Effect of the Inclusion of Natural Pigments on the Performance and Gene Expression of Immune Response and Oxidative Stress of Oreochromis niloticus Cultured in a Biofloc System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Culture Conditions
2.3. Feeding and Carbon Source Incorporation
2.4. Monitoring of Physical and Chemical Water Parameters
2.5. Biometry of Organisms
2.6. Thermal Stress Test
2.7. Evaluation of the Immune Response by Leukocyte Count
2.8. Evaluation of Oxidative and Immune Response Gene Expression
2.9. Muscle Carotenoid Content
2.10. Data Analysis
3. Results
3.1. Physical and Chemical Water Parameters
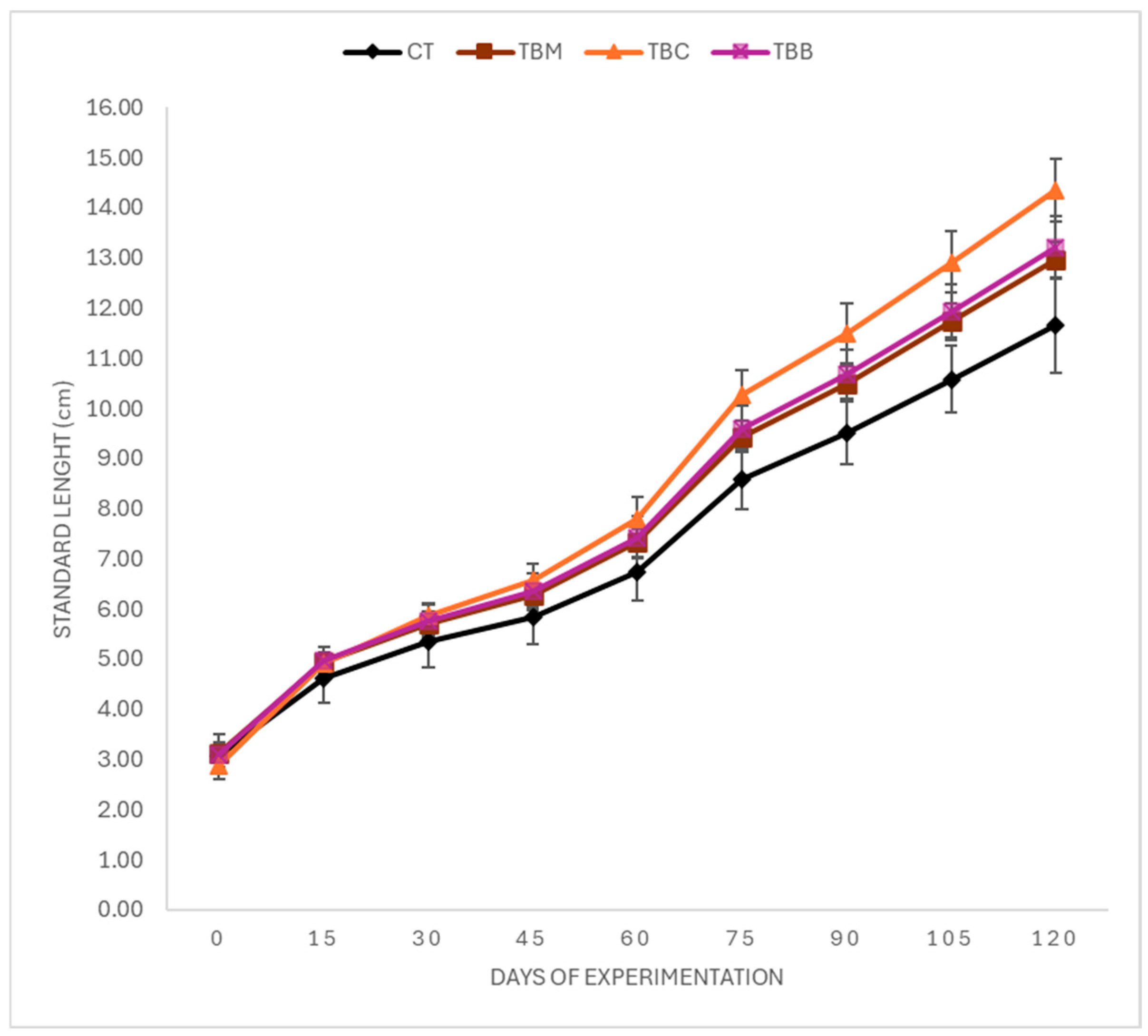
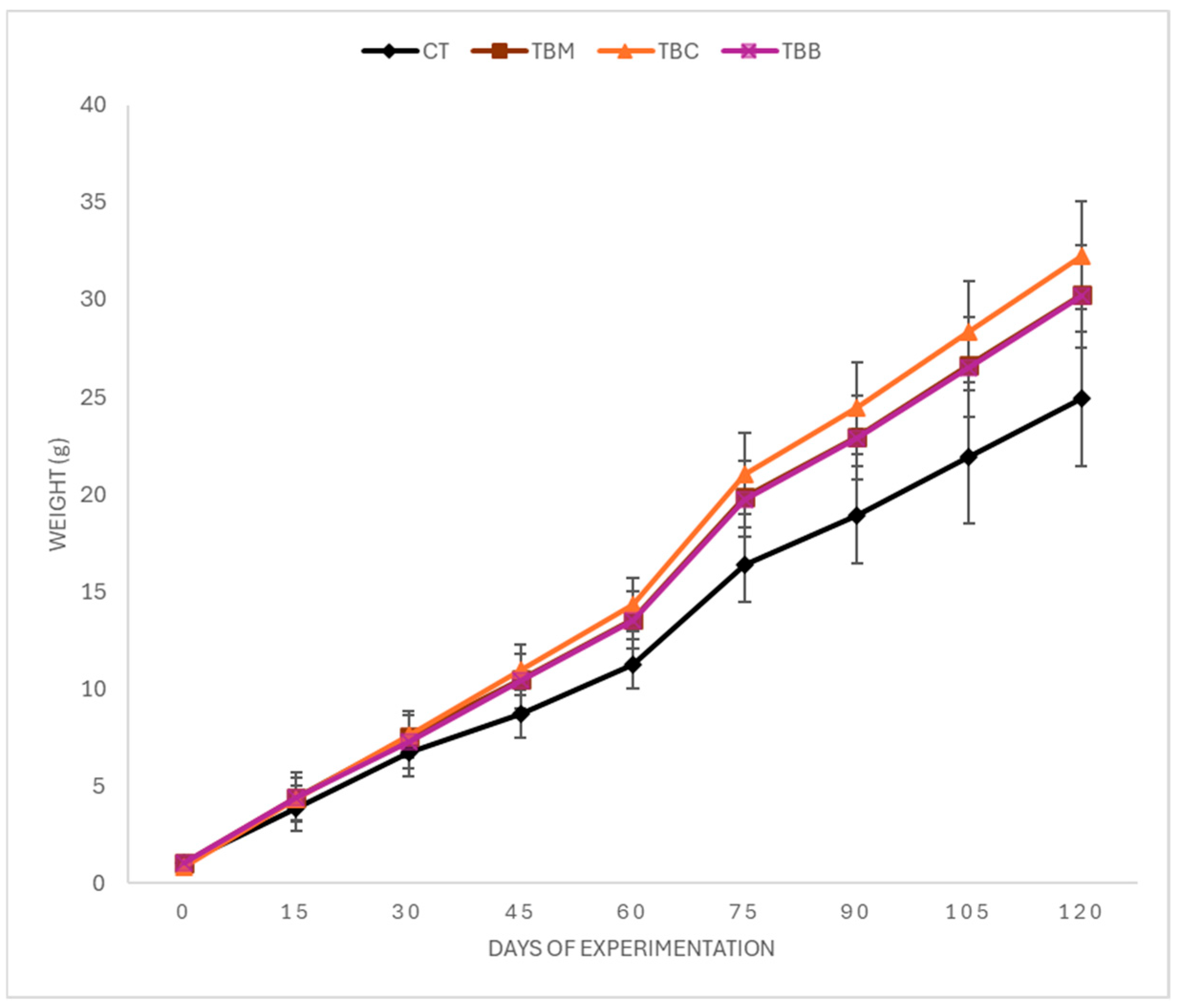
3.2. Growth Performance
3.3. Leukocyte Count
3.4. Relative Expression of Oxidative Stress Genes at 120 Days of Culture
3.5. Relative Expression of Immune Genes at 120 Days of Culture
3.6. Relative Expression of Oxidative Stress Genes After Temperature Challenge
3.7. Relative Expression of Immune System Genes After Temperature Challenge
3.8. Tissue Carotenoid Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WG | Weight gain |
| LG | Length gain |
| SGR | Specific growth rate |
| FCR | Feed conversion rate |
| SR | Survival rate |
| gpx | Glutathione peroxidase |
| sod | Superoxide dismutase, |
| cat | Catalase |
| hsp70 | Heat shock protein 70 |
| tgfb | Transforming growth factor-beta |
| tfr | Transferrin receptor |
| tnfa | Tumor necrosis factor alpha |
| il1b | Interleukin 1 beta |
| β-act (actb) | Beta actin |
References
- Youssuf, H.; Soror, E.I.; Shehab, A.; El-daim, A.M.; Abo-Gamil, Z.H.; Ahmed-Farid, O.; Hamad, A.; Edris, S.; Matter, A.F. Amelioration of hypoxia and cold stress in Nile tilapia: Comparative effect of Chlorella vulgaris and its nanoparticle dietary supplementation on performance, antioxidant, hepatic functions, and meat quality. Aquac. Int. 2025, 33, 66. [Google Scholar] [CrossRef]
- Wang, B.; Thompson, K.D.; Wangkahart, E.; Yamkasem, J.; Bondad-Reantaso, M.G.; Tattiyapong, P.; Jian, J.; Surachetpong, W. Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev. Aquac. 2023, 15, 41–56. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Mohammadi, A.; Emerenciano, M.G.C. Water quality in biofloc technology (BFT): An applied review for an evolving aquaculture. Aquac. Int. 2024, 32, 9321–9374. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Hossein, S.; Harikrishnan, R.; Balasundaram, C.; Jaturasitha, S. Effects of coffee silverskin on growth performance, immune response, and disease resistance of Nile tilapia culture under biofloc system. Aquaculture 2021, 736995, 543. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.; Pulkkinen, J.; Kiuru, T.; Koskela, J.; Vielma, J. Water quality in recirculating aquaculture system using woodchip denitrification and slow sand filtration. Environ. Sci. Pollut. Res. 2020, 27, 17314–17328. [Google Scholar] [CrossRef]
