Molecular Mechanisms and Biomarker-Based Early-Warning Indicators of Heavy Metal Toxicity in Marine Fish
Abstract
1. Introduction
2. Methodology
3. Molecular and Biochemical Mechanisms of Toxicity
3.1. Oxidative Stress and Antioxidant Disruption
3.2. Detoxification Pathways: Metallothioneins, Glutathione Systems, Metallochaperones, and Metal Transporters
3.2.1. Metallothioneins: Metal-Binding Proteins
3.2.2. Glutathione and Antioxidant Systems in Metal Detoxification
3.2.3. Other Mechanisms: Metallochaperones and Metal Transporters
3.3. DNA Damage and Genotoxicity
3.4. Molecular Reprogramming and Stress Adaptation
3.5. Inflammation and Apoptosis
3.6. Neurotoxicity and Cholinergic Disruption
3.7. Endocrine Disruption and Hormonal Dysregulation
3.8. Cellular Dysfunction and Energy Disruption
4. Applications in Biomonitoring and Risk Assessment
- Biomarkers of exposure
- Biomarkers of effect
- Biomarkers of susceptibility or condition
5. Knowledge Gaps and Future Perspectives
- Autophagy and lysosomal integrity, through markers such as LMS or autophagic flux, provide early warning of proteotoxic stress and subcellular dysfunction [247].
6. Conclusions
- (i)
- The integration of emerging endpoints such as microRNAs, epigenetic markers, and metabolomics for higher diagnostic resolution;
- (ii)
- Evaluating the effects of real-world pollutant mixtures, which often involve complex interactions between metals and organics;
- (iii)
- Understanding how environmental variables (e.g., temperature, salinity, hypoxia) influence biomarker responses;
- (iv)
- Validating biomarker responses across life stages and ecological scenarios.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMs | Heavy metals |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| ROS | Reactive oxygen species |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| CAT | Catalase |
| MDA | Malondialdehyde |
| GSH | Reduced glutathione (γ-L-glutamyl-L-cysteinyl-glycine) |
| MyoD | Myogenic differentiation 1 |
| IGF-1 | Insulin-like growth factor 1 |
| HO-1 | Heme oxygenase-1 |
| Hsp32 | Heat shock protein 32 |
| Nrf2/Keap1 | NF-E2-related nuclear factor2/Kelch-like-ECH-associated protein1 |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| MTs | Metallothioneins |
| ATOX1 | Antioxidant protein 1 |
| CCS | Copper chaperone for superoxide dismutase |
| MTF-1 | Metal-responsive transcription factor-1 |
| MRP | Multidrug resistance-associated protein |
| GST | Glutathione S-transferase |
| GR | Glutathione reductase |
| TrxR | Thioredoxin reductase |
| EROD | Ethoxyresorufin-O-deethylase |
| CYP1A | Cytochrome P450 1A |
| AhR | Aryl hydrocarbon receptor |
| COX17 | Cytochrome c oxidase copper chaperone |
| ZIP | Zinc-regulated transporter |
| IRT | Iron-regulated transporter |
| DMT1 | Divalent metal transporters |
| ATP7A/B | Copper-transporting ATPases |
| ZnT | Zn transporters |
| ABC | ATP-binding cassette |
| 8-oxodG | 8-hydroxy-2′-deoxyguanosine |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| p53 | Tumor protein 53 |
| BAX | Bcl-2-associated X protein |
| caspase-3 | Cysteine-aspartic protease 3 |
| HSPs | Heat shock proteins |
| HIF-1α | Hypoxia-inducible factor-1α |
| AMPK | AMP-activated protein kinase |
| FAS | Fatty acid synthase |
| microRNAs or miRNAs | Small non-coding RNAs |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| TERT | Telomerase reverse transcriptase |
| AChE | Acetylcholinesterase |
| Na+/K+-ATPase | Sodium-potassium adenosine triphosphatase |
| Ca2+-ATPase | Calcium adenosine triphosphatase |
| EDCs | Endocrine-disrupting chemicals |
| HPG axis | Hypothalamic–pituitary–gonadal axis |
| GnRH | Gonadotropin-releasing hormone |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| VTG | Vitellogenin |
| 17β-HSD | 17β-hydroxysteroid dehydrogenase |
| CYP19A1 | Cytochrome P450 family 19 subfamily A member 1, commonly known as aromatase |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| ALAD | δ-aminolevulinic acid dehydratase |
| TPO | Thyroid peroxidase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| LMS | Lysosomal membrane stability |
| CA | Carbonic anhydrase |
| IBR | Integrated biomarker response |
| qPCR | quantitative Polymerase Chain Reaction |
| E2/T ratio | 17β-estradiol/testosterone ratio |
| ZPP | Zinc protoporphyrin |
| PUFA | Polyunsaturated fatty acids |
| MnSOD | Manganese superoxide dismutase |
| ARE | Antioxidant response element |
Appendix A
| Study | Metal (s) | Fish Species | Biomarkers/ Mechanisms | Major Findings |
|---|---|---|---|---|
| Field studies | ||||
| Gabriel et al. (2020) (Atlantic Oc.) [47] | Mixed mine tailing metals (As, Cr, Cu, Hg, Zn, etc.) | Demersal fish species (Cathorops spixii, Genidens genidens, Eugerres brasilianus, Diapterus rhombeus, and Mugil sp.) | MT and GSH in liver and muscle | Chronic exposure led to elevated MT and GSH in fish tissues, with levels significantly correlated to tissue metal concentrations; indicates active metal sequestration and antioxidant compensation in wild fish living in an area affected by a mine tailings disaster. |
| Hauser et al. (2021) (Atlantic Oc.) [77] | Broad range of metals (Ag, Al, As, Cd, Co, Cr, Cs, Cu, Fe, Hg, Mn, Ni, Pb, Sb, Sr, Ti, V, and Zn) | Blue shark (Prionace glauca) | MT and GSH in liver and muscle | MT plays a role in detoxifying As, Cd, Cs, Pb, Se, and Zn in both liver and muscle, while for Cu, Hg, and Ti only in liver; GSH appears to be involved in the physiological response to Co and Zn; this was the first report of MT-mediated detox for elements like Ti and Co in this species, providing baseline data for biomonitoring. |
| Filipovic Marijic et al. (2007) (Adriatic Sea) [70] | Cu | Red mullet (Mullus barbatus) | Intestinal MT and cytosolic Cu | Cu concentrations and MT levels in the intestines are significantly higher in the polluted areas, indicating a direct relationship between copper exposure and MT induction. |
| Liu et al. (2023) (Pacific Oc.) [85] | Hg, Cd, As, and Zn | Gobies (Acanthogobius ommaturus) | Liver MT; GPx, GR; MDA; and IBR index | MT, MDA, GPx, and GR in liver were elevated in fish from more polluted sites; an integrated biomarker response (IBR) analysis clearly distinguished heavily polluted stations by their higher combined biomarker scores. This study demonstrates a successful application of multiple detoxification-related biomarkers for environmental risk assessment. |
| Holbert et al. (2023) (Pacific Oc.) [86] | Total mercury (THg) | Bonefish (Albula spp.) Trevally (Caranx spp.) | THg in muscle, liver, kidney; MT (MET) gene expression; and TrxR gene expression | MT gene (MET) expression was elevated in high-Hg fish, supporting MT mRNA as a biomarker for Hg exposure; in contrast, TrxR gene expression was inversely related to Hg in liver, implying that Hg may suppress this antioxidant enzyme’s expression or activity. The study suggests that monitoring these molecular biomarkers can help detect the subclinical effects of mercury in fish before overt toxicity occurs. |
| Moussa et al. (2022) (Mediterranean Sea) [110] | Cd, Pb, Cu, Zn, Fe, and Al | Oreochromis niloticus and Clarias gariepinus | Metal levels; DNA damage (Comet assay, 8-oxodG); and organ indices | Fish chronically exposed to polluted agricultural drain waters accumulated high levels of metals in liver, kidney, and gills. Genotoxicity was evident as fish from the contaminated site had significantly more DNA damage (Comet tail length, fragmentation) than reference fish, proportional to tissue metal burdens. Despite signs of metal tolerance, the persistence of genotoxic effects underscores the long-term risk of exposure, even at environmentally relevant concentrations. |
| Schmitt et al. (2002) (N. America, rivers impacted by smelters) [166] | Pb and Zn | Demersal fish species: catostomids (Hypentelium nigricans, Carpiodes carpio, Catostomus macrocheilus, C. platyrhynchus) salmonids (Oncorhynchus mykiss, Salvelinus fontinalis), carp (Cyprinus carpio), and channel catfish (Ictalurus punctatus) | Erythrocyte ALAD; hemoglobin (Hb); and blood/liver/muscle Pb and Zn; | ALAD activity was significantly inhibited in fish from rivers downstream of smelters compared to upstream reference sites; catostomids showed greater ALAD sensitivity to Pb than salmonids or carp; no ALAD activity was detected in channel catfish at some sites, possibly due to species-specific sensitivity; blood and tissue Pb concentrations correlated with ALAD inhibition; and Zn concentrations showed less variation and may have modulated Pb effects. |
| Schmitt et al. (2007) (N. America, rivers impacted by Pb/Zn mining activities) [167] | Pb, Zn, Cd, Co, Ni, and Fe | Largescale stoneroller (Campostoma oligolepis), Longear sunfish (Lepomis megalotis), and Northern hog sucker (Hypentelium nigricans) | ALAD activity; ZPP; hepatic MT gene expression; lipid peroxidation; hemoglobin (Hb); and HMs in blood. | Blood Pb concentrations were significantly elevated in fish from sites near active and historical mines; ALAD activity was negatively correlated with blood Pb levels across all species; ZPP was only detectable in fish from contaminated sites; MT gene expression showed positive correlation with liver Zn concentrations, although differences between contaminated and reference sites were not statistically significant; and lipid peroxidation levels and MT differences were not significant among groups of fish classified by Pb exposure levels. |
| Laboratory experiments | ||||
| Espinoza et al. (2012) [71] Acute (8–48 h) exposures to low (3.7 ppb) and high (347 ppb) levels of Cd | Cd | Coho Salmon (Oncorhynchus kisutch) | mRNA expression of GST isoforms and MT in liver, gill, and olfactory tissues, using qPCR | While GSTs do respond to cadmium-induced oxidative stress, their inconsistent and transient changes make them less reliable as biomarkers. In contrast, MT mRNA expression, particularly in the olfactory system, shows a more consistent and pronounced response to cadmium exposure. Thus, MT mRNA expression may serve as a more reliable biomarker for assessing short-term cadmium exposure in fish compared to GST isoforms. |
| Taysı et al. (2024) [44] Exposure to three Cd concentrations (1, 3, and 5 mg/L) over three time-points (24, 48, and 96 h) | Cd | Rainbow trout (Oncorhynchus mykiss) (juvenile) | SOD, CAT, GSH, and MDA; 8-oxodG; caspase-3; and histology | Cd exposure induced oxidative stress (SOD increased, GSH, and CATdecreased) and DNA damage (8-oxodG levels rose significantly in liver/kidney); caspase-3 activity increased (apoptosis); and histopathology showed liver and kidney tissue injury. |
