Molecular Mechanisms of Low-Temperature Stress Response in the Muscle of Yellowtail Kingfish (Seriola aureovittata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Low-Temperature Treatment and Sample Collection
2.3. RNA Extraction and Transcriptome Sequencing
2.4. Differential Gene Expression and Bioinformatics Analysis
2.5. Quantitative PCR Validation of RNA-Seq Results
3. Result
3.1. Sequencing and Read Mapping
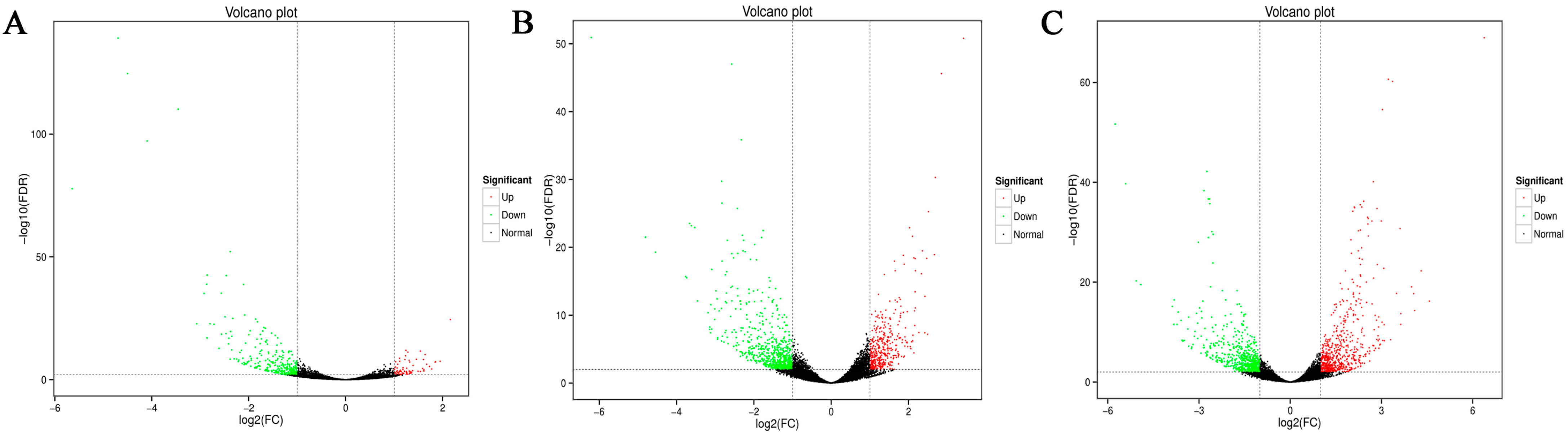
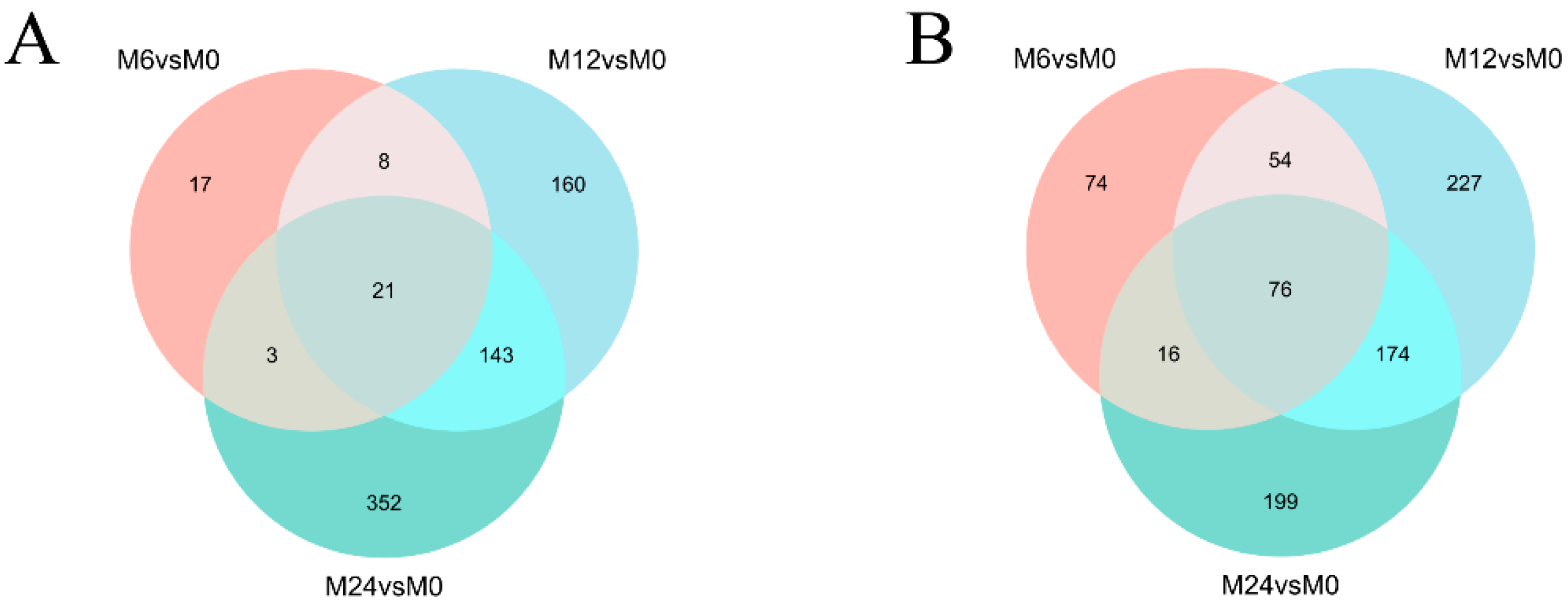
3.2. Analysis of Gene Expression Differences
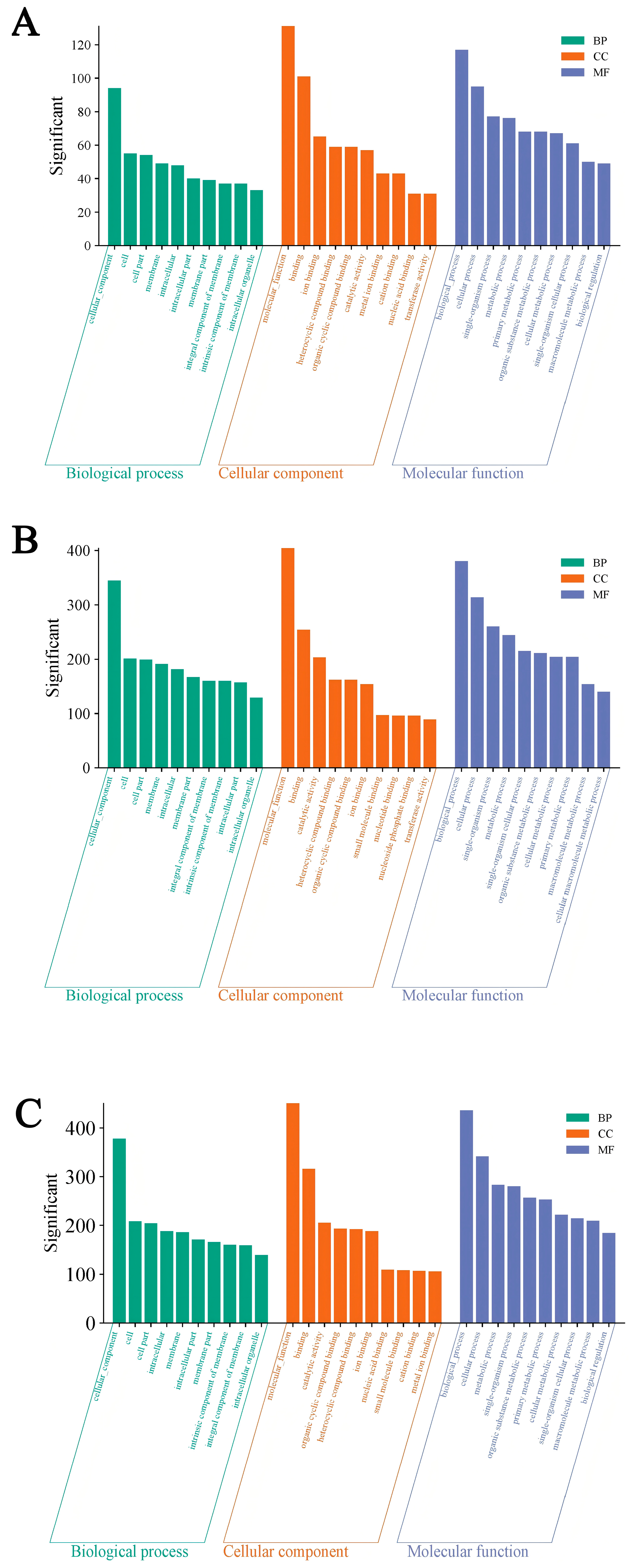
3.3. GO Enrichment Analysis of the DEGs
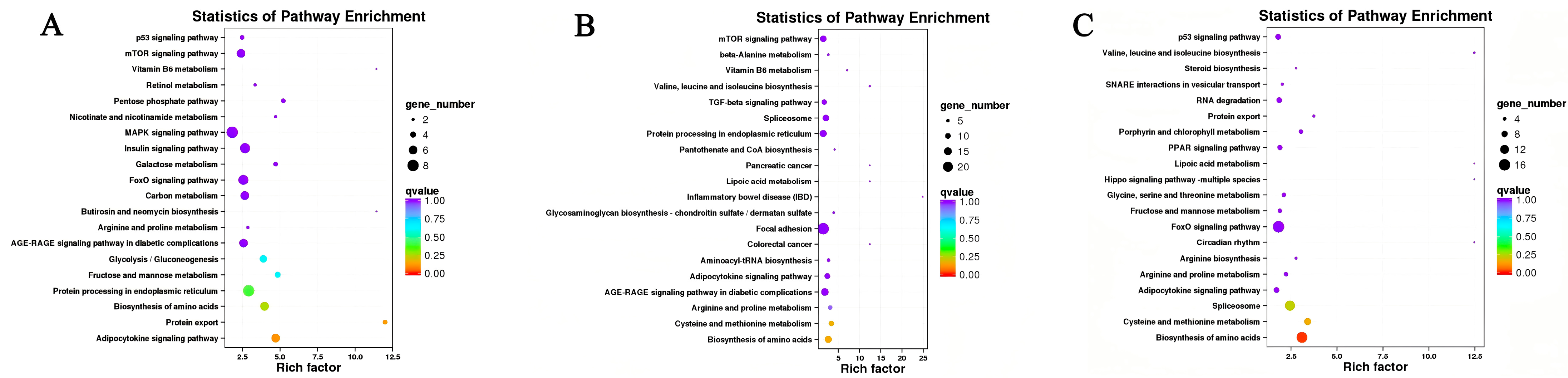
3.4. KEGG Pathway Enrichment Analysis of the DEGs
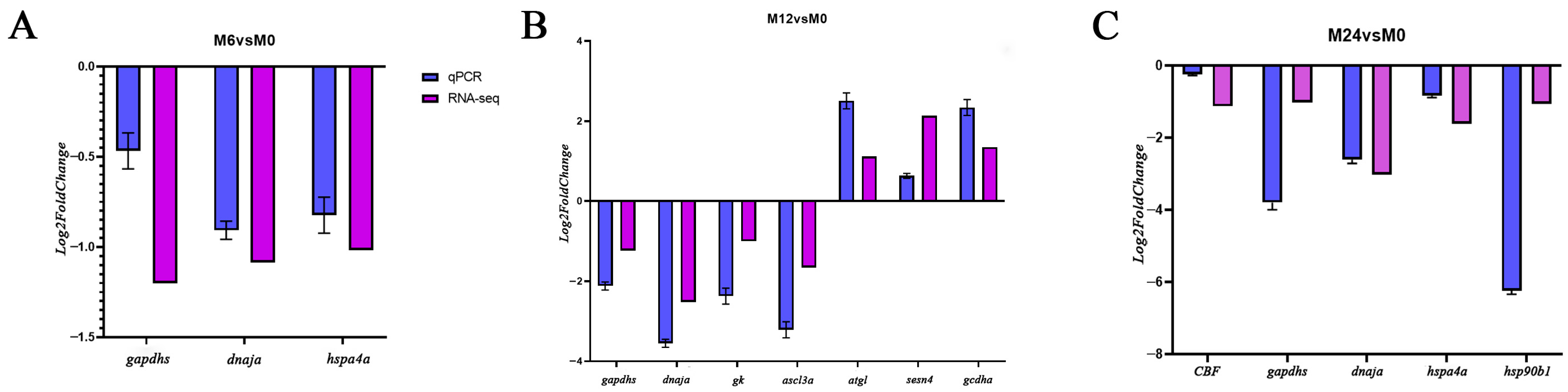
3.5. qPCR Validation of RNA-Seq Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canosa, L.F.; Bertucci, J.I. The effect of environmental stressors on growth in fish and its endocrine control. Front. Endocrinol. 2023, 14, 1109461. [Google Scholar] [CrossRef] [PubMed]
- Soyano, K.; Mushirobira, Y. The mechanism of low-temperature tolerance in fish. Adapt. Mech. Their Appl. 2018, 1081, 149–164. [Google Scholar] [CrossRef]
- Refaey, M.M.; Mehrim, A.I.; El-Komy, M.M.; Zenhom, O.A.; Mansour, A.T. Chronic cold-stress induced histopathological changes, oxidative stress, and alterations in liver functions and nutrient composition of hybrid red tilapia and the potential protection of unsaturated fatty acids. Front. Mar. Sci. 2023, 10, 1148978. [Google Scholar] [CrossRef]
- Ummenhofer, C.C.; Meehl, G.A. Extreme weather and climate events with ecological relevance: A review. Philos. Trans. R. Soc. B 2017, 372, 20160135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ozsolak, F.; Milos, P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Ye, C.X.; Guo, Z.X.; Wang, A.-L. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress. Fish Shellfish Immunol. 