Multifunctional Applications of Biofloc Technology (BFT) in Sustainable Aquaculture: A Review
Abstract
1. Introduction
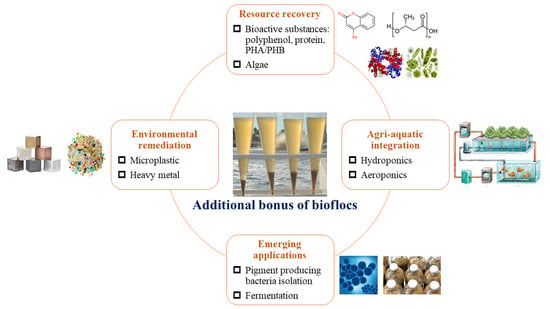
2. Multifunctional Roles of Bioflocs
2.1. Resource Recovery and Value-Added Production
2.1.1. Extraction of Bioactive Substances and Microbial Protein
2.1.2. Algae Cultivation and Nutrient Remediation
2.2. Environmental Remediation and Pollutant Mitigation
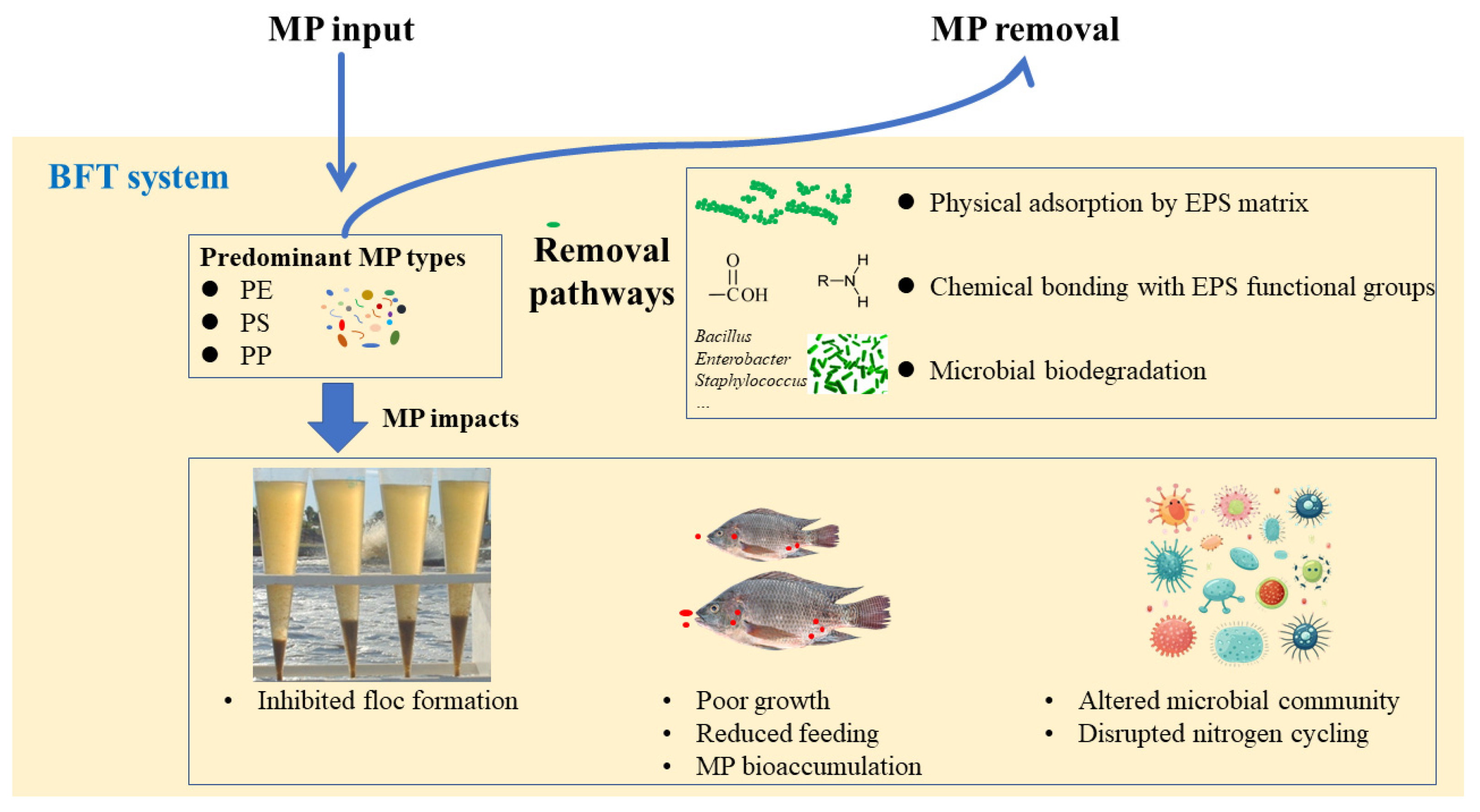
2.2.1. Microplastic Removal
2.2.2. Heavy Metal Removal
2.3. Agri-Aquatic Integration
2.4. Emerging Applications and Byproduct Utilization
3. Current Challenges and Future Direction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dewali, S.; Sharma, N.; Melkani, D.; Arya, M.; Kathayat, N.; Panda, A.K.; Bisht, S.S. Aquaculture: Contributions to Global Food Security. In Emerging Solutions in Sustainable Food and Nutrition Security; Ghosh, S., Kumari Panda, A., Jung, C., Singh Bisht, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 123–139. ISBN 978-3-031-40907-3. [Google Scholar]
- Gao, X.; Ye, C.; Ma, H.; Zhang, Z.; Wang, J.; Zhang, Z.-H.; Zhao, X.; Ho, C.-T. Research Advances in Preparation, Stability, Application, and Possible Risks of Nanoselenium: Focus on Food and Food-Related Fields. J. Agric. Food Chem. 2023, 71, 8731–8745. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Cai, Y.; Liang, S.; Ku, J.; Qin, Y. Numerical Simulation and Analysis of Feeding Uniformity of Viscous Miscellaneous Fish Bait Based on EDEM Software. Agriculture 2023, 13, 356. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Monto, A.R.; Su, K.; Zhang, H.; Shi, T.; Xiong, Z.; Xu, G.; Luo, Y.; Bao, Y.; et al. Metabolomic Profile of Muscles from Tilapia Cultured in Recirculating Aquaculture Systems and Traditional Aquaculture in Ponds and Protein Stability during Freeze-Thaw Cycles. Food Chem. 2024, 451, 139325. [Google Scholar] [CrossRef] [PubMed]
- Anandkumar, A.; Li, J.; Prabakaran, K.; Jia, X.Z.; Leng, Z.; Nagarajan, R.; Du, D. Accumulation of Toxic Elements in an Invasive Crayfish Species (Procambarus clarkii) and Its Health Risk Assessment to Humans. J. Food Compos. Anal. 2020, 88, 103449. [Google Scholar] [CrossRef]
- Shi, B.; Sreeram, V.; Zhao, D.; Duan, S.; Jiang, J. A Wireless Sensor Network-Based Monitoring System for Freshwater Fishpond Aquaculture. Biosyst. Eng. 2018, 172, 57–66. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, A.; Adade, S.Y.-S.S.; Ali, S.; Chen, Q.; Wei, J.; Chen, X.; Jiao, T.; Chen, Q. Ag@Au Core-Shell Nanoparticle-Based Surface-Enhanced Raman Scattering Coupled with Chemometrics for Rapid Determination of Chloramphenicol Residue in Fish. Food Chem. 2024, 438, 138026. [Google Scholar] [CrossRef] [PubMed]
- Angel, D.; Jokumsen, A.; Lembo, G. Aquaculture Production Systems and Environmental Interactions. In Organic Aquaculture; Lembo, G., Mente, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 103–118. ISBN 978-3-030-05602-5. [Google Scholar]
- Shin, H.; Park, T.; Jo, S.-K.; Jung, J.Y. Enhancing Flow-through Aquaculture System Monitoring: A Comparative Study of Machine Learning Algorithms for Missing-Data Imputation. Aquaculture 2025, 601, 742303. [Google Scholar] [CrossRef]
