4.1. Age and Growth
This study generated age, length, and growth data for gray snapper that can be compared with past and future studies in the region. Size data, in particular mean SL (± sd) at the lagoon scale (88.7 ± 28.8 mm) suggests gray snapper in the study region are predominantly later stage juveniles [
4,
8]. With the range of SLs being relatively similar between living shoreline and oyster reef habitats (living shoreline SL range = 26.0–171.0 mm; oyster reef SL range = 43.0–161.0 mm), gray snapper in Mosquito Lagoon range from early- to late-stage juveniles, all of which have yet to reach sexual maturity (~175 mm SL) [
4,
10]. However, gray snapper collected from living shorelines were smaller on average than those collected at oyster reefs (77.4 ± 27.5 mm vs. 97.5 ± 26.7 mm), implying gray snapper foraging at living shorelines are younger than those foraging at oyster reefs, corresponding with habitat preference findings from past studies (e.g., [
4]). This age and size distribution is likely due to the proximity of living shoreline habitats to seagrass, which is a known settlement habitat for larval gray snapper [
2,
5]. In the study region Ponce de Leon Inlet is the only direct connection between Mosquito Lagoon and the Atlantic Ocean (where larval gray snapper originate), and is located north of both oyster reef and living shoreline habitats (
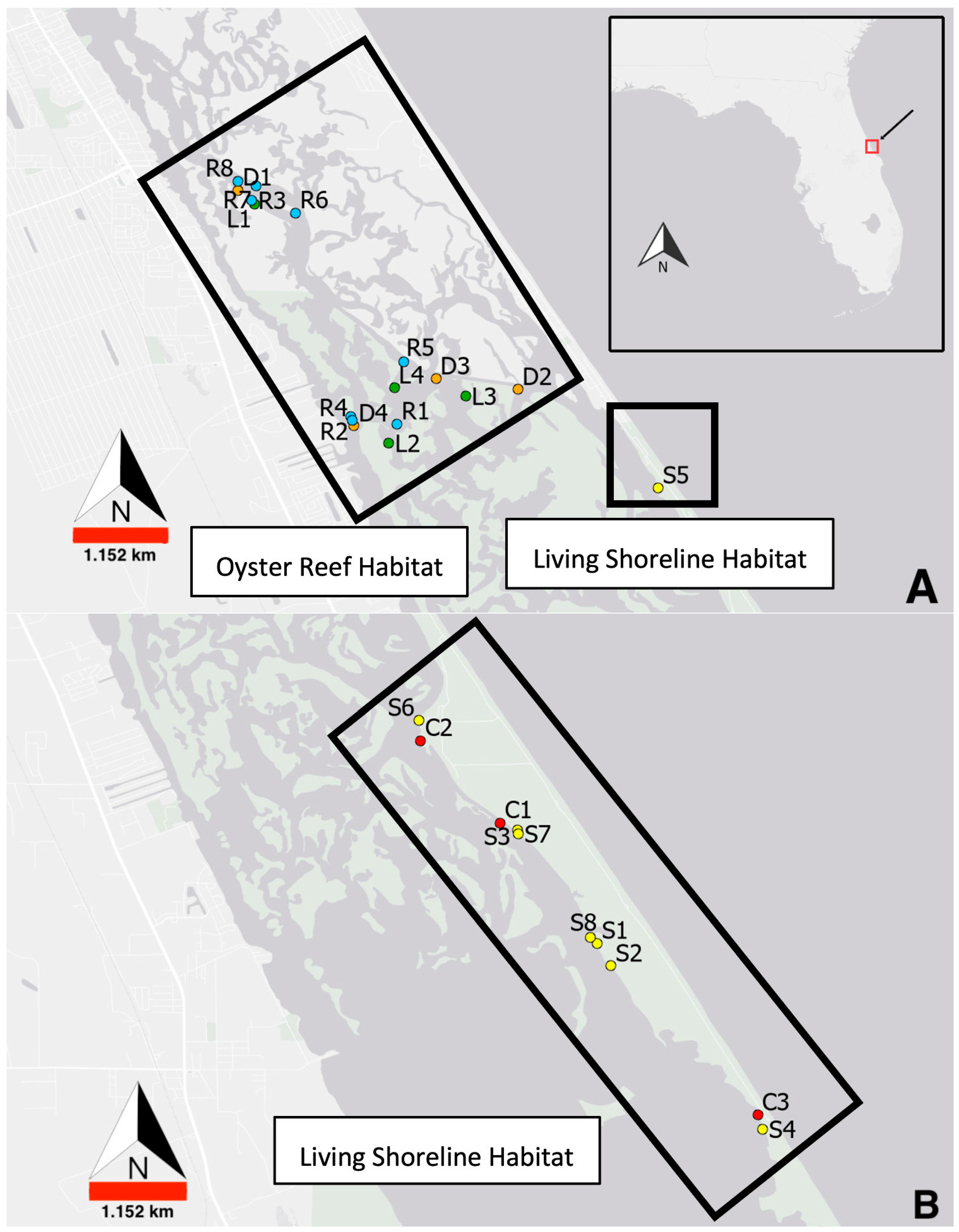
Figure 1). Yet despite living shoreline habitats being farther from Ponce Inlet than oyster reef habitats, a greater proportion of relatively younger, smaller juveniles were collected at living shorelines. This finding implies seagrass adjacent to living shorelines may be providing stronger settlement cues than oyster reef habitats to the north [
23,
25]. These findings further suggest gray snapper in Mosquito Lagoon are generalists capable of foraging in various benthic habitat types and feeding on associated prey communities [
24,
59]. This generalist behavior might be influenced by the relative paucity of seagrass cover in Mosquito Lagoon, which could incline gray snapper to move from seagrass meadows to adjacent living shoreline or oyster reef habitats [
4,
60].