- Wei, G.; Shan, D.; Li, G.; Li, X.; Tian, R.; He, J.; Shao, Z. Prokaryotic communities vary with floc size in a biofloc-technology based aquaculture system. Aquaculture 2020, 735632, 529. [Google Scholar] [CrossRef]
- Fontana, C.M.; Sumon, M.A.A.; Wannavijit, S.; Lubis, A.R.; Khongdee, N.; Linh, N.V.; Phimolsiripol, Y.; Hoseinifar, S.H.; Van Doan, H. Effects of Mango Seed (Mangifera indica) Powder on Growth Performance, Immune Response, Gut Morphology, and Gene Expression of Nile Tilapia (Oreochromis niloticus). Fishes 2024, 9, 514. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology with addition molasses as carbon sources applied to Litopenaeus vannamei juvenile production under the effects of different C/N ratios. Aquac. Int. 2022, 30, 383–397. [Google Scholar] [CrossRef]
- Luo, G.; Chen, X.; Tan, J.; Abakari, G.; Tan, H. Effects of carbohydrate addition strategy and biofloc levels on the establishment of nitrification in biofloc technology aquaculture systems. Aquaculture 2020, 514, 734441. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Eissa, E.S.H.; Tawfik, W.A.; Abd Elnabi, H.E.; Saadony, S.; Bazina, W.K.; Ahmed, R.A. Dietary curcumin nanoparticles promoted the performance, antioxidant activity, and humoral immunity, and modulated the hepatic and intestinal histology of Nile tilapia fingerlings. Fish Physiol. Biochem. 2022, 48, 585–601. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Sabae, S.A.; Mahmoud, A.M.; El-Haroun, E.R. Comparative study on the effect of dietary β-carotene and phycocyanin extracted from Spirulina platensis on immune-oxidative stress biomarkers, genes expression and intestinal enzymes, serum biochemical in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2021, 108, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Yousefi, S.; Capillo, G.; Paknejad, H.; Khalili, M.; Tabarraei, A.; van Doan, S.N.; Faggio, C. Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol. 2018, 83, 232–237. [Google Scholar] [CrossRef] [PubMed]
- AbdAllah, H.M.; Hamed, A.A.S.; Bakry, M.A.; Eldin, I.M.; AbdElal, M.N.; Mohamed, D.T.; Mohamady, S.N. Impact of Spirulina Supplementation on Some Immune and Biochemical Parameters of Nile Tilapia Under Cold Stress. Alex. J. Vet. Sci. 2023, 79, 107–123. [Google Scholar] [CrossRef]