2017, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.P.; Costa, R.; Gisbert, E.; Pinto, P.I.; Andree, K.B.; Estévez, A.; Power, D.M. Thermal imprinting modifies bone homeostasis in cold-challenged sea bream (Sparus aurata). J. Exp. Biol. 2017, 220, 3442–3454. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.A.; Hliwa, P.; Adamek, M.; Steinhagen, D.; Karol, H.; Ciereszko, A. Acclimation to cold and warm temperatures is associated with differential expression of male carp blood proteins involved in acute phase and stress responses, and lipid metabolism. Fish Shellfish Immunol. 2018, 76, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Bacchetta, C.; Cazenave, J. Effect of thermal stress on metabolic and oxidative stress biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol. Indic. 2017, 79, 361–370. [Google Scholar] [CrossRef]
- Somero, G.N. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners ’ and ‘losers’. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, T.; Butail, S.; Porfiri, M. Temperature influences sociality and activity of freshwater fish. Environ. Biol. Fishes 2015, 98, 825–832. [Google Scholar] [CrossRef]
- Symonds, J.; Walker, S.; Pether, S.; Gublin, Y.; McQueen, D.; King, A.; Irvine, G.; Setiawan, A.; Forsythe, J.; Bruce, M. Developing yellowtail kingfish (Seriola lalandi) and hāpuku (Polyprion oxygeneios) for New Zealand aquaculture. N. Z. J. Mar. Freshw. Res. 2014, 48, 371–384. [Google Scholar] [CrossRef]
- Soltanian, S.; Adloo, M.N.; Hafeziyeh, M.; Ghadimi, N. Effect of β-Glucan on cold-stress resistance of striped catfish, Pangasianodon hypophthalmus (Sauvage, 1878). Vet. Med. 2014, 59, 440–446. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Zhang, X.; Zou, C.; Lin, L. Improvement of muscle quality in tilapia (Oreochromis niloticus) with dietary faba bean (Vicia faba L.). Front. Nutr. 2023, 10, 1153323. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.Y.; Payne, N.L. Thermal sensitivity of metabolic rate mirrors biogeographic differences between teleosts and elasmobranchs. Nat. Commun. 2023, 14, 2054. [Google Scholar] [CrossRef] [PubMed]
