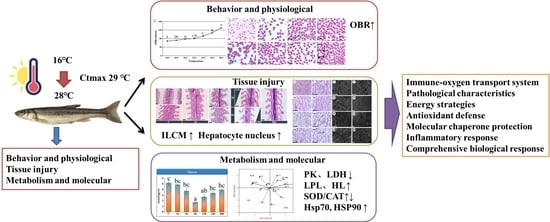
Behavioral, Hematological, Histological, Physiological Regulation and Gene Expression in Response to Heat Stress in Amur Minnow (Phoxinus lagowskii)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
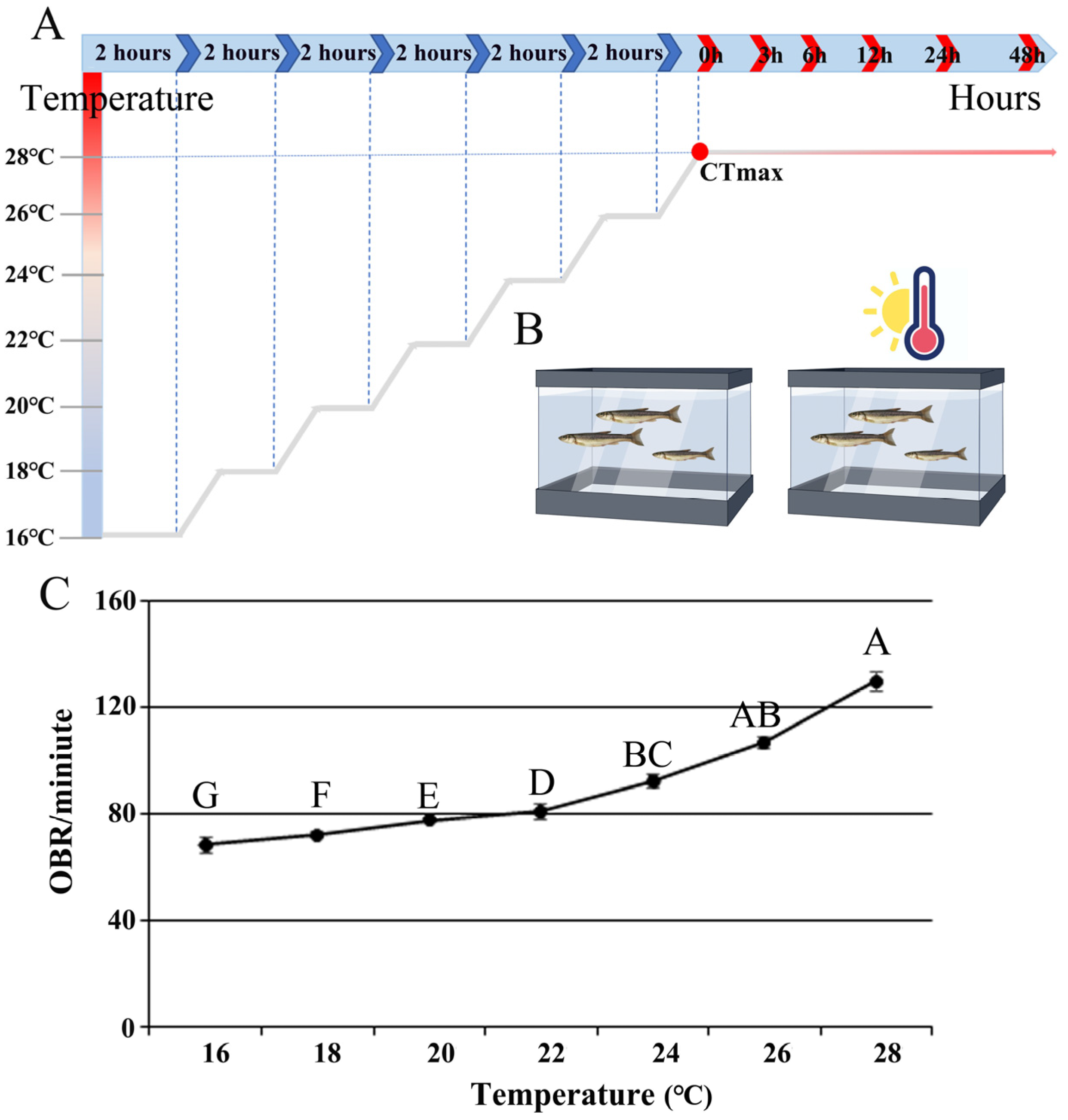
2.2. Measurements of CTmax and Behavioral Studies
2.3. Fish Treatments and Sample Collection
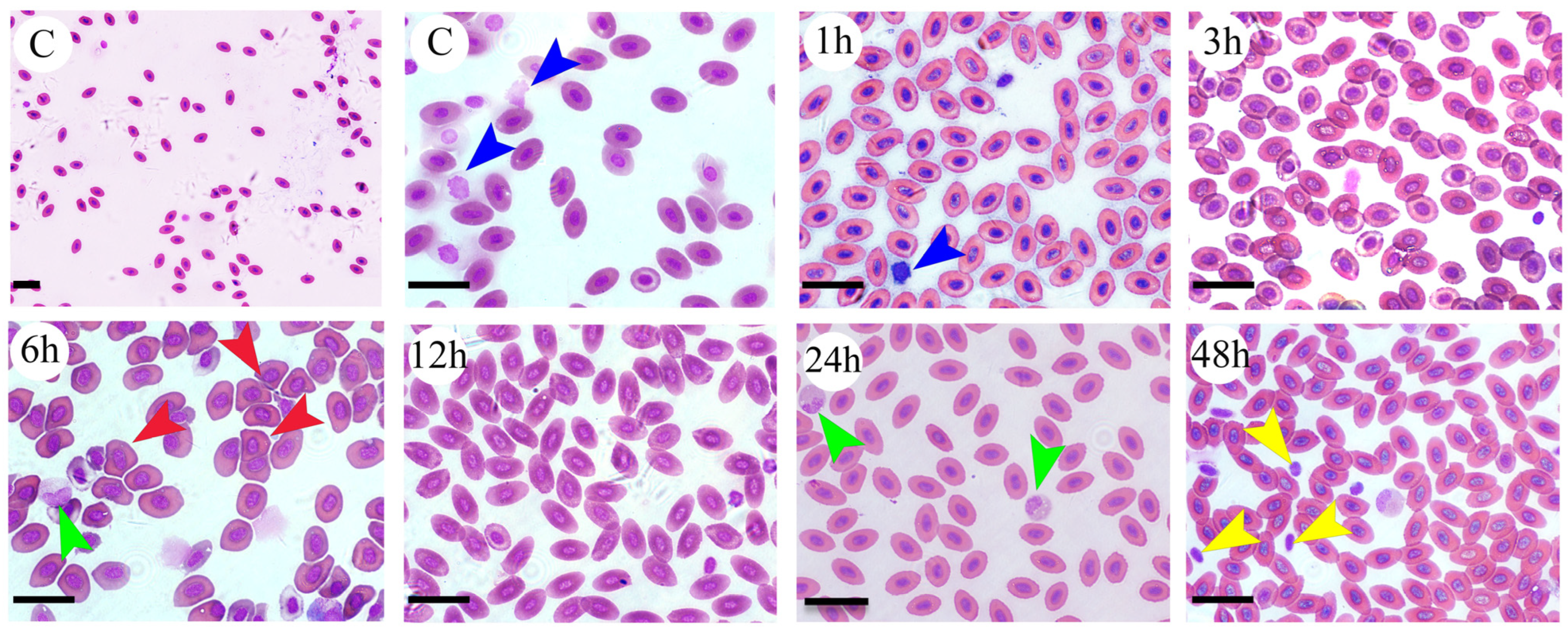
2.4. Hematological Study
2.5. Diff-Quick Staining
2.6. Observation of the Gill and Liver Histopathology
2.7. Determination of Relevant Enzyme Activities
2.8. Integrated Biomarker Response (IBR)
2.9. Gene Expression Analysis with qPCR
2.10. Data Analysis
3. Results
3.1. Changes in Behavioral and Hematological Parameters
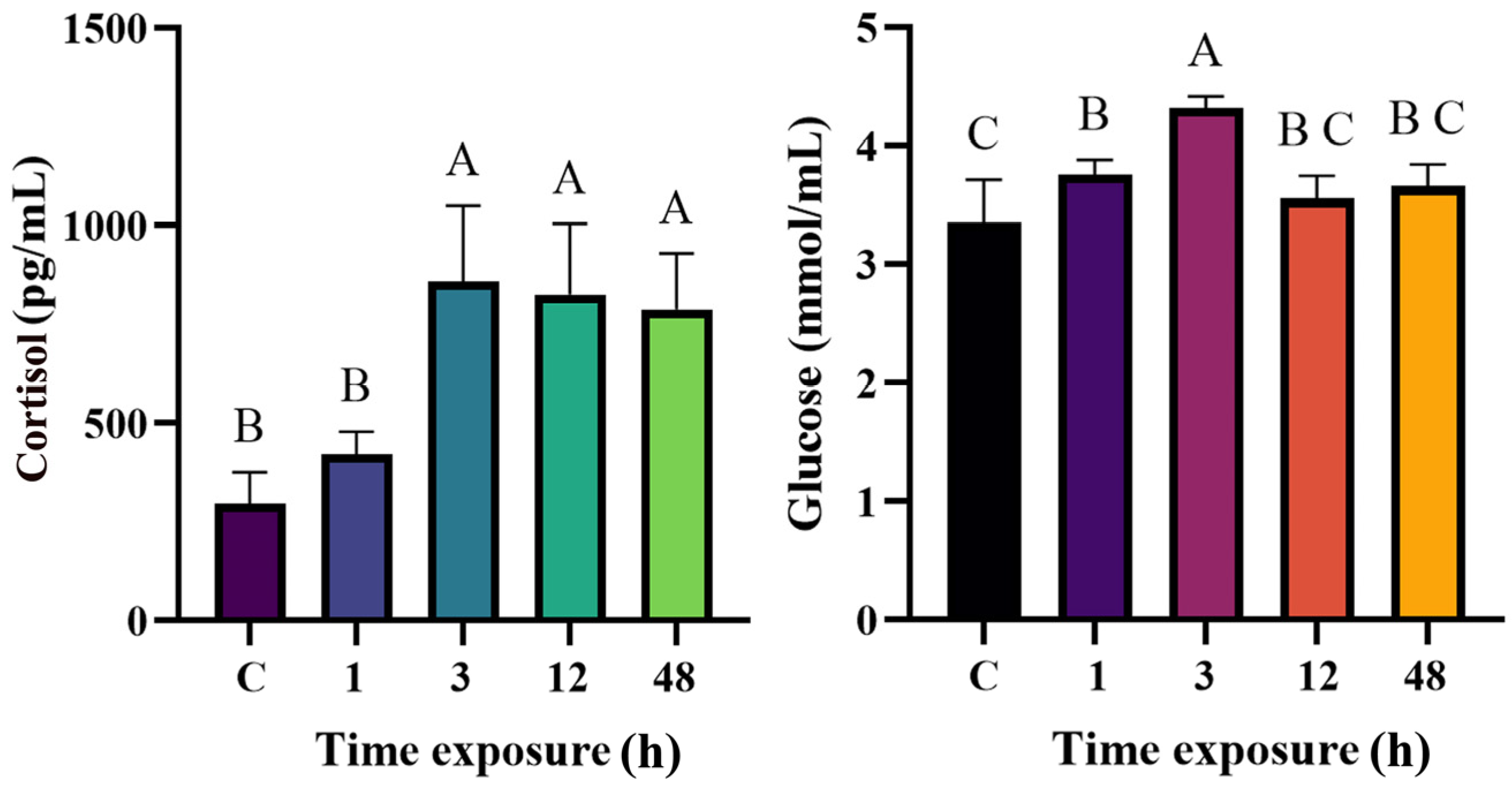
3.2. Effects of Heat Stress on Serum Cortisol and Glucose During 48 h Period
3.3. Effects of Heat Stress on Activity of ATPase in Gills During 48 h Period
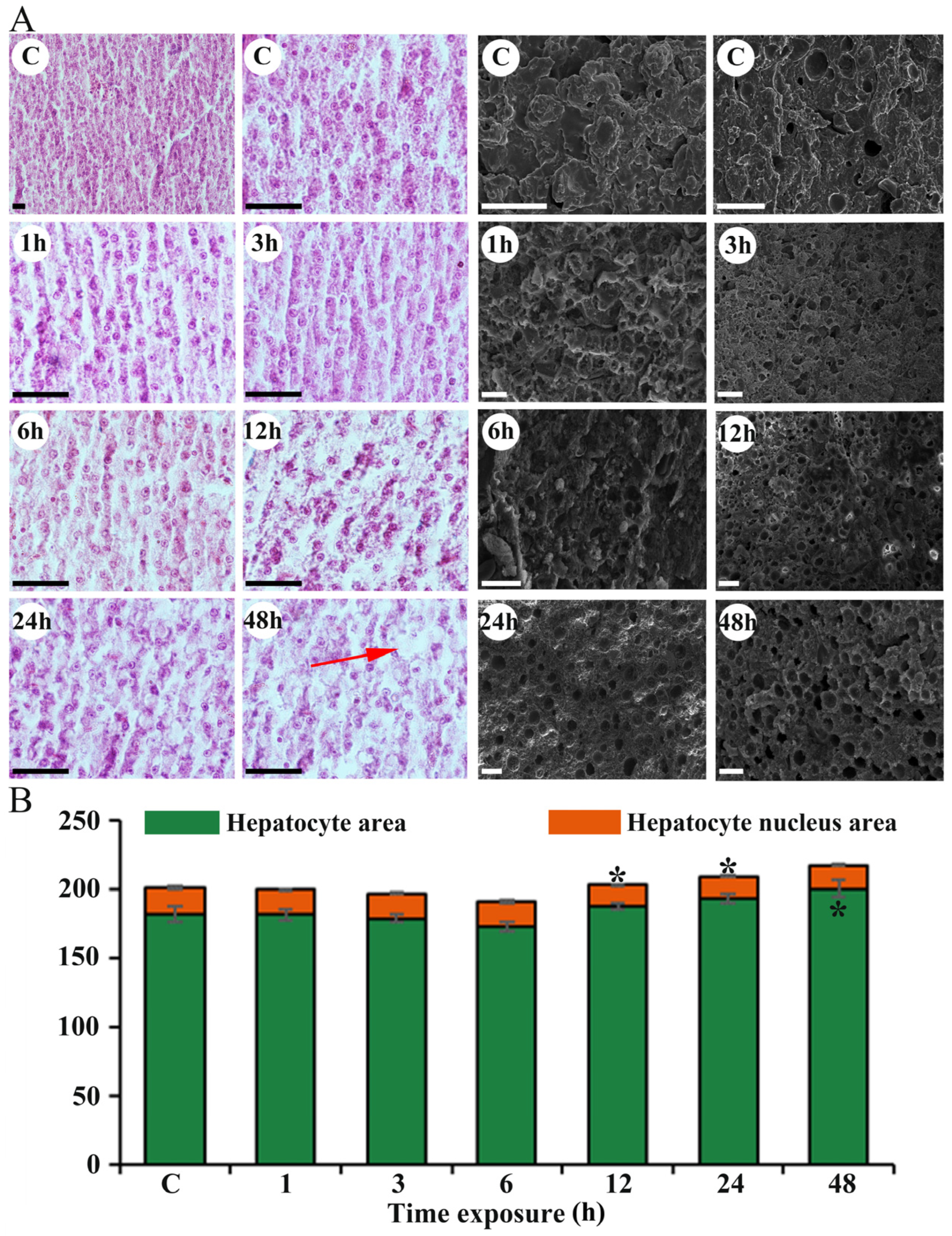
3.4. Effects of Heat Stress Duration on Gill and Liver Damage
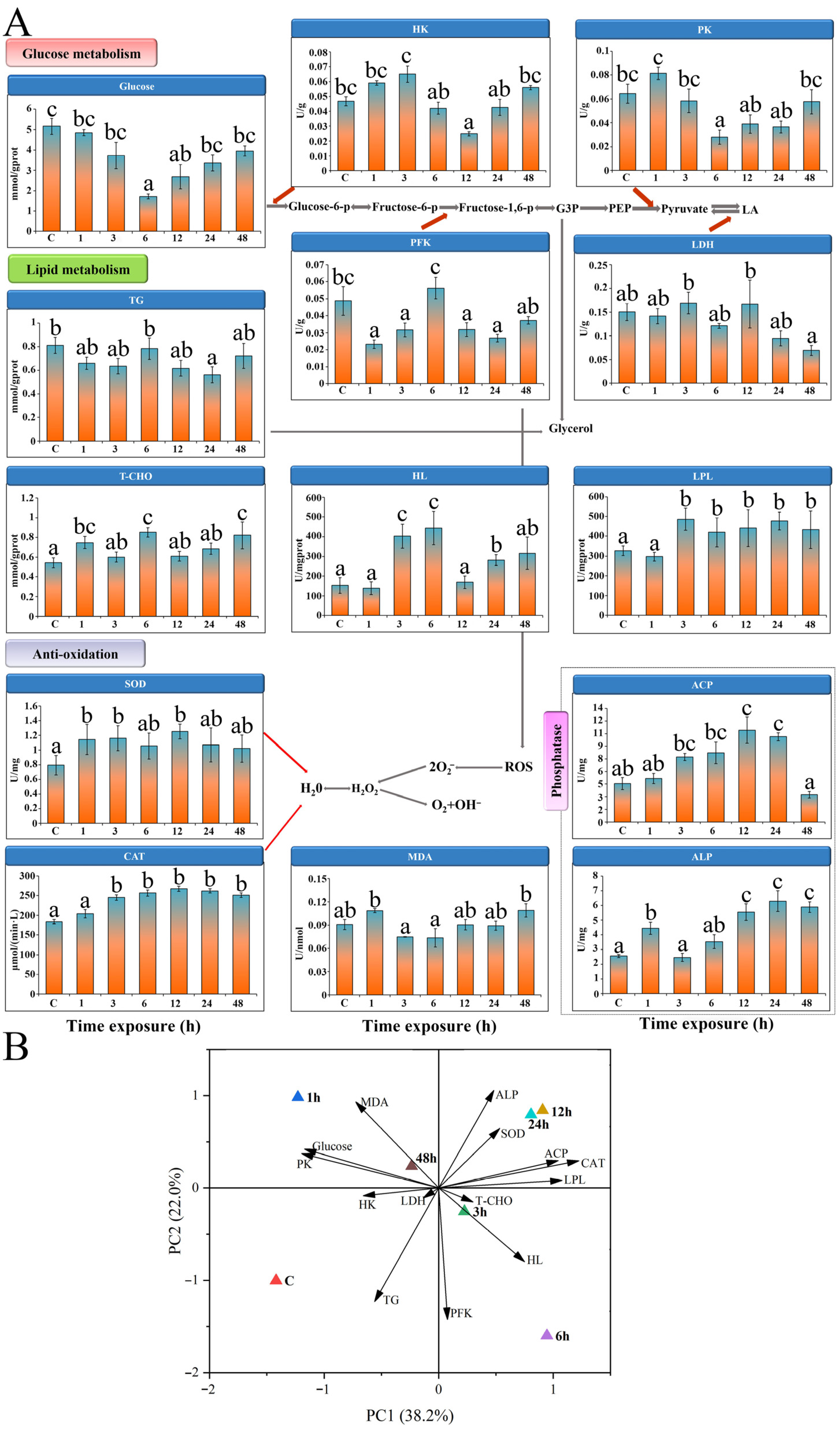
3.5. Enzyme Activities
3.5.1. Glycolytic Enzymes
3.5.2. Lipid Metabolism
3.5.3. Antioxidant Enzyme Activities
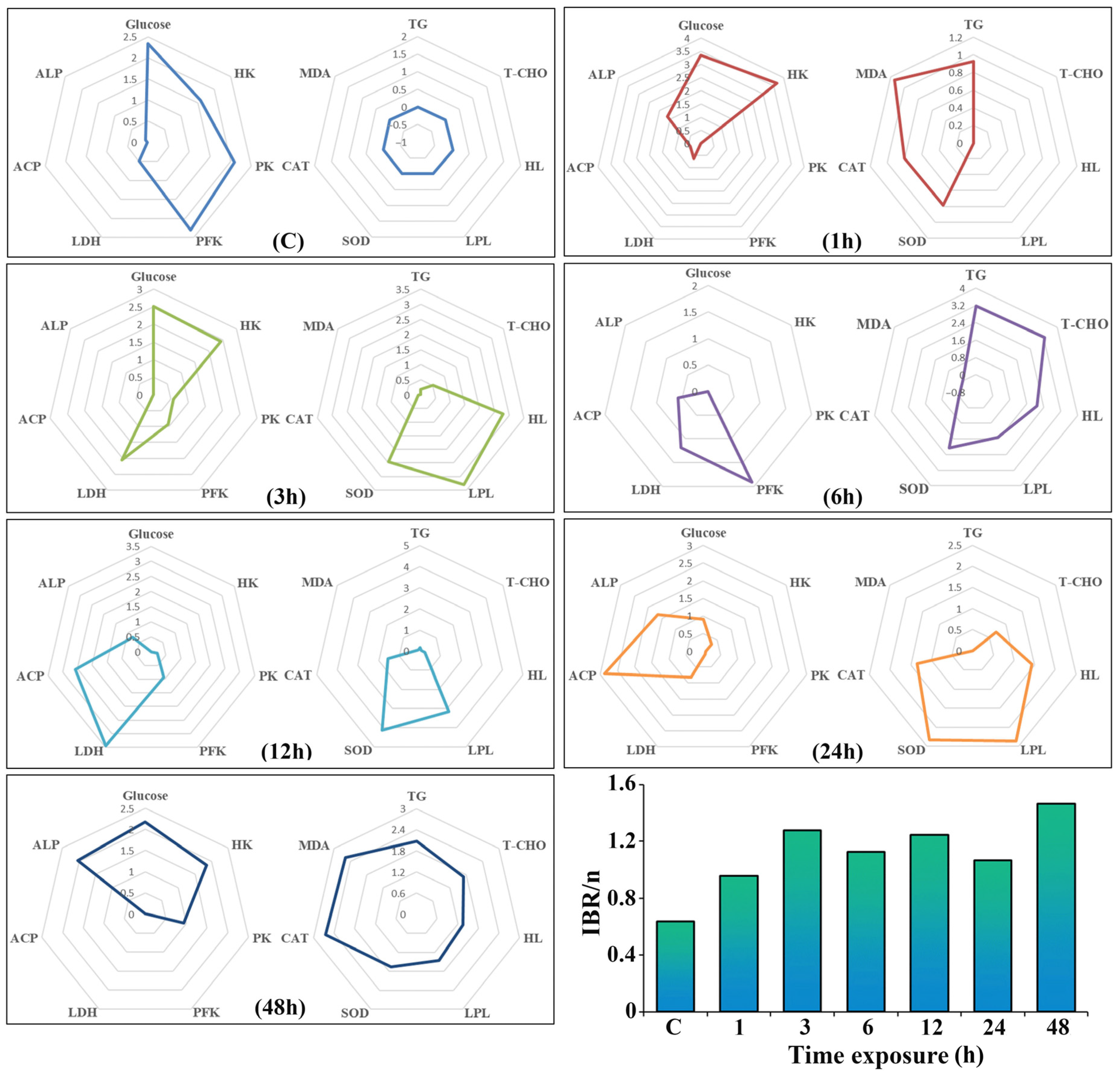
3.6. PCA and IBR Analysis
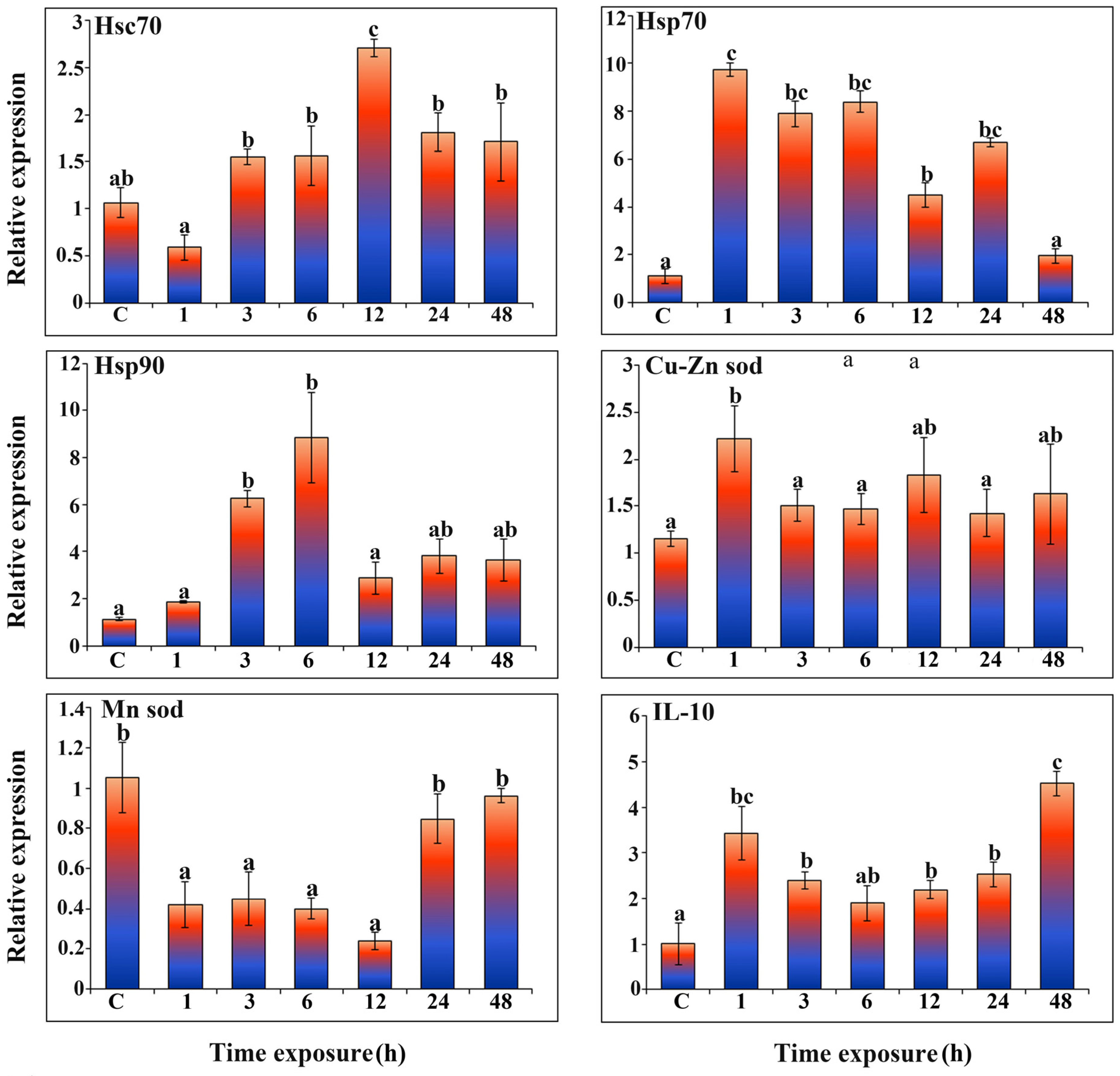
3.7. Expression of Antioxidant-Related Genes and Hsp Member Family Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Wu, X.; Li, P.; Liu, Y.; Song, M.; Li, F.; Ou, J.; Lai, J. Integrated metabolomic and transcriptomic responses to heat stress in a high-altitude fish, Triplophysa siluroides. Fish Shellfish Immunol. 2023, 142, 109118. [Google Scholar] [CrossRef] [PubMed]
- Saravia, J.; Paschke, K.; Oyarzún-Salazar, R.; Cheng, C.C.; Navarro, J.M.; Vargas-Chacoff, L. Effects of warming rates on physiological and molecular components of response to CTMax heat stress in the Antarctic fish Harpagifer antarcticus. J. Therm. Biol. 2021, 99, 103021. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress altered growth performance and metabolism and induced anaemia and liver disorder in Labeo rohita. Aquac. Res. 2020, 51, 1406–1414. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, H.; Huo, Z.; Yan, X. Heat hardening enhances the energy metabolism activity and oxidative defense ability of Manila clam Ruditapes philippinarum under different thermal stress. Aquaculture 2024, 595, 741511. [Google Scholar] [CrossRef]
- Zhao, H.; Ke, H.; Zhang, L.; Zhao, Z.; Lai, J.; Zhou, J.; Huang, Z.; Li, H.; Du, J.; Li, Q. Integrated analysis about the effects of heat stress on physiological responses and energy metabolism in Gymnocypris chilianensis. Sci. Total Environ. 2022, 806, 151252. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Guo, Z.; Zhao, Y.; Gao, Y.; Yu, T.; Chen, Y.; Zhang, D.; Wang, G. Effects of dietary oxidized fish oil on growth performance and antioxidant defense mechanism of juvenile Rhynchocypris lagowski Dybowski. Aquaculture 2019, 512, 734368. [Google Scholar] [CrossRef]
- Bartolini, T.; Butail, S.; Porfiri, M. Temperature influences sociality and activity of freshwater fish. Environ. Biol. Fishes 2015, 98, 825–832. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhu, X.L.; Lu, J.; Cai, W.J.; Ye, Y.-P.; Lv, Y.P. Effect of high temperature stress on heat shock protein expression and antioxidant enzyme activity of two morphs of the mud crab Scylla paramamosain. Comp. Biochem. Phys. A 2018, 223, 10–17. [Google Scholar] [CrossRef]
