Remote Sensing Reveals Multi-Dimensional Functional Changes in Fish Assemblages Under Eutrophication and Hydrological Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Fish Sampling
2.3. Measurements of Environmental Properties
2.4. Statistical Analysis
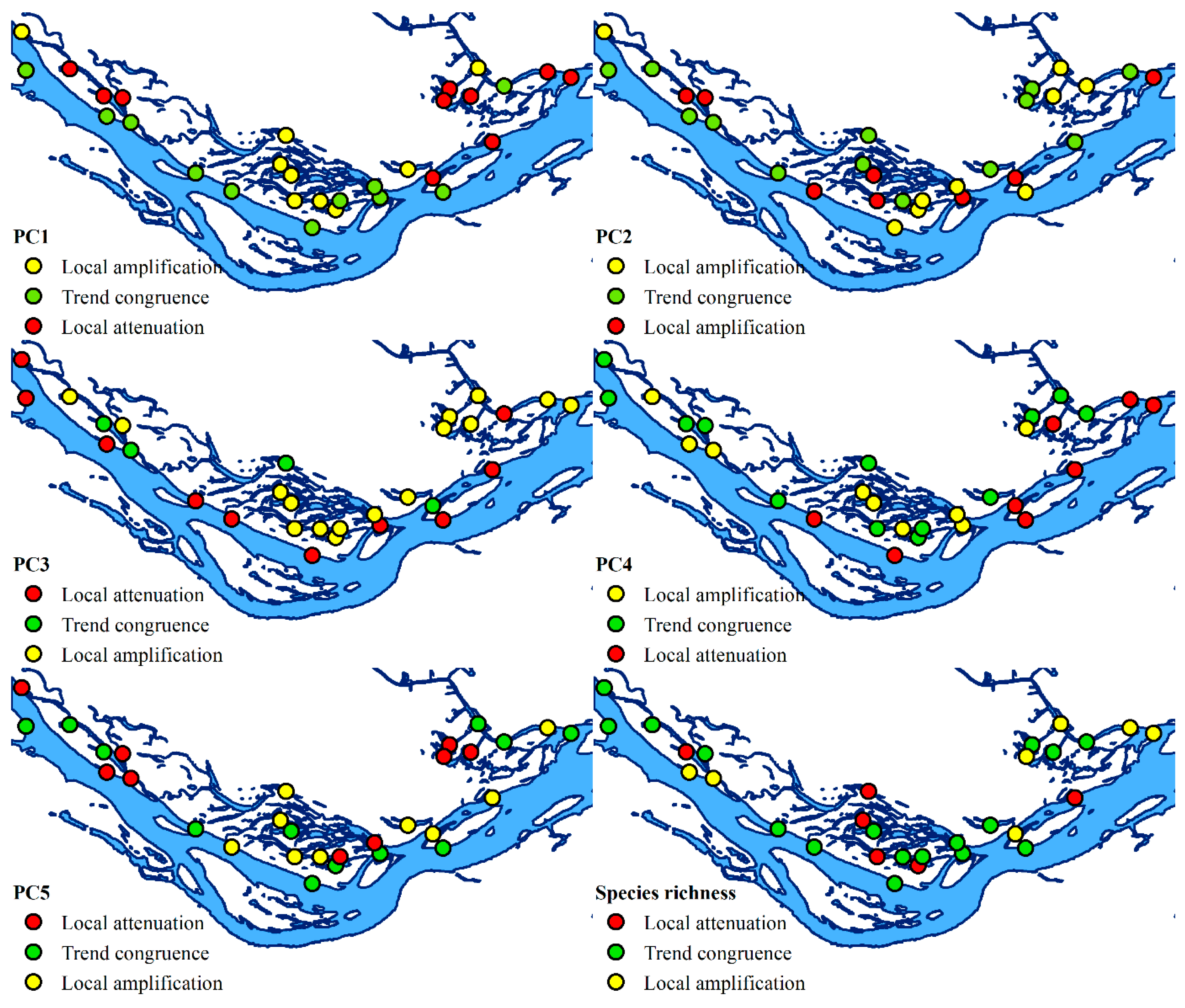
3. Results
3.1. Dynamics of Water Quality Indicators
3.2. Correlation Between Taxonomic and Functional Diversity Indices and Species Richness
3.3. Principal Component Analysis of Diversity Indices
3.4. Interpretation of Principal Components About Functional Traits
3.5. Temporal Trends and the Influence of Environmental Factors on Functional Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerr, M.R.; Ordonez, A.; Riede, F.; Atkinson, J.; Pearce, E.A.; Sykut, M.; Trepel, J.; Svenning, J.C. Widespread Ecological Novelty across the Terrestrial Biosphere. Nat. Ecol. Evol. 2025, 9, 589–598. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine Flood Plains: Present State and Future Trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Larsen, S.; Karaus, U.; Claret, C.; Sporka, F.; Hamerlík, L.; Tockner, K. Flooding and Hydrologic Connectivity Modulate Community Assembly in a Dynamic River-Floodplain Ecosystem. PLoS ONE 2019, 14, e0213227. [Google Scholar] [CrossRef]
- EEA Report. Floodplains: A Natural System to Preserve and Restore; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Tockner, K.; Pusch, M.; Borchardt, D.; Lorang, M.S. Multiple Stressors in Coupled River-Floodplain Ecosystems. Freshw. Biol. 2010, 55, 135–151. [Google Scholar] [CrossRef]
- Opperman, J.J.; Luster, R.; McKenney, B.A.; Roberts, M.; Meadows, A.W. Ecologically Functional Floodplains: Connectivity, Flow Regime, and Scale1. JAWRA J. Am. Water Resour. Assoc. 2010, 46, 211–226. [Google Scholar] [CrossRef]
- Talbot, C.J.; Bennett, E.M.; Cassell, K.; Hanes, D.M.; Minor, E.C.; Paerl, H.; Raymond, P.A.; Vargas, R.; Vidon, P.G.; Wollheim, W.; et al. The Impact of Flooding on Aquatic Ecosystem Services. Biogeochemistry 2018, 141, 439–461. [Google Scholar] [CrossRef]
- Poff, N.L.; Zimmerman, J.K.H. Ecological Responses to Altered Flow Regimes: A Literature Review to Inform the Science and Management of Environmental Flows. Freshw. Biol. 2010, 55, 194–205. [Google Scholar] [CrossRef]
- Zhang, S.; Zhan, A.; Zhao, J.; Yao, M. Metropolitan Pressures: Significant Biodiversity Declines and Strong Filtering of Functional Traits in Fish Assemblages. Sci. Total Environ. 2024, 944, 173885. [Google Scholar] [CrossRef]
- Teichert, N.; Lepage, M.; Lobry, J. Beyond Classic Ecological Assessment: The Use of Functional Indices to Indicate Fish Assemblages Sensitivity to Human Disturbance in Estuaries. Sci. Total Environ. 2018, 639, 465–475. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M. Fishes as Indicators of Environmental and Ecological Changes within Estuaries: A Review of Progress and Some Suggestions for the Future. J. Fish Biol. 2002, 61, 229–250. [Google Scholar] [CrossRef]
- Zymaroieva, A.; Bondarev, D.; Kunakh, O.; Svenning, J.C.; Zhukov, O. Young-of-the-Year Fish as Bioindicators of Eutrophication and Temperature Regime of Water Bodies. Environ. Monit. Assess. 2024, 196, 161. [Google Scholar] [CrossRef]
- Degen, R.; Aune, M.; Bluhm, B.A.; Cassidy, C.; Kędra, M.; Kraan, C.; Vandepitte, L.; Włodarska-Kowalczuk, M.; Zhulay, I.; Albano, P.G.; et al. Trait-Based Approaches in Rapidly Changing Ecosystems: A Roadmap to the Future Polar Oceans. Ecol. Indic. 2018, 91, 722–736. [Google Scholar] [CrossRef]
- Vandewalle, M.; de Bello, F.; Berg, M.P.; Bolger, T.; Dolédec, S.; Dubs, F.; Feld, C.K.; Harrington, R.; Harrison, P.A.; Lavorel, S.; et al. Functional Traits as Indicators of Biodiversity Response to Land Use Changes across Ecosystems and Organisms. Biodivers. Conserv. 2010, 19, 2921–2947. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, F.; Lin, Z.; Yuan, L.; Yao, H.; Duan, G.; Liu, Y.; Liu, Y.; Shi, H.; Wen, Z. Using the Response–Effect Trait Framework to Disentangle the Effects of Environmental Change on the Ecosystem Services. J. Plant Ecol. 2024, 17, rtae024. [Google Scholar] [CrossRef]