| Monteiro et al. (2023) [45] Exposure to metallurgical particles (SePM) (0.0, 0.01, 0.1 and 1.0 g L−1), for 96 h. | Metallife-rous particulate mixture (Al, Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, As, Se, Rb, Sr, Y, Zr, Nb, Mo, Ag, Cd, Sn, Ba, La, Ce, W, Hg, Pb, and Bi) | Fat snook (Centropomus parallelus) | SOD, CAT, GPx, GST, and GSH in gill, liver, and kidney; lipid peroxidation; and tissue lesions | Waterborne exposure to metal-rich particulates caused tissue-specific antioxidant responses: CAT, GST, and GSH increased in kidney, while SOD/CAT decreased in liver, whileGPx increased; no rise in MDA or protein oxidation occurred (effective antioxidant defense), but gill and kidney lesions were observed, indicating partial physiological stress. |
| Xie et al. (2020) [51] Exposure to 0.95 mg Cu/L in water for one week. | Cu | Golden pompano (Trachinotus ovatus) | mRNA expression of Nrf2 and keap1, SOD, CAT, HO-1, NQO1, and GPX | HO-1 upregulated (mRNA increased); Nrf2–HO-1 pathway activated; and SOD, CAT, NQO1, and GPx transcripts significantly increased, indicating oxidative stress response. |
| Dong et al. (2016) [257] Exposure to 0.001–10 µM MeHg, HgCl2, HgS up to 10 days. | MethylHg (MeHg) and inorganic Hg | Medaka fish embryos (Oryzias spp.) | MT and HO-1 gene expression (RT-PCR) | HO-1 upregulated (mRNA increased); HO-1 and MT strongly induced by toxic MeHg and inorganic HgCl2, but not by less toxic HgS forms; And HO-1 induction correlates with mercury toxicity. |
| Bonsignore et al. (2022) [60] Exposure to Hg (4.9 ± 1.2 μg L−1) and Cd (29 ± 5 μg L−1) for 25 days. | Hg and Cd | Gilthead seabream (Sparus aurata) (adults) | Liver fatty acids (polyunsaturated fatty acids, PUFA); MDA; and gene expression (Nrf2, HIF-1α, AMPK, NF-κB, and fatty acid synthase, FAS) | Chronic exposure to Cd and Hg led to bioaccumulation (highest in gill, liver, and kidney) and oxidative stress (MDA increased, liver PUFA decreased); both metals triggered significant upregulation of Nrf2, HIF-1α, and AMPK genes (adaptive metabolic responses); Hg specifically increased NF-κB (suggesting inflammation); and FAS expression dropped, showing altered lipid metabolism under metal stress. |
| Kamila et al. (2024) [258] Exposure to environmentally relevant concentrations of As and Cr for 15, 30 and 60 days. | As (III) + Cr (VI) mixture | Zebrafish (Danio rerio) | MDA, GSH, and CAT; and HO-1 (mRNA), Nrf2, NQO1, and MnSOD | Chronic exposure; elevated MDA level, GSH content, altered CAT activity indicated oxidative stress; upregulation of Nrf2-Keap1-ARE (Antioxidant response element) pathway; HO-1 upregulated (mRNA increased); and sustained elevation of HO-1 (and Nrf2, NQO1, MnSOD) at all time-points during exposure, indicating prolonged antioxidant defense activation under metal co-exposure. |
| Zang et al. (2020) [259] Exposure to 15 mg/L cadmium chloride 24 h | Cd | Swamp eel (Monopterus albus) | HO-1 and Nrf2 mRNA expression in liver. | Acute exposure: HO-1 upregulated (mRNA/protein increased); HO-1 significantly induced under Cd-induced oxidative stress, being a crucial mediator of antioxidant and anti-apoptotic defenses; HO-1 knockdown increased Cd toxicity. |
| Liu et al. (2023) [43] Acute exposure to different concentrations of MnCl2 (0–152.00 mg/L) | Mn | Marine medaka (Oryzias melastigma) (embryos) | Developmental indices (hatching, malformations); SOD, CAT, GPx, and MDA; and gene expression (cardiac genes, p53, TNF-α, and IL-1β) | Mn exposure caused embryotoxicity (delayed hatching, malformations) and oxidative stress (MDA and antioxidant enzyme activities increased dose-dependently); notably, Mn disrupted expression of cardiac development genes and upregulated stress markers (telomerase, p53) and inflammatory cytokines (TNF-α, IL-1β), thus implicating ROS and inflammation in Mn toxicity. |
| Shaw et al., (2022) [133] Exposure to 2 mg L−1 Cr [VI] | Cr(VI) | Zebrafish (Danio rerio) | DNA damage (comet assay); and apoptosis-related gene expression (p53, Bax, Caspase-3, Caspase-9, and Bcl2) | Significant increase in DNA damage index; intrinsic pathway activation (p53, Bax, Caspase-9, Caspase-3); decreased Bcl2. Mechanism: Cr(VI) reduced to Cr(III) in hepatocytes, causing ROS generation followed by DNA damage and apoptosis. |
| Zhang et al. (2024) [48] Exposure to four concentrations: 0, 0.2, 0.4, and 0.6 mg/L Cd2+, for 30 days | Cd | Nile tilapia (Oreochromis niloticus) (juveniles) | Growth performance; digestive enzymes; and antioxidant gene expression (CAT, SOD, GST, and GPx) | Sub-chronic Cd exposure in a marine aquaculture context led to stunted growth and lower muscle protein/lipid content; Cd significantly downregulated hepatic antioxidant genes (CAT, SOD, GST, and GPx), indicating an impaired antioxidant system; digestive enzyme activities dropped, suggesting general physiological stress. This study shows Cd can repress antioxidant defenses and metabolism during prolonged exposure. |
| Sac et al. (2023) [42] Exposure at concentrations of 25, 50, and 75 mg/L As for 96 h | As | Rainbow trout (Oncorhynchus mykiss) (juveniles) | Brain oxidative stress markers (SOD, CAT, GPx, GSH, and MDA); and NF-κB, TNF-α, IL-6, Nrf2, 8-oxodG, AChE, and caspase-3 (using ELISA) | Acute As exposure resulted in pronounced oxidative stress and neurotoxicity; in-brain levels of oxidative stress markers MDA, NF-κB, IL-6, TNF-α, Nrf2, and 8-OHdG were significantly elevated, while antioxidant enzymes (SOD, CAT, and GPx) and GSH were markedly reduced; a significant increase in caspase-3 expression, accompanied by inhibition of AChE activity, indicated activation of apoptotic pathways and neural dysfunction. These results demonstrate that As simultaneously induces oxidative damage, inflammation, and apoptosis in fish brain tissue. |
| Richetti et al. (2011) [146] Exposure to mercury chloride and lead acetate (20 μg/L), zinc chloride (5 mg/L) and cadmium acetate (0.1 mg/L), 24 h, 96 h and 30 days. | Cd, Pb, Hg, and Zn | Adult zebrafish (Danio rerio) | AChE activity; AChE gene expression; and antioxidant capacity (brain) | The findings suggest that Hg and Pb can impair cholinergic neurotransmission by inhibiting AChE activity and that mercury can also compromise antioxidant defenses, potentially leading to neurotoxic effects. |
| Ayllon et al., 2000 [112] Exposure (intraperitoneal injections) to different doses (0.17, 1.7, 2 × 1.7, and 3.4 mg/kg) of cadmium and mercury for 24 h | Cd and Hg | Phoxinus phoxinus and Poecilia latipinna | Micronuclei induction and other nuclear abnormalities | P. phoxinus shows limited sensitivity to HM genotoxicity, but is responsive to other clastogens (e.g., colchicine); and P. latipinna is sensitive to both Cd and Hg and is a valid model for HM genotoxicity testing. |
| Puar et al. (2020) [260] Exposure to 4 μM Zn | Zn exposure HM analyzed: Zn, Ca, Ni, Cu, Mn, Fe, Co, andSe | Danio rerio (zebrafish), early life stages | Whole-body metal content; mRNA expression levels of ZIP transporters via ddPCR; and spatial localization of ZIPs via in situ hybridization | High Zn exposure led to increased Zn body burden and transient reductions in Ca, Ni, and Cu; Zn exposure significantly altered the expression of ZIP transporters: zip1 and zip8 were upregulated while zip4 was downregulated; ZIPs showed distinct spatial distribution, e.g., zip8 localized in the intestinal tract, zip14 in the pronephric tubules and head regions. The study highlights Zn-induced disruption of metal homeostasis and differential ZIP transporter regulation in developing zebrafish. |
Appendix B
| Biomarker/Mechanism | Pollutant Types | Notes |
|---|---|---|
| Metallothioneins (MTs) | Specific to HMs | Classic biomarker of metal exposure. |
| Metal transporters (DMT1, ZIP, and ATP7A/B) | Specific to HMs | Regulate uptake/homeostasis of metals; altered expression under HM stress. |
| Metallochaperones (e.g., ATOX1) | Specific to HMs | Mediate intracellular delivery and compartmentalization of metal ions; disrupted by HM exposure. |
| δ-ALA dehydratase (ALAD) inhibition | Specific to Pb and other HMs | Enzyme activity is used as HM exposure marker. |
| SOD, CAT, GPx, GSH, and MDA | General (HMs, organics, and microplastics) | Core oxidative stress markers across pollutant types. |
| Heme oxygenase-1 (HO-1) | HMs, PAHs, organic EDCs, and oxidative agents | Cytoprotective enzyme upregulated via Nrf2 pathway; early indicator of oxidative stress but broadly inducible by ROS and not exclusive to metals. |
| DNA base oxidation (8-oxodG) | HM, PAHs, pesticides, and oxidative agents | Marker of oxidative DNA damage; induced by multiple ROS-generating pollutants. Useful for genotoxicity screening, but not HM-specific. |
| EROD/CYP1A induction | Primarily organic pollutants (PAHs), but metals in complexes may also trigger it. | Indicates activation of the AhR pathway; reflects mixed exposure to organic pollutants and metal–organic complexes. |
| Comet assay/DNA strand breaks | HMs, PAHs, pesticides, pharmaceuticals, and oxidative agents | Sensitive genotoxicity assay used across pollutant classes; detects single- and double-strand breaks, alkali-labile sites, and DNA crosslinking in individual cells. Not HM specific. |
| MicroRNA (miRNA) expression profiles | Emerging for HMs, organic pollutants, microplastics, and pharmaceuticals | Altered expression of specific miRNAs (e.g., miR-122, miR-155) regulates oxidative stress, apoptosis, and immune pathways; sensitive to various pollutants but not HM specific. |
| Epigenetic alterations (DNA methylation patterns) | HMs, PAHs, EDCs, and hypoxia | Disruption of global or gene-specific methylation affects gene regulation; indicative of chronic and possibly transgenerational stress, but broadly responsive to multiple stressors. |
| Lysosomal membrane stability (LMS) | Common across pollutants | Affects bivalves, fish, in response to multiple stressors. |
| Apoptosis genes, caspase-3, p53 | General cellular stress | Activated in metal and organic toxicity. |
| AChE inhibition | Neurotoxicants: organophosphates, HMs, and microplastics | Common neurotoxicity biomarker. |
| Carbonic anhydrase (CA) inhibition | HM, pesticides, and other pollutants | Sensitive to various pollutants; indicative of general environmental stress, not metal specific. |
| Vitellogenin (VTG) induction | Classical for endocrine disruptors | Induced by both metals (Hg, Cd, and Pb) and EDCs (e.g., BPA, phthalates). |
References
- El-Sharkawy, M.; Alotaibi, M.O.; Li, J.; Du, D.; Mahmoud, E. Heavy Metal Pollution in Coastal Environments: Ecological Implications and Management Strategies: A Review. Sustainability 2025, 17, 701. [Google Scholar] [CrossRef]
- Haidar, Z.; Fatema, K.; Shoily, S.S.; Sajib, A.A. Disease-Associated Metabolic Pathways Affected by Heavy Metals and Metalloid. Toxicol. Rep. 2023, 10, 554–570. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, S.; Jain, A.; Yadav, S.; Dubey, A.; Trivedi, S.P. A Review on Heavy Metal-Induced Toxicity in Fishes: Bioaccumulation, Antioxidant Defense System, Histopathological Manifestations, and Transcriptional Profiling of Genes. J. Trace Elem. Med. Biol. 2024, 83, 127377. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Taslima, K.; Al-Emran, M.; Rahman, M.S.; Hasan, J.; Ferdous, Z.; Rohani, M.F.; Shahjahan, M. Impacts of Heavy Metals on Early Development, Growth and Reproduction of Fish—A Review. Toxicol. Rep. 2022, 9, 858–868. [Google Scholar] [CrossRef]