- Liu, F.; Qu, Y.K.; Wang, A.M.; Yu, Y.B.; Yang, W.P.; Lv, F.; Nie, Q. Effects of carotenoids on the growth performance, biochemical parameters, immune responses and disease resistance of yellow catfish (Pelteobagrus fulvidraco) under high-temperature stress. Aquaculture 2019, 503, 293–303. [Google Scholar] [CrossRef]
- Allison, C.; Muller, C.; Childs, A.R.; Froneman, W.; Bailey, L.A.; Potts, W.M. When cooling is worse than warming: Investigations into the thermal tolerance of an endemic reef fish, Boopsoidea inornata. Afr. J. Mar. Sci. 2021, 43, 239–249. [Google Scholar] [CrossRef]
- Morgan, R.; Finnoen, M.H.; Jutfelt, F. CTmax is repeatable and doesn’t reduce growth in zebrafish. Sci. Rep. 2018, 8, 7099. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, C.; Dias, M.; Roma, J.; Silva, A.; Madeira, D.; Diniz, M.S. Critical thermal maxima of common rocky intertidal fish and shrimps-A preliminary assessment. J. Sea Res. 2013, 81, 10–12. [Google Scholar] [CrossRef]
- Morrison, S.M.; Mackey, T.E.; Durhack, T.; Jeffrey, J.D.; Wiens, L.M.; Mochnacz, N.J.; Hasler, C.T.; Enders, E.C.; Treberg, J.R.; Jeffries, K.M. Sub-lethal temperature thresholds indicate acclimation and physiological limits in brook trout Salvelinus fontinalis. J. Fish Biol. 2020, 97, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Elahee, K.B.; Bhagwant, S. Hematological and gill histopathological parameters of three tropical fish species from a polluted lagoon on the west coast of Mauritius. Ecotoxicol. Environ. Saf. 2007, 68, 361–371. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, Z.; Song, Z.; Xiao, P.; Liu, Y.; Zhang, P.; You, F. Insight into the heat resistance of fish via blood: Effects of heat stress on metabolism, oxidative stress and antioxidant response of olive flounder Paralichthys olivaceus and turbot Scophthalmus maximus. Fish Shellfish Immunol. 2016, 58, 125–135. [Google Scholar] [CrossRef]
- Shah, N.; Khisroon, M.; Shah, S.S.A. Assessment of copper, chromium, and lead toxicity in fish (Ctenopharyngodon idella Valenciennes, 1844) through hematological biomarkers. Environ. Sci. Pollut. Res. Int. 2020, 27, 33259–33269. [Google Scholar] [CrossRef]
- Radoslav, D.; Aleksandar, I.; Rajko, G.; Goran, T.; Danijela, Ć.; Svjetlana, L. Effect of thermal stress of short duration on the red blood cell parameters of Barbus balcanicus Kotlik, Tsigenopulos, Rab, Berrebi, 2002. Afr. J. Biotechnol. 2013, 12, 2484–2491. [Google Scholar]
- Forgati, M.; Kandalski, P.K.; Herrerias, T.; Zaleski, T.; Machado, C.; Souza, M.; Donatti, L. Effects of heat stress on the renal and branchial carbohydrate metabolism and antioxidant system of Antarctic fish. J. Comp. Physiol. B 2017, 187, 1137–1154. [Google Scholar] [CrossRef]
- Harper, C.; Wolf, J.C. Morphologic Effects of the Stress Response in Fish. ILAR J. 2009, 50, 387–396. [Google Scholar] [CrossRef]
- Rombough, P.J.; Garside, E.T. Hypoxial death inferred from thermally induced injuries at upper lethal temperatures, in the banded killifish, Fundulus diaphanus (LeSueur). Can. J. Zool. 1977, 55, 1705–1719. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, D.; Zhao, C.; Wang, W.; Zhang, X.; Liu, X.; Liu, Y.; Xiao, Z.; Xu, S.; Xiao, Y.; et al. Histological and enzymatic responses of Japanese flounder (Paralichthys olivaceus) and its hybrids (P. olivaceus female symbol × P. dentatus male symbol) to chronic heat stress. Fish Physiol. Biochem. 2014, 40, 1031–1041. [Google Scholar]