- Barabás, G.; Parent, C.; Kraemer, A.; Van de Perre, F.; De Laender, F. The Evolution of Trait Variance Creates a Tension between Species Diversity and Functional Diversity. Nat. Commun. 2022, 13, 2521. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Bastazini, V.A.G.; Griffin, J.N. Choosing and Using Multiple Traits in Functional Diversity Research. Environ. Conserv. 2015, 42, 104–107. [Google Scholar] [CrossRef]
- de Bello, F.; Lavorel, S.; Hallett, L.M.; Valencia, E.; Garnier, E.; Roscher, C.; Conti, L.; Galland, T.; Goberna, M.; Májeková, M.; et al. Functional Trait Effects on Ecosystem Stability: Assembling the Jigsaw Puzzle. Trends Ecol. Evol. 2021, 36, 822–836. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Göthe, E.; Larsen, S.E.; O’Hare, M.; Birk, S.; Riis, T.; Friberg, N. Plant Trait Characteristics Vary with Size and Eutrophication in European Lowland Streams. J. Appl. Ecol. 2015, 52, 1617–1628. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiong, Y.; Zhang, J.; Liu, B.; Chen, T.; Liu, S.; Dang, C.; Xu, W.D.; Ahmad, H.A.; Liu, T. Eutrophication Impacts the Distribution and Functional Traits of Viral Communities in Lakes. Sci. Total Environ. 2024, 946, 174339. [Google Scholar] [CrossRef]
- Machado, K.B.; Bini, L.M.; Melo, A.S.; de Andrade, A.T.; de Almeida, M.F.; Carvalho, P.; Teresa, F.B.; Roque, F.d.O.; Bortolini, J.C.; Padial, A.A.; et al. Functional and Taxonomic Diversities Are Better Early Indicators of Eutrophication than Composition of Freshwater Phytoplankton. Hydrobiologia 2023, 850, 1393–1411. [Google Scholar] [CrossRef]
- Murray, N.J.; Keith, D.A.; Bland, L.M.; Ferrari, R.; Lyons, M.B.; Lucas, R.; Pettorelli, N.; Nicholson, E. The Role of Satellite Remote Sensing in Structured Ecosystem Risk Assessments. Sci. Total Environ. 2018, 619–620, 249–257. [Google Scholar] [CrossRef]
- Rocchini, D.; Boyd, D.S.; Féret, J.B.; Foody, G.M.; He, K.S.; Lausch, A.; Nagendra, H.; Wegmann, M.; Pettorelli, N. Satellite Remote Sensing to Monitor Species Diversity: Potential and Pitfalls. Remote Sens. Ecol. Conserv. 2016, 2, 25–36. [Google Scholar] [CrossRef]
- Roni, P.; Hall, J.E.; Drenner, S.M.; Arterburn, D. Monitoring the Effectiveness of Floodplain Habitat Restoration: A Review of Methods and Recommendations for Future Monitoring. Wiley Interdiscip. Rev. Water 2019, 6, e1355. [Google Scholar] [CrossRef]
- Pettorelli, N.; Laurance, W.F.; O’Brien, T.G.; Wegmann, M.; Nagendra, H.; Turner, W. Satellite Remote Sensing for Applied Ecologists: Opportunities and Challenges. J. Appl. Ecol. 2014, 51, 839–848. [Google Scholar] [CrossRef]
- Sims, N.C.; Colloff, M.J. Remote Sensing of Vegetation Responses to Flooding of a Semi-Arid Floodplain: Implications for Monitoring Ecological Effects of Environmental Flows. Ecol. Indic. 2012, 18, 387–391. [Google Scholar] [CrossRef]
- Zhukov, O.; Kunakh, O.; Bondarev, D.; Chubchenko, Y. Extraction of Macrophyte Community Spatial Variation Allows to Adapt the Macrophyte Biological Index for Rivers to the Conditions of the Middle Dnipro River. Limnologica 2022, 97, 126036. [Google Scholar] [CrossRef]
- Fedonenko, E.V.; Kunakh, O.M.; Chubchenko, Y.A.; Zhukov, O.V. Application of Remote Sensing Data for Monitoring Eutrophication of Floodplain Water Bodies. Biosyst. Divers. 2022, 30, 179–190. [Google Scholar] [CrossRef]
- Arsan, О.M.; Davydov, O.Y.; Dyachenko, T.M. Methods of Hydroecological Research of Surface Waters; Romanenko, V.D., Ed.; Logos: Kyiv, Ukraine, 2006. [Google Scholar]
- Zymaroieva, A.; Bondarev, D.; Kunakh, O.; Svenning, J.-C.; Zhukov, O. Which Fish Benefit from the Combined Influence of Eutrophication and Warming in the Dnipro River (Ukraine)? Fishes 2022, 8, 14. [Google Scholar] [CrossRef]
- Žiliukas, V.; Žiliukienė, V.; Repečka, R. Temporal Variation in Juvenile Fish Communities of Kaunas Reservoir Littoral Zone, Lithuania. Cent. Eur. J. Biol. 2012, 7, 858–866. [Google Scholar] [CrossRef]
