Morphometric, Nutritional, and Blood Analyses in Hybrid Striped Bass (Morone chrysops x Morone saxatilis, Walbaum 1972) Reared in a Recirculating Aquaculture System (RAS) Implant in Sicily, Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Morphometric and Biometric Measurements
2.3. Automatic Hematological Analysis
2.4. Proximate Body Composition
2.5. Statistical Analysis
3. Results
3.1. Morphometric and Body Indices
3.2. Hematological and Proximate Body Composition
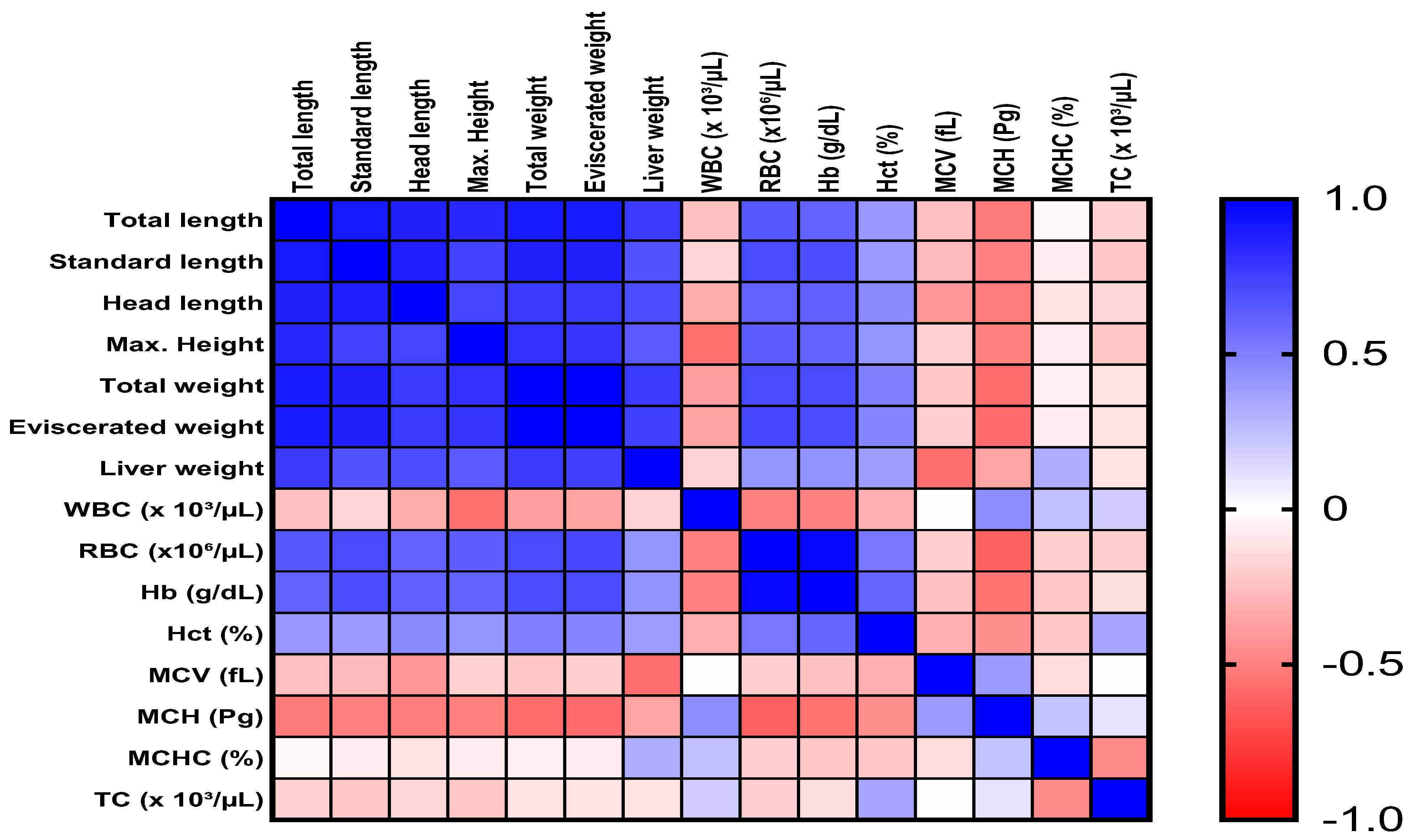
3.3. Correlation Between Morphometric Measurements and Blood Parameters in HSB
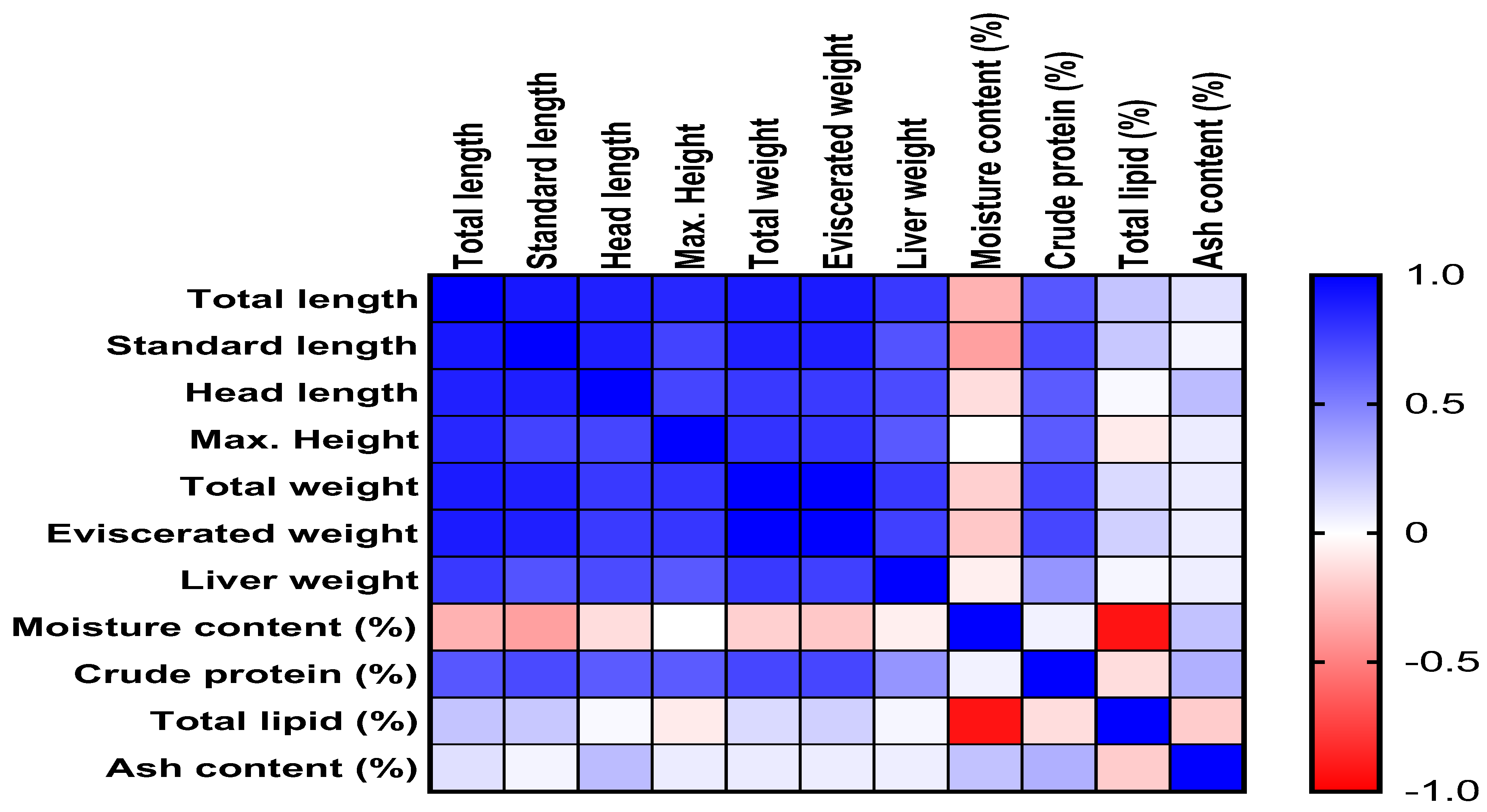
3.4. Correlation Matrix of Morphometric Traits and Proximate Composition in HSB
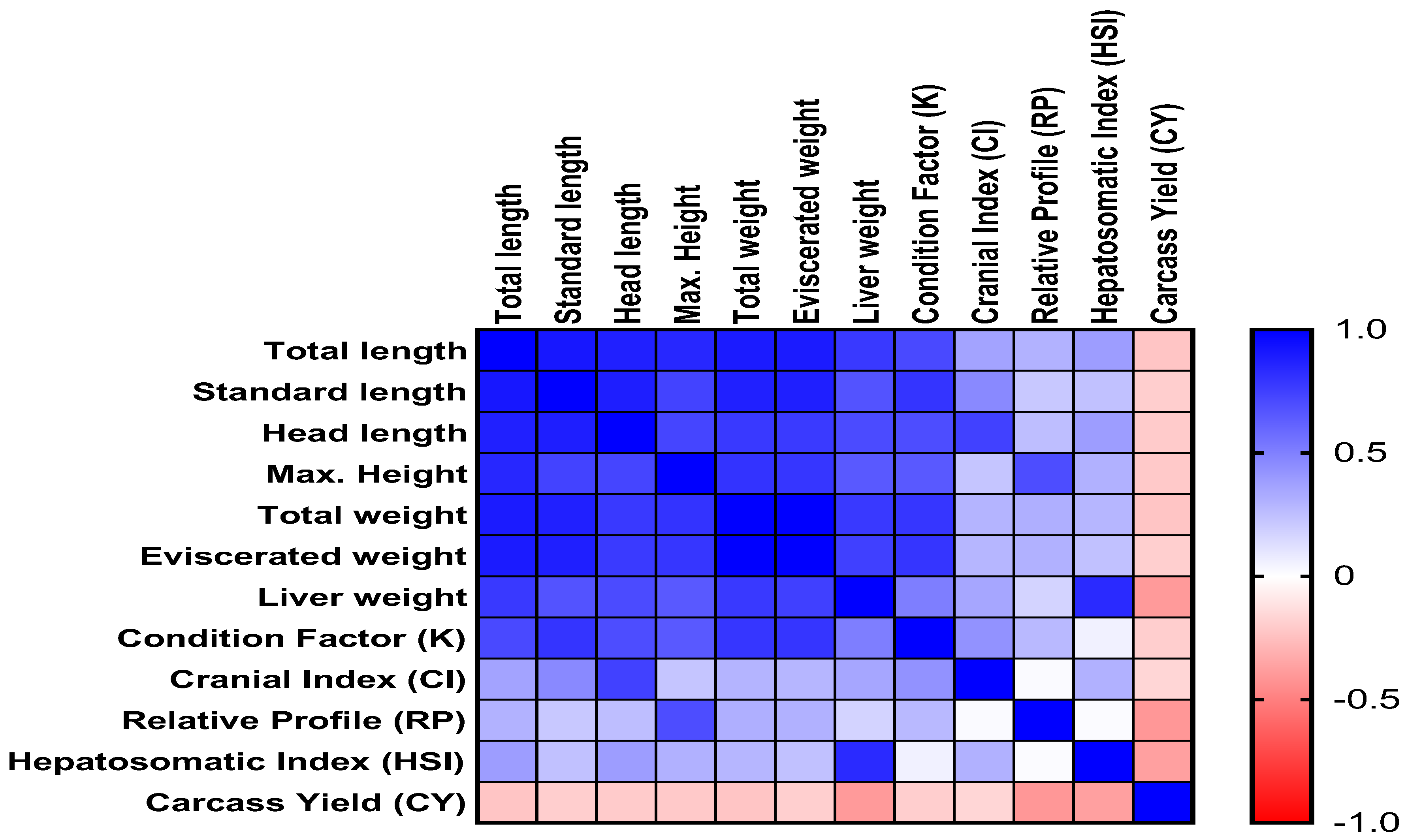
3.5. Correlation Matrix of Morphometric Parameters, Condition Indices, and Carcass Yield in HSB
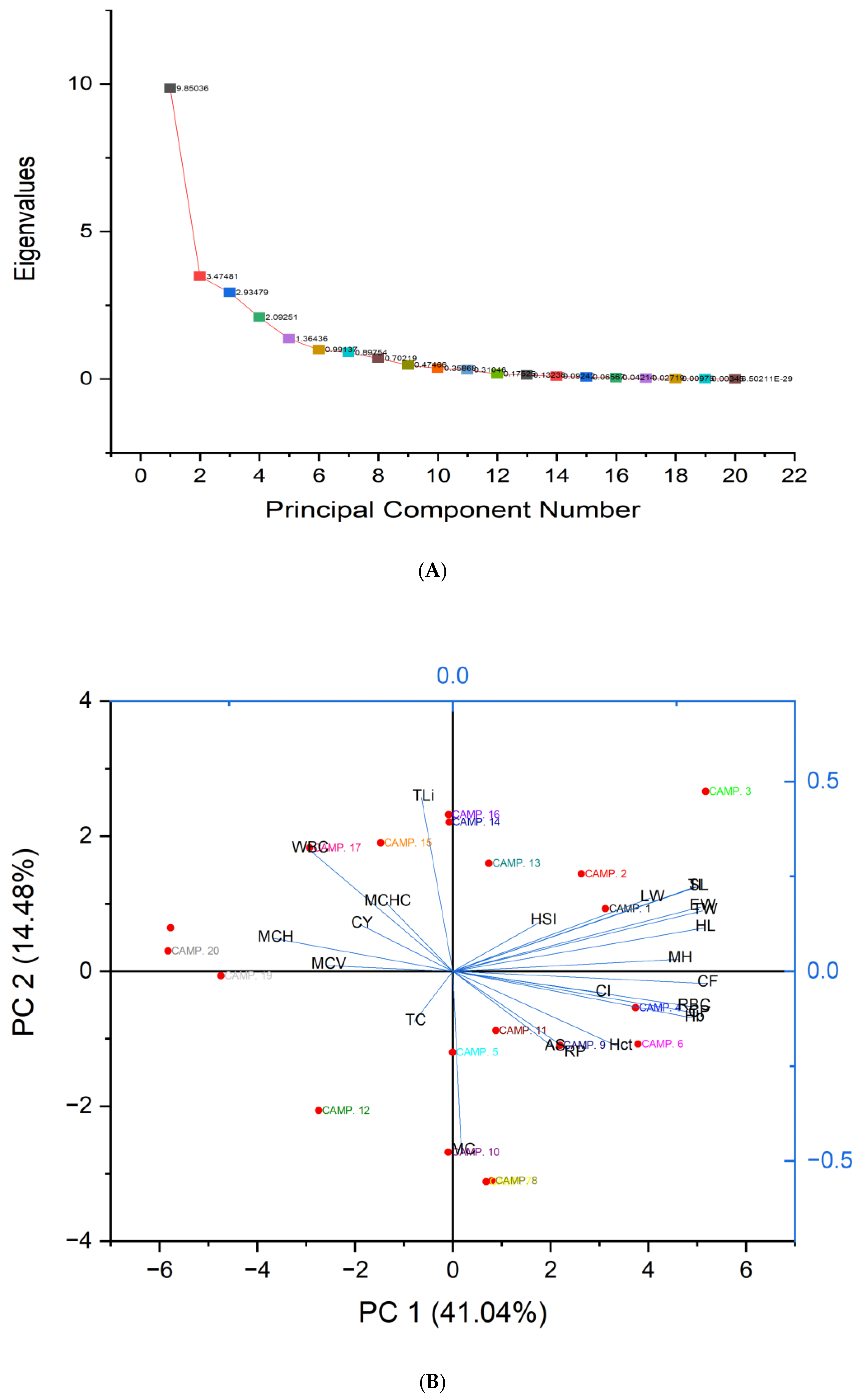
3.6. PCA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.K.; Loftus, M.; Gabriel, N.N.; Fortes-Silva, R.; Watson, T.R.; Rossi, W. Evaluation of Supplemental Carbohydrase and Protease in Plant-Based Diets for Hybrid Striped Bass (Morone saxatilis ♀ × M. chrysops ♂). J. Appl. Aquacult. 2024, 1–20. [Google Scholar] [CrossRef]