- Farkas, T.; Fodor, E.; Kitajka, K.; Halver, J.E. Response of fish membranes to environmental temperature. Aquac. Res. 2001, 32, 645–655. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; El-Sayed, A.M.; Ezzat, A.A.; Essa, M.A.; Helal, A.M. Dietary lipid sources affect cold tolerance of Nile tilapia (Oreochromis niloticus). J. Therm. Biol. 2019, 79, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, M.P.A.; Parrish, C.C.; Gallardi, D.; Nag, K.; Mansour, A. Diet and temperature affect liver lipids and membrane properties in steelhead trout (Oncorhynchus mykiss). Aquac. Nutr. 2021, 27, 734–746. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C.; Zhang, Y.; Wei, Y.; Liang, M. Moderate levels of dietary arachidonic acid reduced lipid accumulation and tended to inhibit cell cycle progression in the liver of Japanese seabass Lateolabrax japonicus. Sci. Rep. 2018, 8, 10682. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Sun, J.; Chang, Z.; Gou, N.-N.; Wu, W.-Y.; Luo, X.-L.; Zhou, J.-S.; Yu, H.-B.; Ji, H. Energy response and fatty acid metabolism in Onychostoma macrolepis exposed to low-temperature stress. J. Therm. Biol. 2020, 94, 102725. [Google Scholar] [CrossRef] [PubMed]
- Winnikoff, J.R.; Haddock, S.H.D.; Budin, I. Depth- and temperature-specific fatty acid adaptations in ctenophores from extreme habitats. J. Exp. Biol. 2021, 224, jeb242800. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P. Structural analysis of hydrated egg lecithin and cholesterol bilayers. I. X-Ray diffraction. J. Mol. Biol. 1976, 100, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, L.; Yang, Y.; Chen, C.; Wang, D.; Wang, H. Acyl-CoA thioesterase 1 prevents cardiomyocytes from Doxorubicin-induced ferroptosis via shaping the lipid composition. Cell Death Dis. 2020, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Chen, Z.; Qin, J.; Huang, Y.; Du, X.; Zhang, C.; Pan, X.; Lin, Y.; Abdelsalam, M. Effects of salinity on muscle nutrition, fatty acid composition, and substance anabolic metabolism of Blue Tilapia Oreochromis aureus. J. Appl. Ichthyol. 