- Bernard, B.; Leguen, I.; Mandiki, S.N.M.; Cornet, V.; Redivo, B.; Kestemont, P. Impact of temperature shift on gill physiology during smoltification of Atlantic salmon smolts (Salmo salar L.). Comp. Biochem. Physiol. A 2020, 244, 110685. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, B.; Xie, J.; Xu, P.; Tsion, H.M.H.; Zhang, Y. The effect of hyperthermia on cell viability, oxidative damage, and heat shock protein expression in hepatic cells of grass carp (Ctenopharyngodon idellus). J. Therm. Biol. 2013, 38, 355–361. [Google Scholar] [CrossRef]
- Yu, J.; Liu, F.; Yin, P.; Zhu, X.; Cheng, G.; Wang, N.; Lu, A.; Luan, W.; Zhang, N.; Li, J.; et al. Integrating miRNA and mRNA expression profiles in response to heat stress-induced injury in rat small intestine. Funct. Integr. Genom. 2011, 11, 203–213. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Ahmad, I.; Maria, V.L.; Oliveira, M.; Serafim, A.; Bebianno, M.J.; Pacheco, M.; Santos, M.A. DNA damage and lipid peroxidation vs. protection responses in the gill of Dicentrarchus labrax L. from a contaminated coastal lagoon (Ria de Aveiro, Portugal). Sci. Total. Environ. 2008, 406, 298–307. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Grim, J.M.; Miles, D.R.; Crockett, E.L. Temperature acclimation alters oxidative capacities and composition of membrane lipids without influencing activities of enzymatic antioxidants or susceptibility to lipid peroxidation in fish muscle. J. Exp. Biol. 2010, 213, 445–452. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Saha, N.C. Consequences of sodium dodecyl sulfate exposure on the antioxidant status and steroidogenesis in fish gonad. Environ. Sci. Pollut. Res. Int. 2021, 28, 19247–19259. [Google Scholar] [CrossRef]
- Subramanian, S.; Ross, N.W.; Mackinnon, S.L. Comparison of the biochemical composition of normal epidermal mucus and extruded slime of hagfish (Myxine glutinosa L.). Fish Shellfish Immunol. 2008, 25, 625–632. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Kassab, M.S.; Zaineldin, A.I.; Amer, A.A.; Gewaily, M.S.; Darwish, S.; Dawood, M.A.O. Mitigation of heat stress in striped catfish (Pangasianodon hypophthalmus) by dietary allicin: Exploring the growth performance, stress biomarkers, antioxidative, and immune responses. Aquac. Res. 2023, 2023, 8292007. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Lu, C.; Qi, D.; Qi, H.; Wang, Y.; Zhao, K.; Tian, F. Fatty acid metabolism and antioxidant capacity in Gymnocypris przewalskii (Kessler, 1876) response to thermal stress. J. Therm. Biol. 2023, 116, 103650. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, M.C.; Savelkoul, H.S.; Pietretti, D.; Wiegertjes, G.F.; Forlenza, M. Carp il10 has anti-inflammatory activities on phagocytes, promotes proliferation of memory T cells, and regulates B cell differentiation and antibody secretion. J. Immunol. 2015, 194, 187–199. [Google Scholar] [CrossRef]
- Zhang, D.C.; Shao, Y.Q.; Huang, Y.Q.; Jiang, S.G. Cloning, characterization and expression analysis of interleukin-10 from the zebrafish (Danio rerion). J. Biochem. Mol. Biol. 2005, 38, 571–576. [Google Scholar] [CrossRef]
- Savan, R.; Igawa, D.; Sakai, M. Cloning, characterization and expression analysis of interleukin-10 from the common carp, Cyprinus carpio L. Eur. J. Biochem. 2003, 270, 4647–4654. [Google Scholar]
- Grayfer, L.; Hodgkinson, J.W.; Hitchen, S.J.; Belosevic, M. Characterization and functional analysis of goldfish (Carassius auratus L.) interleukin-10. Mol. Immunol. 2011, 48, 563–571. [Google Scholar] [CrossRef]
- Ashburner, M.; Bonner, J.J. The induction of gene activity in Drosophila by heat shock. Cell 1979, 17, 241–254. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, C.; Ban, F.X.; Zhu, D.T.; Liu, S.S.; Wang, X.W. Genome-wide identification and characterization of HSP gene superfamily in whitefly (Bemisia tabaci) and expression profiling analysis under temperature stress. Insect Sci. 2019, 26, 44–57. [Google Scholar] [CrossRef]