- Kratochvíl, M.; Čech, M.; Vašek, M.; Kubečka, J.; Hejzlar, J.; Matěna, J.; Peterka, J.; Macháček, J.; Seďa, J. Diel Vertical Migrations of Age 0+ Percids in a Shallow, Well-Mixed Reservoir. J. Limnol. 2010, 69, 305. [Google Scholar] [CrossRef]
- Paradis, Y.; Mingelbier, M.; Brodeur, P.; Magnan, P. Comparisons of Catch and Precision of Pop Nets, Push Nets, and Seines for Sampling Larval and Juvenile Yellow Perch. N. Am. J. Fish Manag. 2008, 28, 1554–1562. [Google Scholar] [CrossRef]
- Pierce, C.L.; Rasmussen, J.B.; Leggett, W.C. Sampling Littoral Fish with a Seine: Corrections for Variable Capture Efficiency. Can. J. Fish. Aquat. Sci. 1990, 47, 1004–1010. [Google Scholar] [CrossRef]
- Treasurer, J.W. Sampling Larval and Juvenile Fish Populations in Freshwater. Aquac. Res. 1978, 9, 6–17. [Google Scholar] [CrossRef]
- Aparicio, E.; Carmona-Catot, G.; Moyle, P.B.; García-Berthou, E. Development and Evaluation of a Fish-Based Index to Assess Biological Integrity of Mediterranean Streams. Aquat. Conserv. 2011, 21, 324–337. [Google Scholar] [CrossRef]
- Northcote, T. Migratory Behaviour of Fish and Its Significance to Movement through Riverine Fish Passage Facilities. In Fish Migration and Fish Bypasses; Jungwirth, M., Schmultz, S., Weiss, S., Eds.; Fishing News Books: Vienna, Austria, 1998; pp. 3–18. [Google Scholar]
- Hondorp, D.W.; Breitburg, D.L.; Davias, L.A. Eutrophication and Fisheries: Separating the Effects of Nitrogen Loads and Hypoxia on the Pelagic-to-Demersal Ratio and Other Measures of Landings Composition. Mar. Coast. Fish. 2010, 2, 339–361. [Google Scholar] [CrossRef]
- Karr, J. Assessment of Biotic Integrity Using Fish Communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Pont, D.; Hugueny, B.; Beier, U.; Goffaux, D.; Melcher, A.; Noble, R.; Rogers, C.; Roset, N.; Schmutz, S. Assessing River Biotic Condition at a Continental Scale: A European Approach Using Functional Metrics and Fish Assemblages. J. Appl. Ecol. 2006, 43, 70–80. [Google Scholar] [CrossRef]
- Powers, S.; Peterson, C.; Christian, R.; Sullivan, E.; Powers, M.; Bishop, M.; Buzzelli, C. Effects of Eutrophication on Bottom Habitat and Prey Resources of Demersal Fishes. Mar. Ecol. Prog. Ser. 2005, 302, 233–243. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Jeppesen, E.; Zanden, M.J.V.; Schierup, H.; Christoffersen, K.; Lodge, D.M. From Greenland to Green Lakes: Cultural Eutrophication and the Loss of Benthic Pathways in Lakes. Limnol. Oceanogr. 2003, 48, 1408–1418. [Google Scholar] [CrossRef]
- Balon, E.K. Reproductive Guilds of Fishes: A Proposal and Definition. J. Fish. Res. Board Can. 1975, 32, 821–864. [Google Scholar] [CrossRef]
- Berkman, H.E.; Rabeni, C.F. Effect of Siltation on Stream Fish Communities. Environ Biol. Fishes 1987, 18, 285–294. [Google Scholar] [CrossRef]
- Kryzhanovsky, S.G. Ecological Groups of Fishes and Principles of Their Development. Russ. Fed. Res. Inst. Fish. Oceanogr. VNIRO 1948, 27, 1–114. [Google Scholar]
- Kvach, Y.; Kutsokon, Y. The Non-Indigenous Fishes in the Fauna of Ukraine: A Potentia Ad Actum. Bioinvasions. Rec. 2017, 6, 269–279. [Google Scholar] [CrossRef]
- Slastenenko, E. The Fishes of the Black Sea Basin; The General Directorate of Meat and Fish Publications: Istanbul, Turley, 1956. [Google Scholar]
- Tammi, J.; Lappalainen, A.; Mannio, J.; Rask, M.; Vuorenmaa, J. Effects of Eutrophication on Fish and Fisheries in Finnish Lakes: A Survey Based on Random Sampling. Fish Manag. Ecol. 1999, 6, 173–186. [Google Scholar] [CrossRef]
- Zambrano, L.; Perrow, M.R.; Sayer, C.D.; Tomlinson, M.L.; Davidson, T.A. Relationships between Fish Feeding Guild and Trophic Structure in English Lowland Shallow Lakes Subject to Anthropogenic Influence: Implications for Lake Restoration. Aquat. Ecol. 2006, 40, 391–405. [Google Scholar] [CrossRef]
- Santos, R.; Poulet, N.; Besnard, A. Life-history Traits Correlate with Temporal Trends in Freshwater Fish Populations for Common European Species. Freshw. Biol. 2021, 66, 317–331. [Google Scholar] [CrossRef]