2024, 2024, 5549406. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Guixà-González, R.; Javanainen, M.; Gómez-Soler, M.; Cordobilla, B.; Domingo, J.C.; Sanz, F.; Pastor, M.; Ciruela, F.; Martinez-Seara, H.; Selent, J. Membrane omega-3 fatty acids modulate the oligomerisation kinetics of adenosine A2A and dopamine D2 receptors. Sci. Rep. 2016, 6, 19839. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.P.; Sathyanarayan, A.; Mashek, D.G. Acyl-CoA thioesterase 1 (ACOT1) regulates PPARα to couple fatty acid flux with oxidative capacity during fasting. Diabetes 2017, 66, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Novgorodtseva, T.P.; Karaman, Y.K.; Zhukova, N.V.; Lobanova, E.G.; Antonyuk, M.V.; Kantur, T.A. Composition of fatty acids in plasma and erythrocytes and eicosanoids level in patients with metabolic syndrome. Lipids Health Dis. 2011, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Jacob, R.F.; Shrivastava, S.; Sherratt, S.C.; Chattopadhyay, A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Skrzynska, A.K.; Maiorano, E.; Bastaroli, M.; Naderi, F.; Míguez, J.M.; Martínez-Rodríguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Impact of Air Exposure on vasotocinergic and isotocinergic systems in gilthead sea bream (Sparus aurata): New insights on fish stress response. Front. Physiol. 2018, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, J.; Zhang, Z.; Shi, H.; Sun, W.; Yi, Q. The role of AMPK in macrophage metabolism, function and polarisation. J. Transl. Med. 2023, 21, 892. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Petyaykina, K.; Tao, R.; Xiong, X.; Dong, X.C.; Liangpunsakul, S. The inhibitory effect of ethanol on Sestrin3 in the pathogenesis of ethanol-induced liver injury. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G58–G65. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Moradi, M.; Razavi, F.; Rabiei, V. Exogenous phenylalanine application promotes chilling tolerance in tomato fruits during cold storage by ensuring supply of NADPH for activation of ROS scavenging systems. Sci. Hortic. 2019, 246, 818–825. [Google Scholar] [CrossRef]