- Majluf, P.; Matthews, K.; Pauly, D.; Skerritt, D.J.; Palomares, M.L.D. A Review of the Global Use of Fishmeal and Fish Oil and the Fish In: Fish Out Metric. Sci. Adv. 2024, 10, eadn5650. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Sharifinia, M. Biofloc Technology as a Promising Tool to Improve Aquaculture Production. Rev. Aquac. 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Soaudy, M.R.; Ghonimy, A.; Greco, L.S.L.; Chen, Z.; Dyzenchauz, A.; Li, J. Total Suspended Solids and Their Impact in a Biofloc System: Current and Potentially New Management Strategies. Aquaculture 2023, 572, 739524. [Google Scholar] [CrossRef]
- Iber, B.T.; Ikyo, B.C.; Nor, M.N.M.; Abdullah, S.R.S.; Shafie, M.S.B.; Manan, H.; Abdullah, M.H.D.I.; Kasan, N.A. Application of Biofloc Technology in Shrimp Aquaculture: A Review on Current Practices, Challenges, and Future Perspectives. J. Agric. Food Res. 2025, 19, 101675. [Google Scholar] [CrossRef]
- Shi, M.; Ruan, Y.; Wu, B.; Ye, Z.; Zhu, S. Performance Evaluation of Hydrodynamic Vortex Separator at Different Hydraulic Retention Times Applied in Recirculating Biofloc Technology System. Trans. ASABE 2017, 60, 1737–1747. [Google Scholar] [CrossRef]
- Shamsuddin, M.; Hossain, M.B.; Rahman, M.; Kawla, M.S.; Shufol, M.B.A.; Rashid, M.M.; Asadujjaman, M.; Rakib, M.R.J. Application of Biofloc Technology for the Culture of Heteropneustes Fossilis (Bloch) in Bangladesh: Stocking Density, Floc Volume, Growth Performance, and Profitability. Aquac. Int. 2022, 30, 1047–1070. [Google Scholar] [CrossRef]
- Bezerra, G.A.; Pires, D.C.; Watanabe, A.L.; Buglione Neto, C.C.; Cardoso, A.J.D.S.; Simões, A.R.P.; Hisano, H. Economic Feasibility and Risk Analysis of Nile Tilapia Juveniles Reared in a Biofloc Technology System. Aquac. J. 2025, 5, 9. [Google Scholar] [CrossRef]
- De Schryver, P.; Verstraete, W. Nitrogen Removal from Aquaculture Pond Water by Heterotrophic Nitrogen Assimilation in Lab-Scale Sequencing Batch Reactors. Bioresour. Technol. 2009, 100, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Megahed, M.E. The Effect of Microbial Biofloc on Water Quality, Survival and Growth of the Green Tiger Shrimp (Penaeus semisulcatus) Fed with Different Crude Protein Levels. J. Arab. Aquac. Soc. 2010, 5, 119–141. [Google Scholar]
- de Ramiro, B.O.; Wasielesky, W.; Pimentel, O.A.L.F.; Sun, T.; McAlhaney, E.; Urick, S.; Gonçalves, F.H.; van Senten, J.; Schwarz, M.H.; Krummenauer, D. Assessment of Water Quality, Growth of Penaeus vannamei, and Partial Budget in Super-Intensive BFT and RAS: A Comparison between Sustainable Aquaculture Systems. Sustainability 2024, 16, 11005. [Google Scholar] [CrossRef]
- López-Olmeda, J.F.; Sánchez-Vázquez, F.J.; Fortes-Silva, R. Biology and Aquaculture of Tilapia, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-003-00413-4. [Google Scholar]
- Emerenciano, M.G.C.; Khanjani, M.H.; Sharifinia, M.; Miranda-Baeza, A. Could Biofloc Technology (BFT) Pave the Way toward a More Sustainable Aquaculture in Line with the Circular Economy? Aquac. Res. 2025, 2025, 1020045. [Google Scholar] [CrossRef]