- Phaenark, C.; Phankamolsil, Y.; Sawangproh, W. Ecological and Health Implications of Heavy Metal Bioaccumulation in Thai Fauna: A Systematic Review. Ecotoxicol. Environ. Saf. 2024, 285, 117086. [Google Scholar] [CrossRef] [PubMed]
- Oros, A. Bioaccumulation and Trophic Transfer of Heavy Metals in Marine Fish: Ecological and Ecosystem-Level Impacts. J. Xenobiotics 2025, 15, 59. [Google Scholar] [CrossRef]
- Santhosh, K.; Kamala, K.; Ramasamy, P.; Musthafa, M.S.; Almujri, S.S.; Asdaq, S.M.B.; Sivaperumal, P. Unveiling the Silent Threat: Heavy Metal Toxicity Devastating Impact on Aquatic Organisms and DNA Damage. Mar. Pollut. Bull. 2024, 200, 116139. [Google Scholar] [CrossRef]
- Maret, W. Metalloproteomics, Metalloproteomes, and the Annotation of Metalloproteins. Metallomics 2010, 2, 117–125. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I.; Rosato, A. Metalloproteomes: A Bioinformatic Approach. Acc. Chem. Res. 2009, 42, 1471–1479. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Tolkou, A.K.; Toubanaki, D.K.; Kyzas, G.Z. Detection of Arsenic, Chromium, Cadmium, Lead, and Mercury in Fish: Effects on the Sustainable and Healthy Development of Aquatic Life and Human Consumers. Sustainability 2023, 15, 16242. [Google Scholar] [CrossRef]
- Naz, S.; Chatha, A.M.M.; Téllez-Isaías, G.; Ullah, S.; Ullah, Q.; Khan, M.Z.; Shah, M.K.; Abbas, G.; Kiran, A.; Mushtaq, R.; et al. A Comprehensive Review on Metallic Trace Elements Toxicity in Fishes and Potential Remedial Measures. Water 2023, 15, 3017. [Google Scholar] [CrossRef]
- Dhaneesh, K.V.; Gopi, M.; Ganeshamurthy, R.; Kumar, T.T.A.; Balasubramanian, T. Bio-Accumulation of Metals on Reef Associated Organisms of Lakshadweep Archipelago. Food Chem. 2012, 131, 985–991. [Google Scholar] [CrossRef]
- Ahmed, I.; Zakiya, A.; Fazio, F. Effects of Aquatic Heavy Metal Intoxication on the Level of Hematocrit and Hemoglobin in Fishes: A Review. Front. Environ. Sci. 2022, 10, 919204. [Google Scholar] [CrossRef]
- Shahjahan, M.; Islam, M.J.; Hossain, M.T.; Mishu, M.A.; Hasan, J.; Brown, C. Blood Biomarkers as Diagnostic Tools: An Overview of Climate-Driven Stress Responses in Fish. Sci. Total Environ. 2022, 843, 156910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Noman, A.; Zhang, C.; AL-Bukhaiti, W.Q.; Abed, S.M. Effect of Fish-Heavy Metals Contamination on the Generation of Reactive Oxygen Species and Its Implications on Human Health: A Review. Front. Mar. Sci. 2024, 11, 1500870. [Google Scholar] [CrossRef]
- Elgendy, M.Y.; Ali, S.E.; Abbas, W.T.; Algammal, A.M.; Abdelsalam, M. The Role of Marine Pollution on the Emergence of Fish Bacterial Diseases. Chemosphere 2023, 344, 140366. [Google Scholar] [CrossRef]
- Zeng, H.; Zhong, Y.; Wei, W.; Luo, M.; Xu, X. Combined Exposure to Microplastics and Copper Elicited Size-Dependent Uptake and Toxicity Responses in Red Swamp Crayfish (Procambarus clarkia). J. Hazard. Mater. 2025, 487, 137263. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Liu, Y.-N.; Liu, J.-H.; Feng, X.-S. Single and Combined Effects of Microplastics and Cadmium on the Cadmium Accumulation, Antioxidant Defence and Innate Immunity of the Discus Fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Banaee, M. Perspective Chapter: Exploring the Toxicity Effect of Heavy Metals on Aquatic Organisms—A Comprehensive Analysis. In Heavy Metals in the Environment—Contamination, Risk, and Remediation; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Gochfeld, M.; Burger, J. Mercury Interactions with Selenium and Sulfur and the Relevance of the Se:Hg Molar Ratio to Fish Consumption Advice. Environ. Sci. Pollut. Res. 2021, 28, 18407–18420. [Google Scholar] [CrossRef]
- Connon, R.E.; Geist, J.; Werner, I. Effect-Based Tools for Monitoring and Predicting the Ecotoxicological Effects of Chemicals in the Aquatic Environment. Sensors 2012, 12, 12741–12771. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhu, A.; Guo, Y.; Liu, G.; Chen, B.; He, B.; Liang, Y.; Yin, Y.; Cai, Y.; Jiang, G. Decreased Bioavailability of Both Inorganic Mercury and Methylmercury in Anaerobic Sediments by Sorption on Iron Sulfide Nanoparticles. J. Hazard. Mater. 2022, 424, 127399. [Google Scholar] [CrossRef] [PubMed]
- McGeer, J.C.; Niyogi, S.; Smith, D.S. Cadmium. In Fish Physiology; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: New York, NY, USA, 2011; Volume 31, pp. 125–184. ISBN 9780123786340. [Google Scholar]
- Ray, S.; Vashishth, R. From Water to Plate: Reviewing the Bioaccumulation of Heavy Metals in Fish and Unraveling Human Health Risks in the Food Chain. Emerg. Contam. 2024, 10, 100358. [Google Scholar] [CrossRef]
- Almashhadany, D.A.; Rashid, R.F.; Altaif, K.I.; Mohammed, S.H.; Mohammed, H.I.; Al-Bader, S.M. Heavy Metal (Loid) Bioaccumulation in Fish and Its Implications for Human Health. Ital. J. Food Saf. 2024, 14, 12782. [Google Scholar] [CrossRef]
- Khushbu; Gulati, R.; Sushma; Kour, A.; Sharma, P. Ecological Impact of Heavy Metals on Aquatic Environment with Reference to Fish and Human Health. J. Appl. Nat. Sci. 2022, 14, 1471–1484. [Google Scholar] [CrossRef]
- Amin Rajizadeh, M.; Pourbabaki, R. Oxidative Stress and Exposure to Metals. In Biochemical and Physiological Response During Oxidative Stress—From Invertebrates to Vertebrates; Cordaro, M., Di Paola, R., Fusco, R., Eds.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Ju, Z.; Ndandala, C.B.; Lei, Y.; Shija, V.M.; Luo, J.; Wang, P.; Wen, C.; Liang, H. Cadmium-Induced Oxidative Stress, Histopathology, and Transcriptomic Changes in the Hepatopancreas of Fenneropenaeus merguiensis. Aquac. Rep. 2024, 36, 102061. [Google Scholar] [CrossRef]
- Schuijt, L.M.; Peng, F.-J.; van den Berg, S.J.P.; Dingemans, M.M.L.; Van den Brink, P.J. (Eco)Toxicological Tests for Assessing Impacts of Chemical Stress to Aquatic Ecosystems: Facts, Challenges, and Future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-Stimulated Reactive Oxygen Species Production and Oxidative Damage in Aquatic Organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of Heavy Metals on Fish Physiology—A Review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, H.; Hwang, U.-K.; Kang, J.-C.; Kang, Y.J.; Kim, K.I.; Kim, J.-H. Toxic Effects of Lead Exposure on Bioaccumulation, Oxidative Stress, Neurotoxicity, and Immune Responses in Fish: A Review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Jo, A.-H.; Lee, D.-C.; Choi, C.Y.; Kang, J.-C.; Kim, J.-H. Review of Cadmium Toxicity Effects on Fish: Oxidative Stress and Immune Responses. Environ. Res. 2023, 236, 116600. [Google Scholar] [CrossRef]
- Lushchak, V.I. Contaminant-Induced Oxidative Stress in Fish: A Mechanistic Approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public. Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khalil, A.A.; Awadallah, S.; Khan, S.A.; Abu-Izneid, T.; Kamran, M.; Hemeg, H.A.; Mubarak, M.S.; Khalid, A.; Wilairatana, P. Reactive Oxygen Species in Biological Systems: Pathways, Associated Diseases, and Potential Inhibitors—A Review. Food Sci. Nutr. 2024, 12, 675–693. [Google Scholar] [CrossRef]
- Saç, H.; Yeltekin, A.Ç. Investigation of Oxidative Stress Status and Apoptotic Markers of Juvenile Trout Exposed to Arsenic Toxicity. Toxicol. Res. 2023, 12, 608–614. [Google Scholar] [CrossRef]
- Liu, K.; Yu, D.; Xin, M.; Lü, F.; Zhang, Z.; Zhou, J.; Liu, T.; Liu, X.; Song, J.; Wu, H. Exposure to Manganese (II) Chloride Induces Developmental Toxicity, Oxidative Stress and Inflammatory Response in Marine Medaka (Oryzias melastigma) Embryos. Aquat. Toxicol. 2023, 261, 106622. [Google Scholar] [CrossRef]
- Taysı, M.R. Assessing the Effects of Cadmium on Antioxidant Enzymes and Histological Structures in Rainbow Trout Liver and Kidney. Sci. Rep. 2024, 14, 27453. [Google Scholar] [CrossRef]
- Monteiro, R.; Souza, I.d.C.; Morozesk, M.; Soares, M.P.; De Angelis, C.F.; Vieira, N.S.; Bendhack, F.; Monferrán, M.V.; Wunderlin, D.A.; Fernandes, M.N. Metalliferous Atmospheric Settleable Particulate Matter Action on the Fat Snook Fish (Centropomus parallelus): Metal Bioaccumulation, Antioxidant Responses and Histological Changes in Gills, Hepatopancreas and Kidneys. Chemosphere 2023, 330, 138715. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ma, Y.; Li, Q.; Chen, N.; Wen, S. Stressful Effects of Individual and Combined Exposure to Low-Concentration Polylactic Acid Microplastics and Chromium on Marine Medaka Larvae (Oryzias melastigma). Toxics 2024, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, F.Â.; Hauser-Davis, R.A.; Soares, L.; Mazzuco, A.C.A.; Rocha, R.C.C.; Saint Pierre, T.D.; Saggioro, E.; Correia, F.V.; Ferreira, T.O.; Bernardino, A.F. Contamination and Oxidative Stress Biomarkers in Estuarine Fish Following a Mine Tailing Disaster. PeerJ 2020, 8, e10266. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, Y.; Qin, R.; Huang, E.; Zhang, Z.; Zhou, J.; Liu, D.; Meng, L.; Liu, Y.; Tong, T. Effects of Cadmium on the Growth, Muscle Composition, Digestion, Gene Expression of Antioxidant and Lipid Metabolism in Juvenile Tilapia (Oreochromis niloticus). Front. Mar. Sci. 2024, 11, 1443484. [Google Scholar] [CrossRef]
- Shaalan, W.M. Hazardous Effects of Heavy Metal Pollution on Nile Tilapia in the Aquatic Ecosystem of the Eastern Delta in Egypt. BMC Vet. Res. 2024, 20, 585. [Google Scholar] [CrossRef] [PubMed]
- Jancsó, Z.; Hermesz, E. Impact of Acute Arsenic and Cadmium Exposure on the Expression of Two Haeme Oxygenase Genes and Other Antioxidant Markers in Common Carp (Cyprinus carpio). J. Appl. Toxicol. 2015, 35, 310–318. [Google Scholar] [CrossRef]
- Xie, J.; He, X.; Fang, H.; Liao, S.; Liu, Y.; Tian, L.; Niu, J. Identification of Heme Oxygenase-1 from Golden Pompano (Trachinotus ovatus) and Response of Nrf2/HO-1 Signaling Pathway to Copper-Induced Oxidative Stress. Chemosphere 2020, 253, 126654. [Google Scholar] [CrossRef]
- Dey, K.K.; Kamila, S.; Das, T.; Chattopadhyay, A. Lead Induced Genotoxicity and Hepatotoxicity in Zebrafish (Danio rerio) at Environmentally Relevant Concentration: Nrf2-Keap1 Regulated Stress Response and Expression of Biomarker Genes. Environ. Toxicol. Pharmacol. 2024, 107, 104396. [Google Scholar] [CrossRef]
- Zheng, F.; Gonçalves, F.M.; Abiko, Y.; Li, H.; Kumagai, Y.; Aschner, M. Redox Toxicology of Environmental Chemicals Causing Oxidative Stress. Redox Biol. 2020, 34, 101475. [Google Scholar] [CrossRef]
