [2]. Functionally, ScDF overexpression during the early infection (6–24 hpi) significantly suppressed GCRV capsid gene expression, with a 68.8% decrease in VP2, a 61.2% reduction in VP5, and a 54.3% decline in VP7 at 24 hpi (p < 0.01) (Figure 5C). This explosive early expression enables immune activation before viral establishment. However, the transient suppression of GCRV genes (VP2, VP5, and VP7) through ScDF overexpression at 24 hpi—absent at 48 hpi—suggests viral adaptation or host immune response fatigue. GCRV may deploy immune-evasion mechanisms (e.g., VP35-mediated IFN suppression) by 48 hpi, overwhelming early defenses. Future studies should examine viral countermeasures against the complement pathways.
Synergistic coordination of multiple pathways through ScDF amplifies antiviral states. Recombinant ScDF protein (signal peptide-truncated) incubation directly triggered substantial upregulation of interferon pathway genes (3.21-fold upregulation of IFN-1, 2.85-fold induction of IFN-2, and 3.75-fold increase in ISG15) and complement component C3 (3.21-fold elevation of C3). This mirrors the pronounced induction of IRF9 in overexpression experiments. As a typical member of the IRF family, IRF9 plays a unique signal transduction hub role in antiviral immune response [34]. IRF9 plays an irreplaceable role in maintaining the body’s sustained antiviral state through the STAT-IRF9 signal amplification loop [34]. Our team identified the IRF9 gene in barbel chub in the early stage and found that it can realize the immune response by regulating the expression of interferon and mediating the production of interferon [34]. It belongs to the JAK/STAT signaling pathway and plays an important role in inhibiting viral disease [35,36]. The results suggest that ScDF amplifies immune responses through cross-talk between interferon signaling and complement cascades.
Such synergy holds significant evolutionary implications for teleosts, wherein the complement system serves as the primary antiviral barrier due to underdeveloped adaptive immunity [26]. Concurrent elevation of inflammatory cytokines (TNF-α and IL-8) aligns with zebrafish (Danio rerio) studies demonstrating TNF-α-mediated viral suppression via NF-κB modulation [37], and Nile tilapia (Oreochromis niloticus) research, where IL-8 potentiates inflammatory responses [38]. Moreover, ScDF-induced C3 upregulation may synergize with membrane attack complex (MAC)-mediated pathogen lysis (executed via terminal complement components C6–C9), to enhance antiviral efficacy. However, direct validation of ScDF’s activation efficiency in the alternative pathway (e.g., via Bf cleavage or C3 convertase activity assays) remains unaddressed, necessitating future in vitro enzymatic studies to clarify its canonical complement functions.
Notably, recombinant ScDF protein directly induces interferon pathway genes (IFN-1/2 and ISG15), complement C3, and inflammatory factor TNF-α (Figure 6), revealing a novel function beyond the classical complement cascade; as a potential immunomodulatory signaling molecule, secreted ScDF may directly activate cellular immune responses via unknown receptors. This synergistic activation of multiple pathways (interferon antiviral status, complement effect, and inflammatory recruitment) constructs a hierarchical early defense network that explains the core mechanism of ScDF’s potent antiviral activity in barbel chub. Future work will need to decipher the molecular basis of this novel immunomodulatory function by elucidating ScDF’s cell surface receptor and its downstream signaling pathways, such as the toll-like receptor pathway.
Collectively, ScDF’s rapid early induction (peaking at 6 hpi) and multi-pathway coordination may provide key molecular insights into the barbel chub’s GCRV resistance: its expression peak coincides with the sharp upregulation of viral genes and drives a preemptive defense strategy (e.g., >10-fold IRF9 induction)—distinct from the lagged response of CiDF in grass carp (168 hpi). This mechanism likely underlies the rarity of GCRV outbreaks in natural barbel chub populations. Given ScDF’s nodal role in antiviral immunity, it represents a promising molecular target for grass carp breeding programs. Strategies such as CRISPR/Cas9-mediated gene editing or ectopic expression to enhance its early-response capacity hold substantial translational potential for disease control in aquaculture.