- Schreiber, R.; Xie, H.; Schweiger, M. Of mice and men: The physiological role of adipose triglyceride lipase (ATGL). Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Reyes-Zurita, F.J.; García-Salguero, L.; Peragón, J.; de la Higuera, M.; Lupiáñez, J.A. NADPH production, a growth marker, is stimulated by maslinic acid in gilthead sea bream by increased NADP-IDH and ME expression. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 187, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Chapter 3-Energy metabolism in the brain. In From Molecules to Networks, 3rd ed.; Byrne, J.H., Heidelberger, R., Waxham, M.N., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 53–117. [Google Scholar]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.; Carter, J.F. Stable carbon isotope diagnostics of mammalian metabolism, a high-resolution isotomics approach using amino acid carboxyl groups. PLoS ONE 2019, 14, e0224297. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, K.; Haigis, M.C. Nitrogen Metabolism in Cancer and Immunity. Trends Cell Biol. 2020, 30, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Hayamizu, K. 21-Amino acids and energy metabolism: An overview. Sustain. Energy Enhanc. Hum. Funct. Act. 2017, 339–349. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128054130000211 (accessed on 14 April 2025).
- Robert, J. Evolution of heat shock protein and immunity. Dev. Comp. Immunol. 2003, 27, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Reed, J.C.; Homma, S. Heat-shock proteins as regulator of apoptosis. Oncogene 2004, 22, 9041–9047. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2020, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Kapila, R.; Pant, R.; Gaur, A.K.; Mahanta, P.C. Effect of low temperature on metabolic enzymes and HSP-70 expression of coldwater fish Barilius bendelisis. Asian Fish. Sci. 2009, 22, 125–136. [Google Scholar] [CrossRef]