- Ni, M.; Li, X.; Yin, Z.; Jiang, H.; Sidoryk-Węgrzynowicz, M.; Milatovic, D.; Cai, J.; Aschner, M. Methylmercury Induces Acute Oxidative Stress, Altering Nrf2 Protein Level in Primary Microglial Cells. Toxicol. Sci. 2010, 116, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Xiao, Y.; Wang, Y.; Wan, Y.; Li, X.; Li, Q.; Tang, X.; Cai, D.; Ran, B.; et al. Curcumin Ameliorates Mercuric Chloride-Induced Liver Injury via Modulating Cytochrome P450 Signaling and Nrf2/HO-1 Pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111426. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, J.; Dong, P.; Li, L.; Gu, Z.; Yuan, J. Antioxidant and Anti-Apoptotic Properties of Heme Oxygenase-1 in Red Swamp Crayfish Procambarus Clarkii. Fish Shellfish. Immunol. 2025, 162, 110348. [Google Scholar] [CrossRef]
- Lee, J.-W.; Jo, A.-H.; Kang, Y.-J.; Lee, D.; Choi, C.-Y.; Kang, J.-C.; Kim, J.-H. Review of Cadmium Bioaccumulation in Fish Exposed to Cadmium. Toxics 2024, 13, 7. [Google Scholar] [CrossRef]
- Yachie, A. Heme Oxygenase-1 Deficiency and Oxidative Stress: A Review of 9 Independent Human Cases and Animal Models. Int. J. Mol. Sci. 2021, 22, 1514. [Google Scholar] [CrossRef]
- Bilgin, M.; Uluturhan-Suzer, E.; Darılmaz, E. Integrated Assessment with Biomarker Responses and Metal Concentrations on Some Fish Species from İzmir Bay: A Preliminary Investigation. Ege J. Fish. Aquat. Sci. 2022, 39, 284–292. [Google Scholar] [CrossRef]
- Bonsignore, M.; Maria, M.C.; Antonio, B.; Simona, M.; Rosaria, A.; Andrea, S.; Giulia, M.; Marianna, D.C.; Mario, S. Chemical and Biochemical Responses to Sub−lethal Doses of Mercury and Cadmium in Gilthead Seabream (Sparus aurata). Chemosphere 2022, 307, 135822. [Google Scholar] [CrossRef]
- Mills, M.G.; Gallagher, E.P. A Targeted Gene Expression Platform Allows for Rapid Analysis of Chemical-Induced Antioxidant MRNA Expression in Zebrafish Larvae. PLoS ONE 2017, 12, e0171025. [Google Scholar] [CrossRef]
- Chen, D.S.; Chan, K.M. Differentially Expressed Proteins in Zebrafish Liver Cells Exposed to Copper. Aquat. Toxicol. 2011, 104, 270–277. [Google Scholar] [CrossRef]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef]
- Lavradas, R.T.; Hauser-Davis, R.A.; Lavandier, R.C.; Rocha, R.C.C.; Saint’ Pierre, T.D.; Seixas, T.; Kehrig, H.A.; Moreira, I. Metal, Metallothionein and Glutathione Levels in Blue Crab (Callinectes sp.) Specimens from Southeastern Brazil. Ecotoxicol. Environ. Saf. 2014, 107, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kehrig, H.A.; Hauser-Davis, R.A.; Seixas, T.G.; Pinheiro, A.B.; Di Beneditto, A.P.M. Mercury Species, Selenium, Metallothioneins and Glutathione in Two Dolphins from the Southeastern Brazilian Coast: Mercury Detoxification and Physiological Differences in Diving Capacity. Environ. Pollut. 2016, 213, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.K.; Anderson, M.E.; Meister, A. Glutathione, a First Line of Defense against Cadmium Toxicity. FASEB J. 1987, 1, 220–223. [Google Scholar] [CrossRef]
- O’Halloran, T.V.; Culotta, V.C. Metallochaperones, an Intracellular Shuttle Service for Metal Ions. J. Biol. Chem. 2000, 275, 25057–25060. [Google Scholar] [CrossRef]
- Portnoy, M.; Schmidt, P.; Rogers, R.; Culotta, V. Metal Transporters that Contribute Copper to Metallochaperones in Saccharomyces Cerevisiae. Mol. Genet. Genom. 2001, 265, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xia, Z.; Wang, F. Zebrafish in the Sea of Mineral (Iron, Zinc, and Copper) Metabolism. Front. Pharmacol. 2014, 5, 33. [Google Scholar] [CrossRef]
- Filipović Marijić, V.; Raspor, B. Metallothionein in Intestine of Red Mullet, Mullus Barbatus as a Biomarker of Copper Exposure in the Coastal Marine Areas. Mar. Pollut. Bull. 2007, 54, 935–940. [Google Scholar] [CrossRef]
- Espinoza, H.M.; Williams, C.R.; Gallagher, E.P. Effect of Cadmium on Glutathione S-Transferase and Metallothionein Gene Expression in Coho Salmon Liver, Gill and Olfactory Tissues. Aquat. Toxicol. 2012, 110–111, 37–44. [Google Scholar] [CrossRef]
- De Boeck, G.; Ngo, T.T.H.; Van Campenhout, K.; Blust, R. Differential Metallothionein Induction Patterns in Three Freshwater Fish during Sublethal Copper Exposure. Aquat. Toxicol. 2003, 65, 413–424. [Google Scholar] [CrossRef]
- Le Croizier, G.; Lacroix, C.; Artigaud, S.; Le Floch, S.; Raffray, J.; Penicaud, V.; Coquillé, V.; Autier, J.; Rouget, M.-L.; Le Bayon, N.; et al. Significance of Metallothioneins in Differential Cadmium Accumulation Kinetics between Two Marine Fish Species. Environ. Pollut. 2018, 236, 462–476. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Bastos, F.F.; Tuton, B.; Chávez Rocha, R.; Pierre, T.S.; Ziolli, R.L.; Arruda, M.A.Z. Bile and Liver Metallothionein Behavior in Copper-Exposed Fish. J. Trace Elem. Med. Biol. 2014, 28, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, C.S.V.; da Silva, E.G.P.; Hauser-Davis, R.A.; Dias, F.; Amorim, F.A.C.; de Jesus, R.M.; Novaes, C.G.; dos Santos, A.M.P.; Saint’Pierre, T.D. Determination and Evaluation of Metallothionein and Metals in Mugil Cephalus (Mullet) from Pontal Bay, Brazil. Bull. Environ. Contam. Toxicol. 2017, 98, 84–90. [Google Scholar] [CrossRef]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish Bioaccumulation and Biomarkers in Environmental Risk Assessment: A Review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Rocha, R.C.C.; Saint’Pierre, T.D.; Adams, D.H. Metal Concentrations and Metallothionein Metal Detoxification in Blue Sharks, Prionace glauca L. from the Western North Atlantic Ocean. J. Trace Elem. Med. Biol. 2021, 68, 126813. [Google Scholar] [CrossRef] [PubMed]
- Benedicto, J.; Martínez-Gómez, C.; Campillo, J. Induction of Metallothioneins in Mullus Barbatus as Specific Biomarker of Metal Contamination: A Field Study in the Western Mediterranean. Cienc. Mar. 2005, 31, 265–274. [Google Scholar] [CrossRef]
- Bakiu, R.; Pacchini, S.; Piva, E.; Schumann, S.; Tolomeo, A.M.; Ferro, D.; Irato, P.; Santovito, G. Metallothionein Expression as a Physiological Response against Metal Toxicity in the Striped Rockcod Trematomus hansoni. Int. J. Mol. Sci. 2022, 23, 12799. [Google Scholar] [CrossRef]
- Ladhar-Chaabouni, R.; Machreki-Ajmi, M.; Hamza-Chaffai, A. Use of Metallothioneins as Biomarkers for Environmental Quality Assessment in the Gulf of Gabès (Tunisia). Environ. Monit. Assess. 2012, 184, 2177–2192. [Google Scholar] [CrossRef] [PubMed]
- Davidova, S.; Milushev, V.; Satchanska, G. The Mechanisms of Cadmium Toxicity in Living Organisms. Toxics 2024, 12, 875. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic Effects of Cadmium on Fish. Toxics 2022, 10, 622. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Massarsky, A.; Kozal, J.S.; Di Giulio, R.T. Glutathione and Zebrafish: Old Assays to Address a Current Issue. Chemosphere 2017, 168, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, H.; Zou, Q.; Fang, F.; Sun, S.; Zhao, Y.; He, X.; Bo, Y.; Yao, L.; Fang, Y. Assessment of Heavy Metal Pollution in Laizhou Bay (China) Using the Ecological Risk Index and the Integrated Biomarker Response of the Goby Acanthogobius ommaturus. J. Oceanol. Limnol. 2023, 41, 1519–1536. [Google Scholar] [CrossRef]
- Holbert, S.S.; Bryan, C.E.; Korsmeyer, K.E.; Jensen, B.A. Mercury Accumulation and Biomarkers of Exposure in Two Popular Recreational Fishes in Hawaiian Waters. Ecotoxicology 2023, 32, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A Review of Toxicity and Mechanisms of Individual and Mixtures of Heavy Metals in the Environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Whyte, J.J.; Jung, R.E.; Schmitt, C.J.; Tillitt, D.E. Ethoxyresorufin-O-Deethylase (EROD) Activity in Fish as a Biomarker of Chemical Exposure. Crit. Rev. Toxicol. 2000, 30, 347–570. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 and Chemical Toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef]
- Bury, N.R.; Walker, P.A.; Glover, C.N. Nutritive Metal Uptake in Teleost Fish. J. Exp. Biol. 2003, 206, 11–23. [Google Scholar] [CrossRef]
- La Fontaine, S.; Ackland, M.L.; Mercer, J.F.B. Mammalian Copper-Transporting P-Type ATPases, ATP7A and ATP7B: Emerging Roles. Int. J. Biochem. Cell Biol. 2010, 42, 206–209. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, J.; Reis-Henriques, M.A. ABC Transporters in Fish Species: A Review. Front. Physiol. 2014, 5, 266. [Google Scholar] [CrossRef]
- Lange, A.; Segner, H. The Role of Glutathione and Sulfhydryl Groups in Cadmium Uptake by Cultures of the Rainbow Trout RTG-2 Cell Line. Cells 2023, 12, 2720. [Google Scholar] [CrossRef]
- Johnston, C.U.; Kennedy, C.J. A Review of P-Glycoprotein Function and Regulation in Fish. Fishes 2024, 9, 51. [Google Scholar] [CrossRef]
- Bieczynski, F.; Painefilú, J.C.; Venturino, A.; Luquet, C.M. Expression and Function of ABC Proteins in Fish Intestine. Front. Physiol. 2021, 12, 791834. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hu, J.; Deng, X.; Zheng, Y.; Tian, J. ABC Transporter-Mediated MXR Mechanism in Fish Embryos and Its Potential Role in the Efflux of Nanoparticles. Ecotoxicol. Environ. Saf. 2023, 263, 115397. [Google Scholar] [CrossRef]
- Wang, W.-X.; Rainbow, P.S. Significance of Metallothioneins in Metal Accumulation Kinetics in Marine Animals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 152, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ponton, D.E.; Caron, A.; Hare, L.; Campbell, P.G.C. Hepatic Oxidative Stress and Metal Subcellular Partitioning Are Affected by Selenium Exposure in Wild Yellow Perch (Perca flavescens). Environ. Pollut. 2016, 214, 608–617. [Google Scholar] [CrossRef]
- Walker, P.A.; Kille, P.; Hurley, A.; Bury, N.R.; Hogstrand, C. An In Vitro Method to Assess Toxicity of Waterborne Metals to Fish. Toxicol. Appl. Pharmacol. 2008, 230, 67–77. [Google Scholar] [CrossRef]
- O’Shields, B.; McArthur, A.G.; Holowiecki, A.; Kamper, M.; Tapley, J.; Jenny, M.J. Inhibition of Endogenous MTF-1 Signaling in Zebrafish Embryos Identifies Novel Roles for MTF-1 in Development. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 1818–1833. [Google Scholar] [CrossRef]
- Le Saux, A.; David, E.; Betoulle, S.; Bultelle, F.; Rocher, B.; Barjhoux, I.; Cosio, C. New Insights into Cellular Impacts of Metals in Aquatic Animals. Environments 2020, 7, 46. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; de Campos, R.C.; Ziolli, R.L. Fish Metalloproteins as Biomarkers of Environmental Contamination. In Reviews of Environmental Contamination and Toxicology; Whitacre, D., Ed.; Springer: Boston, MA, USA, 2012; Volume 218, pp. 101–123. [Google Scholar]
- Beg, M.U.; Al-Jandal, N.; Al-Subiai, S.; Karam, Q.; Husain, S.; Butt, S.A.; Ali, A.; Al-Hasan, E.; Al-Dufaileej, S.; Al-Husaini, M. Metallothionein, Oxidative Stress and Trace Metals in Gills and Liver of Demersal and Pelagic Fish Species from Kuwaits’ Marine Area. Mar. Pollut. Bull. 2015, 100, 662–672. [Google Scholar] [CrossRef]