Regarding the relevance of size findings to local fisheries, the size range of collected gray snapper (32.0–207.0 mm TL) individuals from Mosquito Lagoon does not meet the minimum size limit for gray snapper in Florida’s recreational (254 mm maximum TL) or commercial fisheries (304.8 mm maximum TL) [
61,
62]. This means collected gray snapper in the current study are undersized, and have not recruited to local fisheries. These findings highlight the importance of Mosquito Lagoon providing critical benthic habitats for juvenile gray snapper, and their likely functioning as nursery habitat facilitating the survival of juveniles to adulthood [
16,
27]. The lower proportion of early-stage or recently settled juveniles in the current study suggests Mosquito Lagoon’s oyster reef and living shoreline habitats primarily support mid-stage juvenile gray snapper, with smaller individuals inhabiting the southern mangrove shoreline habitats nearer to seagrass meadows, while relatively larger juveniles inhabit the northern oyster reef habitats closer to Ponce De Leon inlet [
2,
5,
23]. The capture of relatively larger juveniles in the northern portions of Mosquito Lagoon suggests that future studies should focus on collection sites closer to the inlet and potentially include nearshore and offshore collection sites to better understand ontogenetic habitat shifts of gray snapper in the study region.
The current population status of gray snapper from the Atlantic coast of Florida, including sexually mature adults collected from offshore habitats, remains unknown due to the lack of stock assessments in this region (see Bacheler et al. [
63]). Fishery-independent surveys, such as the current study, provide important information on the early life history of gray snapper that can inform potential stock assessments. Therefore, natural resource managers should continue monitoring Mosquito Lagoon’s juvenile gray snapper and their relation to local environmental factors to help (1) understand potential changes in local juvenile gray snapper development in relation to habitat conditions and food availability, (2) determine whether stricter regulations on boating activity (i.e., human impacts) are necessary to protect local benthic habitats, and (3) assess the need for additional restoration efforts on dead oyster reefs and destabilized and/or eroding shorelines [
22,
24,
35]. For abundance and length data, the SouthEast Data, Assessment, and Review 75 process highlights the importance of fishery-independent surveys for gray snapper populations, indicating said surveys contribute unbiased length data on gray snapper below minimum size limits for recreational and commercial fisheries, and help determine if gray snapper populations in the Gulf of Mexico are being overfished based on continuously updated life history data [
15,
16]. A large-scale assessment of gray snapper from Florida’s estuarine habitats, including Mosquito Lagoon, was conducted using data collected between 1996 and 2009 [
21]. Gold et al. [
64] found genetic differences between gray snapper populations in east and west Florida, emphasizing the need for an east Florida stock assessment to determine how coast-specific genetics relate to gray snapper population status. Together, such assessments are important to ensure gray snapper along the east coast of Florida are not at a higher risk of being overfished, which could manifest in reduced sizes and decreased abundance [
65]. Gray snapper are a vital resource for the state’s recreational fishing sector, as Florida’s recreational fisheries represented ~90% of the take for gray snapper in the Southeastern United States, with 2 million pounds of gray snapper recreational landings recorded in 2022 along the east coast of Florida [
66,
67]. Despite the average size of juvenile gray snapper increasing at lower latitudes [
3], studies conducted in east and west Florida found adult gray snapper collected from northern Florida reached larger maximum sizes and ages compared to individuals collected from southern Florida [
11,
12]. These studies suggest increased fishing pressure in southern Florida could be driving size-selective mortality, which would result in fewer larger and older gray snapper within local populations. However, the findings above emphasize the need for continuous monitoring efforts across benthic habitats located along both coasts of Florida to ensure optimal survival and recruitment rates for local gray snapper populations and to reduce the probability of overfishing [
68].