- Audzijonyte, A.; Richards, S.A.; Stuart-Smith, R.D.; Pecl, G.; Edgar, G.J.; Barrett, N.S.; Payne, N.; Blanchard, J.L. Fish Body Sizes Change with Temperature but Not All Species Shrink with Warming. Nat. Ecol. Evol. 2020, 4, 809–814. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D. Functional Organization of Stream Fish Assemblages in Relation to Hydrological Variability. Ecology 1995, 76, 606–627. [Google Scholar] [CrossRef]
- Shevchenko, P.G.; Shcherbukha, A.Y.; Pilipenko, Y.V.; Martsenyuk, N.O.; Khalturin, M.B.; Cherednichenko, I.S. Identification Key of Fish of Continental Water Bodies and Streams of Ukraine; Oldi Plus: Kherson, Ukraine, 2020. [Google Scholar]
- Schmidt-Kloiber, A.; Hering, D. Www.Freshwaterecology.Info—An Online Tool That Unifies, Standardises and Codifies More than 20,000 European Freshwater Organisms and Their Ecological Preferences. Ecol. Indic. 2015, 53, 271–282. [Google Scholar] [CrossRef]
- Free, G.; Bresciani, M.; Pinardi, M.; Ghirardi, N.; Luciani, G.; Caroni, R.; Giardino, C. Detecting Climate Driven Changes in Chlorophyll-a in Deep Subalpine Lakes Using Long Term Satellite Data. Water 2021, 13, 866. [Google Scholar] [CrossRef]
- Geological Survey (U.S.). EROS Data Center. Earth Explorer. Available online: https://earthexplorer.usgs.gov/ (accessed on 25 June 2025).
- Vanhellemont, Q. Adaptation of the Dark Spectrum Fitting Atmospheric Correction for Aquatic Applications of the Landsat and Sentinel-2 Archives. Remote Sens. Env. 2019, 225, 175–192. [Google Scholar] [CrossRef]
- Alawadi, F. Detection of Surface Algal Blooms Using the Newly Developed Algorithm Surface Algal Bloom Index (SABI). In Proceedings Volume 7825, Remote Sensing of the Ocean, Sea Ice, and Large Water Regions 2010; Bostater, C.R., Jr., Mertikas, S.P., Neyt, X., Velez-Reyes, M., Eds.; SPIE Remote Sensing: Toulouse, France, 2010; p. 782506. [Google Scholar]
- Mansaray, A.S.; Dzialowski, A.R.; Martin, M.E.; Wagner, K.L.; Gholizadeh, H.; Stoodley, S.H. Comparing PlanetScope to Landsat-8 and Sentinel-2 for Sensing Water Quality in Reservoirs in Agricultural Watersheds. Remote Sens. 2021, 13, 1847. [Google Scholar] [CrossRef]
- Yin, Z.; Li, J.; Liu, Y.; Zhang, F.; Wang, S.; Xie, Y.; Gao, M. Decline of Suspended Particulate Matter Concentrations in Lake Taihu from 1984 to 2020: Observations from Landsat TM and OLI. Opt. Express 2022, 30, 22572. [Google Scholar] [CrossRef]
- Oyama, Y.; Matsushita, B.; Fukushima, T. Distinguishing Surface Cyanobacterial Blooms and Aquatic Macrophytes Using Landsat/TM and ETM + Shortwave Infrared Bands. Remote Sens. Environ. 2015, 157, 35–47. [Google Scholar] [CrossRef]
- Lacaux, J.P.; Tourre, Y.M.; Vignolles, C.; Ndione, J.A.; Lafaye, M. Classification of Ponds from High-Spatial Resolution Remote Sensing: Application to Rift Valley Fever Epidemics in Senegal. Remote Sens. Environ. 2007, 106, 66–74. [Google Scholar] [CrossRef]
- Martins, S.; Chokmani, K.; Alcântara, E.; Ogashawara, I.; El-Alem, A. Mapping the Coloured Dissolved Organic Matter Absorption Coefficient in a Eutrophic Reservoir Using Remotely Sensed Images. Inland Waters 2018, 8, 488–504. [Google Scholar] [CrossRef]
- Song, K.; Ma, J.; Wen, Z.; Fang, C.; Shang, Y.; Zhao, Y.; Wang, M.; Du, J. Remote Estimation of Kd (PAR) Using MODIS and Landsat Imagery for Turbid Inland Waters in Northeast China. ISPRS J. Photogramm. Remote Sens. 2017, 123, 159–172. [Google Scholar] [CrossRef]
- Chamberlain, S. Rnoaa: “NOAA” Weather Data from R.; R Package Version 1.2.0; Data Camp: New York, NY, USA, 2020. [Google Scholar]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional Richness, Functional Evenness and Functional Divergence: The Primary Components of Functional Diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Legendre, P.; Galzin, R.; Harmelin-Vivien, M.L. Relating Behavior to Habitat: Solutions to Thefourth-Corner Problem. Ecology 1997, 78, 547–562. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Bilgili, M.; Tokmakci, M. Climate Change and Trends in Europe and Globally over the Period 1970–2023. Phys. Chem. Earth Parts A/B/C 2025, 139, 103928. [Google Scholar] [CrossRef]