- Kennedy, C.J. Toxicology | The Toxicology of Metals in Fishes. Encycl. Fish Physiol. 2011, 3, 2061–2068. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Das, J.N.; Saikia, S. Metal Induced Oxidative Stress in Fishes: A Review. J. Adv. Zool. 2024, 45, 434–449. [Google Scholar] [CrossRef]
- Bhat, R.A.; Alam, A.; Jha, D.N.; Kumar, V.; Kumar, J.; Thakur, V.R.; Das, B.K. Fate and Effects of Heavy Metals in Fishes: Antioxidant Defense System, MiRNA/Gene Expression Response, and Histopathological Reproductive Manifestations. Biol. Trace Elem. Res. 2024, 203, 4326–4346. [Google Scholar] [CrossRef]
- Rodríguez-Ariza, A.; Alhama, J.; Díaz-Méndez, F.M.; López-Barea, J. Content of 8-OxodG in Chromosomal DNA of Sparus aurata Fish as Biomarker of Oxidative Stress and Environmental Pollution. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 438, 97–107. [Google Scholar] [CrossRef]
- Oliveira, M.; Maria, V.L.; Ahmad, I.; Teles, M.; Serafim, A.; Bebianno, M.J.; Pacheco, M.; Santos, M.A. Golden Grey Mullet and Sea Bass Oxidative DNA Damage and Clastogenic/Aneugenic Responses in a Contaminated Coastal Lagoon. Ecotoxicol. Environ. Saf. 2010, 73, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.A.; Mohamed, H.R.H.; Abdel-Khalek, A.A. Metal Accumulation and DNA Damage in Oreochromis niloticus and Clarias Gariepinus After Chronic Exposure to Discharges of the Batts Drain: Potential Risk to Human Health. Bull. Environ. Contam. Toxicol. 2022, 108, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Ayllon, F.; Garcia-Vazquez, E. Induction of Micronuclei and Other Nuclear Abnormalities in European Minnow Phoxinus Phoxinus and Mollie Poecilia Latipinna: An Assessment of the Fish Micronucleus Test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 467, 177–186. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Villar, S.; Acuña Plavan, A. Micronucleus Test in Fishes as Indicators of Environmental Quality in Subestuaries of the Río de La Plata (Uruguay). Mar. Pollut. Bull. 2015, 91, 518–523. [Google Scholar] [CrossRef]
- Hemmaphan, S.; Bordeerat, N.K. Genotoxic Effects of Lead and Their Impact on the Expression of DNA Repair Genes. Int. J. Environ. Res. Public Health 2022, 19, 4307. [Google Scholar] [CrossRef]
- Sarkar, O.; Dey, K.K.; Islam, S.; Chattopadhyay, A. Lead and Aquatic Ecosystems, Biomarkers, and Implications for Humankind. In Biomarkers in Toxicology; Springer: Cham, Switzerland, 2023; pp. 961–988. [Google Scholar]
- Brander, S.M.; Biales, A.D.; Connon, R.E. The Role of Epigenomics in Aquatic Toxicology. Environ. Toxicol. Chem. 2017, 36, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Gavery, M.R.; Roberts, S.B. Epigenetic Considerations in Aquaculture. PeerJ 2017, 5, e4147. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish. In Fish Physiology; Academic Press: New York, NY, USA, 2016; Volume 35, pp. 1–34. [Google Scholar] [CrossRef]
- Kumar, N.; Thorat, S.T.; Gunaware, M.A.; Kumar, P.; Reddy, K.S. Unraveling Gene Regulation Mechanisms in Fish: Insights into Multistress Responses and Mitigation through Iron Nanoparticles. Front. Immunol. 2024, 15, 1410150. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, W.R.; Park, E.G.; Lee, D.H.; Kim, J.; Jeong, H.; Roh, H.-Y.; Choi, Y.H.; Srivastava, V.; Mishra, A.; et al. Phenotypic and Gene Expression Alterations in Aquatic Organisms Exposed to Microplastics. Int. J. Mol. Sci. 2025, 26, 1080. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.-S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef]
- Osman, A.G.M.; Wuertz, S.; Mohammed-Geba, K. Lead-Induced Heat Shock Protein (HSP70) and Metallothionein (MT) Gene Expression in the Embryos of African Catfish Clarias Gariepinus (Burchell, 1822). Sci. Afr. 2019, 3, e00056. [Google Scholar] [CrossRef]
- Causey, D.R.; Kim, J.-H.; Devlin, R.H.; Martin, S.A.M.; Macqueen, D.J. The AMPK System of Salmonid Fishes Was Expanded through Genome Duplication and Is Regulated by Growth and Immune Status in Muscle. Sci. Rep. 2019, 9, 9819. [Google Scholar] [CrossRef]
- Madesh, S.; Gopi, S.; Sau, A.; Rajagopal, R.; Namasivayam, S.K.R.; Arockiaraj, J. Chemical Contaminants and Environmental Stressors Induced Teratogenic Effect in Aquatic Ecosystem—A Comprehensive Review. Toxicol. Rep. 2024, 13, 101819. [Google Scholar] [CrossRef]
- Elkin, E.R.; Higgins, C.; Aung, M.T.; Bakulski, K.M. Metals Exposures and DNA Methylation: Current Evidence and Future Directions. Curr. Environ. Health Rep. 2022, 9, 673–696. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef]
- Martelli, A.; Rousselet, E.; Dycke, C.; Bouron, A.; Moulis, J.-M. Cadmium Toxicity in Animal Cells by Interference with Essential Metals. Biochimie 2006, 88, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Abdelnour, S.A.; Alotaibi, M.A.; AlGabbani, Q.; Naiel, M.A.E.; Shokrollahi, B.; Noreldin, A.E.; Jahejo, A.R.; Shah, M.A.; Alagawany, M.; et al. MicroRNAs Mediated Environmental Stress Responses and Toxicity Signs in Teleost Fish Species. Aquaculture 2022, 546, 737310. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, X.; Su, Y.; Gao, B.; Liu, P.; Lv, J. Immunoregulatory Mechanisms and Cross-Kingdom Bacteriostatic Effects of MicroRNAs in Crustacean. Int. J. Biol. Macromol. 2025, 311, 144079. [Google Scholar] [CrossRef] [PubMed]
- Abo-Al-Ela, H.G.; Faggio, C. MicroRNA-Mediated Stress Response in Bivalve Species. Ecotoxicol. Environ. Saf. 2021, 208, 111442. [Google Scholar] [CrossRef]
- Cione, E.; Abrego Guandique, D.M.; Caroleo, M.C.; Luciani, F.; Colosimo, M.; Cannataro, R. Liver Damage and MicroRNAs: An Update. Curr. Issues Mol. Biol. 2022, 45, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Z.; Li, P.; Liu, L.; Li, Z.-H. Transcriptomic and Proteomic Analysis of Chinese Rare Minnow (Gobiocypris rarus) Larvae in Response to Acute Waterborne Cadmium or Mercury Stress. Aquat. Toxicol. 2022, 246, 106134. [Google Scholar] [CrossRef]
- Shaw, P.; Mondal, P.; Dey Bhowmik, A.; Bandyopadhyay, A.; Sudarshan, M.; Chakraborty, A.; Chattopadhyay, A. Environmentally Relevant Hexavalent Chromium Disrupts Elemental Homeostasis and Induces Apoptosis in Zebrafish Liver. Bull. Environ. Contam. Toxicol. 2022, 108, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, M.; Chen, Y.; Liu, D.; Xie, S.; Zou, J.; Tang, H.; Li, Q.; Zhou, A. Expression of Genes Related to Antioxidation, Immunity, and Heat Stress in Gambusia affinis Exposed to the Heavy Metals Cu and Zn. Ecotoxicol. Environ. Saf. 2022, 247, 114269. [Google Scholar] [CrossRef]
- der Maur, A.A.; Belser, T.; Elgar, G.; Georgiev, O.; Schaffner, W. Characterization of the Transcription Factor MTF-1 from the Japanese Pufferfish (Fugu rubripes) Reveals Evolutionary Conservation of Heavy Metal Stress Response. Biol. Chem. 1999, 380, 175–185. [Google Scholar] [CrossRef]
- Liu, L.-L.; Song, C.-C.; Abu-Elala, N.; Tan, X.-Y.; Zhao, T.; Zheng, H.; Yang, H.; Luo, Z. Transcriptional Regulation of Znt Family Members Znt4, Znt5 and Znt10 and Their Function in Zinc Transport in Yellow Catfish (Pelteobagrus fulvidraco). Biochim. Et Biophys. Acta (BBA)—Gene Regul. Mech. 2024, 1867, 195041. [Google Scholar] [CrossRef]
- Schlezinger, J.J.; Blickarz, C.E.; Mann, K.K.; Doerre, S.; Stegeman, J.J. Identification of NF-ΚB in the Marine Fish Stenotomus Chrysops and Examination of Its Activation by Aryl Hydrocarbon Receptor Agonists. Chem.-Biol. Interact. 2000, 126, 137–157. [Google Scholar] [CrossRef]
- Kataoka, C.; Kashiwada, S. Ecological Risks due to Immunotoxicological Effects on Aquatic Organisms. Int. J. Mol. Sci. 2021, 22, 8305. [Google Scholar] [CrossRef]
- Biller, J.D.; Takahashi, L.S. Oxidative Stress and Fish Immune System: Phagocytosis and Leukocyte Respiratory Burst Activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef]
- Ching, B.; Chen, X.L.; Yong, J.H.A.; Wilson, J.M.; Hiong, K.C.; Sim, E.W.L.; Wong, W.P.; Lam, S.H.; Chew, S.F.; Ip, Y.K. Increases in Apoptosis, Caspase Activity and Expression of p53 and bax, and the Transition between Two Types of Mitochondrion-Rich Cells, in the Gills of the Climbing Perch, Anabas testudineus, during a Progressive Acclimation from Freshwater to Seawater. Front. Physiol. 2013, 4, 135. [Google Scholar] [CrossRef]
- Xu, J.; Lian, L.; Wu, C.; Wang, X.; Fu, W.; Xu, L. Lead Induces Oxidative Stress, DNA Damage and Alteration of P53, Bax and Bcl-2 Expressions in Mice. Food Chem. Toxicol. 2008, 46, 1488–1494. [Google Scholar] [CrossRef]
- Tait, S.W.G.; Green, D.R. Mitochondrial Regulation of Cell Death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Fulton, M.H.; Key, P.B. Acetylcholinesterase Inhibition in Estuarine Fish and Invertebrates as an Indicator of Organophosphorus Insecticide Exposure and Effects. Environ. Toxicol. Chem. 2001, 20, 37–45. [Google Scholar] [CrossRef]
- Dias Assis, C.R.; Souza, R.; Carvalho, L.B., Jr. Fish Cholinesterases as Biomarkers of Organophosphorus and Carbamate Pesticides. In Pesticides in the Modern World—Pests Control and Pesticides Exposure and Toxicity Assessment; InTech: Vienna, Austria, 2011. [Google Scholar]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Bogo, M.R.; Bonan, C.D. Acetylcholinesterase Activity and Antioxidant Capacity of Zebrafish Brain Is Altered by Heavy Metal Exposure. Neurotoxicology 2011, 32, 116–122. [Google Scholar] [CrossRef]
- Paduraru, E.; Iacob, D.; Rarinca, V.; Plavan, G.; Ureche, D.; Jijie, R.; Nicoara, M. Zebrafish as a Potential Model for Neurodegenerative Diseases: A Focus on Toxic Metals Implications. Int. J. Mol. Sci. 2023, 24, 3428. [Google Scholar] [CrossRef]
- Haverinen, J.; Vornanen, M. Dual Effect of Metals on Branchial and Renal Na,K-ATPase Activity in Thermally Acclimated Crucian Carp (Carassius carassius) and Rainbow Trout (Oncorhynchus mykiss). Aquat. Toxicol. 2023, 254, 106374. [Google Scholar] [CrossRef]
- Kaya, H.; Akbulut, M. Effects of Waterborne Lead Exposure in Mozambique Tilapia: Oxidative Stress, Osmoregulatory Responses, and Tissue Accumulation. J. Aquat. Anim. Health 2015, 27, 77–87. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl Groups as Targets of Mercury Toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.; Seeger, R.; Puntel, R.; Vargas Barbosa, N. Role of Calcium and Mitochondria in MeHg-Mediated Cytotoxicity. J. Biomed. Biotechnol. 2012, 2012, 248764. [Google Scholar] [CrossRef] [PubMed]
- Dyer, C.A. Heavy Metals as Endocrine-Disrupting Chemicals. In Endocrine-Disrupting Chemicals; Humana Press: Totowa, NJ, USA, 2007; pp. 111–133. [Google Scholar]
- Vinanthi Rajalakshmi, K.S.; Liu, W.-C.; Balamuralikrishnan, B.; Meyyazhagan, A.; Sattanathan, G.; Pappuswamy, M.; Joseph, K.S.; Paari, K.A.; Lee, J.-W. Cadmium as an Endocrine Disruptor that Hinders the Reproductive and Developmental Pathways in Freshwater Fish: A Review. Fishes 2023, 8, 589. [Google Scholar] [CrossRef]
- Bera, T.; Kumar, S.V.; Devi, M.S.; Kumar, V.; Behera, B.K.; Das, B.K. Effect of Heavy Metals in Fish Reproduction: A Review. J. Environ. Biol. 2022, 43, 631–642. [Google Scholar] [CrossRef]