- Schveitzer, R.; Baccarat, R.F.C.; Gaona, C.A.P.; Wasielesky, W.; Arantes, R. Concentration of Suspended Solids in Superintensive Culture of the Pacific White Shrimp Litopenaeus vannamei with Biofloc Technology (BFT): A Review. Rev. Aquac. 2024, 16, 785–795. [Google Scholar] [CrossRef]
- Durigon, E.G.; Schneider, T.L.S.; Marasca, S.; Hermes, L.B.; Battisti, E.K.; Finamor, I.A.; Pavanato, M.A.; Lazzari, R. Biofloc Meal for Tilapia Feeding: Growth and Oxidative Parameters. Aquac. Int. 2024, 32, 4955–4969. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Chen, Y.; Zhang, S.; Dai, L.; Zhu, W.; Chen, Y. Optimized Utilization of Organic Carbon in Aquaculture Biofloc Systems: A Review. Fishes 2023, 8, 465. [Google Scholar] [CrossRef]
- Wei, X.-F.; Meng, S.-T.; Wang, Y.-T.; Li, L.; Zhu, R.; Li, D.-L.; Liu, S.-Y.; Wu, L.-F. Effects of Replacing Fish Meal with Biofloc Meal on Growth Performance, Nutrients Metabolism, Immune Response and Intestinal Microbiota of Common Carp (Cyprinus carpio). Aquaculture 2024, 591, 741124. [Google Scholar] [CrossRef]
- Hawkins, S.; de Fonseca, I.B.F.C.; Lima da Silva, R.J.; Quirino, R.L. Aquaculture Waste: Potential Synthesis of Polyhydroxyalkanoates. ACS Omega 2021, 6, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Mozanzadeh, M.T.; Sharifinia, M.; Emerenciano, M.G.C. Biofloc: A Sustainable Dietary Supplement, Nutritional Value and Functional Properties. Aquaculture 2023, 562, 738757. [Google Scholar] [CrossRef]
- Müller-Santos, M.; Koskimäki, J.J.; Alves, L.P.S.; de Souza, E.M.; Jendrossek, D.; Pirttilä, A.M. The Protective Role of PHB and Its Degradation Products against Stress Situations in Bacteria. FEMS Microbiol. Rev. 2021, 45, fuaa058. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.-J.; Zhu, L.; Xu, X.-Y. Study on the Flocs Poly-β-Hydroxybutyrate Production and Process Optimization in the Bio-Flocs Technology System. Bioresour. Technol. 2011, 102, 7599–7602. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, X.-F.; Yang, Z.-Y.; Zhu, R.; Li, D.-L.; Shang, G.-J.; Wang, H.-T.; Meng, S.-T.; Wang, Y.-T.; Liu, S.-Y.; et al. Alleviative Effect of Poly-β-Hydroxybutyrate on Lipopolysaccharide-Induced Oxidative Stress, Inflammation and Cell Apoptosis in Cyprinus carpio. Int. J. Biol. Macromol. 2023, 253, 126784. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Li, X.; Li, J.; Zhang, M.; Shen, Y.; Zhao, Z.; Zhang, Y.; Qi, Z.; Chen, P.; Sun, Y.; et al. An Eco-Friendly Conversion of Aquaculture Suspended Solid Wastes into High-Quality Fish Food by Improving Poly-β-Hydroxybutyrate Production. Front. Physiol. 2022, 13, 797625. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.M.M.; Ramírez, J.R.B.; Buitrago, A.M.; Colombo, G.M.; Pereira, A.C.; Roselet, F.; Ramos, D.F.; Bernardi, F.; Monserrat, J.M. Biofloc Residue Conversion from Shrimp Production: Optimizing Polyphenol Extraction for Silver Nanoparticles Synthesis with Antibacterial and Antibiofilm Properties. Aquaculture 2024, 585, 740719. [Google Scholar] [CrossRef]