- Nimma, D.; Devi, O.R.; Laishram, B.; Ramesh, J.V.N.; Boddupalli, S.; Ayyasamy, R.; Tirth, V.; Arabil, A. Implications of Climate Change on Freshwater Ecosystems and Their Biodiversity. Desalination Water Treat. 2025, 321, 100889. [Google Scholar] [CrossRef]
- Palmer, M.A.; Lettenmaier, D.P.; Poff, N.L.; Postel, S.L.; Richter, B.; Warner, R. Climate Change and River Ecosystems: Protection and Adaptation Options. Environ. Manag. 2009, 44, 1053–1068. [Google Scholar] [CrossRef]
- Loures, R.C.; Pompeu, P.S. Temporal Changes in Fish Diversity in Lotic and Lentic Environments along a Reservoir Cascade. Freshw. Biol. 2019, 64, 1806–1820. [Google Scholar] [CrossRef]
- El Aoula, R.; Mhammdi, N.; Dezileau, L.; Mahe, G.; Kolker, A.S. Fluvial Sediment Transport Degradation after Dam Construction in North Africa. J. Afr. Earth Sci. 2021, 182, 104255. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Zhou, X.; Huang, J.; Dong, X.; Zhu, S.; Shen, Y. Fish Community Diversity and Spatiotemporal Dynamics in the Downstream of the Fujiang River Based on Environmental DNA. Fishes 2025, 10, 43. [Google Scholar] [CrossRef]
- Marenkov, O.N. Transformation of Dnipro (Zaporizhia) Reservoir’s Fish Fauna: Retrospective Review and Current Status. Ecol. Noospherology 2016, 27, 70–76. [Google Scholar] [CrossRef][Green Version]
- Ganassin, M.J.M.; Muñoz-Mas, R.; de Oliveira, F.J.M.; Muniz, C.M.; dos Santos, N.C.L.; García-Berthou, E.; Gomes, L.C. Effects of Reservoir Cascades on Diversity, Distribution, and Abundance of Fish Assemblages in Three Neotropical Basins. Sci. Total Environ. 2021, 778, 146246. [Google Scholar] [CrossRef]
- Feng, K.; Deng, W.; Zhang, Y.; Tao, K.; Yuan, J.; Liu, J.; Li, Z.; Lek, S.; Wang, Q.; Hugueny, B. Eutrophication Induces Functional Homogenization and Traits Filtering in Chinese Lacustrine Fish Communities. Sci. Total Environ. 2023, 857, 159651. [Google Scholar] [CrossRef]
- Ricotta, C.; Bacaro, G.; Maccherini, S.; Pavoine, S. Functional Imbalance Not Functional Evenness Is the Third Component of Community Structure. Ecol. Indic. 2022, 140, 109035. [Google Scholar] [CrossRef]
- Dehling, D.M.; Stouffer, D.B. Bringing the Eltonian Niche into Functional Diversity. Oikos 2018, 127, 1711–1723. [Google Scholar] [CrossRef]
- Mao, Z.; Gu, X.; Cao, Y.; Luo, J.; Zeng, Q.; Chen, H.; Jeppesen, E. How Does Fish Functional Diversity Respond to Environmental Changes in Two Large Shallow Lakes? Sci. Total Environ. 2021, 753, 142158. [Google Scholar] [CrossRef]
- van Treeck, R.; Van Wichelen, J.; Wolter, C. Fish Species Sensitivity Classification for Environmental Impact Assessment, Conservation and Restoration Planning. Sci. Total Environ. 2020, 708, 135173. [Google Scholar] [CrossRef]
- Riza, M.; Ehsan, M.N.; Pervez, M.N.; Khyum, M.M.O.; Cai, Y.; Naddeo, V. Control of Eutrophication in Aquatic Ecosystems by Sustainable Dredging: Effectiveness, Environmental Impacts, and Implications. Case Stud. Chem. Environ. Eng. 2023, 7, 100297. [Google Scholar] [CrossRef]
- Hitchman, S.M.; Mather, M.E.; Smith, J.M.; Fencl, J.S. Identifying Keystone Habitats with a Mosaic Approach Can Improve Biodiversity Conservation in Disturbed Ecosystems. Glob. Chang. Biol. 2018, 24, 308–321. [Google Scholar]
- Soares, M.G.M.; Menezes, N.A.; Junk, W.J. Adaptations of Fish Species to Oxygen Depletion in a Central Amazonian Floodplain Lake. Hydrobiologia 2006, 568, 353–367. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of Plant Diversity to Ecosystems: Immediate, Filter and Founder Effects. J. Ecol. 1998, 86, 902–910. [Google Scholar]
- Mouillot, D.; Villéger, S.; Parravicini, V.; Kulbicki, M.; Arias-González, J.E.; Bender, M.; Chabanet, P.; Floeter, S.R.; Friedlander, A.; Vigliola, L.; et al. Functional Over-Redundancy and High Functional Vulnerability in Global Fish Faunas on Tropical Reefs. Proc. Natl. Acad. Sci. USA 2014, 111, 13757–13762. [Google Scholar] [CrossRef]
- Teichert, N.; Lepage, M.; Sagouis, A.; Borja, A.; Chust, G.; Ferreira, M.T.; Pasquaud, S.; Schinegger, R.; Segurado, P.; Argillier, C. Functional Redundancy and Sensitivity of Fish Assemblages in European Rivers, Lakes and Estuarine Ecosystems. Sci. Rep. 2017, 7, 17611. [Google Scholar] [CrossRef]