- Paschoalini, A.L.; Savassi, L.A.; Arantes, F.P.; Rizzo, E.; Bazzoli, N. Heavy Metals Accumulation and Endocrine Disruption in Prochilodus argenteus from a Polluted Neotropical River. Ecotoxicol. Environ. Saf. 2019, 169, 539–550. [Google Scholar] [CrossRef]
- Kleinkauf, A.; Scott, A.P.; Stewart, C.; Simpson, M.G.; Leah, R.T. Abnormally Elevated VTG Concentrations in Flounder (Platichthys flesus) from the Mersey Estuary (UK)—A Continuing Problem. Ecotoxicol. Environ. Saf. 2004, 58, 356–364. [Google Scholar] [CrossRef]
- Hecker, M.; Tyler, C.R.; Hoffmann, M.; Maddix, S.; Karbe, L. Plasma Biomarkers in Fish Provide Evidence for Endocrine Modulation in the Elbe River, Germany. Environ. Sci. Technol. 2002, 36, 2311–2321. [Google Scholar] [CrossRef]
- Langston, W.J. Endocrine Disruption and Altered Sexual Development in Aquatic Organisms: An Invertebrate Perspective. J. Mar. Biol. Assoc. U. K. 2020, 100, 495–515. [Google Scholar] [CrossRef]
- Kar, S.; Sangem, P.; Anusha, N.; Senthilkumaran, B. Endocrine Disruptors in Teleosts: Evaluating Environmental Risks and Biomarkers. Aquac. Fish 2021, 6, 1–26. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Ying, G.-G.; Liang, Y.-Q.; Liu, S.-S.; Liu, Y.-S. Expression Patterns of Metallothionein, Cytochrome P450 1A and Vitellogenin Genes in Western Mosquitofish (Gambusia affinis) in Response to Heavy Metals. Ecotoxicol. Environ. Saf. 2014, 105, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hinfray, N.; Porcher, J.-M.; Brion, F. Inhibition of Rainbow Trout (Oncorhynchus mykiss) P450 Aromatase Activities in Brain and Ovarian Microsomes by Various Environmental Substances. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 252–262. [Google Scholar] [CrossRef]
- Mehinto, A.C.; Prucha, M.S.; Colli-Dula, R.C.; Kroll, K.J.; Lavelle, C.M.; Barber, D.S.; Vulpe, C.D.; Denslow, N.D. Gene Networks and Toxicity Pathways Induced by Acute Cadmium Exposure in Adult Largemouth Bass (Micropterus salmoides). Aquat. Toxicol. 2014, 152, 186–194. [Google Scholar] [CrossRef]
- Brar, N.K.; Waggoner, C.; Reyes, J.A.; Fairey, R.; Kelley, K.M. Evidence for Thyroid Endocrine Disruption in Wild Fish in San Francisco Bay, California, USA. Relationships to Contaminant Exposures. Aquat. Toxicol. 2010, 96, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, J.G.; Klaren, P.H.M.; Bouquegneau, J.-M.; Das, K. Environmental Factors Affecting Thyroid Function of Wild Sea Bass (Dicentrarchus labrax) from European Coasts. Chemosphere 2012, 87, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Konuk, M.; Ciğerci, İ.H.; Korcan, S.E. ALAD (δ-Aminolevulinic Acid Dehydratase) as Biosensor for Pb Contamination. In Intelligent and Biosensors; InTech: Vienna, Austria, 2010. [Google Scholar]
- Schmitt, C.J.; Caldwell, C.A.; Olsen, B.; Serdar, D.; Coffey, M. Inhibition of Erythrocyte δ-aminolevulinic Acid Dehydratase (ALAD) Activity in Fish from Waters Affected by Lead Smelters. Environ. Monit. Assess. 2002, 77, 99–119. [Google Scholar] [CrossRef]
- Schmitt, C.J.; Whyte, J.J.; Roberts, A.P.; Annis, M.L.; May, T.W.; Tillitt, D.E. Biomarkers of Metals Exposure in Fish from Lead-Zinc Mining Areas of Southeastern Missouri, USA. Ecotoxicol. Environ. Saf. 2007, 67, 31–47. [Google Scholar] [CrossRef]
- Barra, R.O.; Chiang, G.; Saavedra, M.F.; Orrego, R.; Servos, M.R.; Hewitt, L.M.; McMaster, M.E.; Bahamonde, P.; Tucca, F.; Munkittrick, K.R. Endocrine Disruptor Impacts on Fish From Chile: The Influence of Wastewaters. Front. Endocrinol. 2021, 12, 611281. [Google Scholar] [CrossRef]
- Gashkina, N.A. Metal Toxicity: Effects on Energy Metabolism in Fish. Int. J. Mol. Sci. 2024, 25, 5015. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Shi, L.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H. Heavy Metals Induced Mitochondrial Dysfunction in Animals: Molecular Mechanism of Toxicity. Toxicology 2022, 469, 153136. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Rolshausen, G.; Uren Webster, T.M.; Tyler, C.R. Adaptive Capabilities and Fitness Consequences Associated with Pollution Exposure in Fish. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160042. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, Q.; Laffaille, P.; Perrault, A.; Cousseau, M.; Jean, S.; Jacquin, L. Adaptive Plastic Responses to Metal Contamination in a Multistress Context: A Field Experiment in Fish. Environ. Sci. Pollut. Res. 2023, 30, 55678–55698. [Google Scholar] [CrossRef]
- Shah, N.; Khan, A.; Ali, R.; Marimuthu, K.; Uddin, M.N.; Rizwan, M.; Rahman, K.U.; Alam, M.; Adnan, M.; Muhammad; et al. Monitoring Bioaccumulation (in Gills and Muscle Tissues), Hematology, and Genotoxic Alteration in Ctenopharyngodon idella Exposed to Selected Heavy Metals. Biomed. Res. Int. 2020, 2020, 6185231. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, S.; Al-Ghanim, K.A.; Al-Balawi, H.F.; Al-Misned, F.; Ahmed, Z. Toxicological Effects of Heavy Metals on Histological Alterations in Various Organs in Nile Tilapia (Oreochromis niloticus) from Freshwater Reservoir. J. King Saud Univ. Sci. 2020, 32, 970–973. [Google Scholar] [CrossRef]
- Chiarelli, R.; Martino, C.; Agnello, M.; Bosco, L.; Roccheri, M.C. Autophagy as a Defense Strategy against Stress: Focus on Paracentrotus Lividus Sea Urchin Embryos Exposed to Cadmium. Cell Stress Chaperones 2016, 21, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Roccheri, M.C. Heavy Metals and Metalloids as Autophagy Inducing Agents: Focus on Cadmium and Arsenic. Cells 2012, 1, 597–616. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Y.; Wang, R.; Liu, R.; Ba, Y.; Huang, H. Recent Insights into Autophagy and Metals/Nanoparticles Exposure. Toxicol. Res. 2023, 39, 355–372. [Google Scholar] [CrossRef]
- Moore, M.N.; Koehler, A.; Lowe, D.; Viarengo, A. Lysosomes and Autophagy in Aquatic Animals. In Methods in Enzymology; Academic Press: New York, NY, USA, 2008; pp. 581–620. ISBN 9780123745484. [Google Scholar]
- Reinheckel, T.; Tholen, M. Low-level Lysosomal Membrane Permeabilization for Limited Release and Sublethal Functions of Cathepsin Proteases in the Cytosol and Nucleus. FEBS Open Bio 2022, 12, 694–707. [Google Scholar] [CrossRef]
- Tresguerres, M. Novel and Potential Physiological Roles of Vacuolar-Type H+-ATPase in Marine Organisms. J. Exp. Biol. 2016, 219, 2088–2097. [Google Scholar] [CrossRef]
- Tamás, M.; Sharma, S.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids As a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Izagirre, U.; Marigómez, I. Lysosomal Enlargement and Lysosomal Membrane Destabilisation in Mussel Digestive Cells Measured by an Integrative Index. Environ. Pollut. 2009, 157, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Holth, T.F.; Beckius, J.; Zorita, I.; Cajaraville, M.P.; Hylland, K. Assessment of Lysosomal Membrane Stability and Peroxisome Proliferation in the Head Kidney of Atlantic Cod (Gadus morhua) Following Long-Term Exposure to Produced Water Components. Mar. Environ. Res. 2011, 72, 127–134. [Google Scholar] [CrossRef]
- Skaggs, H.S.; Henry, R.P. Inhibition of Carbonic Anhydrase in the Gills of Two Euryhaline Crabs, Callinectes Sapidus and Carcinus Maenas, by Heavy Metals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.; Caricato, R.; Giordano, M.; Schettino, T. The Complex Relationship between Metals and Carbonic Anhydrase: New Insights and Perspectives. Int. J. Mol. Sci. 2016, 17, 127. [Google Scholar] [CrossRef]
- Soyut, H.; Beydemir, Ş.; Hisar, O. Effects of Some Metals on Carbonic Anhydrase from Brains of Rainbow Trout. Biol. Trace Elem. Res. 2008, 123, 179–190. [Google Scholar] [CrossRef]
- Sousa, C.S.V.; Sun, J.; Mestre, N.C. Potential Biomarkers of Metal Toxicity in Deep-Sea Invertebrates—A Critical Review of the Omics Data. Sci. Total Environ. 2024, 951, 175628. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, S. Heavy Metal Contamination in Fish: Sources, Mechanisms and Consequences. Aquat. Sci. 2024, 86, 107. [Google Scholar] [CrossRef]
- Sanchez, W.; Porcher, J.-M. Fish Biomarkers for Environmental Monitoring within the Water Framework Directive of the European Union. TrAC Trends Anal. Chem. 2009, 28, 150–158. [Google Scholar] [CrossRef]
- El-SiKaily, A.; Shabaka, S. Biomarkers in Aquatic Systems: Advancements, Applications and Future Directions. Egypt. J. Aquat. Res. 2024, 50, 169–182. [Google Scholar] [CrossRef]
- Sarkar, A.; Ray, D.; Shrivastava, A.N.; Sarker, S. Molecular Biomarkers: Their Significance and Application in Marine Pollution Monitoring. Ecotoxicology 2006, 15, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhang, H.; Li, T.; He, L.; Zong, J.; Shan, H.; Huang, L.; Zhang, Y.; Liu, H.; Jiang, J. Profiling MiRNAs of Teleost Fish in Responses to Environmental Stress: A Review. Biology 2023, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, F.Y.; Onishi, K.; Ralha, T.R.; Silva, L.F.O.; Deda, B.; Pessali, T.Y.C.; Souza, C.; Oliveira Ribeiro, C.A.; Abessa, D.M.S. Earlier Biomarkers in Fish Evidencing Stress Responses to Metal and Organic Pollution along the Doce River Basin. Environ. Pollut. 2023, 329, 121720. [Google Scholar] [CrossRef]
- Vergani, L.; Grattarola, M.; Borghi, C.; Dondero, F.; Viarengo, A. Fish and Molluscan Metallothioneins. FEBS J. 2005, 272, 6014–6023. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Nuño, S.; Carbonell, T.; Ibarz Valls, A. Redox Balance Affects Fish Welfare. In Redox; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Petitjean, Q.; Jean, S.; Gandar, A.; Côte, J.; Laffaille, P.; Jacquin, L. Stress Responses in Fish: From Molecular to Evolutionary Processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef]
- Schlenk, D. Necessity of Defining Biomarkers for Use in Ecological Risk Assessments. Mar. Pollut. Bull. 1999, 39, 48–53. [Google Scholar] [CrossRef]
- Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, 164, 19–40. Available online: https://eur-lex.europa.eu/eli/dir/2008/56/oj/eng (accessed on 2 June 2025).
- OSPAR Commission. JAMP Guidelines for Contaminant-Specific Biological Effects; OSPAR Commission: London, UK, 2008. [Google Scholar]
- Davies, I.M.; Vethaak, D. Integrated Marine Environmental Monitoring of Chemicals and Their Effects; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2012. [Google Scholar]
- Burgeot, T.; Mauffret, A.; Anderson, J.; Brooks, S.; Assuncao, M.; Bellas Bereijo, J.; Bignell, J.; Campillo, J.A.; Coorman, K.; Förlin, L.; et al. Integrated Biological Effects and Chemical Contaminants Approach: A Case Study. In OSPAR, 2023: The 2023 Quality Status Report for the North-East Atlantic; OSPAR Commission: London, UK, 2022. [Google Scholar]
- Stephensen, E. Biochemical Indicators of Pollution Exposure in Shorthorn Sculpin (Myoxocephalus scorpius), Caught in Four Harbours on the Southwest Coast of Iceland. Aquat. Toxicol. 2000, 48, 431–442. [Google Scholar] [CrossRef]