- Somero, G.H.A.G. Evidence for protein damage at environmental temperatures: Seasonal changesin levels of ubiquitin conjugates and hsp70 in the intertidal mussel Mytilus trossulus. J. Exp. Biol. 1995, 198, 1509–1518. [Google Scholar]
- Susan, C.; Fader, Z.; Spotila, J.R. Seasonal variation in heat shock proteins (hsp 70) in stream fish under natural conditions. J. Therm. Biol. 1994, 19, 335–341. [Google Scholar]
- Dong, Y.; Dong, S.; Meng, X. Effects of thermal and osmotic stress on growth, osmoregulation and Hsp70 in sea cucumber (Apostichopus japonicus Selenka). Aquaculture 2008, 276, 179–186. [Google Scholar] [CrossRef]
- Mu, W.; Wen, H.; Li, J.; He, F. Cloning and expression analysis of a HSP70 gene from Korean rockfish (Sebastes schlegeli). Fish Shellfish Immunol. 2013, 35, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, D.; Wei, Q. The complete mitochondrial gemone of Phoxinus lagowskii (Teleostei, Cypriniformes: Cyprinidae). Mitochondrial DNA 2014, 27, 830–831. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.; Adineh, H.; Kulikov, E.; Vatnikov, Y.; Telezhenkova, A.; Yousefi, M. Effects of dietary autolyzed yeast and quercetin on growth performance, antioxidant and immunological parameters, and resistance to heat stress in rainbow trout. Aquaculture 2025, 600, 742257. [Google Scholar] [CrossRef]
- van der Walt, K.A.; Porri, F.; Potts, W.M.; Duncan, M.I.; James, N.C. Thermal tolerance, safety margins and vulnerability of coastal species: Projected impact of climate change induced cold water variability in a temperate African region. Mar. Environ. Res. 2021, 169, 105346. [Google Scholar] [CrossRef]
- Martinez-Palacios, C.; Tovar, E.; Taylor, J.; Ríos-Durán, M.; Ross, L. Effect of temperature on growth and survival of Chirostoma estor estor, Jordan 1879, monitored using a simple video technique for remote measurement of length and mass of larval and juvenile fishes. Aquaculture 2002, 209, 369–377. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Guo, H.; Zhang, X. Effect of environmental enrichment on the stress response of juvenile black rockfish Sebastes schlegelii. Aquaculture 2021, 533, 736088. [Google Scholar] [CrossRef]
- Brown, C.; Gardner, C.; Braithwaite, V. Differential stress responses in fish from areas of high- and low-predation pressure. J. Comp. Physiol. B 2005, 175, 305–312. [Google Scholar] [CrossRef]
- Fan, Z.; Mao, J.; Wang, Y.; Tang, A.; Hang, Y.; Tian, Y.; Wang, X.; Hao, Z.; Ding, J.; Chang, Y. Transcriptomic WGCNA analyses reveal endoplasmic reticulum response of Patinopecten yessoensis under acute heat stress. Aquaculture 2024, 589, 740938. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Wang, J.; Lyu, F.; Xu, K.; Mu, W. Histopathological, hematological, and biochemical changes in high-latitude fish Phoxinus lagowskii exposed to hypoxia. Fish Physiol. Biochem. 2021, 47, 919–938. [Google Scholar] [CrossRef]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Wang, C.; Pan, X.; Jin, W.; Wang, Y.; Shi, B. TiO2 nanoparticles and multi-walled carbon nanotubes monitoring and bioremediation potential using ciliates Pseudocohnilembus persalinus. Ecotoxicol. Environ. Saf. 2020, 187, 109825. [Google Scholar]
- Wang, J.; Yang, Y.; Wang, Z.; Xu, K.; Xiao, X.; Mu, W. Comparison of effects in sustained and diel-cycling hypoxia on hypoxia tolerance, histology, physiology and expression of clock genes in high latitude fish Phoxinus lagowskii. Comp. Biochem. Phys. A 2021, 260, 111020. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lee, C.G.; Farrell, A.P.; Lotto, A.; MacNutt, M.J.; Hinch, S.G.; Healey, M.C. The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J. Exp. Biol. 2003, 206, 3239–3251. [Google Scholar] [CrossRef]