- Gomes, R.M.M.; Ramírez, J.R.B.; Araujo, A.C.S.; Pereira, A.C.; Couto, C.M.O.; Rojas, C.; Pinto, L.A.; Junior, T.; Ramos, D.F.; Monserrat, J.M. Biovalorization of Aquaculture Biofloc Waste through Polyphenol Extraction by Alkaline Hydrolysis and Green Nanoparticle Synthesis Optimization. Processes 2025, 13, 29. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, M.; Zhou, S.; Li, L.; Song, K. Increasing Fish Production in Recirculating Aquaculture System by Integrating a Biofloc-Worm Reactor for Protein Recovery. Water Res. X 2024, 24, 100246. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Dai, Z.; Song, K.; Wang, Y.; He, X. Integrating Microbial Protein Production and Harvest Systems into Pilot-Scale Recirculating Aquaculture Systems for Sustainable Resource Recovery: Linking Nitrogen Recovery to Microbial Communities. Environ. Sci. Technol. 2021, 55, 16735–16746. [Google Scholar] [CrossRef] [PubMed]
- Khoa, T.N.D.; Tao, C.T.; Van Khanh, L.; Hai, T.N. Super-Intensive Culture of White Leg Shrimp (Litopenaeus vannamei) in Outdoor Biofloc Systems with Different Sunlight Exposure Levels: Emphasis on Commercial Applications. Aquaculture 2020, 524, 735277. [Google Scholar] [CrossRef]
- Brito, L.O.; dos Santos, I.G.S.; de Abreu, J.L.; de Araújo, M.T.; Severi, W.; Gàlvez, A.O. Effect of the Addition of Diatoms (Navicula spp.) and Rotifers (Brachionus plicatilis) on Water Quality and Growth of the Litopenaeus vannamei Postlarvae Reared in a Biofloc System. Aquac. Res. 2016, 47, 3990–3997. [Google Scholar] [CrossRef]
- Abreu, J.L.; Brito, L.O.; Lima, P.C.M.; Silva, S.M.B.C.D.; Severi, W.; Gálvez, A.O. Effects of Addition of Navicula sp. (Diatom) in Different Densities to Postlarvae of Shrimp Litopenaeus vannamei Reared in a BFT System: Growth, Survival, Productivity and Fatty Acid Profile. Aquac. Res. 2019, 50, 2231–2239. [Google Scholar] [CrossRef]
- Molina-Cárdenas, C.A.; Sánchez-Saavedra, M. del P. Inhibitory Effect of Benthic Diatom Species on Three Aquaculture Pathogenic Vibrios. Algal Res. 2017, 27, 131–139. [Google Scholar] [CrossRef]
- Afrin, E.; Akter, T.; Baidya, A.; Hossain, M.A.; Islam, M.R.; Das, M.; Fatema, U.K.; Alam, M.S.; Iqbal, M.A. Biofloc Wastewater for Microalgae (Chlorella ellipsoidea) Production: An Approach to Algal Biomass Production and Nutrient Remediation. J. Appl. Aquac. 2025, 37, 86–106. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Le, T.V.A.; Show, P.L.; Nguyen, T.T.; Tran, M.H.; Tran, T.N.T.; Lee, S.Y. Bioflocculation Formation of Microalgae-Bacteria in Enhancing Microalgae Harvesting and Nutrient Removal from Wastewater Effluent. Bioresour. Technol. 2019, 272, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Potential of Microalgae Cultivation Using Nutrient-Rich Wastewater and Harvesting Performance by Biocoagulants/Bioflocculants: Mechanism, Multi-Conversion of Biomass into Valuable Products, and Future Challenges. J. Clean. Prod. 2022, 365, 132806. [Google Scholar] [CrossRef]
- Carvalho, A.; Braga, Í.; Chaar, F.; Cardozo, A.P.; Monserrat, J.M.; Ramírez, J.R.B.; Wasielesky, W.; Poersch, L.H. Production of the Macroalgae Ulva Lactuca Integrated with the Shrimp Penaeus vannamei in a Biofloc System: Effect of Total Suspended Solids and Nutrient Concentrations. Phycology 2023, 4, 37–52. [Google Scholar] [CrossRef]