- Stagg, R.; McIntosh, A.; Gubbins, M.J. Determination of CYP1A-Dependent Monooxygenase Activity in Dab by Fluorimetric Measurement of EROD Activity in S9 or Microsomal Liver Fractions; ICES Techniques in Marine Environmental Sciences: Copenhagen, Denmark, 2016. [Google Scholar]
- O’Connor, T.P.; Ehler, C.N. Results from the NOAA National Status and Trends Program on Distribution and Effects of Chemical Contamination in the Coastal and Estuarine United States. Environ. Monit. Assess. 1991, 17, 33–49. [Google Scholar] [CrossRef]
- Goldberg, E.D.; Bertine, K.K. Beyond the Mussel Watch—New Directions for Monitoring Marine Pollution. Sci. Total Environ. 2000, 247, 165–174. [Google Scholar] [CrossRef]
- Lehtonen, K.K.; Sundelin, B.; Lang, T.; Strand, J. Development of Tools for Integrated Monitoring and Assessment of Hazardous Substances and Their Biological Effects in the Baltic Sea. Ambio 2014, 43, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. Biomarkers Based Tools to Assess Environmental and Chemical Stressors in Aquatic Systems. Ecol. Indic. 2021, 122, 107207. [Google Scholar] [CrossRef]
- da Luz, J.Z.; Gorshkov, V.; Miranda, R.R.; de Souza, T.L.; Rodrigues Ribeiro, L.; Duan, X.; Huang, Y.; Oliveira Ribeiro, C.A.d.; Xu, E.G.; Kjeldsen, F.; et al. Metallothionein as a Biomarker of Aquatic Contamination in Fish: An In Silico and In Vitro Approach Using Zebrafish as Experimental Model Organism. Chemosphere 2025, 376, 144316. [Google Scholar] [CrossRef] [PubMed]
- Kessabi, K.; Navarro, A.; Casado, M.; Saïd, K.; Messaoudi, I.; Piña, B. Evaluation of Environmental Impact on Natural Populations of the Mediterranean Killifish Aphanius Fasciatus by Quantitative RNA Biomarkers. Mar. Environ. Res. 2010, 70, 327–333. [Google Scholar] [CrossRef]
- Srikanth, K.; Pereira, E.; Duarte, A.C.; Ahmad, I. Glutathione and Its Dependent Enzymes’ Modulatory Responses to Toxic Metals and Metalloids in Fish—A Review. Environ. Sci. Pollut. Res. 2013, 20, 2133–2149. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.V.; Kumar, A.; Middha, S.K.; Paital, B.; Mathur, S.; Johnson, R.; Kademan, A.; Usha, T.; Hemavathi, K.N.; Dayal, S.; et al. Water Physicochemical Factors and Oxidative Stress Physiology in Fish, a Review. Front. Environ. Sci. 2023, 11, 1240813. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The Role of Biomarkers in the Assessment of Aquatic Ecosystem Health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef]
- Adorno, H.A.; Souza, I.d.C.; Monferrán, M.V.; Wunderlin, D.A.; Fernandes, M.N.; Monteiro, D.A. A Multi-Biomarker Approach to Assess the Sublethal Effects of Settleable Atmospheric Particulate Matter from an Industrial Area on Nile Tilapia (Oreochromis niloticus). Sci. Total Environ. 2023, 856, 159168. [Google Scholar] [CrossRef]
- Oliveira, J.; Oliva-Teles, A.; Couto, A. Tracking Biomarkers for the Health and Welfare of Aquaculture Fish. Fishes 2024, 9, 289. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Varela, Z.; Franco, D.; Fernández, J.A.; Aboal, J.R. Can Proteomics Contribute to Biomonitoring of Aquatic Pollution? A Critical Review. Environ. Pollut. 2020, 267, 115473. [Google Scholar] [CrossRef]
- Kadim, M.K.; Risjani, Y. Biomarker for Monitoring Heavy Metal Pollution in Aquatic Environment: An Overview toward Molecular Perspectives. Emerg. Contam. 2022, 8, 195–205. [Google Scholar] [CrossRef]
- Truchet, D.M.; Villagran, D.M.; Menone, M.L. Pollution Biomarkers in Latin American and Caribbean Marine Environments: A Review to Identify Gaps in Passive Biomonitoring Studies. J. Hazard. Mater. Adv. 2025, 17, 100554. [Google Scholar] [CrossRef]
- Mariu, A.; Chatha, A.M.M.; Naz, S.; Khan, M.F.; Safdar, W.; Ashraf, I. Effect of Temperature, PH, Salinity and Dissolved Oxygen on Fishes. J. Zool. Syst. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Goussen, B.; Rendal, C.; Sheffield, D.; Butler, E.; Price, O.R.; Ashauer, R. Bioenergetics Modelling to Analyse and Predict the Joint Effects of Multiple Stressors: Meta-Analysis and Model Corroboration. Sci. Total Environ. 2020, 749, 141509. [Google Scholar] [CrossRef]
- Earhart, M.L.; Blanchard, T.S.; Harman, A.A.; Schulte, P.M. Hypoxia and High Temperature as Interacting Stressors: Will Plasticity Promote Resilience of Fishes in a Changing World? Biol. Bull. 2022, 243, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of Temperature in Thermal and Oxidative Stress Responses in Estuarine Fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dragun, Z.; Podrug, M.; Raspor, B. The Assessment of Natural Causes of Metallothionein Variability in the Gills of European Chub (Squalius cephalus L.). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 209–217. [Google Scholar] [CrossRef]
- Shek, A.C.S.; Chan, K.M. Effects of Salinity on Metal Uptake and Metallothionein MRNA Levels in the Organs of Tilapia Exposed to Cadmium, Copper, and Zinc Ions. Arch. Environ. Contam. Toxicol. 2015, 68, 622–635. [Google Scholar] [CrossRef]
- Pérez-Iglesias, J.M.; Bach, N.C.; Colombetti, P.L.; Acuña, P.; Colman-Lerner, J.E.; González, S.P.; Brodeur, J.C.; Almeida, C.A. Biomonitoring of Alterations in Fish that Inhabit Anthropic Aquatic Environments in a Basin from Semi-Arid Regions. Toxics 2023, 11, 73. [Google Scholar] [CrossRef]
- Kroon, F.; Streten, C.; Harries, S. A Protocol for Identifying Suitable Biomarkers to Assess Fish Health: A Systematic Review. PLoS ONE 2017, 12, e0174762. [Google Scholar] [CrossRef]
- Nos, D.; Navarro, J.; Solé, M. The Influence of Ecological Factors in the Modulation of Pollution Biomarkers of Two Small Pelagic Marine Fish. Mar. Pollut. Bull. 2023, 188, 114717. [Google Scholar] [CrossRef]
- Natnan, M.E.; Low, C.-F.; Chong, C.-M.; Bunawan, H.; Baharum, S.N. Integration of Omics Tools for Understanding the Fish Immune Response due to Microbial Challenge. Front. Mar. Sci. 2021, 8, 668771. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Pollution Biomarkers in the Framework of Marine Biodiversity Conservation: State of Art and Perspectives. Water 2021, 13, 1847. [Google Scholar] [CrossRef]
- Hagger, J.A.; Jones, M.B.; Leonard, D.P.; Owen, R.; Galloway, T.S. Biomarkers and Integrated Environmental Risk Assessment: Are There More Questions than Answers? Integr. Environ. Assess. Manag. 2006, 2, 312–329. [Google Scholar] [CrossRef]
- Hartl, M.G.J.; Baumann, L.M.; Sweetman, A.K. At-Sea Application of the Comet Assay to a Deep-Sea Fish. Deep Sea Res. Part I: Oceanogr. Res. Pap. 2024, 208, 104298. [Google Scholar] [CrossRef]
- Regoli, F.; d’Errico, G.; Nardi, A.; Mezzelani, M.; Fattorini, D.; Benedetti, M.; Di Carlo, M.; Pellegrini, D.; Gorbi, S. Application of a Weight of Evidence Approach for Monitoring Complex Environmental Scenarios: The Case-Study of Off-Shore Platforms. Front. Mar. Sci. 2019, 6, 377. [Google Scholar] [CrossRef]
- Hernández-Jerez, A.; Hougaard Bennekou, S.; Hoogenboom, L.; Mcardle, H.; Pieper, C.; Schwerdtle, T.; Van Loveren, H.; Al Harraq, Z.; Croera, C.; Christodoulidou, A.; et al. Conceptual Basis for the Development of Guidance for the Use of Biomarkers of Effect in Regulatory Risk Assessment of Chemicals. EFSA J. 2024, 22, e9153. [Google Scholar] [CrossRef]
- Borja, A.; Elliott, M.; Andersen, J.H.; Berg, T.; Carstensen, J.; Halpern, B.S.; Heiskanen, A.-S.; Korpinen, S.; Lowndes, J.S.S.; Martin, G.; et al. Overview of Integrative Assessment of Marine Systems: The Ecosystem Approach in Practice. Front. Mar. Sci. 2016, 3, 20. [Google Scholar] [CrossRef]
- Borja, A.; Berg, T.; Gundersen, H.; Hagen, A.G.; Hancke, K.; Korpinen, S.; Leal, M.C.; Luisetti, T.; Menchaca, I.; Murray, C.; et al. Innovative and Practical Tools for Monitoring and Assessing Biodiversity Status and Impacts of Multiple Human Pressures in Marine Systems. Environ. Monit. Assess. 2024, 196, 694. [Google Scholar] [CrossRef]
- Leprêtre, M.; Geffard, A.; Palos Ladeiro, M.; Dedourge-Geffard, O.; David, E.; Delahaut, L.; Bonnard, I.; Barjhoux, I.; Nicolaï, M.; Noury, P.; et al. Determination of Biomarkers Threshold Values and Illustration of Their Use for the Diagnostic in Large-Scale Freshwater Biomonitoring Surveys. Environ. Sci. Eur. 2022, 34, 115. [Google Scholar] [CrossRef]
- OSPAR Commission. Background Documents and Technical Annexes for Biological Effects Monitoring; Monitoring and Assessment Series; OSPAR Commission: London, UK, 2013. [Google Scholar]
- Catteau, A.; Le Guernic, A.; Palos Ladeiro, M.; Dedourge-Geffard, O.; Bonnard, M.; Bonnard, I.; Delahaut, L.; Bado-Nilles, A.; Porcher, J.-M.; Lopes, C.; et al. Integrative Biomarker Response—Threshold (IBR-T): Refinement of IBRv2 to Consider the Reference and Threshold Values of Biomarkers. J. Environ. Manag. 2023, 341, 118049. [Google Scholar] [CrossRef]
- Van Aggelen, G.; Ankley, G.T.; Baldwin, W.S.; Bearden, D.W.; Benson, W.H.; Chipman, J.K.; Collette, T.W.; Craft, J.A.; Denslow, N.D.; Embry, M.R.; et al. Integrating Omic Technologies into Aquatic Ecological Risk Assessment and Environmental Monitoring: Hurdles, Achievements, and Future Outlook. Environ. Health Perspect. 2010, 118, 1–5. [Google Scholar] [CrossRef]
- Qian, X.; Ba, Y.; Zhuang, Q.; Zhong, G. RNA-Seq Technology and Its Application in Fish Transcriptomics. Omics 2014, 18, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Sidira, M.; Agriopoulou, S.; Smaoui, S.; Varzakas, T. Omics-Integrated Approach (Metabolomics, Proteomics and Lipidomics) to Assess the Quality Control of Aquatic and Seafood Products. Appl. Sci. 2024, 14, 10755. [Google Scholar] [CrossRef]
- Lin, T.; Meegaskumbura, M. Fish MicroRNA Responses to Thermal Stress: Insights and Implications for Aquaculture and Conservation Amid Global Warming. Animals 2025, 15, 624. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Woldemariam, N.T.; Caballero-Solares, A.; Umasuthan, N.; Fast, M.D.; Taylor, R.G.; Rise, M.L.; Andreassen, R. Dietary Immunostimulant CpG Modulates MicroRNA Biomarkers Associated with Immune Responses in Atlantic Salmon (Salmo salar). Cells 2019, 8, 1592. [Google Scholar] [CrossRef]
- Bancel, S.; Cachot, J.; Bon, C.; Rochard, É.; Geffard, O. A Critical Review of Pollution Active Biomonitoring Using Sentinel Fish: Challenges and Opportunities. Environ. Pollut. 2024, 360, 124661. [Google Scholar] [CrossRef]
- Terrazas-Salgado, L.; García-Gasca, A.; Betancourt-Lozano, M.; Llera-Herrera, R.; Alvarado-Cruz, I.; Yáñez-Rivera, B. Epigenetic Transgenerational Modifications Induced by Xenobiotic Exposure in Zebrafish. Front. Cell Dev. Biol. 2022, 10, 832982. [Google Scholar] [CrossRef]
- Brandão, F.; Cappello, T.; Raimundo, J.; Santos, M.A.; Maisano, M.; Mauceri, A.; Pacheco, M.; Pereira, P. Unravelling the Mechanisms of Mercury Hepatotoxicity in Wild Fish (Liza aurata) through a Triad Approach: Bioaccumulation, Metabolomic Profiles and Oxidative Stress. Metallomics 2015, 7, 1352–1363. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Maisey, K.; Reyes-Lpez, F.; Toro-Ascuy, D.; Mara, A.; Imarai, M. Fish Cytokines and Immune Response. In New Advances and Contributions to Fish Biology; InTech: Vienna, Austria, 2012. [Google Scholar]