- Hernández, A.; García, B.G.; Jordán, M.; Hernández, M. Improved conservation of gilthead seabream (Sparus aurata) in ice storage. The influence of doses of rosemary extract added to feed. Aquaculture 2024, 426, 31–40. [Google Scholar] [CrossRef]
- Hansen, O.J.; Puvanendran, V.; Bangera, R. Broodstock diet with water and astaxanthin improve condition and egg output of brood fish and larval survival in Atlantic cod, Gadus morhua L. Aquac. Res. 2016, 47, 819–829. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarundin, M.S. Dietary astaxanthin augments disease resistance of Asian seabass, Lates calcarifer (Bloch, 1790), against Vibrio alginolyticus infection. Fish Shellfish Immunol. 2021, 114, 90–101. [Google Scholar] [CrossRef]
- Nakano, T. Stress in fish and application of carotenoid for aquafeed as an antistress supplement. In Encyclopedia of Marine Biotechnology; Kim, S.K., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 2999–3019. [Google Scholar] [CrossRef]
- Castro-Castellón, A.E.; Monroy-Dosta, M.D.C.; Castro-Mejía, J.; Castro-Mejía, G.; López-García, E.; Martínez-Meingüer, A.M. Evaluation of growth development and pigmentation of Heros severus cultured in a biofloc system with enriched pigment diets. Lat. Am. J. Aquat. Res. 2023, 51, 88–97. [Google Scholar] [CrossRef]
- Emerenciano, M.; Córdova, L.; Porchas, M.; Baeza, A. Biofloc Technology (BFT): A Tool for Water Quality Management in Aquaculture. In Water Quality; Tutu, H., Grover, B., Eds.; InTech: Rijeka, Croatia, 2017; pp. 91–109. [Google Scholar]
- Mansour, A.T.; Esteban, M.Á. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 64, 202–209. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ponce, G.C.V.; Monroy, D.M.C.; Ramírez, T.J.A.; Ocampo, C.J.A.; Castro, M.J. Rhodococcus sp. as probiotic bacteria for increase the survival, growth and coloration of fish Puntius conchonius. Sci. J. Anim. Sci. 2016, 5, 370–375. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent progress towards the application of biofloc technology for tilapia farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Martins, G.B.; da Rosa, C.E.; Tarouco, F.D.M.; Robaldo, R.B. Growth, water quality and oxidative stress of Nile tilapia Oreochromis niloticus (L.) in biofloc technology system at different pH. Aquac. Res. 2019, 50, 1030–1039. [Google Scholar] [CrossRef]
- Fleckenstein, L.J.; Tierney, T.W.; Ray, A.J. Comparing biofloc, clear-water, and hybrid recirculating nursery systems (Part II): Tilapia (Oreochromis niloticus) production and water quality dynamics. Aquac. Eng. 2018, 82, 80–85. [Google Scholar] [CrossRef]