Quality Assurance and Quality Control (QA/QC) procedures indicate relatively good concordance between readers aging fish using daily growth rings in gray snapper otoliths (
Table 2) [
46]. The secondary reader found higher daily age estimates than the primary reader (i.e., age range from 103 to 290 days old vs. 106 to 317 days old) due to the presence of an annulus in nine of the randomized samples. While this suggests current enumerated and calculated age data skew slightly younger, the primary reader achieved an individual APE below 10% for all 49 otolith samples, indicating high precision in age estimates for analyses. Although 2 of the 16 QA/QC otoliths had an APE slightly over 10% between both readers (10.3% and 12%), their enumerated ages did not appear to skew analyses, and were deemed sufficiently precise for use in analyses. However, there was complexity/difficulty in accurately estimating daily age due to individual annulus formations. A consistent QA/QC protocol should be employed for future studies on gray snapper growth [
11,
13,
14]. QA/QC measures, such as creating reference collections and calculating APE between readers (i.e., methods applied in the current study) can help mitigate over- and underestimations of age, and help to prevent inaccurate population assessments [
46].
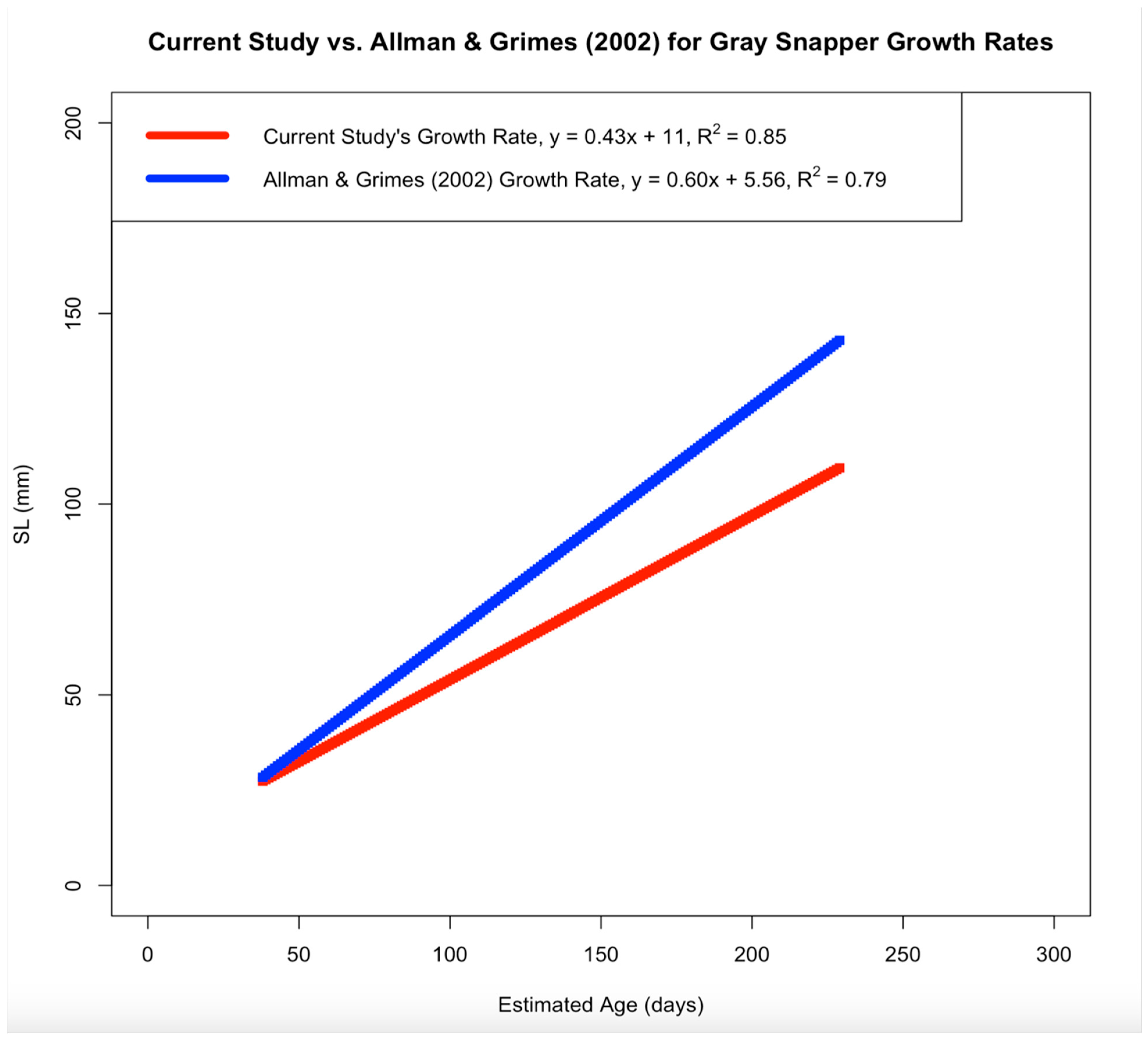
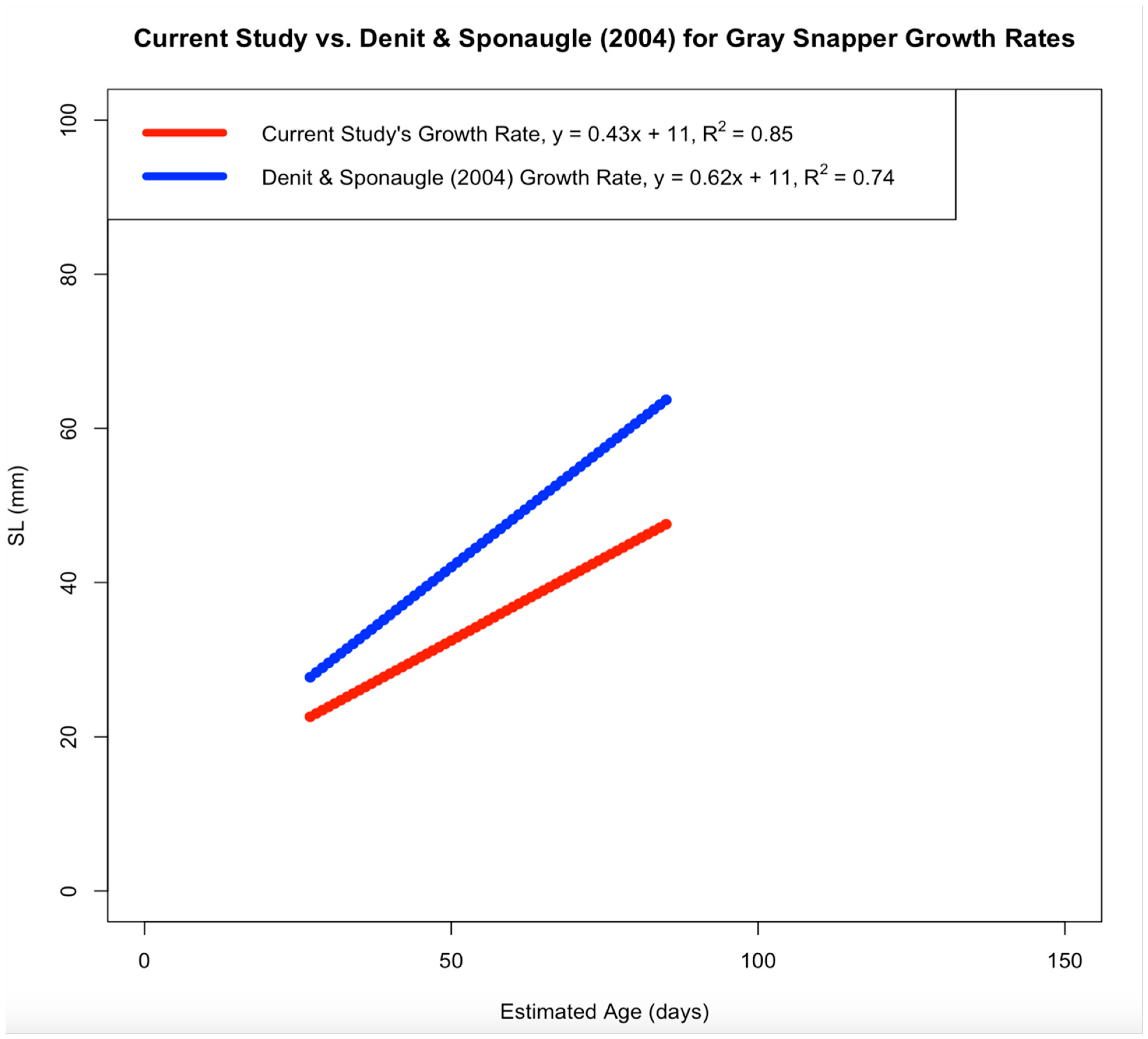
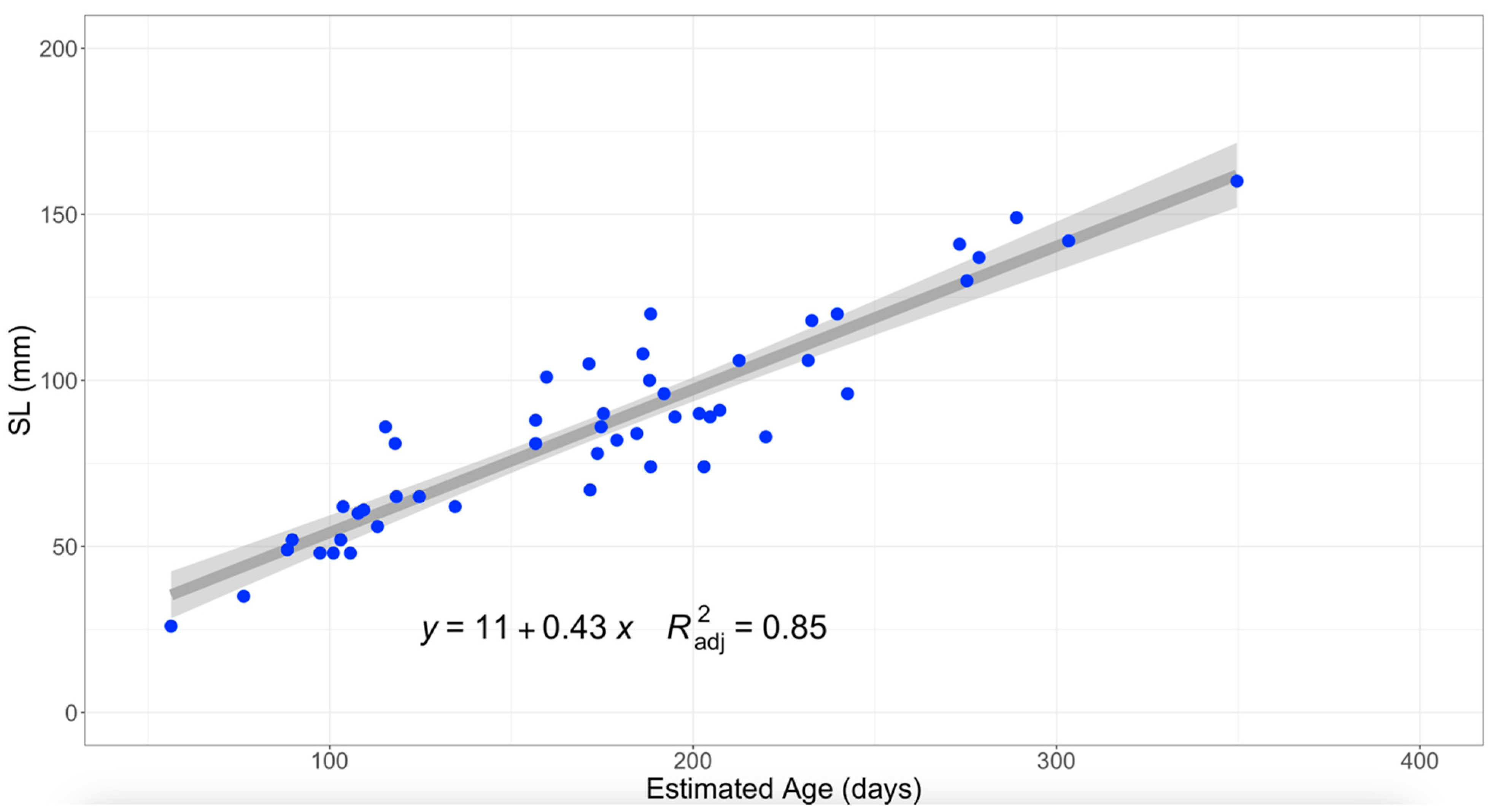
Age and length data from Mosquito Lagoon’s gray snapper were modeled using a linear growth model. Current results indicate the linear growth model to be the most parsimonious function when compared to the von Bertalanffy, Gompertz and logistic functions. This result may be due to the von Bertalanffy growth function typically being applied to fish across a wider age span (in years) than fish sampled here (in days). In contrast, linear models better analyze growth for fish less than 1 year old [
3,
8]. When comparing growth rates between the current study and two previous studies [
8,
14], gray snapper in this study had smaller calculated sizes despite comparisons using the lowest growth rates from the other studies. Growth patterns for separate juvenile gray snapper populations are similar within the Southeastern United States [
3,
8], with lower growth rates (1) indicating a presence of larger and older juveniles in collected samples [
4], and (2) suggesting that gray snapper grow slower as they age. The minimum estimates for linear growth rate from North Carolina (0.62–0.72 mm SL/day) [
3] and the west coast of Florida (0.60–1.02 mm SL/day) [
8] were lower than those from the east coast of Florida (0.68–0.88 mm SL/day) [
3]. However, the growth rate calculated in the current study (0.43 mm SL/day) was the lowest when compared to the above studies, which is most likely due to the presence of older juvenile samples in the current study (discussed below;
Figure A1 and
Figure A2 in
Appendix C). For future studies in Mosquito Lagoon, a greater sample size of gray snapper with complementary otolith age data is recommended to create habitat-specific growth models. While the lagoon-scale linear growth model has established the first growth analysis of gray snapper in Mosquito Lagoon, future analyses should aim to obtain larger sample sizes from oyster reefs, living shorelines, and seagrass beds to create habitat-specific growth models [
69]. Habitat-specific growth models would allow resource managers to investigate quantitative differences in gray snapper growth between benthic habitat types, along with how they relate to potential correlations between gray snapper size and environmental factors.