- Pounder, K.C.; Mitchell, J.L.; Thomson, J.S.; Pottinger, T.G.; Buckley, J.; Sneddon, L.U. Does environmental enrichment promote recovery from stress in rainbow trout? Appl. Anim. Behav. Sci. 2016, 176, 136–142. [Google Scholar] [CrossRef]
- de Araújo, E.R.L.; da Silva e Silva, J.; Lopes, L.M.; Torres, M.F.; da Costa, B.M.P.A.; do Amarante, C.B.; Hamoy, M.; Barbas, L.A.L.; Sampaio, L.A. Geraniol and citronellol as alternative and safe phytoconstituents to induce immobilization and facilitate handling of fish. Aquaculture 2021, 537, 736517. [Google Scholar] [CrossRef]
- Berame, J.S. Physiological adaptations and behavioral responses of Janitor fish (Ancistrus sp. orange) to warming temperature. Int. J. Environ. Sci. Nat. Resour. 2019, 16, 58–63. [Google Scholar] [CrossRef]
- Bellgraph, B.J.; McMichael, G.A.; Mueller, R.P.; Monroe, J.L. Behavioural response of juvenile Chinook salmon Oncorhynchus tshawytscha during a sudden temperature increase and implications for survival. J. Therm. Biol. 2010, 35, 6–10. [Google Scholar] [CrossRef]
- Dagoudo, M.; Mutebi, E.T.; Qiang, J.; Tao, Y.F.; Zhu, H.J.; Ngoepe, T.K.; Xu, P. Effects of acute heat stress on haemato-biochemical parameters, oxidative resistance ability, and immune responses of hybrid yellow catfish (Pelteobagrus fulvidraco × P. vachelli) juveniles. Vet. Res. Commun. 2023, 47, 1217–1229. [Google Scholar] [CrossRef]
- Bao, J.; Qiang, J.; Tao, Y.; Li, H.; He, J.; Xu, P.; Chen, D. Responses of blood biochemistry, fatty acid composition and expression of microRNAs to heat stress in genetically improved farmed tilapia (Oreochromis niloticus). J. Therm. Biol. 2018, 73, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Q.; Dai, Z.; Li, J.; Li, W.; Fu, C.; Wang, Q.; Yin, W. Structural optimization and prospect of constructing hemoglobin oxygen carriers based on hemoglobin. Heliyon 2023, 9, e19430. [Google Scholar] [CrossRef]
- Basu, N.; Nakano, T.; Grau, E.G.; Iwama, G.K. The effects of cortisol on heat shock protein 70 levels in two fish species. Gen. Comp. Endocrinol. 2001, 124, 97–105. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; El-Sharawy, M.E.; Awad, S.S.; Van Doan, H.; Paray, B.A. Dietary white button mushroom improved the growth, immunity, antioxidative status and resistance against heat stress in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 523, 735229. [Google Scholar] [CrossRef]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Tzaneva, V.; Bailey, S.; Perry, S.F. The interactive effects of hypoxemia, hyperoxia, and temperature on the gill morphology of goldfish (Carassius auratus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1344–R1351. [Google Scholar] [CrossRef]
- Tzaneva, V.; Perry, S.F. The control of breathing in goldfish (Carassius auratus) experiencing thermally induced gill remodelling. J. Exp. Biol. 2010, 213, 3666–3675. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, E.; Li, C.; Pan, C.; Zhao, X.; Wang, Y.; Ling, Q. Effects of heat stress on histopathology, antioxidant enzymes, and transcriptomic profiles in gills of pikeperch Sander lucioperca. Aquaculture 2021, 534, 736277. [Google Scholar] [CrossRef]
- Li, P.; Liu, Q.; Li, J.; Wang, F.; Wen, S.; Li, N. Transcriptomic responses to heat stress in gill and liver of endangered Brachymystax lenok tsinlingensis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 38, 100791. [Google Scholar] [CrossRef]