- Martins, G.B.; Tarouco, F.; Rosa, C.E.; Robaldo, R.B. The utilization of sodium bicarbonate, calcium carbonate or hydroxide in biofloc system: Water quality, growth performance and oxidative stress of Nile tilapia (Oreochromis niloticus). Aquaculture 2017, 468, 10–17. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef]
- Rind, K.H.; Habib, S.S.; Ujan, J.A.; Fazio, F.; Naz, S.; Batool, A.I.; Ullah, M.; Attaullah, S.; Khayyam, K.; Khan, K. The effects of different carbon sources on water quality, growth performance, hematology, immune, and antioxidant status in cultured Nile Tilapia with biofloc technology. Fishes 2023, 8, 512. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Li, C.; Deng, Y.; Tadda, M.A.; Lan, L.; Zhu, S.; Liu, D. Effects of different solid carbon sources on water quality, biofloc quality and gut microbiota of Nile tilapia (Oreochromis niloticus) larvae. Aquaculture 2018, 495, 919–931. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Alizadehe, M.; Mohammedi, M. Water quality and immune function of Nile tilapia (Oreochromis niloticus) fingerlings under the influence of different times of carbohydrate addition in a limited water exchange system. ISFJ 2021, 30, 1–12. [Google Scholar]
- Emerenciano, M.; Ballester, E.L.; Cavalli, R.O.; Wasielesky, W. Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquac. Res. 2012, 43, 447–457. [Google Scholar] [CrossRef]
- Tibaldi, E.; Zittelli, G.C.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freeze-dried Isochrysis sp.(T-ISO) biomass as a source of protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture 2015, 440, 60–68. [Google Scholar] [CrossRef]
- Elashry, M.A.; Mohammady, E.Y.; Soaudy, M.R.; Ali, M.M.; El-Garhy, H.S.; Ragaza, J.A.; Hassaan, M.S. Growth, health, and immune status of Nile tilapia Oreochromis niloticus cultured at different stocking rates and fed algal β-carotene. Aquac. Rep. 2024, 35, 101987. [Google Scholar] [CrossRef]
- Judan Cruz, K.G.; Landingin, E.P.; Gajeton, M.B.; Fernando, S.I.D.; Watanabe, K. Carotenoid coloration and coloration-linked gene expression in red tilapia (Oreochromis sp.) tissues. BMC Vet. Res. 2021, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Mamauag, R.E.P. Interactive effects of vitamin C and E supplementation on growth performance, fatty acid composition and reduction of oxidative stress in juvenile Japanese flounder Paralichthys olivaceus fed dietary oxidized fish oil. Aquaculture 2014, 422, 84–90. [Google Scholar] [CrossRef]
- Chen, Y.J.; Yuan, R.M.; Liu, Y.J.; Yang, H.J.; Liang, G.Y.; Tian, L.X. Dietary vitamin C requirement and its effects on tissue antioxidant capacity of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2015, 435, 431–436. [Google Scholar] [CrossRef]