Caution should be taken when comparing results from the previous and current study due to size variations between collected gray snapper; the largest sample collected from North Carolina was 65 mm SL, while the largest sample collected from west Florida was 150 mm SL (current study largest SL = 171 mm) [
3,
8]. Additionally, the lack of studies focusing on juvenile gray snapper growth indicates a knowledge gap in early gray snapper development outside of Florida and North Carolina, along with how early gray snapper development is affected by habitat restoration outside of Mosquito Lagoon [
23]. Expanding research in this area could enhance understanding of habitat use by juvenile gray snapper and their developmental responses to restored benthic habitats. Furthermore, while most studies on early-stage gray snapper growth date back to the early 2000s and may contain outdated population data, current findings indicate Mosquito Lagoon’s gray snapper have the slowest growth rates compared to samples collected from other estuarine habitats. Lagoon-scale age and length data for gray snapper also reveals individual variation in length-at-age. Although this specific topic has not yet been examined for gray snapper, Wimberger [
70] suggests variation in size among similarly aged fish may be relative to differences in foraging behavior. Mosquito Lagoon’s gray snapper primarily consume macroinvertebrates; however, although prey fish are less common in their diet, they become more prevalent as gray snapper grow larger [
23]. This finding suggests larger gray snapper may exhibit increased mobility and energy expenditure to forage, which could lead to shifts in diet composition and potential differences in length-at-age [
23]. Combined with the high explanatory power of the current study’s linear growth model (R
2 = 0.85), both findings could help explain variation in gray snapper length-at-age.
4.2. Model Analyses
Investigating correlations between gray snapper size and local environmental factors in Mosquito Lagoon provided insight into the environmental factors potentially influencing gray snapper growth in the study region. While daily age estimates for Mosquito Lagoon’s gray snapper were relatively accurate and precise for growth function analyses, length was more accurate and precise as a response variable for models investigating correlations between gray snapper size and environmental factors in Mosquito Lagoon.
The results suggest lagged salinity and year of restoration, namely 2017, influence gray snapper size. Before investigating the effects and model outputs of environmental variables on gray snapper size, unsurprisingly the most parsimonious models identified body mass as a more influential biological variable, as compared to models that included calculated age. This finding supports previous studies of gray snapper that demonstrate a strong positive correlation between length and mass [
13,
14]. Similarly, current models incorporating mass demonstrated strong explanatory power (conditional R
2 = 0.99). For models with the greatest predictive power, predictive ability should be interpreted with caution, as inconsistencies (i.e., data outliers) were detected when testing model assumptions. While inconsistencies did not heavily impact predictive ability of said models, a higher sample size could reduce the relative number of potential outliers and provide more robust results for gray snapper size-based models [
71]. Future analyses should continue to explore if age has a greater effect on gray snapper size than mass, as previous studies have used gray snapper age to determine life stage [
2,
4,
10].
Gray snapper can survive in a broad range of salinity levels (0–60 ppt) [
72]. Higher salinity levels are related to increased feeding frequency, and if prey remains sufficiently high, this could result in greater growth and size [
20]. Current results show a positive correlation between gray snapper size and lagged salinity levels in Mosquito Lagoon, indicating that salinity levels over a 4-week period prior to the date of capture were more influential in affecting gray snapper size than salinity recorded on the date of capture. This finding provides new insight into lagged relationships between gray snapper size and local environmental conditions, as past research has only found lagged relationships between gray snapper growth and sea surface temperature [
40]. While gray snapper typically prefer a salinity range of 9–23 ppt to optimize osmoregulation, Serrano et al. [
73] suggest that proximity to offshore habitats takes priority over the need to minimize osmoregulatory energetic costs. This is evident in Mosquito Lagoon, where despite elevated salinity levels (mean of 33.91 ppt in oyster reefs and 33.56 ppt in living shorelines) [
26], gray snapper continue to inhabit these areas. Sampled juvenile gray snapper were relatively older and larger in Mosquito Lagoon’s oyster reef habitats than living shorelines possibly due to local oyster reefs (1) having slightly higher salinity levels, and (2) being relatively closer to the Ponce de Leon Inlet, which corresponds with known ontogenetic movements from inshore nursery habitats to offshore habitats as gray snapper age [
26]. Model results here indicate habitat suitability in Mosquito Lagoon similarly relates to gray snapper age and size, with older and larger individuals found in greater numbers at oyster reefs, while relatively younger and smaller individuals inhabit living shorelines.