- Holanda, M.; Besold, C.; Sempere, F.L.; Abreu, P.C.; Poersch, L. Treatment of Effluents from Marine Shrimp Culture with Biofloc Technology: Production of Arthrospira (Spirulina) platensis (Cyanobacteria) and Nutrient Removal. J. World Aquac. Soc. 2022, 53, 669–680. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Waiho, K.; Fazhan, H.; Azwar, E.; Shu-Chien, A.C.; Hersi, M.A.; Kasan, N.A.; Foo, S.S.; Wong, K.Y.; Draman, A.S.; et al. Emerging Paradigms in Sustainable Shellfish Aquaculture: Microalgae and Biofloc Technologies for Wastewater Treatment. Aquaculture 2024, 587, 740835. [Google Scholar] [CrossRef]
- Hossain, S.; Manan, H.; Shukri, Z.N.A.; Othman, R.; Kamaruzzan, A.S.; Rahim, A.I.A.; Khatoon, H.; Minhaz, T.M.; Islam, Z.; Kasan, N.A. Microplastics Biodegradation by Biofloc-Producing Bacteria: An Inventive Biofloc Technology Approach. Microbiol. Res. 2023, 266, 127239. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Castañeda, G.; Medina-López, J.A.; Frías-Espericueta, M.G.; Páez-Osuna, F. Farmed Stage (Age)-Dependent Accumulation and Size of Microplastics in Litopenaeus vannamei Shrimp Reared in a Super-Intensive Controlled System. Sci. Total Environ. 2024, 918, 170575. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.R.; Urbina, M.A.; Corr, S.; Lewis, C.; Galloway, T.S. Ingestion of Plastic Microfibers by the Crab Carcinus Maenas and Its Effect on Food Consumption and Energy Balance. Environ. Sci. Technol. 2015, 49, 14597–14604. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Meng, L.-J.; Liu, H.-D.; Guo, Y.-S.; Liu, W.-C.; Tan, H.-X.; Luo, G.-Z. Impacts of Nile Tilapia (Oreochromis niloticus) Exposed to Microplastics in Bioflocs System. Sci. Total Environ. 2023, 901, 165921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yue, Y.; Dong, X.; Zhang, M.; Gan, L.; Shao, J. Size Dependent Effects of Nanoplastics and Microplastics on the Nitrogen Cycle of Microbial Flocs. Chemosphere 2023, 324, 138351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, X.; Zhang, M.; Gan, L.; Shao, J.; Sun, W. Effects of Environmentally Relevant Concentrations of Micro(Nano)Plastics on Aquatic Microorganisms: Changes in Potential Function but Not in Overall Composition. Water Biol. Secur. 2024, 3, 100233. [Google Scholar] [CrossRef]
- Meng, L.-J.; Hu, X.; Wen, B.; Liu, Y.-H.; Luo, G.-Z.; Gao, J.-Z.; Chen, Z.-Z. Microplastics Inhibit Biofloc Formation and Alter Microbial Community Composition and Nitrogen Transformation Function in Aquaculture. Sci. Total Environ. 2023, 866, 161362. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, S.; Zhang, X.; Zhang, Y.; Li, B.; Jin, K.; Feng, X.; Hong, J.; Huang, X.; Cao, H.; et al. Sustainable Microplastic Remediation with Record Capacity Unleashed via Surface Engineering of Natural Fungal Mycelium Framework. Adv. Funct. Mater. 2023, 33, 2212570. [Google Scholar] [CrossRef]