- Lozano, A.R.; Borges, P.; Robaina, L.; Betancor, M.; Hernandez-Cruz, C.M.; García, J.R.; Caballero, M.J.; Izquierdo, M. Effect of different dietary vitamin E levels on growth, fish composition, fillet quality and liver histology of meagre (Argyrosomus regius). Aquaculture 2017, 468, 175–183. [Google Scholar] [CrossRef]
- Liu, H.P.; Wen, B.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.; Zhang, Y.C.; Wang, Z.X.; Peng, Y. Effects of dietary vitamin C and vitamin E on the growth, antioxidant defence and digestive enzyme activities of juvenile discus fish (Symphysodon haraldi). Aquac. Nutr. 2019, 25, 176–183. [Google Scholar] [CrossRef]
- Sánchez, E.G.T.; Fuenmayor, C.A.; Mejía, S.M.V.; Díaz-Moreno, C.; Mahecha, H.S. Effect of bee pollen extract as a source of natural carotenoids on the growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 514, 734490. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.O.H.S.E.N.; Ahmad, M.H.; Abdel-Hadi, Y.M.; Seden, M.E. Use of Spirulina (Arthrospir platensis) as a growth and immunity promoter for nile tilapia, Oreochromis niloticus (L.) fry challenged with pathogenic Aeromonas hydrophila. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 1015–1032. [Google Scholar]
- Taalab, H.A.; Mohammady, E.Y.; Hassan, T.M.; Abdella, M.M.; Hassaan, M.S. β-Carotene of Arthrospira platensis versus vitamin C and vitamin E as a feed supplement: Effects on growth, haemato-biochemical, immune-oxidative stress and related gene expression of Nile tilapia fingerlings. Aquac. Res. 2022, 53, 4832–4846. [Google Scholar] [CrossRef]
- Chow, E.P.Y.; Liong, K.H.; Schoeters, E. The effect of dietary carotenoids of different forms: Microemulsified and non-microemulsified on the growth performance, pigmentation and hematological parameters in hybrid catfish (Clarias macrocephalus × Clarias gariepinus). J. Aquac. Res. Development 2016, 7, 2. [Google Scholar] [CrossRef]
- Panase, P.; Vongkampang, T.; Wangkahart, E.; Sutthi, N. Impacts of astaxanthin-enriched Paracoccus carotinifaciens on growth, immune responses, and reproduction performance of broodstock Nile tilapia during winter season. Fish Physiol. Biochem. 2024, 50, 1205–1224. [Google Scholar] [CrossRef]
- Zhu, X.; Hao, R.; Zhang, J.; Tian, C.; Hong, Y.; Zhu, C.; Li, G. Dietary astaxanthin improves the antioxidant capacity, immunity, and disease resistance of coral trout (Plectropomus leopardus). Fish Shellfish Immunol. 