In Mosquito Lagoon’s restored oyster reefs and stabilized shorelines, Loch & Cook [
23] found gray snapper size to be equal to, and at times larger than, those collected from positive or negative control sites. Current model outputs using the same dataset support this finding, with gray snapper being relatively larger at oyster reefs restored in 2017 compared to control living shorelines, suggesting benthic habitat restoration has a positive relationship with gray snapper size. This finding is supported by Loch et al. [
22], which found gray snapper abundance was generally higher at restored (2017) oyster reef sites compared to positive and negative control sites. Combined with current results indicating gray snapper size is slightly larger at restored (2017 and 2018) and live oyster reefs than at dead oyster reefs, the general presence of oyster reefs may aid growth by providing foraging and sheltering opportunities for local gray snapper as well as prey fish and macroinvertebrates [
18,
22,
23,
24,
26]. Gray snapper are a generalist species [
59,
74], and current results suggest habitat quality and availability influences gray snapper size. Thus, location and habitat type could be used to predict gray snapper size in Mosquito Lagoon.
Based on the study findings, environmental conditions in Mosquito Lagoon appear to influence gray snapper size. Across all scales, lagged salinity and location or site status were correlated with gray snapper size. Oyster reef and living shoreline habitats in Mosquito Lagoon are relatively close in proximity (i.e., within kilometers), which may explain why similar-sized juvenile gray snapper were found at both habitat types. On the Mosquito Lagoon and oyster reef scale, oyster reefs restored in 2017 were found to have larger gray snapper than control living shorelines, indicating there is some benefit to gray snapper provided by restoration projects in Mosquito Lagoon [
23,
24]. Further analyses should be conducted to investigate if living shoreline stabilization provides additional size-appropriate ecological services (i.e., prey and shelter) for recently settled gray snapper. Local water properties should also be monitored to investigate potential changes in their correlation with gray snapper size and survivorship, along with environmental conditions and potential lags [
75,
76]. Of all the water property measurements taken during fish sampling, lagged salinity was found to be positively related to gray snapper growth. Overall, further post-restoration monitoring of potential changes in ecological community and habitat conditions/site status should be conducted to analyze the long-term impacts of oyster reef/living shoreline restoration on the body size of Mosquito Lagoon’s gray snapper.
Local water properties and their effects on Mosquito Lagoon’s oyster reef and living shoreline habitats should be monitored to assess potential shifts in the relationship between gray snapper size and habitat properties. Additionally, habitat-specific variables such as vegetative coverage, oyster reef height (as a proxy for structural complexity), and density should be recorded alongside gray snapper size to determine whether both are influenced by environmental properties at the time of sampling (non-lagged) or by shifts in environmental properties prior to sampling (lagged). Due to insufficient data on habitat-type specific variables in this study, consistent sampling of these variables is recommended for future studies to bolster investigations of gray snapper and their surrounding environment in Mosquito Lagoon. Gradual shifts in environmental conditions due to climate change could potentially reduce gray snapper size by affecting available habitat and prey communities [
26,
75]. For example, salinity in Mosquito Lagoon has risen over the past two and a half decades, from a mean of 30 ± 5 ppt (range: 31–38 ppt) in the late 1990s to a mean of 34 ± 4 ppt (range: 24–45 ppt) as of the late 2010s [
26]. These increases have been gradual, suggesting changes in habitat coverage for oyster reef and living shoreline habitats may occur over decadal time scales. For local oyster populations, salinity levels > 35 ppt could increase oyster mortality, and potentially decrease survival rates for local populations [
77]. For living shorelines, increasing salinity levels (>30–35 ppt) inhibit the growth and development of mangroves (e.g.,
R. mangle,
L. racemosa) and reduce germination rates for smooth cordgrass (
S. alterniflora) [
78,
79,
80]. Results in the current study indicate larger juvenile gray snapper are more common in oyster reef habitats, suggesting a potential reduction in gray snapper length-at-age could occur via the reduction in size-appropriate foraging and sheltering habitat [
4]. Thus, management in Mosquito Lagoon should continue to monitor the size structure of local gray snapper populations and investigate how they adapt to gradual changes in habitat quality and availability. Ensuring the continued use of local habitats and prey communities by gray snapper will help to optimize their recruitment to adult populations offshore.