- Mottola, G.; Nikinmaa, M.; Anttila, K. Hsp70s transcription-translation relationship depends on the heat shock temperature in zebrafish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 240, 110629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qian, Z.; Ji, J.; Wang, T.; Yin, S.; Zhang, K. Characterization of HSP70 and HSP90 gene family in Takifugu fasciatus and their expression profiles on biotic and abiotic stresses response. Genes 2024, 15, 1445. [Google Scholar] [CrossRef] [PubMed]
- Al-Whaibi, M.H. Plant heat-shock proteins: A mini review. J. King Saud. Univ.-Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef]
- Turan, M. Genome-wide analysis and characterization of HSP gene families (HSP20, HSP40, HSP60, HSP70, HSP90) in the yellow fever mosquito (Aedes aegypti) (Diptera: Culicidae). J. Insect Sci. 2023, 23, 27. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Primer Sequence (5′–3′) |
|---|---|
| 18S | F:TACCACATCCAAAGAAGGCA |
| R:TCGATCCCGAGATCCAACTA | |
| gcdha | F:GGATATTGCCAGACAAGCCAGAGAC |
| R:GTATGTGTTGACAGCCTCCAGGTTC | |
| acsl3a | F:TCACCTTCCTGCCTTACCACCTC |
| R:CTCTTGGCTCGCTCCTCCTCTG | |
| gk | F:AACGCCAGGAAGGAATAAGAACCAC |
| R:TCCATCAGCCAGCGAAGTTTCAC | |
| CBF | F:CGGCTTGGTACTGGCACAGAATC |
| R:AGCAGCATCCATGTCCACTCAAAG | |
| hspa4a | F:GCCCTTCACCCTTGATGCTTACTAC |
| R:GACGCCTGAGGAACCACATTCTG | |
| dnaja | F:GGAAGAGGCGTACAAGTCAAGGTG |
| R:GTCCCTGGCAGTCTGAACACATG | |
| sesn4 | F:CACATAACACGACGACGGTCTCTG |
| R:TTCTCTCTTCTCACGCTCCTCCTG | |
| gapdhs | F:GGCATCTCCCTCAACGACAACTTC |
| R:GTACATCAGCAGGTCAGCGACAC | |
| hsp90b1 | F:GAAGACTGTGTGGGATTGGGAACTG |
| R:ACTCATCCTCCTCAACCTCCTTAGC | |
| atg1 | F:CATCGTGTCGCCCTACTCTTTAGC |
| R:TGTCACTGTCCTCCGCTCTGTC |
| Sample | Clean Reads | Clean Bases | Q20 | Q30 | GC pct | Mapped Reads | Mapped Ratio |
|---|---|---|---|---|---|---|---|