2022, 122, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zu, L.; Wang, Z.; Cheng, Y.; Yang, Y.; Wu, X. Micro-algal astaxanthin could improve the antioxidant capability, immunity and ammonia resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2020, 102, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ettefaghdoost, M.; Haghighi, H. Impact of different dietary lutein levels on growth performance, biochemical and immuno-physiological parameters of oriental river prawn (Macrobrachium nipponense). Fish Shellfish Immunol. 2021, 115, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Wiegertjes, G. Properties of Carotenoids in Fish Fitness: A Review. Marine Drugs 2020, 18, 568. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Karim, M.; Natrah, F.M.I. Carotenoids modulate stress tolerance and immune responses in aquatic animals. Rev. Aquac. 2023, 15, 872–894. [Google Scholar] [CrossRef]
- Ming, J.; Xie, J.; Xu, P.; Liu, W.; Ge, X.; Liu, B.; He, Y.; Cheng, Y.; Zhou, Q.; Pan, L. Molecular cloning and expression of two HSP70 genes in the Wuchang bream (Megalobrama amblycephala Yih). Fish Shellfish Immunol. 2010, 28, 407–418. [Google Scholar] [CrossRef]
- Niu, J.; Wen, H.; Li, C.H.; Liu, Y.J.; Tian, L.X.; Chen, X.U.; Huang, Z.; Lin, H.Z. Comparison effect of dietary astaxanthin and β-carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture 2014, 422, 8–17. [Google Scholar] [CrossRef]
- Al-Deriny, S.H.; Dawood, M.A.; Elbialy, Z.I.; El-Tras, W.F.; Mohamed, R.A. Selenium nanoparticles and spirulina alleviate growth performance, hemato-biochemical, immune-related genes, and heat shock protein in Nile tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2020, 198, 661–668. [Google Scholar] [CrossRef]
- Liu, D.; Lazado, C.C.; Pedersen, L.F.; Straus, D.L.; Meinelt, T. Antioxidative, histological and immunological responses of rainbow trout after periodic and continuous exposures to a peracetic acid-based disinfectant. Aquaculture 2020, 520, 734956. [Google Scholar] [CrossRef]
- Li, M.Y.; Guo, W.Q.; Guo, G.L.; Zhu, X.M.; Niu, X.T.; Shan, X.F.; Tian, J.X.; Wang, G.Q.; Zhang, D.M. Effects of dietary astaxanthin on lipopolysaccharide-induced oxidative stress, immune responses and glucocorticoid receptor (GR)-related gene expression in Channa argus. Aquaculture 2020, 517, 734816. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, K.; Li, S.; Ma, H.; Zheng, H. Genome-wide analysis of TRAF gene family and its response to bacterial infection in noble scallop Chlamys nobilis with different carotenoids content. Aquaculture 2021, 535, 736309. [Google Scholar] [CrossRef]
- Eldessouki, E.A.A.; Elshopakey, G.E.; Elbahnaswy, S.; Shakweer, M.S.; Abdelwarith, A.A.; Younis, E.M.; Davies, S.J.; Mili, A.; Abd El-Aziz, Y.; Abdelnour, S.A.; et al. Influence of astaxanthin-enriched Haematococcus pluvialis microalgae on the growth efficacy, immune response, antioxidant capacity, proinflammatory cytokines, and tissue histomorphology of hybrid red tilapia. Aquac. Int. 2024, 32, 7447–7468. [Google Scholar] [CrossRef]