- Garofalo, F.; Santovito, G.; Amelio, D. Morpho-functional effects of heat stress on the gills of Antarctic, T. bernacchii and C. hamatus. Mar. Pollut. Bull. 2019, 141, 194–204. [Google Scholar] [CrossRef]
- Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotoxicol. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.; Miranda, A.; Allinson, G.; Nugegoda, D. Evaluating the non-lethal effects of organophosphorous and carbamate insecticides on the yabby (Cherax destructor) using cholinesterase (AChE, BChE), Glutathione S-Transferase and ATPase as biomarkers. Ecotoxicol. Environ. Saf. 2017, 143, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Lian-Tien, S.; Chen, G.-R.; Chang, C.-F. Characteristics of blood parameters and gill Na+-K+-ATPase in chilled comatose tilapia cultured in various salinities. Camp. Eiochem. Physiol. 1994, 107A, 641–646. [Google Scholar] [CrossRef]
- Yoloğlu, E. Assesment of Na+/K+-ATPase Mg2+-ATPase Ca2+-ATPase, and total-ATPase activities in gills of freshwater mussels exposed to penconazole. Commagene J. Biol. 2019, 3, 88–92. [Google Scholar] [CrossRef]
- Mustafa, S.A.; Karieb, S.S.; Davies, S.J.; Jha, A.N. Assessment of oxidative damage to DNA, transcriptional expression of key genes, lipid peroxidation and histopathological changes in carp Cyprinus carpio L. following exposure to chronic hypoxic and subsequent recovery in normoxic conditions. Mutagenesis 2015, 30, 107–116. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Pan, C.; Liu, E.; Zhao, X.; Ling, Q. Alterations to transcriptomic profile, histopathology, and oxidative stress in liver of pikeperch (Sander lucioperca) under heat stress. Fish Shellfish Immunol. 2019, 95, 659–669. [Google Scholar] [CrossRef]
- Leung, L.Y.; Woo, N.Y. Influence of dietary carbohydrate level on endocrine status and hepatic carbohydrate metabolism in the marine fish Sparus sarba. Fish Physiol. Biochem. 2012, 38, 543–554. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.P.; Pal, A.K.; Subramanian, S.; Priyadarshi, H.; Kumar, V. High dietary protein combats the stress of Labeo rohita fingerlings exposed to heat shock. Fish Physiol. Biochem. 2011, 37, 1005–1019. [Google Scholar] [CrossRef]
- Quan, J.; Kang, Y.; Li, L.; Zhao, G.; Sun, J.; Liu, Z. Proteome analysis of rainbow trout (Oncorhynchus mykiss) liver responses to chronic heat stress using DIA/SWATH. J. Proteom. 2021, 233, 104079. [Google Scholar] [CrossRef]
- Montero, D.; Izquierdo, M.S.; Tort, L.; Robaina, L.; Vergara, J.M. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol. Biochem. 1999, 20, 53–60. [Google Scholar] [CrossRef]
- Weber, J.M. Metabolic fuels: Regulating fluxes to select mix. J. Exp. Biol. 2011, 214, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Qi, C.; Li, E.; Du, Z.; Qin, J.G.; Chen, L. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 2018, 495, 187–195. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Hu, X.; Gong, J.; Hwang, H.; Mai, K. Effects of temperature on non-specific immune parameters in two scallop species: Argopecten irradians (Lamarck 1819) and Chlamys farreri (Jones & Preston 1904). Aquac. Res. 2004, 35, 678–682. [Google Scholar]
- Naveed, A.; Janaiah, C.; Venkateshwarlu, P. The effects of lihocin toxicity on protein metabolism of the fresh water edible fish, Channa punctatus (Bloch). J. Toxicol. Environ. Health Sci 2010, 3, 18–23. [Google Scholar]
- Chen, M.; Yang, H.; Delaporte, M.; Zhao, S. Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture 2007, 271, 479–487. [Google Scholar] [CrossRef]