- Stefani, F.; Fasola, E.; Marziali, L.; Tirozzi, P.; Schiavon, A.; Bocchi, S.; Gomarasca, S. Response of Functional Diversity of Fish Communities to Habitat Alterations in Small Lowland Rivers. Biodivers. Conserv. 2024, 33, 1439–1458. [Google Scholar] [CrossRef]
- Zeng, Z.; Cheung, W.W.L.; Lai, H.; Yi, H.; Bi, S.; Li, H.; Chen, X.; Su, Y.; Liu, X.; Chen, Q.; et al. Species and Functional Dynamics of the Demersal Fish Community and Responses to Disturbances in the Pearl River Estuary. Front. Mar. Sci. 2022, 9, 921595. [Google Scholar] [CrossRef]
- Novitskyi, R.; Hapich, H.; Maksymenko, M.; Kutishchev, P.; Gasso, V. Losses in Fishery Ecosystem Services of the Dnipro River Delta and the Kakhovske Reservoir Area Caused by Military Actions in Ukraine. Front. Environ. Sci. 2024, 12, 1301435. [Google Scholar] [CrossRef]
- Danet, A.; Giam, X.; Olden, J.D.; Comte, L. Past and Recent Anthropogenic Pressures Drive Rapid Changes in Riverine Fish Communities. Nat. Ecol. Evol. 2024, 8, 442–453. [Google Scholar] [CrossRef]
- de Sousa Gomes-Gonçalves, R.; Silva de Aguiar, F.; Costa de Azevedo, M.C.; Araújo, F.G. Functional Stability despite Anthropogenic Influences on the Ichthyofauna of a Tropical Bay. Mar. Environ. Res. 2020, 159, 105016. [Google Scholar] [CrossRef]
- Ruiz-Navarro, A.; Jackson, M.C.; Almeida, D.; Britton, J.R. Influence of Nutrient Enrichment on the Growth, Recruitment and Trophic Ecology of a Highly Invasive Freshwater Fish. Aquat. Ecol. 2020, 54, 1029–1039. [Google Scholar] [CrossRef]
- Akimov, I.A.; Radchenko, V. Red Data Book of Ukraine. Animals.; Globalconsulting: Kyiv, Ukraine, 2009. [Google Scholar]
- Pakhomov, O.E.; Gasso, V.Y.; Goloborodko, K.K.; Polyakov, M.V.; Gritsan, Y.I.; Bulakhov, V.L.; Brigadirenko, V.V.; Klyuchko, Z.F.; Mezhherin, S.V.; Novitsky, R.O.; et al. Red Book of Dnipropetrovska Oblast. Animal World; Dnipro National University Press: Dnipro, Ukraine, 2011. [Google Scholar]
- Bondarev, D.; Fedushko, M.; Hubanova, N.; Novitskiy, R.; Kunakh, O.; Zhukov, O. Temporal Dynamics of the Fish Communities in the Reservoir: The Influence of Eutrophication on Ecological Guilds Structure. Ichthyol. Res. 2023, 70, 21–39. [Google Scholar] [CrossRef]

| Variable | PC 1, λ = 6.0, Explained Variance = 54.4% | PC 2, λ = 1.4, Explained Variance = 12.7% | PC 3, λ = 1.1, Explained Variance = 9.2% |
|---|---|---|---|
| NDVI | 0.96 | − | − |
| FAI | 0.93 | − | − |
| SABI | 0.94 | − | − |
| Chl-a | 0.94 | − | − |
| SPM | −0.74 | − | − |
| FAI | −0.93 | − | 0.24 |
| NDTI | − | 0.51 | − |
| CDOM | 0.89 | − | − |
| Kd(PAR) | 0.40 | 0.72 | − |
| TURBIDITY | − | 0.58 | 0.77 |
| Maximal temperature | − | −0.47 | 0.50 |
| Diversity Index | Parameters of Linear Regression Analysis of Functional Diversity Indices Regarding Species Richness | Principal Components | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | K (Regression Slope) | p-Value | PC1, λ = 4.1, 24.1% | PC2, λ = 3.2, 19.0% | PC3, λ = 2.3, 13.6% | PC4, λ = 1.7, 10.0% | PC5, λ = 1.4, 8.3% | |
| Species diversity indices | ||||||||
| Species richness | − | − | − | − | − | − | − | − |
| Gini–Simpson (Gsimpson) | 0.36 | 0.11 | <0.001 | 0.39 | − | − | −0.36 | 0.15 |
| Functional diversity indices | ||||||||
| Functional mean pairwise distance (Fmpd) | 0.01 | 0.01 | 0.050 | −0.31 | − | −0.30 | −0.20 | 0.15 |
| Functional divergence (Fdiv) | 0.02 | 0.03 | 0.001 | − | 0.22 | − | 0.34 | 0.44 |
| Functional redundancy (FunRedundancy) | 0.05 | 0.01 | <0.001 | − | −0.31 | − | − | 0.24 |
| Functional specialization (Fspe) | 0.01 | −0.01 | 0.018 | −0.40 | − | − | −0.28 | 0.26 |
| Functional dispersion (Fdis) | 0.16 | 0.08 | <0.001 | − | 0.33 | − | −0.29 | 0.32 |
| Rao’s quadratic entropy (FunRao) | 0.38 | 0.10 | <0.001 | 0.37 | 0.18 | − | −0.35 | − |
| B correlation coefficient (CorB) | 0.01 | 0.03 | 0.074 | − | 0.35 | 0.28 | − | − |
| Standardized effect size (SESB) | 0.00 | −0.16 | 0.093 | − | 0.39 | − | − | −0.15 |