5. Conclusions
This study comprehensively investigated the molecular characteristics and antiviral functions of complement factor D (ScDF) in barbel chub (Squaliobarbus curriculus) against grass carp reovirus (GCRV). The research successfully cloned the full-length ScDF cDNA, revealing a unique N-glycosylation site at Asn139 in the ScDF protein, which is absent in grass carp DF (CiDF). Expression profiling demonstrated that ScDF exhibits hepatic dominance and peaks rapidly at 6 hpi in GCRV-challenged cells, significantly earlier than the delayed response of CiDF observed in grass carp. Functional assays confirmed that ScDF overexpression suppresses viral replication by downregulating key capsid genes (VP2, VP5, and VP7) and upregulating interferon regulator IRF9. Furthermore, recombinant ScDF protein incubation synergistically activated interferon pathway genes (IFN-1, IFN-2, and ISG15) and complement component C3, highlighting its role in orchestrating a multi-pathway antiviral defense network.
This work bridges a critical knowledge gap by revealing ScDF’s coordination of multi-pathway antiviral defenses, offering foundational insights for developing targeted disease-resistance strategies in aquaculture. To further advance these findings, future studies could prioritize validating ScDF’s immunomodulatory functions in vivo and decipher glycosylation-dependent mechanisms through advanced structural and functional approaches. Such efforts could optimize the application of ScDF in breeding programs to enhance antiviral resilience in economically important fish species.
Author Contributions
Conceptualization, Y.L. and Z.L.; methodology, Y.X. and Y.W.; software, Y.X.; validation, Y.X., Y.W. and M.Z.; formal analysis, Y.X., H.Y. and Y.L.; investigation, Y.X., Y.W., M.Z. and H.Y.; resources, T.X.; data curation, Y.X., Y.W. and Y.L.; writing—original draft preparation, Y.W., M.Z., H.Y. and Y.L.; writing—review and editing, all authors; visualization, Y.X. and Y.W.; supervision, Z.L. and C.H.; project administration, Z.L. and C.H.; funding acquisition, T.X., Z.L. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the Yuelushan Laboratory Talent Program (2024RC2020).
Institutional Review Board Statement
The animal experiment in this study was approved by the Ethics Committee of Hunan Agricultural University, Changsha, China; Approval Code: 202402481; Approval Date: 3 August 2024.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Q.; Zeng, W.; Liu, C.; Zhang, C.; Wang, Y.; Shi, C.; Wu, S. Complete genome sequence of a reovirus isolated from grass carp, indicating different genotypes of GCRV in China. J. Virol. 2012, 86, 12466. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Zhang, M.; Xu, Y.; Qin, B.; Yang, H.; Wei, R.; Xiao, T. Structural and functional characteristics of TLR19 in barbel chub compared to TLR19 in grass carp. Int. J. Mol. Sci. 2025, 26, 3103. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Y.; Ding, C.H.; Xu, B.H.; Lv, Z.; Xiao, T. Serum proteomic profiling of grass carp survived from GCRV infection. Acta Hydrobiol. Sin. 2024, 48, 304–314. [Google Scholar] [CrossRef]
- Caputo, M.B.; Elias, J.; Cesar, G.; Alvarez, M.G.; Laucella, S.A.; Albareda, M.C. Role of the complement system in the modulation of T-cell responses in chronic chagas disease. Front. Cell Infect. Microbiol. 2022, 12, 910854. [Google Scholar] [CrossRef]
- Dobó, J.; Kocsis, A.; Farkas, B.; Demeter, F.; Cervenak, L.; Gál, P. The lectin pathway of the complement system-activation, regulation, disease connections and interplay with other (proteolytic) systems. Int. J. Mol. Sci. 2024, 25, 1566. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Sander, B.; Jensen, R.K.; Pedersen, J.S.; Golas, M.M.; Jensenius, J.C.; Hansen, A.G.; Thiel, S.; Andersen, G.R. Structure and activation of C1, the complex initiating the classical pathway of the complement cascade. Proc. Natl. Acad. Sci. USA 2017, 114, 986–991. [Google Scholar] [CrossRef]