- Ahmad Shukri, Z.N.; Che Engku Chik, C.E.N.; Hossain, S.; Othman, R.; Endut, A.; Lananan, F.; Terkula, I.B.; Kamaruzzan, A.S.; Abdul Rahim, A.I.; Draman, A.S.; et al. A Novel Study on the Effectiveness of Bioflocculant-Producing Bacteria Bacillus enclensis, Isolated from Biofloc-Based System as a Biodegrader in Microplastic Pollution. Chemosphere 2022, 308, 136410. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Shukri, Z.N.A.; Waiho, K.; Ibrahim, Y.S.; Kamaruzzan, A.S.; Rahim, A.I.A.; Draman, A.S.; Wahab, W.; Khatoon, H.; Kasan, N.A. Biodegradation of Polyethylene (PE), Polypropylene (PP), and Polystyrene (PS) Microplastics by Floc-Forming Bacteria, Bacillus cereus Strain SHBF2, Isolated from a Commercial Aquafarm. Environ. Sci. Pollut. Res. 2024, 31, 32225–32245. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.; Al-Omari, J.; Szerdahelyi, G.S.; Farkas, M.; Al-Omari, Y.; Szabó, P.M.; Sebők, R.; Griffitts, J.; Kriszt, B.; Szoboszlay, S. In Situ Investigation of Plastic-Associated Bacterial Communities in a Freshwater Lake of Hungary. Water Air Soil Pollut. 2021, 232, 493. [Google Scholar] [CrossRef]
- Tang, C.; Wang, L.; Sun, J.; Chen, G.; Shen, J.; Wang, L.; Han, Y.; Luo, J.; Li, Z.; Zhang, P.; et al. Degradable Living Plastics Programmed by Engineered Spores. Nat. Chem. Biol. 2024, 21, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Haque, M.R.; Siddique, M.A.B.; Akbor, M.A.; Hasan, M.; Rahman, M.M. Is Biofloc Fish a Safe Alternative to Conventionally Cultivated Fish Regarding Metal Bioaccumulation in Bangladesh? Environ. Chall. 2023, 11, 100704. [Google Scholar] [CrossRef]
- Deswati, D.; Khairiyah, K.; Safni, S.; Yusuf, Y.; Refinel, R.; Pardi, H. Environmental Detoxification of Heavy Metals in Flood & Drain Aquaponic System Based on Biofloc Technology. Int. J. Environ. Anal. Chem. 2022, 102, 7155–7164. [Google Scholar] [CrossRef]
- Rind, K.H.; Arshad, M.; Majeed, S.; Habib, S.S.; Al-Rejaie, S.S.; Mohany, M.; Aragona, F.; Fazio, F. Impact of Heavy Metals on Health and Quality of Oreochromis niloticus Cultured in Biofloc and Earthen Pond Systems. J. Environ. Sci. Health Part B 2025, 60, 129–137. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy Et, M.A.; Ashry, O.A.; Abbas, E.M.; Sharawy, Z.; Kelany, M.S. Potential Application of Agriculture By-Products in Heavy Metals Bioremediation. Egypt. J. Aquat. Biol. Fish 2022, 26, 53–67. [Google Scholar] [CrossRef]
- Abo-Alkasem, M.I.; Hassan, N.H.; Abo Elsoud, M.M. Microbial Bioremediation as a Tool for the Removal of Heavy Metals. Bull. Natl. Res. Cent. 2023, 47, 31. [Google Scholar] [CrossRef]
- Pandey, M.; Shabuddhin, S.; Tsunoji, N.; Das, S.; Bandyopadhyay, M. Extraction of Heavy Metals from Wastewater Using Amine-Modified Mesoporous Silica. Environ. Sci. Pollut. Res. 2023, 30, 113409–113423. [Google Scholar] [CrossRef] [PubMed]
- Keshta, B.E.; Gemeay, A.H.; Kumar Sinha, D.; Elsharkawy, S.; Hassan, F.; Rai, N.; Arora, C. State of the Art on the Magnetic Iron Oxide Nanoparticles: Synthesis, Functionalization, and Applications in Wastewater Treatment. Results Chem. 2024, 7, 101388. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Tiwari, P.; Upadhyay, M.K.; Srivastava, S.; Dwivedi, S.; Dhankher, O.P.; Tripathi, R.D. Biotechnological Strategies for Remediation of Arsenic-Contaminated Soils to Improve Soil Health and Sustainable Agriculture. Soil Environ. Health 2024, 2, 100061. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; Alardhi, S.M.; Al Omar, M.; Aljumaily, M.M.; Al Saadi, M.A.; Fayaed, S.S.; Ahmed, S.N.; Salman, A.D.; Abdalsalm, A.H.; Jabbar, N.M.; et al. A Comprehensive Review on Modelling the Adsorption Process for Heavy Metal Removal from Waste Water Using Artificial Neural Network Technique. Heliyon 2023, 9, e15455. [Google Scholar] [CrossRef] [PubMed]