- Xie, W.; Deng, H.; Li, K.; Ma, Y.; Gao, M.; Duan, H.; Sui, L. Dietary supplementation of archaeal carotenoids improved antioxidative capacity and regulated immune related gene expression of golden trout Oncorhynchus mykiss against challenge. Aquac. Res. 2022, 53, 5053–5062. [Google Scholar] [CrossRef]
- Ebeneezar, S.; Prabu, D.L.; Chandrasekar, S.; Tejpal, C.S.; Madhu, K.; Sayooj, P.; Vijayagopal, P. Evaluation of dietary oleoresins on the enhancement of skin coloration and growth in the marine ornamental clown fish, Amphiprion ocellaris (Cuvier, 1830). Aquaculture 2020, 529, 735728. [Google Scholar] [CrossRef]
- Wang, H.; Bai, B.; Wang, Y.; Bai, T.; Shi, W.; Wang, X.; Wang, W.; Yang, J.; Pan, S. Current trends and perspectives on the color of fish during low-temperature preservation: A focus on evaluation methods, discoloration mechanism, and protection methods. Food Chemistry 2025, 474, 143199. [Google Scholar] [CrossRef]
- Yilmaz, S.; Sebahattin, E.; Soytas, N. Enhancement of Growth Performance and Pigmentation in Red Oreochromis mossambicus Associated with Dietary Intake of Astaxanthin, Paprika, or Capsicum. Isr. J. Aquac. 2013, 65, 7. [Google Scholar] [CrossRef]
- Jorjani, M.; Rohani, M.S.; Rostami, A.M.; Ako, H.; Hwai, A.T.S. Pigmentation and growth performance in the blue gourami, Trichogaster trichopterus, fed Marigold, Calendula officinalis, powder, a natural carotenoid source. J. World Aquac. Soc. 2018, 50, 789–799. [Google Scholar] [CrossRef]




| Gen (Symbol) | Fwd Sequence (5′-3′) | Rev Sequence (5′-3′) | GenBank |
|---|---|---|---|
| gpx | GGAACGACAACCAGGGACTA | TCCCTGGACGGACATACTTC | NM_001279711.1 |
| sod | GACGTGACAACACAGGTTGC | TACAGCCACCGTAACAGCAG | JF801727.1 |
| cat | GGCCGGGTTTCTAAAAGAAG | GCTGTAAACGTGCAAAGTGG | XM_019361816.2 |
| hsp70 | CAAGATCACCATCACCAACG | TCTTGTCCTCCTCGCTGATT | NM_001279671.2 |
| tgfb | CGAGCAGCTGTCCAATATGA | AGGTCCATGGCTTAATGTGC | NM_001311325.1 |
| tfr | GAGCATCGTCCATTCCCTTA | CTCTGGCATTCAATGGAGGT | DQ272465.1 |
| tnfa | TCTGGAGTGGAGGAATGGTC | TCTGAGTAGCGCCAGATCCT | XM_025902124.1 |
| il1b | TTTTGGATCCTCAGGACAGG | GTAGCAGAACATTGGCAGCA | XM_005457887.3 |
| actb | GAGCGTGGCTACTCCTTCAC | GCAGGATTCCATACCAAGGA | EF026001.1 |
| Treatment | NH4 (mg L−1) | NO2 (mg L−1) | NO3 (mg L−1) | pH |
|---|---|---|---|---|
| CT | 0.37 ± 0.05 | 2.17 ± 0.52 | 79.16 ± 10.46 | 8.2 ± 0.3 |
| TBM | 0.41 ± 0.17 | 1.86± 0.90 | 76.66 ±6.67 | 8.3 ± 0.2 |
| TBC | 0.31 ± 0.07 | 2.18 ± 1.95 | 77.49 ± 8.24 | 8.2 ± 0.3 |
| TBB | 0.39 ± 0.17 | 2.48 ± 1.35 | 83.33 ± 9.48 | 8.1 ± 0.2 |