- Naqshbandi, A.K.M.; Rizwan, S.; Yusufi, A.N.K.; Khan, F. Studies on the protective effect of fish oil against cisplatin induced hepatotoxicity. Biol. Med 2011, 3, 86–97. [Google Scholar]
- Pereira, D.M.C.; Resende, A.C.; Schleger, I.C.; Neundorf, A.K.A.; Romão, S.; de Souza, M.R.D.P.; Herrerias, T.; Donatti, L. Integrated biomarker response index as an ally in the observation of metabolic biomarkers in muscle of Astyanax lacustris exposed to thermal variation. Biochimie 2023, 210, 3–13. [Google Scholar] [CrossRef]
- Madeira, C.; Madeira, D.; Diniz, M.S.; Cabral, H.N.; Vinagre, C. Thermal acclimation in clownfish: An integrated biomarker response and multi-tissue experimental approach. Ecol. Indic. 2016, 71, 280–292. [Google Scholar] [CrossRef]
- Prego-Faraldo, M.V.; Vieira, L.R.; Eirin-Lopez, J.M.; Mendez, J.; Guilhermino, L. Transcriptional and biochemical analysis of antioxidant enzymes in the mussel Mytilus galloprovincialis during experimental exposures to the toxic dinoflagellate Prorocentrum lima. Mar. Environ. Res. 2017, 129, 304–315. [Google Scholar] [CrossRef]
- Prego-Faraldo, M.V.; Méndez, J.; Laffon, B.; Valdiglesias, V.J.E.R.J. Cellular and molecular biomarkers for assessing the harmful effects of marine toxins in bivalve mollusks. Environ. Res. J. 2016, 10, 337–357. [Google Scholar]
- Chang, C.H.; Mayer, M.; Rivera-Ingraham, G.; Blondeau-Bidet, E.; Wu, W.Y.; Lorin-Nebel, C.; Lee, T.H. Effects of temperature and salinity on antioxidant responses in livers of temperate (Dicentrarchus labrax) and tropical (Chanos Chanos) marine euryhaline fish. J. Therm. Biol. 2021, 99, 103016. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M. Mitochondrial biogenesis in cold-bodied fishes. J. Exp. Biol. 2011, 214, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xu, D.; Tian, L.; Chen, R.; Wang, L.; Tan, P.; You, Q. Overwinter mortality in yellow drum (Nibea albiflora): Insights from growth and immune responses to cold and starvation stress. Fish Shellfish Immunol. 2019, 92, 341–347. [Google Scholar] [CrossRef]
- Chang, C.H.; Wang, Y.C.; Lee, T.H. Hypothermal stress-induced salinity-dependent oxidative stress and apoptosis in the livers of euryhaline milkfish, Chanos chanos. Aquaculture 2021, 534, 736280. [Google Scholar] [CrossRef]
- Igor, N.; Zelko, T.J.M.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 3, 337–349. [Google Scholar]
- Xu, K.; Xu, H.; Han, Z. Genome-wide identification of hsp70 genes in the large yellow croaker (Larimichthys crocea) and their regulated expression under cold and heat stress. Genes 2018, 9, 590. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Boutet, I.; Tanguy, A.; Rousseau, S.; Auffret, M.; Moraga, D. Molecular identification and expression of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperones 2003, 8, 76–85. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Wang, G.; Chen, Y.; Guo, J.; Pan, C.; Liu, E.; Ling, Q. Physicochemical changes in liver and Hsc70 expression in pikeperch Sander lucioperca under heat stress. Ecotoxicol. Environ. Saf. 2019, 181, 130–137. [Google Scholar] [CrossRef]
- Cheng, C.H.; Yang, F.F.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L.; Tan, J.W.; Chen, X.Y. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef]