- van der Wiel, B.Z.; Weijma, J.; van Middelaar, C.E.; Kleinke, M.; Buisman, C.J.N.; Wichern, F. Restoring Nutrient Circularity: A Review of Nutrient Stock and Flow Analyses of Local Agro-Food-Waste Systems. Resour. Conserv. Recycl. X 2019, 3, 100014. [Google Scholar] [CrossRef]
- de Morais, A.P.M.; Santos, I.L.; Carneiro, R.F.S.; Routledge, E.A.B.; Hayashi, L.; de Lorenzo, M.A.; do Nascimento Vieira, F. Integrated Multitrophic Aquaculture System Applied to Shrimp, Tilapia, and Seaweed (Ulva ohnoi) Using Biofloc Technology. Aquaculture 2023, 572, 739492. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Xiao, D.; Zhang, S.; Li, Z.; Luo, K.; Luo, G.; Tan, H. A Novel Multitrophic Biofloc Technology for Duckweed and Megalobrama amblycephala Integrated Culture: Improving Nutrient Utilization and Animal Welfare. Sci. Total Environ. 2024, 934, 173239. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.M.; de Lima, J.P.; Tarigan, N.B.; David, L.H.; Portella, M.C.; Keesman, K.J. Modelling FLOCponics Systems: Towards Improved Water and Nitrogen Use Efficiency in Biofloc-Based Fish Culture. Biosyst. Eng. 2023, 229, 96–115. [Google Scholar] [CrossRef]
- Legarda, E.C.; Da Silva, D.; Miranda, C.S.; Pereira, P.K.M.; Martins, M.A.; Machado, C.; De Lorenzo, M.A.; Hayashi, L.; Do Nascimento Vieira, F. Sea Lettuce Integrated with Pacific White Shrimp and Mullet Cultivation in Biofloc Impact System Performance and the Sea Lettuce Nutritional Composition. Aquaculture 2021, 534, 736265. [Google Scholar] [CrossRef]
- El-Kady, A.F.Y.; Suloma, A. Sediment Management in Integrated Aquaculture-Agriculture Systems: Sustainable Use of Fish Culture Sludge for Organic Fertilization of Terminalia Arjuna. Environ. Sci. Pollut. Res. 2025, 32, 4273–4287. [Google Scholar] [CrossRef] [PubMed]
- Ayipio, E.; Wells, D.E.; Smith, M.; Blanchard, C. Performance of Greenhouse-Grown Beit Alpha Cucumber in Pine Bark and Perlite Substrates Fertigated with Biofloc Aquaculture Effluent. Horticulturae 2021, 7, 144. [Google Scholar] [CrossRef]
- Saseendran, S.; Dube, K.; Chandrakant, M.H.; Haridas, H.; Rani, A.M.B. Production Performance of Tilapia and Bell Pepper in FLOC Ponics, a Bio-Integrated System of Biofloc Technology with Aquaponics. EEC 2024, 30, S101–S111. [Google Scholar] [CrossRef]
- Rodrigues, A.B.; Pinheiro, I.C.; Nonato, T.C.M.; Ferenhof, E.A.; Sens, M.L. Valorization of Reverse Osmosis Concentrate in the Production of Litopenaeus vannamei and New Zealand Spinach in an Aquaponic System with Biofloc. Desalination 2023, 549, 116330. [Google Scholar] [CrossRef]
- Pinho, S.M.; David, L.H.C.; Goddek, S.; Emerenciano, M.G.C.; Portella, M.C. Integrated Production of Nile Tilapia Juveniles and Lettuce Using Biofloc Technology. Aquac. Int. 2021, 29, 37–56. [Google Scholar] [CrossRef]