- Dawood, M.A.; Madkour, K.; Sewilam, H. Polyculture of European Seabass and Nile Tilapia in the Recirculating Aquaculture System with Brackish Water: Effects on the Growth Performance, Feed Utilization, and Health Status. Aquacult. Fish. 2023, 10, 298–304. [Google Scholar] [CrossRef]
- Fuller, S.A. Minimizing Time Fed Rotifers Maximizes Hybrid Striped Bass Larval Growth in Recirculating Aquaculture Systems. N. Am. J. Aquacult. 2020, 82, 208–214. [Google Scholar] [CrossRef]
- Zoli, M.; Rossi, L.; Fronte, B.; Aubin, J.; Jaeger, C.; Wilfart, A.; Bibbiani, C.; Bacenetti, J. Environmental Impact of Different Mediterranean Technological Systems for European Sea Bass (Dicentrarchus labrax) and Gilthead Sea Bream (Sparus aurata) Farming. Aquacult. Eng. 2024, 107, 102457. [Google Scholar] [CrossRef]
- Rosati, S.; Maiuro, L.; Lombardi, S.J.; Iaffaldano, N.; Di Iorio, M.; Cariglia, M.; Lopez, F.; Cofelice, M.; Tremonte, P.; Sorrentino, E. Integrated Biotechnological Strategies for the Sustainability and Quality of Mediterranean Sea Bass (Dicentrarchus labrax) and Sea Bream (Sparus aurata). Foods 2025, 14, 1020. [Google Scholar] [CrossRef]