- Parsell, D.A.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. 1993, 27, 437–496. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yang, M.; Zhao, T.; Wang, X.; Zhou, H. Functional expression and characterization of grass carp IL-10: An essential mediator of TGF-β1 immune regulation in peripheral blood lymphocytes. Mol. Immunol. 2013, 53, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kamota, S.; Ito, K.; Yoshiura, Y.; Ototake, M.; Moritomo, T.; Nakanishi, T. Molecular cloning and expression analysis of rainbow trout (Oncorhynchus mykiss) interleukin-10 cDNAs. Fish Shellfish Immunol. 2005, 18, 335–344. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Primer Sequences (5′–3′) | TM (℃) | Product (bp) |
|---|---|---|---|
| hsp90 | F: CCCAGTTCATCGGATACCCAATCAC R: TTCCTCCTTCTCAGCCTTCTCCTC | 60.0 | 101 |
| hsc70 | F: CGCACTTTGTCCTCCAGCACTC R: CCACGGAAGAGATCGGCATTGAG | 61.5 | 122 |
| Hsp70 | F: GCCCTCATCAAACGCAAC R: CCCTCTCTCCCTCATACACCT | 55 | 109 |
| Cu-Zn SOD | F: TGGTAATGTGACGGCTGGTGAAAG R: TCCCTTACCCAAGTCATCCTCCTTC | 55.5 | 136 |
| Mn SOD | F: TGACGACCCAAGTCTCCCTTCAG R: AGCTCACCCTGTGGTTCTCCTC | 60.7 | 118 |
| IL-10 | F: GAGTGTTGCTCATTTGTGGAA R: TGGTTCTAAGTCGTCATTGGA | 53.7 | 105 |
| β-actin | F: CGGATTCGCTGGAGATGATGCTC R: TGGTGACAATACCGTGCTCAATGG | 60.5 | 176 |
| Control Groups (16 °C) | Heat Stress Groups (28 °C) | ||||||
|---|---|---|---|---|---|---|---|
| C | 1 h | 3 h | 6 h | 12 h | 24 h | 48 h | |
| WBC (1 × 106 cells mg−1) | 4.97 ± 1.03 | 5.31 ± 0.21 | 9.96 ± 1.13 | 8.06 ± 1.45 | 9.26 ± 1.81 | 11.26 ± 2.32 * | 19.02 ± 4.95 * |
| RBC (1 × 109 cells mg−1) | 2.63 ± 0.18 | 2.52 ± 0.21 | 2.14 ± 0.28 | 2.56 ± 0.27 | 3.26 ± 0.50 * | 3.68 ± 0.22 * | 2.59 ± 0.31 |
| HGB (gL−1) | 116.83 ± 9.29 | 96.66 ± 6.92 | 87.16 ± 12.79 * | 103.83 ± 12.03 | 128.66 ± 21.91 | 133.66 ± 4.98 * | 119.16 ± 8.36 |
| HCT (%) | 45.04 ± 1.58 | 41.63 ± 1.23 * | 44.7 ± 2.39 | 45.1 ± 3.65 | 55.2 ± 2.28 * | 49.7 ± 1.81 | 43.57 ± 4.87 * |
| Control Groups (16 °C) | Heat Stress Groups (28 °C) | ||||||
|---|---|---|---|---|---|---|---|
| C | 1 h | 3 h | 6 h | 12 h | 24 h | 48 h | |
| PL thickness (μm) | 24.25 ± 0.59 | 20.17 ± 0.73 * | 20.58 ± 0.63 * | 24.31 ± 0.57 | 21.56 ± 0.57 | 21.30 ± 0.66 | 21.70 ± 0.52 |
| PL height (μm) | 113.14 ± 3.13 | 96.52 ± 2.26 * | 106.80 ± 2.27 | 125.61 ± 2.58 * | 108.35 ± 1.60 | 110.33 ± 2.06 | 102.37 ± 1.76 |
| PL basal length (μm) | 203.30 ± 2.11 | 164.34 ± 3.14 * | 217.93 ± 2.66 | 214.15 ± 4.53 | 233.00 ± 4.57 * | 210.87 ± 2.38 | 207.26 ± 3.29 |
| Distance between adjacent lamella (μm) | 20.91 ± 0.55 | 20.98 ± 0.78 | 22.26 ± 0.54 | 23.17 ± 0.68 * | 21.21 ± 0.44 | 20.31 ± 0.64 | 20.29 ± 1.06 |
| ILCM height (μm) | 81.54 ± 1.82 | 85.67 ± 1.93 | 82.54 ± 2.18 | 87.21 ± 1.20 | 84.96 ± 1.12 | 90.54 ± 1.40 * | 91.29 ± 0.83 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, W.; Wang, J.; Zhou, Y.; Feng, S.; Huang, Y.; Li, Q. Behavioral, Hematological, Histological, Physiological Regulation and Gene Expression in Response to Heat Stress in Amur Minnow (Phoxinus lagowskii). Fishes 2025, 10, 335. https://doi.org/10.3390/fishes10070335
Mu W, Wang J, Zhou Y, Feng S, Huang Y, Li Q. Behavioral, Hematological, Histological, Physiological Regulation and Gene Expression in Response to Heat Stress in Amur Minnow (Phoxinus lagowskii). Fishes. 2025; 10(7):335. https://doi.org/10.3390/fishes10070335
Chicago/Turabian StyleMu, Weijie, Jing Wang, Yanyan Zhou, Shibo Feng, Ye Huang, and Qianyu Li. 2025. "Behavioral, Hematological, Histological, Physiological Regulation and Gene Expression in Response to Heat Stress in Amur Minnow (Phoxinus lagowskii)" Fishes 10, no. 7: 335. https://doi.org/10.3390/fishes10070335
APA StyleMu, W., Wang, J., Zhou, Y., Feng, S., Huang, Y., & Li, Q. (2025). Behavioral, Hematological, Histological, Physiological Regulation and Gene Expression in Response to Heat Stress in Amur Minnow (Phoxinus lagowskii). Fishes, 10(7), 335. https://doi.org/10.3390/fishes10070335