- Moore, M.N.; Allen, J.I.; McVeigh, A.; Shaw, J. Lysosomal and Autophagic Reactions as Predictive Indicators of Environmental Impact in Aquatic Animals. Autophagy 2006, 2, 217–220. [Google Scholar] [CrossRef]
- Colin, N.; Porte, C.; Fernandes, D.; Barata, C.; Padrós, F.; Carrassón, M.; Monroy, M.; Cano-Rocabayera, O.; de Sostoa, A.; Piña, B.; et al. Ecological Relevance of Biomarkers in Monitoring Studies of Macro-Invertebrates and Fish in Mediterranean Rivers. Sci. Total Environ. 2016, 540, 307–323. [Google Scholar] [CrossRef]
- Machuca-Sepúlveda, J.; Miranda, J.; Lefin, N.; Pedroso, A.; Beltrán, J.F.; Farias, J.G. Current Status of Omics in Biological Quality Elements for Freshwater Biomonitoring. Biology 2023, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Jones, O.A.H.; Bose, U.; Broadbent, J.A.; Walsh, T.K.; van de Kamp, J.; Bissett, A. Omics-Based Ecosurveillance for the Assessment of Ecosystem Function, Health, and Resilience. Emerg. Top. Life Sci. 2022, 6, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Bourlat, S.J.; Borja, A.; Gilbert, J.; Taylor, M.I.; Davies, N.; Weisberg, S.B.; Griffith, J.F.; Lettieri, T.; Field, D.; Benzie, J.; et al. Genomics in Marine Monitoring: New Opportunities for Assessing Marine Health Status. Mar. Pollut. Bull. 2013, 74, 19–31. [Google Scholar] [CrossRef]
- Sadaiappan, B.; Balakrishnan, P.; Vishal, C.R.; Vijayan, N.T.; Subramanian, M.; Gauns, M.U. Applications of Machine Learning in Chemical and Biological Oceanography. ACS Omega 2023, 8, 15831–15853. [Google Scholar] [CrossRef]
- Miller, T.; Michoński, G.; Durlik, I.; Kozlovska, P.; Biczak, P. Artificial Intelligence in Aquatic Biodiversity Research: A PRISMA-Based Systematic Review. Biology 2025, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Meivelu, M.; Praburaman, L.; Mujahid Alam, M.; Al-Sehemi, A.G.; K, A. Integrating AI in Food Contaminant Analysis: Enhancing Quality and Environmental Protection. J. Hazard. Mater. Adv. 2024, 16, 100509. [Google Scholar] [CrossRef]
- Ou, L.; Lu, L.; Qian, W.; Liu, B. Application of Artificial Intelligence in Fish Information Identification: A Scientometric Perspective. Front. Mar. Sci. 2025, 12, 1575523. [Google Scholar] [CrossRef]
- Manochkumar, J.; Cherukuri, A.K.; Kumar, R.S.; Almansour, A.I.; Ramamoorthy, S.; Efferth, T. A Critical Review of Machine-Learning for “Multi-Omics” Marine Metabolite Datasets. Comput. Biol. Med. 2023, 165, 107425. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Wei, L.; Jingfeng, Y.; Chernick, M.; Hinton, D.E. Developmental Toxicity from Exposure to Various Forms of Mercury Compounds in Medaka Fish (Oryzias latipes) Embryos. PeerJ 2016, 4, e2282. [Google Scholar] [CrossRef]
- Kamila, S.; Dey, K.K.; Islam, S.; Chattopadhyay, A. Arsenic and Chromium Induced Hepatotoxicity in Zebrafish (Danio rerio) at Environmentally Relevant Concentrations: Mixture Effects and Involvement of Nrf2-Keap1-ARE Pathway. Sci. Total Environ. 2024, 921, 171221. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zheng, S.; Tang, F.; Yang, L.; Wei, X.; Kong, D.; Sun, W.; Li, W. Heme Oxygenase 1 Plays a Crucial Role in Swamp Eel Response to Oxidative Stress Induced by Cadmium Exposure or Aeromonas hydrophila Infection. Fish Physiol. Biochem. 2020, 46, 1947–1963. [Google Scholar] [CrossRef] [PubMed]
- Puar, P.; Niyogi, S.; Kwong, R.W.M. Regulation of Metal Homeostasis and Zinc Transporters in Early-Life Stage Zebrafish Following Sublethal Waterborne Zinc Exposure. Aquat. Toxicol. 2020, 225, 105524. [Google Scholar] [CrossRef] [PubMed]


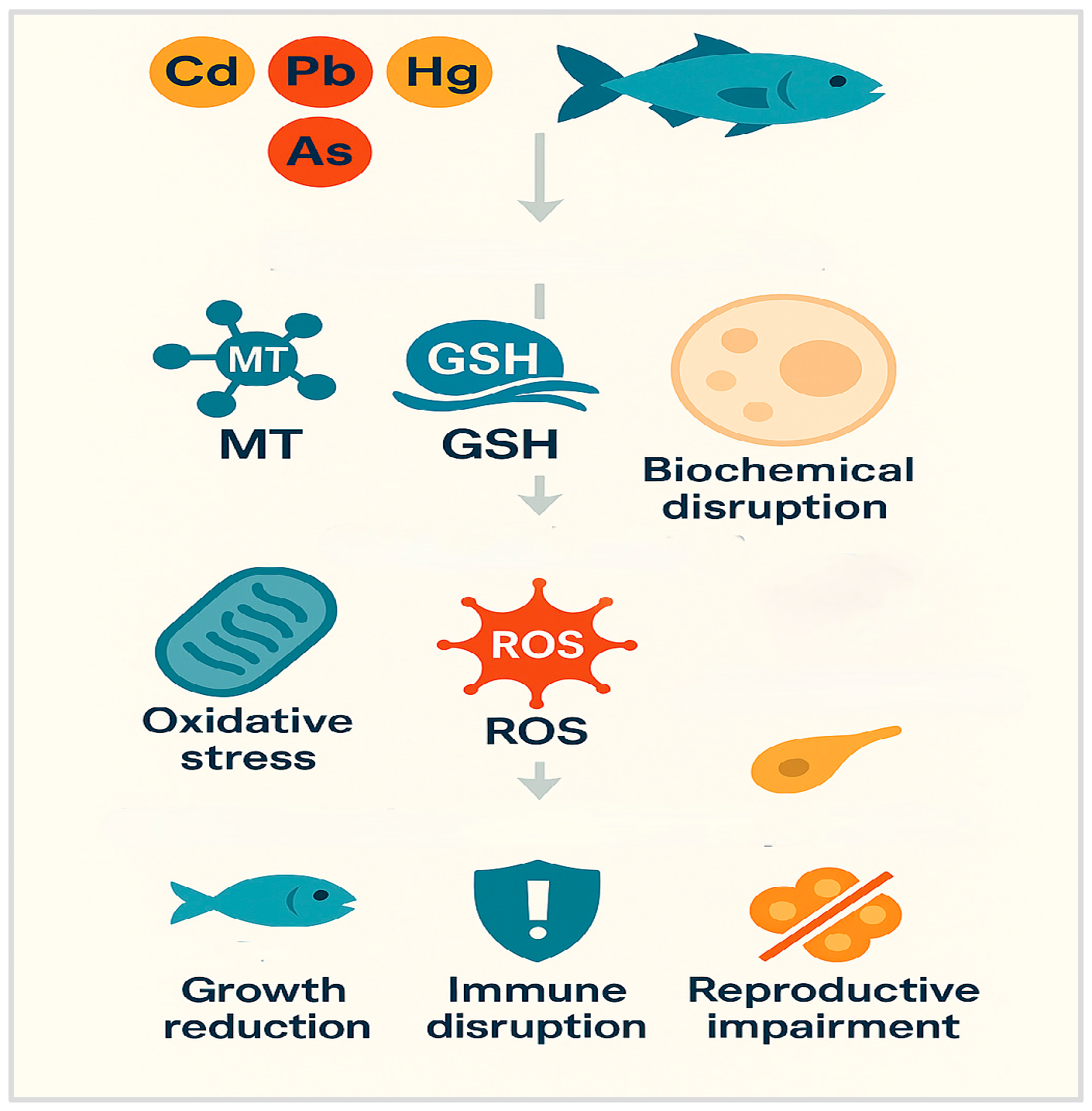
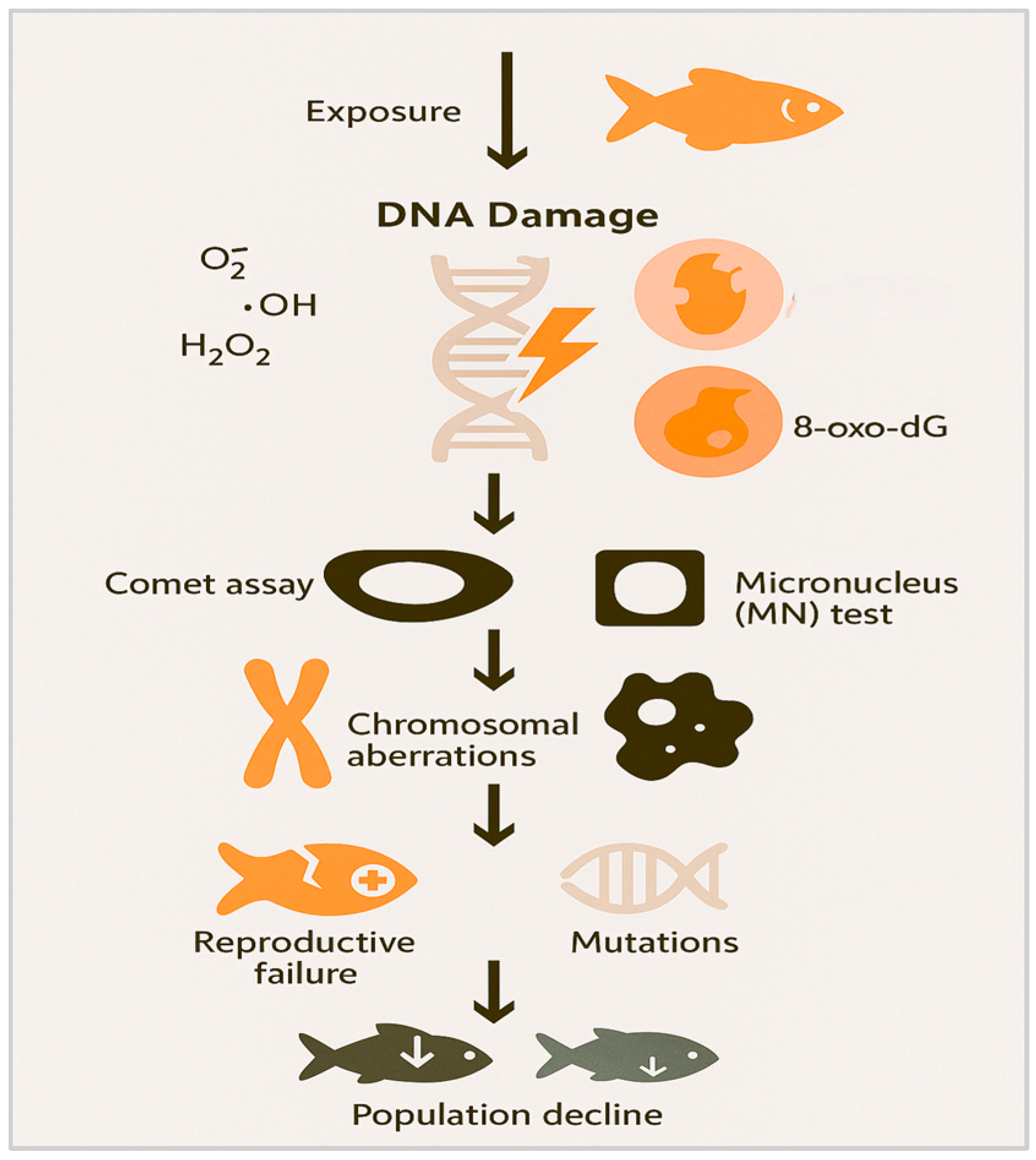

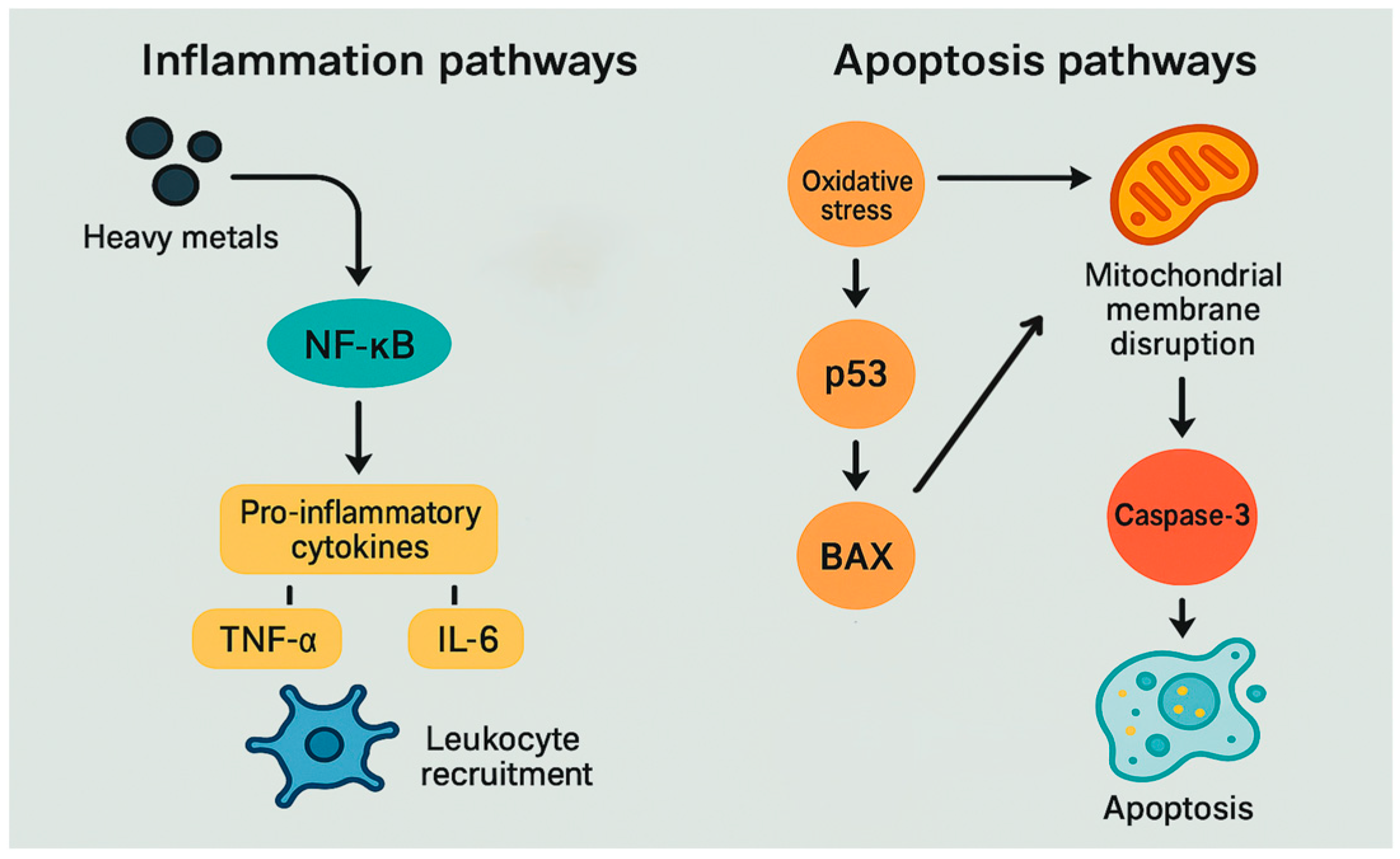
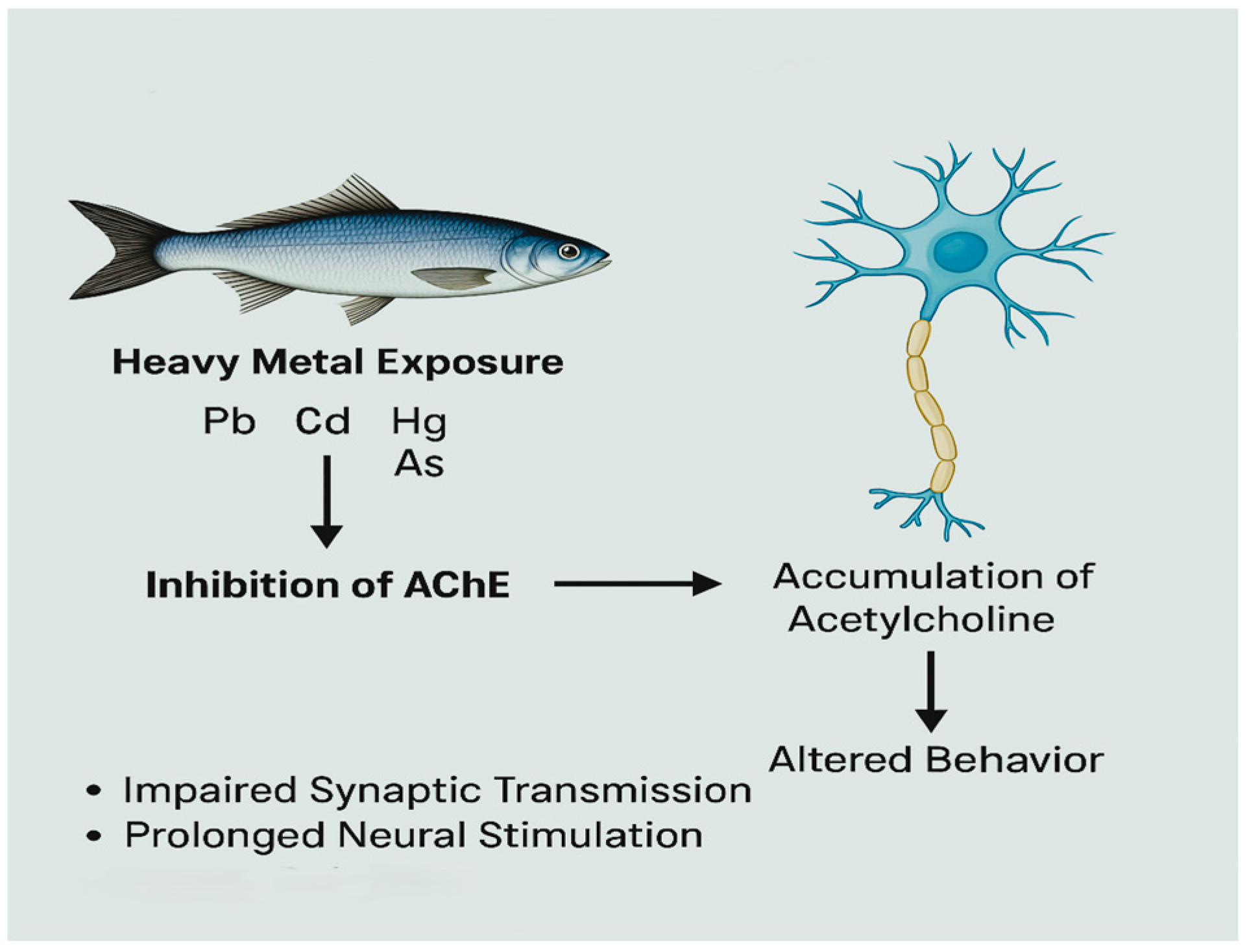
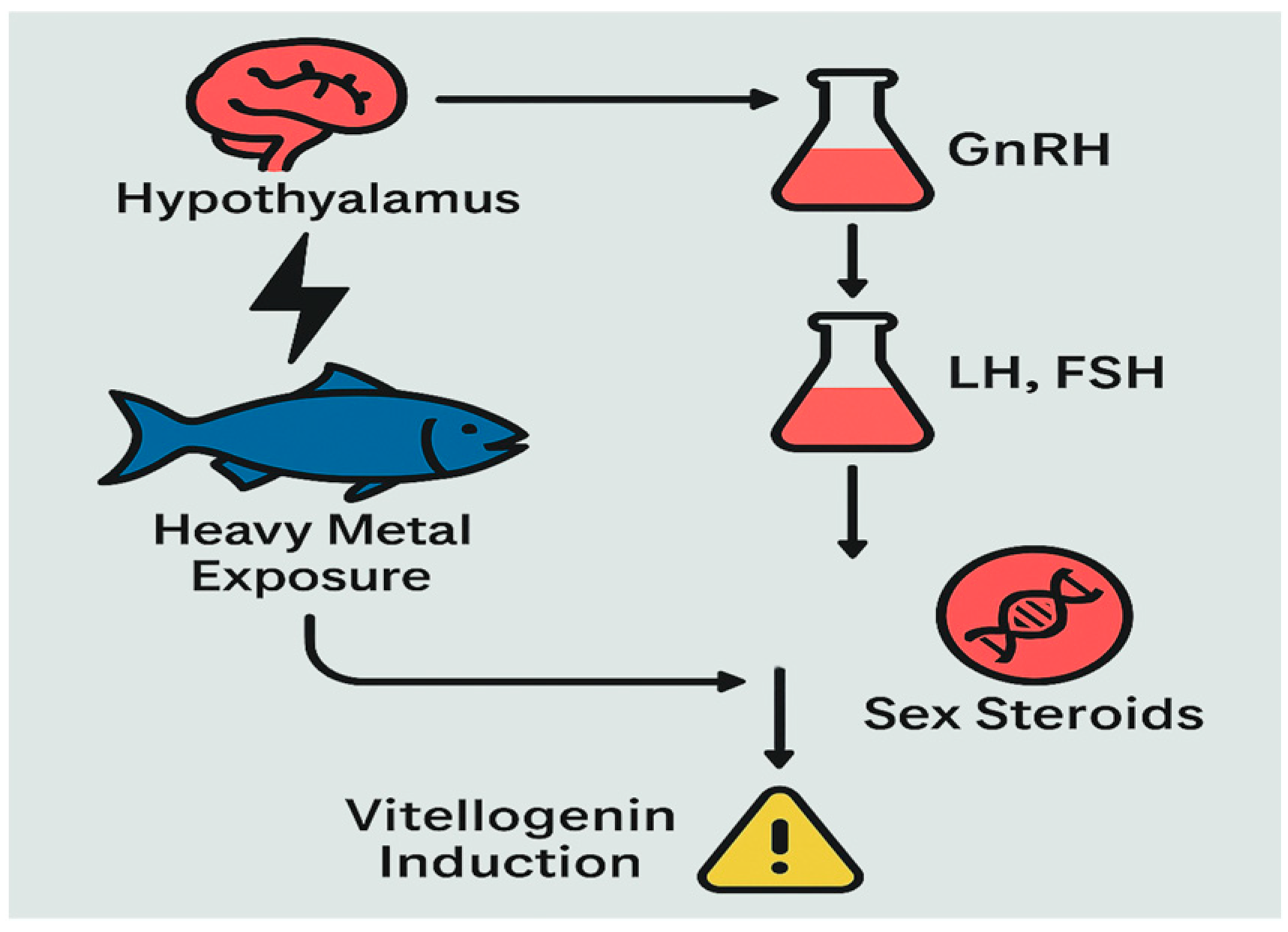
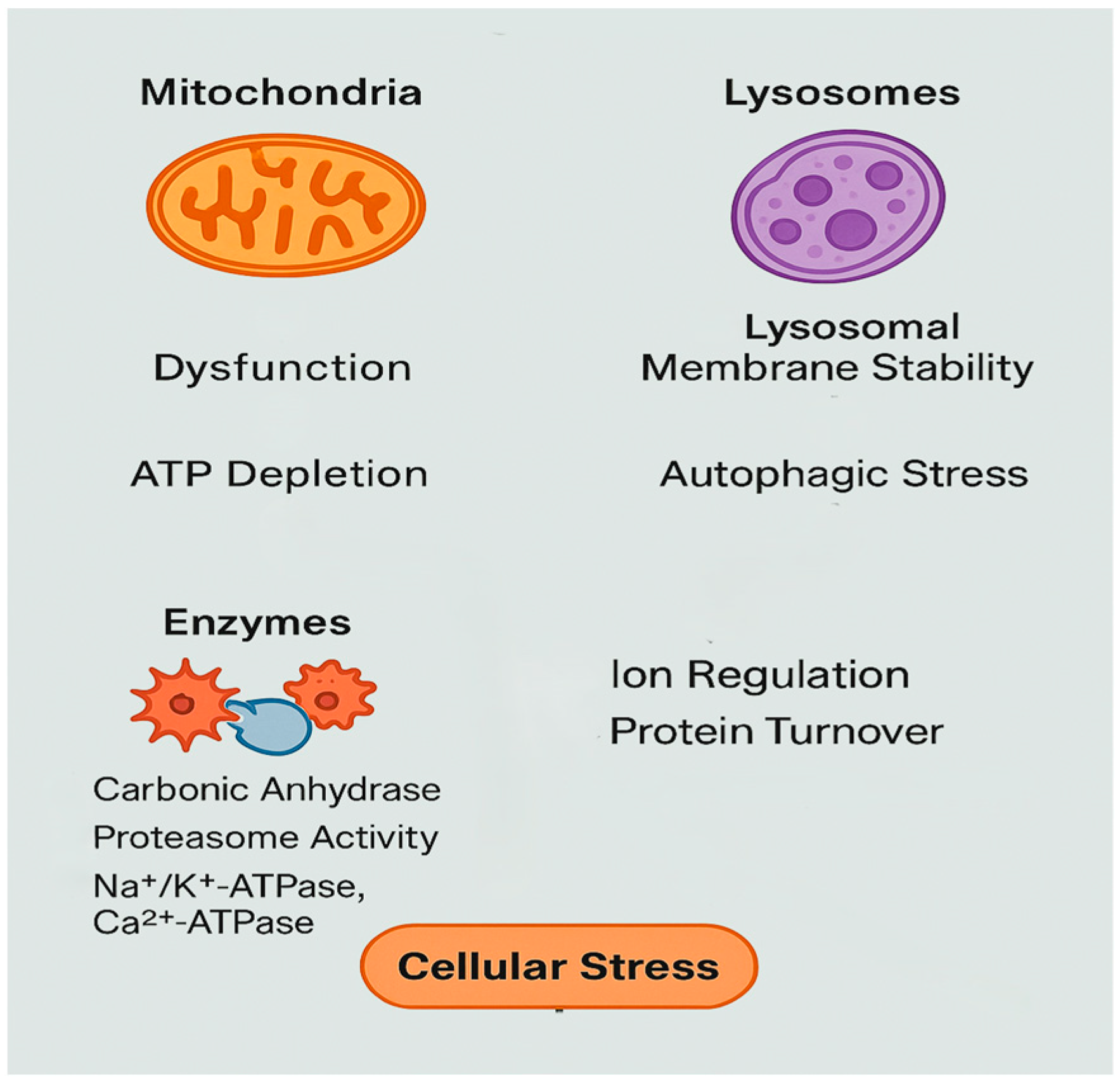
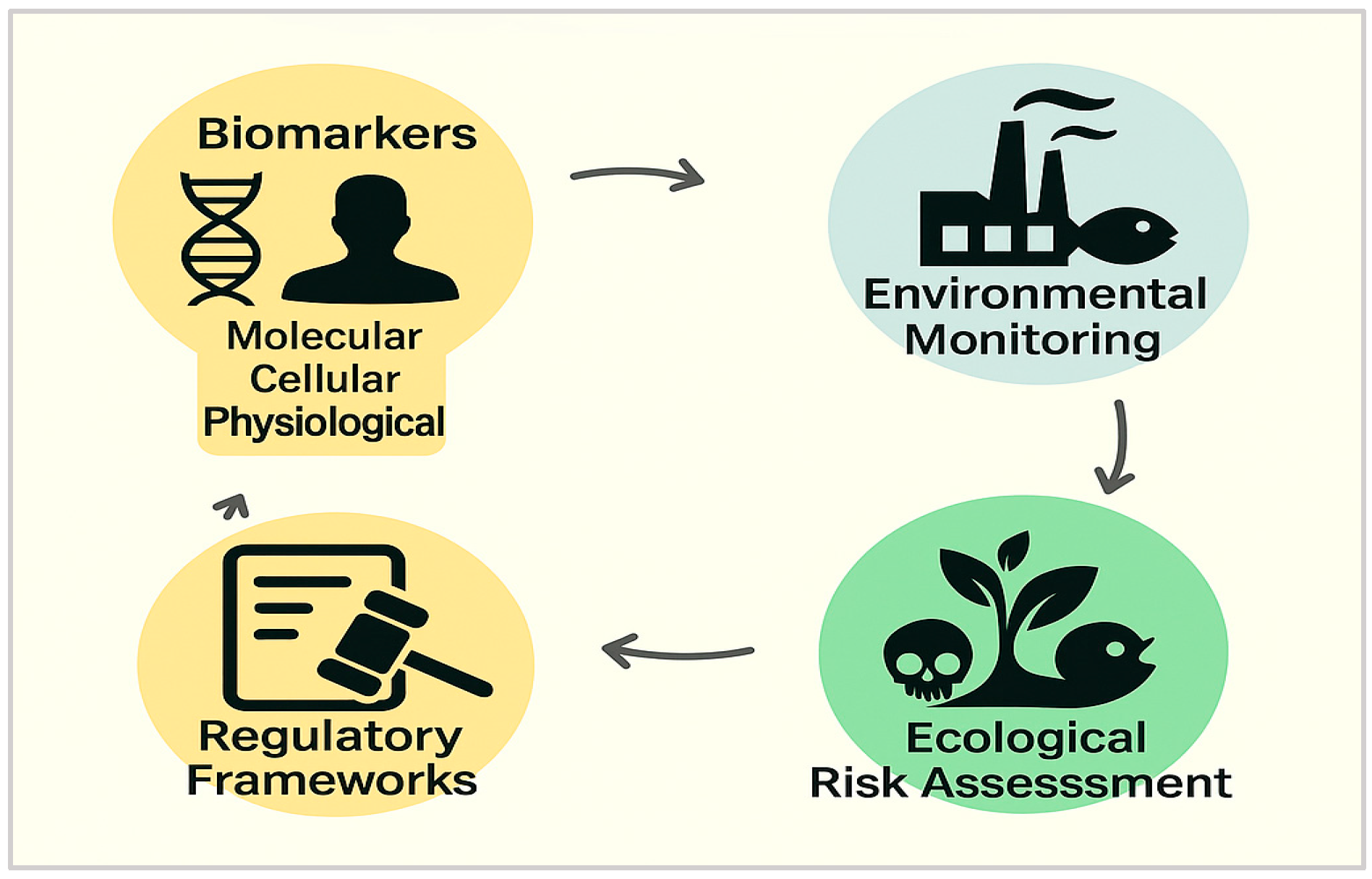
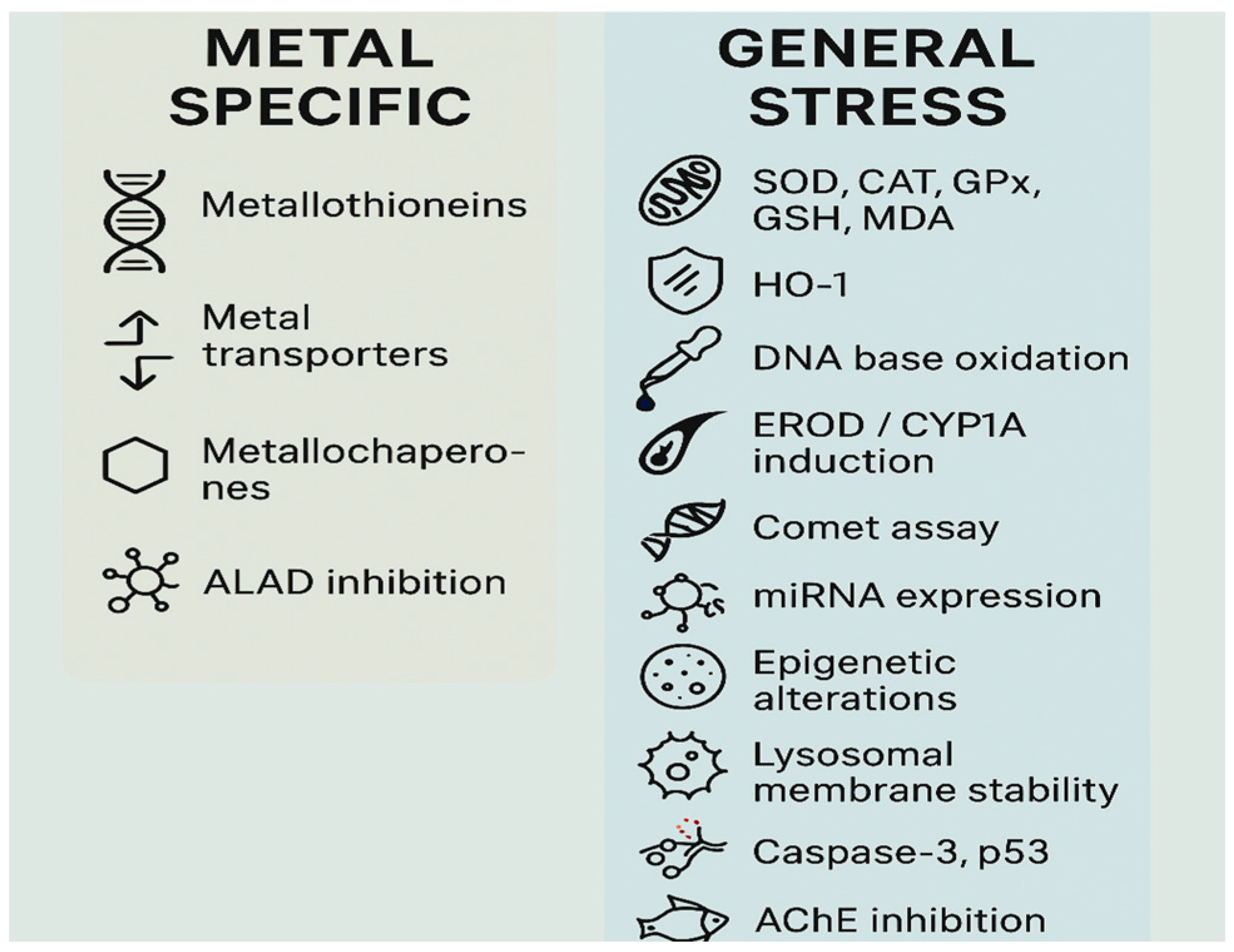
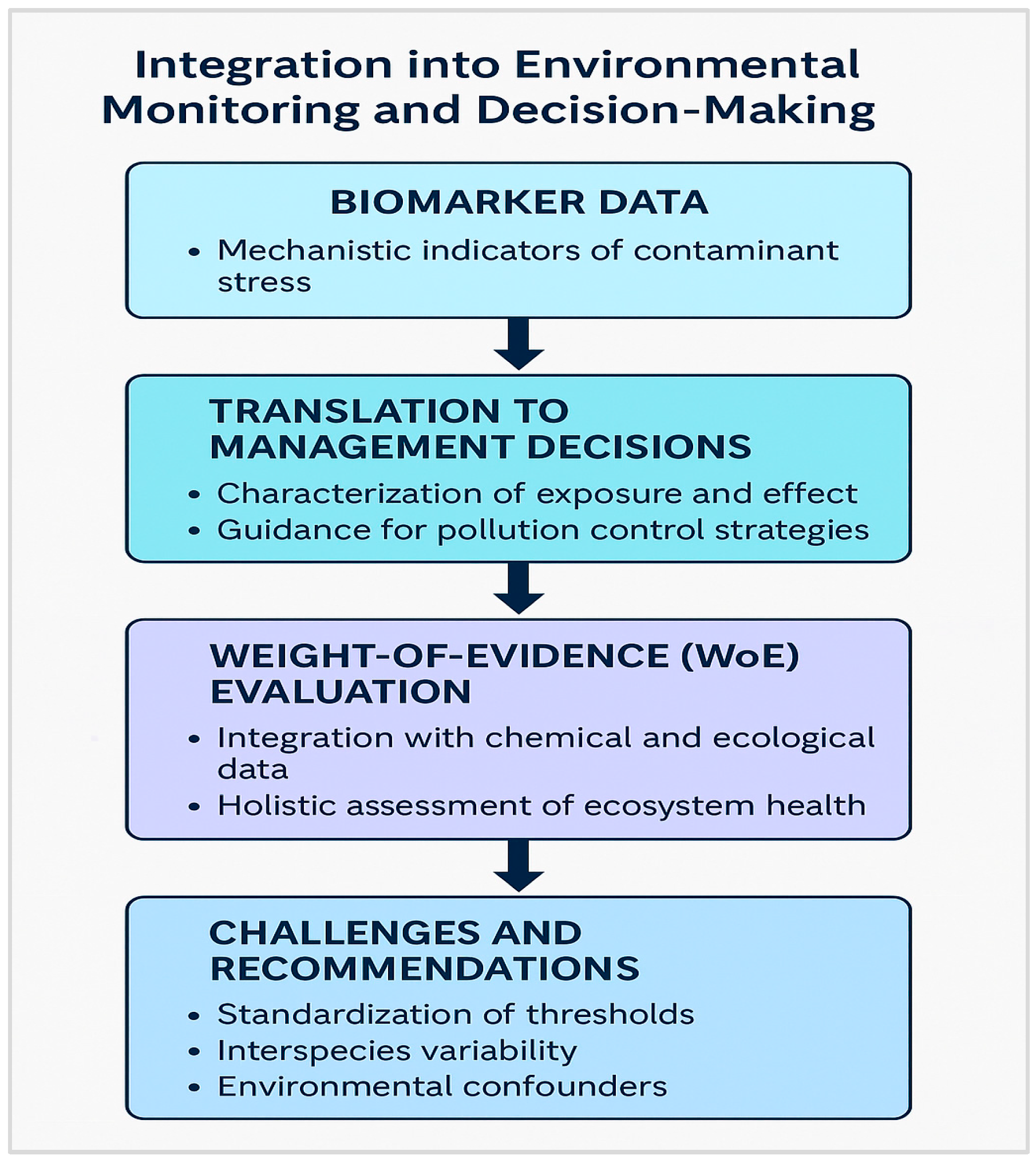
| Biomarker/ Mechanism | Mechanistic Category | Indicative Effect | Specificity to Metals | Biomarker Type | Early-Warning Potential | Limitations |
|---|---|---|---|---|---|---|
| Metallothioneins (MTs) | Detoxification | Metal sequestration | High | Exposure | High | Widely used in marine monitoring. Baseline varies by species, tissue, and environmental conditions. |
| Metal transporters (DMT1, ZIP, and ZnT ATP7A/B) Metallochaperones (ATOX1, CCS, andCOX17) | Detoxification | Metal homeostasis | High | Exposure | High | Baseline variability and limited routine application. |
| ALAD inhibition | Enzymatic inhibition | Disrupted heme synthesis | High (Pb-specific) | Effect | Medium | Inhibited by metals (Pb); also affected by diet and nutritional status. |
| Heme Oxygenase-1 (HO-1) | Oxidative Stress | Cytoprotective response | Moderate | Effect | High | Induced by multiple ROS-generating agents. |
| Malondialdehyde (MDA) and protein carbonyls | Oxidative Stress | Oxidative membrane damage | Moderate | Effect | Medium | Induced by various pollutants and stressors. |
| Mitochondrial function (membrane potential and ATP levels) | Cellular and energy disruption | Bioenergetic failure | Moderate | Effect | Medium | Not specific to HMs; technical demands for measurement. |
| Proteasome activity | Cellular and energy disruption | Protein turnover | Moderate | Effect | Medium | Sensitive but non-specific. |
| Metabolomics/Lipidomics profiles | Cellular/molecular changes | Metabolic health | Moderate | Effect | Medium | Expensive, high complexity, and not routine. |
| Epigenetic alterations (DNA methylation and histone acetylation) | Genotoxicity | Altered gene regulation | Moderate | Effect | Low | Complex and not standardized for routine use. |
| Aromatase and 17β-HSD expression | Endocrine disruption | Steroidogenesis disruption | Moderate | Effect | Low | Species- and sex-dependent expression; limited field use. |
| SOD, CAT, GPx, and GSH | Oxidative stress | Antioxidant defense | Moderate | Effect | Moderate | Induced by multiple stressors. |
| MicroRNA (miRNAs) | Gene regulation | Post-transcriptional modulation | Low to moderate | Effect | High | Not yet standardized for field use. |
| Glutathione system (GSH, GST, GPx, and GR) | Detoxification | Antioxidant defense | Low to moderate | Effect | Medium | Modulated by multiple stressors. |
| DNA strand breaks and 8-oxodG | Genotoxicity | DNA damage | Low | Effect | Medium | Not metal-specific; general genotoxicity. |
| Lysosomal membrane stability (LMS) | Cellular integrity | Subcellular dysfunction | Low | Effect | Medium | Sensitive, but not specific; influenced by many factors. |
| Micronucleus test (MN) | Genotoxicity | Chromosomal damage | Low | Effect | Medium | Not metal-specific; general genotoxicity. |
| HSPs and HIF-1α | Stress adaptation | Stress adaptation | Low | Effect | Medium | Broadly inducible; lacks exposure specificity. |
| Cytokine expression (NF-κB, TNF-α, IL-6) | Inflammation | Immune activation | Low | Effect | Medium | Not unique to metals; can reflect disease and other stress. |
| Na+/K+-ATPase, Ca2+-ATPase | Neurotoxicity | Ion regulation disruption | Low | Effect | Medium | Activity influenced by multiple environmental factors. |
| Caspase-3, p53, BAX | Apoptosis | Programmed cell death | Low | Effect | Low | Activation occurs under multiple stress types; not metal specific. May indicate irreversible damage. |
| AChE inhibition | Neurotoxicity | Neuro- transmission impairment | Low | Effect | Low | Inhibited by metals and pesticides. |
| Carbonic anhydrase (CA) | Ion regulation | pH/ion imbalance | Low | Effect | Low | Influenced by salinity, pH, and other contaminants. |
| Vitellogenin (VTG) and plasma E2/T | Endocrine disruption | Estrogenic response | Moderate (Cd and As) | Condition | Low | Induced by metals and organic EDCs. |
| EROD, CYP1A, and GST | Detoxification and biotransformation | Phase I/II detoxification and CYP1A induction | Low | Effect | Low | EROD is more relevant to organics; not HM-specific. |
| AMPK | Stress adaptation | Energy metabolism shift | Low | Effect | Low | Still experimental; ecological significance not fully established. |
| IL-1β, CRP | Inflammation | Chronic inflammation | Low | Effect | Low | Requires co-assessment with other immune parameters for interpretation. |
| Brain cytokines and 8-oxodG | Neurotoxicity | Neuro-inflammation and damage | Low | Effect | Low | Limited baseline data; currently experimental. |
| ALT and AST leakage | Cellular and energy disruption | Membrane integrity loss | Low | Effect | Low | Non-specific. |
| Lysozyme activity, leukocyte counts, and immunoglobulins | Immunological and hematological | Immune function and physiological stress response | Low | Effect | Low | Broad; not specific for metals. |
| Gut microbiota composition | Systemic/metabolic health | Metabolic health | Low | Effect | Low | Field application limited and not yet standardized. |
| Histopathological lesions | Tissue alteration | Structural damage to gill, liver, and kidney | Low | Condition | Low | Requires expert interpretation; not specific to HMs. |
| Gonadosomatic Index (GSI) | Reproductive indicator | Reproductive impairment | Low | Condition | Low | Sensitive to seasonal and reproductive cycles; low specificity to metal stress. |
| Hepatosomatic Index (HSI) | Metabolic indicator | Liver enlargement from stress | Low | Condition | Low | Affected by nutritional status and multiple stressors; non-specific. |
| Growth and reproduction endpoints | Life-history traits | Growth inhibition and reduced fecundity | Low | Condition | Low | Integrative but slow-response indicators; influenced by multiple environmental and biological variables. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oros, A.; Coatu, V.; Damir, N.; Danilov, D.; Ristea, E.; Lazar, L. Molecular Mechanisms and Biomarker-Based Early-Warning Indicators of Heavy Metal Toxicity in Marine Fish. Fishes 2025, 10, 339. https://doi.org/10.3390/fishes10070339
Oros A, Coatu V, Damir N, Danilov D, Ristea E, Lazar L. Molecular Mechanisms and Biomarker-Based Early-Warning Indicators of Heavy Metal Toxicity in Marine Fish. Fishes. 2025; 10(7):339. https://doi.org/10.3390/fishes10070339
Chicago/Turabian StyleOros, Andra, Valentina Coatu, Nicoleta Damir, Diana Danilov, Elena Ristea, and Luminita Lazar. 2025. "Molecular Mechanisms and Biomarker-Based Early-Warning Indicators of Heavy Metal Toxicity in Marine Fish" Fishes 10, no. 7: 339. https://doi.org/10.3390/fishes10070339
APA StyleOros, A., Coatu, V., Damir, N., Danilov, D., Ristea, E., & Lazar, L. (2025). Molecular Mechanisms and Biomarker-Based Early-Warning Indicators of Heavy Metal Toxicity in Marine Fish. Fishes, 10(7), 339. https://doi.org/10.3390/fishes10070339