- Yadav, M.K.; Maharana, J.; Yadav, R.; Saha, S.; Sarma, P.; Soni, C.; Singh, V.; Saha, S.; Ganguly, M.; Li, X.X.; et al. Molecular basis of anaphylatoxin binding, activation, and signaling bias at complement receptors. Cell 2023, 186, 4956–4973. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Smith, S.L. Complement system of bony and cartilaginous fish. Fish Shellfish Immunol. 2000, 10, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Bavia, L.; Santiesteban-Lores, L.E.; Carneiro, M.C.; Prodocimo, M.M. Advances in the complement system of a teleost fish, Oreochromis niloticus. Fish Shellfish Immunol. 2022, 123, 61–74. [Google Scholar] [CrossRef]
- Boshra, H.; Li, J.; Sunyer, J.O. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006, 20, 239–262. [Google Scholar] [CrossRef]
- Dang, Y.F.; Shen, Y.B.; Xu, X.Y.; Wang, S.T.; Meng, X.Z.; Li, L.S.; Zhang, M.; Hu, M.Y.; Lv, L.Q.; Wang, R.Q.; et al. Mannan-binding lectin-associated serine protease-1 (MASP-1) mediates immune responses against Aeromonas hydrophila in vitro and in vivo in grass carp. Fish Shellfish Immunol. 2017, 66, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mutsuro, J.; Tanaka, N.; Kato, Y.; Dodds, A.W.; Yano, T.; Nakao, M. Two divergent isotypes of the fourth complement component from a bony fish, the common carp (Cyprinus carpio). J. Immunol. 2005, 175, 4508–4517. [Google Scholar] [CrossRef]
- Dang, Y.; Shen, Y.; Xu, X.; Wang, S.; Meng, X.; Zhang, M.; Lv, L.; Wang, R.; Li, J. Complement component Bf/C2b gene mediates immune responses against Aeromonas hydrophila in grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2018, 74, 509–516. [Google Scholar] [CrossRef]
- Risitano, A.M.; Frieri, C.; Urciuoli, E.; Marano, L. The complement alternative pathway in paroxysmal nocturnal hemoglobinuria: From a pathogenic mechanism to a therapeutic target. Immunol. Rev. 2023, 313, 262–278. [Google Scholar] [CrossRef]
- Goshima, M.; Sekiguchi, R.; Matsushita, M.; Nonaka, M. The complement system of elasmobranches revealed by liver transcriptome analysis of a hammerhead shark, Sphyrna zygaena. Dev. Comp. Immunol. 2016, 61, 13–24. [Google Scholar] [CrossRef]
- Biesma, D.H.; Hannema, A.J.; van Velzen-Blad, H.; Mulder, L.; van Zwieten, R.; Kluijt, I.; Roos, D. A family with complement factor D deficiency. J. Clin. Investig. 2001, 108, 233–240. [Google Scholar] [CrossRef]
- Qi, H.; Wei, J.; Gao, Y.; Yang, Y.; Li, Y.; Zhu, H.; Su, L.; Su, X.; Zhang, Y.; Yang, R. Reg4 and complement factor D prevent the overgrowth of E. coli in the mouse gut. Commun. Biol. 2020, 3, 483. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Fan, J.; Wang, W.; Wang, H.; Liu, H. Molecular characterization, expression and antimicrobial activity of complement factor D in Megalobrama amblycephala. Fish Shellfish Immunol. 2019, 89, 43–51. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, H.; Liu, S.; Sun, F.; Peatman, E.; Kucuktas, H.; Kaltenboeck, L.; Feng, T.; Zhang, H.; Niu, D.; et al. Alternative complement pathway of channel catfish (Ictalurus punctatus): Molecular characterization, mapping and expression analysis of factors Bf/C2 and Df. Fish Shellfish Immunol. 2012, 32, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Godahewa, G.I.; Perera, N.C.; Bathige, S.D.; Nam, B.H.; Noh, J.K.; Lee, J. Complement factor D homolog involved in the alternative complement pathway of rock bream (Oplegnathus fasciatus): Molecular and functional characterization and immune responsive mRNA expression analysis. Fish Shellfish Immunol. 2016, 55, 423–433. [Google Scholar] [CrossRef]
- Byon, J.Y.; Ohira, T.; Hirono, I.; Aoki, T. Comparative immune responses in Japanese flounder, Paralichthys olivaceus after vaccination with viral hemorrhagic septicemia virus (VHSV) recombinant glycoprotein and DNA vaccine using a microarray analysis. Vaccine 2006, 24, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Xiao, T.; Qin, B.; Xu, B.; Lv, Z.; Wang, H. Functional identification of complement factor D and analysis of its expression during GCRV infection in grass carp (Ctenopharyngodon idella). Int. J. Mol. Sci. 2021, 22, 12011. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, A.; Pei, Y.; Chu, P.; Li, Y.; Huang, R.; Liao, L.; Zhu, Z.; Wang, Y. Differences in responses of grass carp to different types of grass carp reovirus (GCRV) and the mechanism of hemorrhage revealed by transcriptome sequencing. BMC Genom. 2017, 18, 452. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, M.; Ding, M.; Zhong, M.; Li, B.; Wang, Y.; Fu, S.; Yin, X.; Guo, Z.; Ye, J. C1r and C1s from Nile tilapia (Oreochromis niloticus): Molecular characterization, transcriptional profiling upon bacterial and IFN-gamma inductions and potential role in response to bacterial infection. Fish Shellfish Immunol. 2017, 70, 240–251. [Google Scholar] [CrossRef]