| Normalized Q (QB) | 0.01 | −0.04 | 0.041 | − | 0.35 | − | − | −0.16 |
| Functional originality (Fori) | 0.01 | 0.02 | 0.022 | 0.27 | 0.21 | −0.16 | − | −0.28 |
| Functional mean nearest neighbour distance (Fnnd) | 0.10 | −0.08 | <0.001 | − | 0.26 | −0.17 | − | −0.38 |
| Functional evenness (Feve) | 0.00 | −0.01 | 0.450 | 0.13 | − | − | − | 0.16 |
| Functional richness (Fric) | 0.67 | 0.29 | <0.001 | − | − | −0.51 | −0.38 | −0.22 |
| Functional identity axes | ||||||||
| Fide 1 | 0.01 | −0.01 | 0.083 | − | −0.26 | 0.31 | −0.29 | −0.20 |
| Fide 2 | 0.07 | 0.04 | <0.001 | 0.24 | 0.22 | − | 0.35 | − |
| Fide 3 | 0.11 | 0.11 | <0.001 | 0.37 | − | −0.24 | − | − |
| Fide 4 | 0.00 | 0.00 | 0.733 | − | 0.17 | 0.40 | − | − |
| Fide 5 | 0.01 | 0.01 | 0.069 | − | − | 0.41 | −0.17 | −0.31 |
| Trait * | Principal Components Extracted Based on the Analysis of Variability in Functional Indices | Species Richness | |||||
|---|---|---|---|---|---|---|---|
| Category | Level | PC1 | PC2 | PC3 | PC4 | PC5 | |
| M | dia | − | − | − | 0.07 ± 0.03 | − | 0.09 ± 0.05 |
| nom | −0.25 ± 0.11 | − | − | − | − | − | |
| pot | − | − | − | − | − | − | |
| H | bpl | − | − | − | − | − | − |
| dem | − | − | − | − | − | − | |
| pel | − | − | − | −0.06 ± 0.03 | − | − | |
| R | eur | − | − | − | − | − | − |
| lim | −0.19 ± 0.11 | − | 0.27 ± 0.10 | − | −0.12 ± 0.06 | 0.09 ± 0.05 | |
| rhe | − | − | −0.37 ± 0.02 | − | 0.13 ± 0.06 | − | |
| FH | ben | − | − | − | − | − | − |
| wat | − | − | − | − | − | − | |
| RH | lit | − | − | −0.25 ± 0.10 | − | 0.12 ± 0.06 | − |
| oth | − | − | − | − | − | − | |
| phy | − | − | − | − | − | − | |
| pli | − | − | − | − | − | − | |
| S | fbm | − | − | − | − | − | − |
| fbr | − | − | − | − | 0.13 ± 0.06 | − | |
| fre | − | − | − | − | − | − | |
| FD | car | − | − | − | − | − | − |
| inv | − | − | − | − | − | 0.11 ± 0.05 | |
| omn | − | − | − | − | − | −0.10 ± 0.05 | |
| pis | − | − | − | − | − | − | |
| LS | ls1 | − | − | − | 0.06 ± 0.03 | − | − |
| ls2 | − | − | − | − | − | − | |
| ls3 | − | − | − | −0.07 ± 0.03 | − | − | |
| BL | bl1 | 0.20 ± 0.11 | − | − | 0.08 ± 0.01 | − | 0.10 ± 0.05 |
| bl2 | −0.19 ± 0.11 | − | − | −0.07 ± 0.01 | − | −0.10 ± 0.05 | |
| bl3 | − | − | − | − | − | − | |
| SH | sh1 | − | −0.14 ± 0.07 | − | − | − | − |
| sh2 | − | − | − | −0.11 ± 0.03 | − | − | |
| sh3 | − | − | − | − | − | − | |
| sh4 | − | − | − | − | − | − | |
| SF | sw1 | − | − | − | − | − | − |
| sw2 | − | − | − | − | − | − | |
| sw3 | − | 0.16 ± 0.06 | − | − | − | − | |
| FM | ma1 | − | − | − | − | − | − |
| ma2 | − | − | − | − | − | − | |
| ma3 | − | − | − | − | − | − | |
| ma4 | − | − | − | − | − | − | |
| ma5 | −0.31 ± 0.10 | − | − | − | − | −0.13 ± 0.05 | |
| ST | st1 | − | − | − | − | − | − |
| st2 | − | − | − | − | 0.11 ± 0.06 | − | |
| st3 | − | −0.15 ± 0.06 | − | − | −0.13 ± 0.06 | − | |
| Ip | ip1 | − | − | − | − | − | − |
| ip2 | − | − | − | − | − | − | |
| ip3 | − | − | − | − | − | − | |
| Fc | fe1 | − | − | − | − | − | − |
| fe2 | − | − | −0.25 ± 0.09 | − | − | − | |
| fe3 | − | − | − | − | − | − | |
| rFc | fr1 | − | − | − | − | −0.16 ± 0.06 | − |
| fr2 | − | − | − | − | − | − | |
| fr3 | − | − | − | − | 0.12 ± 0.06 | − | |
| Eg | ed1 | − | − | − | − | − | − |
| ed2 | 0.25 ± 0.01 | − | − | − | − | − | |
| ed3 | −0.30 ± 0.02 | 0.15 ± 0.06 | − | − | − | −0.10 ± 0.05 | |
| LL | ll1 | − | − | − | − | − | − |
| ll2 | − | − | − | − | − | − | |
| ll3 | −0.18 ± 0.11 | − | − | − | − | − | |
| PC | nnh | −0.24 ± 0.10 | − | − | − | − | −0.13 ± 0.05 |
| nop | − | − | − | − | − | − | |
| pnh | − | − | − | − | − | 0.10 ± 0.05 | |
| LD | ld1 | − | − | − | − | − | − |
| ld2 | − | − | − | − | − | − | |
| ld3 | − | − | − | 0.06 ± 0.03 | − | − | |
| HD | im | − | − | − | − | − | − |
| intol | − | − | −0.19 ± 0.10 | − | − | − | |
| tol | − | − | − | − | − | − | |
| QT | im | − | − | − | − | − | − |
| intol | −0.30 ± 0.02 | 0.15 ± 0.06 | − | − | − | −0.10 ± 0.05 | |
| tol | − | −0.16 ± 0.06 | − | − | − | − | |
| OT | im | 0.20 ± 0.11 | − | − | − | − | 0.10 ± 0.05 |