| Parameter | CT | TBM | TBC | TBB |
|---|---|---|---|---|
| WG (g) | 23.88 ± 0.31 a | 29.20 ± 0.16 ab | 31.43 ± 0.13 b | 29.12 ± 0.25 ab |
| LG (cm) | 8.60 ± 0.45 a | 9.84 ± 0.17 ab | 11.47 ± 0.06 b | 10.11 ± 0.25 ab |
| SGR (%) | 3.23 ± 0.31 a | 3.40 ± 0.23 ab | 3.73 ± 0.24 b | 3.43 ± 0.33 ab |
| FCR | 1.73 ± 0.01 a | 1.51 ± 0.02 b | 1.34 ± 0.02 c | 1.45 ± 0.03 d |
| SR (%) | 73.33 | 96.67 | 90.00 | 93.33 |
| K | 1.09 ± 0.2 a | 1.39 ± 0.1 ab | 1.57 ± 0.1 b | 1.30 ± 0.1 ab |
| CT | TBM | TBC | TBB | |
|---|---|---|---|---|
| Leukocyte (×104/μL) | 4.50 ± 0.40 a | 5.97 ± 0.31 bc | 6.37 ± 0.45 b | 5.65 ± 0.45 c |
| CT | TBM | TBC | TBB | |
|---|---|---|---|---|
| Optical density (500 nm) | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.02 | 0.23 ± 0.02 |
| Carotenoid content (µg) | 5.45 ± 0.6 b | 5.71 ± 0.19 b | 6.33 ± 1.13 b | 9.4 ± 1.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Castellon, A.E.; Monroy-Dosta, M.d.C.; Hamdan-Partida, A.; Hernandez-Vergara, M.P.; Castro-Mejía, J.; Castro-Mejia, G.; Martinez-Meingüer, A.M.; Mata-Sotres, J.A. Effect of the Inclusion of Natural Pigments on the Performance and Gene Expression of Immune Response and Oxidative Stress of Oreochromis niloticus Cultured in a Biofloc System. Fishes 2025, 10, 282. https://doi.org/10.3390/fishes10060282
Castro-Castellon AE, Monroy-Dosta MdC, Hamdan-Partida A, Hernandez-Vergara MP, Castro-Mejía J, Castro-Mejia G, Martinez-Meingüer AM, Mata-Sotres JA. Effect of the Inclusion of Natural Pigments on the Performance and Gene Expression of Immune Response and Oxidative Stress of Oreochromis niloticus Cultured in a Biofloc System. Fishes. 2025; 10(6):282. https://doi.org/10.3390/fishes10060282
Chicago/Turabian StyleCastro-Castellon, Andres Elias, Maria del Carmen Monroy-Dosta, Aida Hamdan-Partida, Martha Patricia Hernandez-Vergara, Jorge Castro-Mejía, German Castro-Mejia, Arnulfo Misael Martinez-Meingüer, and José Antonio Mata-Sotres. 2025. "Effect of the Inclusion of Natural Pigments on the Performance and Gene Expression of Immune Response and Oxidative Stress of Oreochromis niloticus Cultured in a Biofloc System" Fishes 10, no. 6: 282. https://doi.org/10.3390/fishes10060282
APA StyleCastro-Castellon, A. E., Monroy-Dosta, M. d. C., Hamdan-Partida, A., Hernandez-Vergara, M. P., Castro-Mejía, J., Castro-Mejia, G., Martinez-Meingüer, A. M., & Mata-Sotres, J. A. (2025). Effect of the Inclusion of Natural Pigments on the Performance and Gene Expression of Immune Response and Oxidative Stress of Oreochromis niloticus Cultured in a Biofloc System. Fishes, 10(6), 282. https://doi.org/10.3390/fishes10060282