- Liao, Z.; Wan, Q.; Yuan, G.; Su, J. The systematic identification and mRNA expression profiles post viral or bacterial challenge of complement system in grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2019, 86, 107–115. [Google Scholar] [CrossRef]
- Chen, G.; Xiong, L.; Wang, Y.; He, L.; Huang, R.; Liao, L.; Zhu, Z.; Wang, Y. Different responses in one-year-old and three-year-old grass carp reveal the mechanism of age restriction of GCRV infection. Fish Shellfish Immunol. 2019, 86, 702–712. [Google Scholar] [CrossRef]
- Sasawatari, S.; Okamoto, Y.; Kumanogoh, A.; Toyofuku, T. Blockade of N-glycosylation promotes antitumor immune response of T cells. J. Immunol. 2020, 204, 1373–1385. [Google Scholar] [CrossRef]
- Blouin, C.M.; Hamon, Y.; Gonnord, P.; Boularan, C.; Kagan, J.; Viaris de Lesegno, C.; Ruez, R.; Mailfert, S.; Bertaux, N.; Loew, D.; et al. Glycosylation-dependent IFN-gammaR partitioning in lipid and actin nanodomains is critical for JAK activation. Cell 2016, 166, 920–934. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.; Meng, B.; Gao, Y.; Xue, Z.; He, M.; Jiang, Y.; Dai, X.; Yan, D.; Fang, X. Identification of dysregulated complement activation pathways driven by N-glycosylation alterations in T2D patients. Front. Chem. 2021, 9, 677621. [Google Scholar] [CrossRef]
- Pascoal, C.; Francisco, R.; Mexia, P.; Pereira, B.L.; Granjo, P.; Coelho, H.; Barbosa, M.; Dos Reis Ferreira, V.; Videira, P.A. Revisiting the immunopathology of congenital disorders of glycosylation: An updated review. Front. Immunol. 2024, 15, 1350101. [Google Scholar] [CrossRef]
- Šoić, D.; Keser, T.; Štambuk, J.; Kifer, D.; Pociot, F.; Lauc, G.; Morahan, G.; Novokmet, M.; Gornik, O. High-throughput human complement C3 N-glycoprofiling identifies markers of early onset type 1 diabetes mellitus in children. Mol. Cell Proteom. 2022, 21, 100407. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Naruse, K.; Shima, A.; Nonaka, M.; Sasaki, M. Molecular cloning and linkage analysis of complement C3 and C4 genes of the Japanese medaka fish. Immunogenetics 2000, 51, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, R.; Li, Y.; Xiao, T. Molecular cloning and functional characterization of interferon regulatory factor 7 of the barbel chub, Squaliobarbus curriculus. Fish Shellfish Immunol. 2017, 69, 185–194. [Google Scholar] [CrossRef]
- Ungrová, L.; Geryk, J.; Kohn, M.; Kučerová, D.; Krchlíková, V.; Hron, T.; Pečenka, V.; Pajer, P.; Gáliková, E.; Pecnová, Ľ.; et al. Avian interferon regulatory factor (IRF) family reunion: IRF3 and IRF9 found. BMC Biol. 2025, 23, 180. [Google Scholar] [CrossRef]
- Guo, C.; Yang, X.; Wu, Y.; Yang, L.; Mi, S.; Liu, Z.; Jia, K.; Huang, Y.; Weng, S.; Yu, X.; et al. Involvement of caveolin-1 in the JAK–STAT signaling pathway and infectious spleen and kidney necrosis virus infection in mandarin fish (Siniperca chuatsi). Mol. Immunol. 2011, 48, 992–1000. [Google Scholar] [CrossRef]
- Wang, W.L.; Liu, W.; Gong, H.Y.; Hong, J.R.; Lin, C.C.; Wu, J.L. Activation of cytokine expression occurs through the TNFalpha/NF-kappaB-mediated pathway in birnavirus-infected cells. Fish Shellfish Immunol. 2011, 31, 10–21. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Y.; Gao, Y.; Wang, X.; Zhou, H.; Zhang, A. Insights into the functional role of grass carp IL-8 in head kidney leukocytes: Pro-inflammatory effects and signalling mechanisms. J. Fish Biol. 2022, 100, 192–202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).