- Menaga, M.; Felix, S.; Mohanasundari, C.; Charulatha, M. Characterisation of Pigment Producing Bacteria Isolated from Bio Floc Ponds and Its Colour Enhancement Effect in Xiphophorus helleri. Indian J. Anim. Res. 2021, 57, 1415–1421. [Google Scholar] [CrossRef]
- Lyu, X.; Kuang, H.; Liu, W.; Tan, H.; Luo, G.; Hu, X.; Zhao, Z. Evaluation of Short-Term Anaerobic Fermentation Using Bioflocs Waste and Performance Assessment. Aquaculture 2023, 577, 739929. [Google Scholar] [CrossRef]
- Ribeiro, T.F.; Trevisan, V.; Fabregat, T.E.H.P.; Skoronski, E. Characterization of a Byproduct Generated by the Treatment of Water from a Biofloc Technology System Using a Plant Tannin-Based Coagulant. Aquac. Eng. 2022, 99, 102297. [Google Scholar] [CrossRef]

| Plant Species | Farmed Species | Key Results | Reference |
|---|---|---|---|
| Duckweed (Lemna minor) | Megalobrama amblycephala | Nutrient utilization (N, P, C) and animal welfare were improved | [69] |
| Seaweed (Ulva ohnoi) | Shrimp (Penaeus vannamei) and tilapia (Oreochromis niloticus) | Seaweed benefited the performance of all species by recycling N, P and increasing overall productivity | [68] |
| Bell pepper (Capsicum annum) | Oreochromis niloticus | Fish stocking density of 150/m3 and plant density of 12 no./m2 is optimum | [74] |
| New Zealand spinach | Litopenaeus vannamei | Shrimp survival was 18% due to high nitrite level using reverse osmosis concentrate | [75] |
| Sea lettuce (Ulva fasciata) | Shrimp (Litopenaeus vannamei) and mullet (Mugil liza) | Enhanced total yield and N, P recovery | [71] |
| Terminalia arjuna | Nile tilapia (Oreochromis niloticus) | Biofloc sludge served as an effective organic fertilizer for ornamental medicinal tree production | [72] |
| Lettuce (Lactuca sativa) | Nile tilapia (Oreochromis niloticus) juvenile | Integration did not improve the plants’ growth and nutrient uptake | [76] |
| Lettuce (Lactuca sativa) | Nile tilapia (Oreochromis niloticus) juvenile | Integration improved water and nutrient use efficiencies, and reduced solids disposal | [70] |
| Beit Alpha cucumber (Cucumis sativus) | Nile tilapia (Oreochromis niloticus) | Substrate evaluation of pine bark and perlite in cucumber aeroponics fertigated with biofloc aquaculture effluent | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Dai, L. Multifunctional Applications of Biofloc Technology (BFT) in Sustainable Aquaculture: A Review. Fishes 2025, 10, 353. https://doi.org/10.3390/fishes10070353
Li C, Dai L. Multifunctional Applications of Biofloc Technology (BFT) in Sustainable Aquaculture: A Review. Fishes. 2025; 10(7):353. https://doi.org/10.3390/fishes10070353
Chicago/Turabian StyleLi, Changwei, and Limin Dai. 2025. "Multifunctional Applications of Biofloc Technology (BFT) in Sustainable Aquaculture: A Review" Fishes 10, no. 7: 353. https://doi.org/10.3390/fishes10070353
APA StyleLi, C., & Dai, L. (2025). Multifunctional Applications of Biofloc Technology (BFT) in Sustainable Aquaculture: A Review. Fishes, 10(7), 353. https://doi.org/10.3390/fishes10070353