| M0-1 | 20418646 | 6110127080 | 98.52% | 95.37% | 52.15% | 17950128 | 87.91% |
| M0-2 | 20870180 | 6244328712 | 98.39% | 95.05% | 51.85% | 18273735 | 87.56% |
| M0-3 | 23491560 | 7029978414 | 98.42% | 95.13% | 52.32% | 20779018 | 88.45% |
| M6-1 | 21792688 | 6517959582 | 98.35% | 95.02% | 52.06% | 19014430 | 87.25% |
| M6-2 | 24179882 | 7238394824 | 98.56% | 95.51% | 52.06% | 21687276 | 89.69% |
| M6-3 | 22025406 | 6597172226 | 98.51% | 95.36% | 52.11% | 19659153 | 89.26% |
| M12-1 | 25662854 | 7677953448 | 98.52% | 95.35% | 52.44% | 22918827 | 89.31% |
| M12-2 | 21675610 | 6488164660 | 98.50% | 95.33% | 52.92% | 19357986 | 89.31% |
| M12-3 | 21820189 | 6535430448 | 98.42% | 95.11% | 52.27% | 19519478 | 89.46% |
| M24-1 | 20407423 | 6113048992 | 98.36% | 94.91% | 52.45% | 18102155 | 88.70% |
| M24-2 | 21918055 | 6563166642 | 98.48% | 95.26% | 52.17% | 19514485 | 89.03% |
| M24-3 | 20115769 | 6011936510 | 98.46% | 95.23% | 51.77% | 17521666 | 87.10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zhang, R.; Wu, B.; Ji, M.; Li, X.; Cao, X.; Jiang, C. Molecular Mechanisms of Low-Temperature Stress Response in the Muscle of Yellowtail Kingfish (Seriola aureovittata). Fishes 2025, 10, 355. https://doi.org/10.3390/fishes10070355
Tian Y, Zhang R, Wu B, Ji M, Li X, Cao X, Jiang C. Molecular Mechanisms of Low-Temperature Stress Response in the Muscle of Yellowtail Kingfish (Seriola aureovittata). Fishes. 2025; 10(7):355. https://doi.org/10.3390/fishes10070355
Chicago/Turabian StyleTian, Yushun, Ruonan Zhang, Bingxin Wu, Mingxin Ji, Xinyang Li, Xinyu Cao, and Chen Jiang. 2025. "Molecular Mechanisms of Low-Temperature Stress Response in the Muscle of Yellowtail Kingfish (Seriola aureovittata)" Fishes 10, no. 7: 355. https://doi.org/10.3390/fishes10070355
APA StyleTian, Y., Zhang, R., Wu, B., Ji, M., Li, X., Cao, X., & Jiang, C. (2025). Molecular Mechanisms of Low-Temperature Stress Response in the Muscle of Yellowtail Kingfish (Seriola aureovittata). Fishes, 10(7), 355. https://doi.org/10.3390/fishes10070355