Local management should consider long-term monitoring for potential changes in gray snapper size and environmental factors in Mosquito Lagoon. Outside of Loch & Cook [
23,
24], past research in Mosquito Lagoon has yet to explore the effects of habitat restoration on gray snapper size [
22,
24]. However, new findings from the current study could provide additional guidance for natural resource managers working in similar inshore nursery environments, and help identify environmental factors conducive to optimal habitat quality and gray snapper growth. Specifically, these results could inform the design of future size- and location-specific structures that promote early gray snapper survivorship both within and beyond Mosquito Lagoon. In the current study, model outputs found oyster reefs restored in 2017 had a positive correlation with gray snapper size, indicating restoration efforts in Mosquito Lagoon have found some success in supporting relatively larger juvenile gray snapper. However, given no effect on gray snapper size was observed from stabilized living shorelines and oyster reefs restored in 2018, it is possible that additional time or long-term monitoring is needed for these restoration projects to fully develop before their influence is detectable in growth models. Specifically, continued monitoring of local fish and macroinvertebrate community responses to restored oyster reefs or stabilized shorelines is recommended to ensure the long-term stability of Mosquito Lagoon’s ecological functions, which ultimately supports the survival of juvenile gray snapper and their recruitment into adult populations. Recent findings from Smith et al. [
81] and Smith & Castorani [
82] found restored oyster reefs need to be established for 6 to 8 years before local fish and macroinvertebrate abundance can consistently match quantities found in natural oyster reefs. For stabilized living shorelines, Gittman et al. [
37] and Guthrie et al. [
83] found nekton abundance from stabilized living shorelines matched that of natural (control) living shorelines after 2 to 8 years post-restoration. For future restoration studies, monitoring fish and macroinvertebrate assemblages on a quarterly or semi-annual basis for 7–8 years following habitat restoration is recommended. This sampling frequency should capture initial changes to the biotic community, and any chance disturbance events (e.g., hurricanes or algal blooms) that may influence the biotic community. Thereafter, annual monitoring may suffice to quantify long-term population trends and restoration outcomes.
Current results show no correlation between gray snapper size and prey richness/abundance in Mosquito Lagoon. Prey availability affects a fish’s size and behavior [
84], and prey availability and feeding opportunities influence metabolic rates, which in turn influence digestion, growth, and locomotion [
85], suggesting fish will seek habitats with higher prey availability to maintain individual fitness and reach optimal length-at-age. At the species-specific level, studies have shown gray snapper length-at-age declines when prey availability is low, prompting them to switch prey based on location and prey quality [
20,
59,
77,
86]. In the current study, ~83% of the gray snapper sampled had consumed prey, indicating a majority of gray snapper in Mosquito Lagoon had access to prey prior to capture. Thus, continued monitoring of Mosquito Lagoon’s oyster reef and living shoreline habitats can help identify habitat-specific changes in species diversity and composition of local prey fish or macroinvertebrate communities that may influence size structure of relatively higher trophic level gray snapper [
23,
24,
33]. Specifically, a negative correlation between gray snapper size and nekton abundance could indicate a degradation of oyster reef and living shoreline sites, and warrant further assessment of local site status and their environmental conditions (e.g., changes in salinity). With climate change driving persistent shifts in environmental conditions and local prey communities [
26,
31], gray snapper could face metabolic challenges in the future.
Biometric and related age and growth analyses provide greater understanding of gray snapper life history. Therefore, fishery managers should continue assessing size-specific habitat requirements and environmental conditions critical to the growth and survivorship of gray snapper in Mosquito Lagoon. Previous studies have found habitat restoration in Mosquito Lagoon improves foraging/sheltering opportunities for local gray snapper [
22,
23,
24]. As habitat quality is related to environmental conditions [
77,
80], continued monitoring of abiotic conditions could inform resource managers about regional opportunities to increase gray snapper landings by implementing strategies to better support the recruitment of juveniles into adult stocks offshore. Wuenschel et al. [
87] found adult gray snapper distributions corresponded with the survival of nearby juvenile gray snapper populations, however gray snapper age and length data have yet to be quantified from inshore and offshore habitats near Mosquito Lagoon [
21,
63]. Thus, gray snapper in nearshore and offshore water near Mosquito Lagoon should be monitored to quantify connectivity among benthic habitats, and help establish spatial boundaries for potential future stock assessments.