| intol | − | − | − | − | − | − | |
| tol | −0.20 ± 0.11 | − | − | − | − | −0.10 ± 0.05 | |
| TT | im | − | − | − | − | − | − |
| intol | − | − | − | − | − | − | |
| tol | − | − | − | − | − | − | |
| TrL | − | − | 0.23 ± 0.10 | − | − | − | |
| Res | − | −0.13 ± 0.06 | − | −0.07 ± 0.03 | − | − | |
| Vuln | − | − | − | −0.08 ± 0.01 | − | − | |
| Effect | Sum of Squares | Degrees of Freedom | Mean Square | F-Statistic | p-Value | β-Coefficient ± Std. Error |
|---|---|---|---|---|---|---|
| F1 (Radj2 = 0.54, F = 21.2, p < 0.001) | ||||||
| Intercept | 43.4 | 1 | 43.4 | 23.3 | <0.001 | − |
| Time | 43.3 | 1 | 43.3 | 23.3 | <0.001 | −0.14 ± 0.03 |
| N*Time | 1073.8 | 30 | 35.8 | 19.3 | <0.001 | − * |
| ENV1 | 40.2 | 1 | 40.2 | 21.6 | <0.001 | −0.24 ± 0.05 |
| ENV2 | 6.2 | 1 | 6.2 | 3.3 | 0.07 | − |
| ENV3 | 2.6 | 1 | 2.6 | 1.4 | 0.24 | − |
| Error | 1030.0 | 554 | 1.9 | − | − | − |
| F2 (Radj2 = 0.37, F = 11.2, p < 0.001) | ||||||
| Intercept | 212.0 | 1 | 212.0 | 105.8 | <0.001 | − |
| Time | 212.0 | 1 | 212.0 | 105.8 | <0.001 | −0.36 ± 0.04 |
| N*Time | 503.8 | 30 | 16.8 | 8.4 | <0.001 | − * |
| ENV1 | 16.2 | 1 | 16.2 | 8.1 | 0.005 | −0.16 ± 0.06 |
| ENV2 | 0.3 | 1 | 0.3 | 0.2 | 0.69 | − |
| ENV3 | 0.1 | 1 | 0.1 | 0.1 | 0.82 | − |
| Error | 1110.3 | 554 | 2.0 | − | − | − |
| F3 (Radj2 = 0.68, F = 37.6, p < 0.001) | ||||||
| Intercept | 8.9 | 1 | 8.9 | 12.1 | 0.001 | − |
| Time | 8.9 | 1 | 8.9 | 12.1 | 0.001 | 0.08 ± 0.02 |
| N*Time | 744.9 | 30 | 24.8 | 33.5 | <0.001 | − * |
| ENV1 | 0.4 | 1 | 0.4 | 0.5 | 0.46 | − |
| ENV2 | 0.0 | 1 | 0.0 | 0.0 | 0.97 | − |
| ENV3 | 2.0 | 1 | 2.0 | 2.7 | 0.10 | − |
| Error | 410.0 | 554 | 0.7 | − | − | − |
| F4 (Radj2 = 0.32, F = 9.0, p < 0.001) | ||||||
| Intercept | 9.0 | 1 | 9.0 | 7.8 | 0.005 | − |
| Time | 9.0 | 1 | 9.0 | 7.8 | 0.005 | −0.10 ± 0.03 |
| N*Time | 326.9 | 30 | 10.9 | 9.4 | <0.001 | − * |
| ENV1 | 23.7 | 1 | 23.7 | 20.5 | <0.001 | 0.28 ± 0.06 |
| ENV2 | 11.0 | 1 | 11.0 | 9.5 | 0.002 | 0.12 ± 0.04 |
| ENV3 | 0.3 | 1 | 0.3 | 0.2 | 0.618 | − |
| Error | 640.9 | 554 | 1.2 | − | − | − |
| F5 (Radj2 = 0.47, F = 16.2, p < 0.001) | ||||||
| Intercept | 15.2 | 1 | 15.2 | 20.5 | <0.001 | − |
| Time | 15.2 | 1 | 15.2 | 20.5 | <0.001 | 0.14 ± 0.03 |
| N*Time | 333.2 | 30 | 11.1 | 15.0 | <0.001 | − * |
| ENV1 | 3.4 | 1 | 3.4 | 4.6 | 0.033 | −0.12 ± 0.06 |
| ENV2 | 19.0 | 1 | 19.0 | 25.6 | <0.001 | 0.17 ± 0.05 |
| ENV3 | 6.0 | 1 | 6.0 | 8.1 | 0.005 | −0.10 ± 0.03 |
| Error | 411.5 | 554 | 0.7 | − | − | − |
| Species richness (Radj2 = 0.47, F = 16.2, p < 0.001) | ||||||
| Intercept | 290.3 | 1 | 290.3 | 94.4 | <0.001 | − |
| Time | 324.5 | 1 | 324.5 | 105.5 | <0.001 | 0.33 ± 0.03 |
| N*Time | 1111.6 | 30 | 37.1 | 12.1 | <0.001 | − * |
| ENV1 | 18.0 | 1 | 18.0 | 5.9 | 0.016 | −0.13 ± 0.05 |
| ENV2 | 26.3 | 1 | 26.3 | 8.6 | 0.004 | 0.10 ± 0.03 |
| ENV3 | 15.8 | 1 | 15.8 | 5.1 | 0.024 | 0.08 ± 0.03 |
| Error | 1703.5 | 554 | 3.1 | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zymaroieva, A.; Bondarev, D.; Kunakh, O.; Svenning, J.-C.; Zhukov, O. Remote Sensing Reveals Multi-Dimensional Functional Changes in Fish Assemblages Under Eutrophication and Hydrological Stress. Fishes 2025, 10, 338. https://doi.org/10.3390/fishes10070338
Zymaroieva A, Bondarev D, Kunakh O, Svenning J-C, Zhukov O. Remote Sensing Reveals Multi-Dimensional Functional Changes in Fish Assemblages Under Eutrophication and Hydrological Stress. Fishes. 2025; 10(7):338. https://doi.org/10.3390/fishes10070338
Chicago/Turabian StyleZymaroieva, Anastasiia, Dmytro Bondarev, Olga Kunakh, Jens-Christian Svenning, and Oleksander Zhukov. 2025. "Remote Sensing Reveals Multi-Dimensional Functional Changes in Fish Assemblages Under Eutrophication and Hydrological Stress" Fishes 10, no. 7: 338. https://doi.org/10.3390/fishes10070338
APA StyleZymaroieva, A., Bondarev, D., Kunakh, O., Svenning, J.-C., & Zhukov, O. (2025). Remote Sensing Reveals Multi-Dimensional Functional Changes in Fish Assemblages Under Eutrophication and Hydrological Stress. Fishes, 10(7), 338. https://doi.org/10.3390/fishes10070338