- Macente, B.I.; Khan, K.U.; do Nascimento, T.M.T.; da Silva, E.P. Morphometric Growth Characteristics and Body Composition of Fish and Amphibians. In New Insights into Morphometry Studies; InTechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Brosset, P.; Averty, A.; Mathieu-Resuge, M.; Schull, Q.; Soudant, P.; Lebigre, C. Fish Morphometric Body Condition Indices Reflect Energy Reserves but Other Physiological Processes Matter. Ecol. Indic. 2023, 154, 110860. [Google Scholar] [CrossRef]
- Habib, S.S.; Fazio, F.; Naz, S.; Arfuso, F.; Piccione, G.; Rehman, H.U.; Achakzai, W.M.; Uddin, M.N.; Rind, K.H.; Rind, N.A. Seasonal Variations in Haematological Parameters and Body Composition of Labeo rohita (Rohu) and Cirrhinus mrigala (Mrigal Carp) in River Indus, District Dera Ismail Khan, Pakistan. Turk. J. Fish. Aquat. Sci. 2021, 21, 435–441. [Google Scholar] [CrossRef]
- Pan, S.; Zou, J.; Mao, H.; Hu, Z.; Sun, S.; Wu, W.; Yang, J.; An, Z.; Wang, C. Available Phosphorus Levels Modulate Growth Performance, Serum Indices, Metabolome, Rumen Fermentation, and Microorganism in Hu Lambs. Anim. Feed Sci. Technol. 2025, 322, 116259. [Google Scholar] [CrossRef]
- Dandi, S.O.; Abarike, E.D.; Ampofo-Yeboah, A. Bitter Leaf Vernonia amygdalina Extract Enhances Growth, Hematology, Heat Stress Response, and Resistance to Aeromonas hydrophila in Nile Tilapia. N. Am. J. Aquacult. 2022, 84, 432–441. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological Methods in Fish–Not Only for Beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Wang, K.; Chen, J.; Jin, K.; Peng, K.; Chen, X.; Liu, Z.; Ouyang, J.; Wang, Y.; et al. Effects of Litsea cubeba Essential Oil on Growth Performance, Blood Antioxidation, Immune Function, Apparent Digestibility of Nutrients, and Fecal Microflora of Pigs. Front. Pharmacol. 2023, 14, 1166022. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Mirghaed, A.T.; Iri, Y.; Hoseinifar, S.H.; Van Doan, H.; Reverter, M. Effects of dietary Russian olive, Elaeagnus angustifolia, leaf extract on growth, hematological, immunological, and antioxidant parameters in common carp, Cyprinus carpio. Aquaculture 2021, 536, 736461. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Cardinaletti, G.; Sigelaki, I.; Polzonetti-Magni, A. Comparative Efficacy of Clove Oil and 2-Phenoxyethanol as Anesthetics in the Aquaculture of European Sea Bass (Dicentrarchus labrax) and Gilthead Sea Bream (Sparus aurata) at Different Temperatures. Aquaculture 2005, 246, 467–481. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Costa, G.; Zumbo, A.; Piccione, G.; Parrino, V. Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): A new hematological approach. Aquaculture 2019, 513, 734398. [Google Scholar] [CrossRef]
- Fazio, F.; Faggio, C.; Torre, A.; Sanfilippo, M.; Piccione, G. Effect of water quality on hematological and biochemical parameters of Gobius niger caught in Faro lake (Sicily). Iran J. Fish. Sci. 2013, 12, 219–231. [Google Scholar]
- Fazio, F.; Saoca, C.; Casella, S.; Fortino, G.; Piccione, G. Relationship between blood parameters and biometric indices of Sparus aurata and Dicentrarcus labrax cultured in onshore tanks. Mar. Freshw. Behav. Physiol. 2015, 48, 289–296. [Google Scholar] [CrossRef]
- Fazio, F.; Arfuso, F.; Levanti, M.; Saoca, C.I.; Piccione, G. High stocking density and water salinity levels influence haematological and serum protein profiles in mullet Mugil cephalus, Linnaeus, 1758. Cah. Biol. Mar. 2017, 58, 331–339. [Google Scholar]
- Fazio, F.; Saoca, C.; Vazzana, I.; Piccione, G. Influence of body size on blood hemogram in rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Vet. Med. Open J. 2017, 2, 91–94. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Roy, D.; Saha, P.K.; Sarker, S.; Parves, M.A.; Zavyalov, A.P.; Latifa, G.A. Culture Practice of Oreochromis niloticus through Recirculating Aquaculture System (RAS): A Modern and Growth-Optimized Approach of Fish Culture by Maintaining Water Quality with Proper Fish Stocking Density in Bangladesh. Environ. Sci. Pollut. Res. 2025, 32, 11749–11766. [Google Scholar] [CrossRef]
- Anil, M.K.; Gomathi, P.; Raheem, P.K.; Raju, B.; Philipose, K.K.; Gopalakrishnan, A. Captive broodstock development, breeding and seed production of Anthid fish (family: Serranidae) Marcia’s anthias, Pseudanthias marcia, in recirculation aquaculture system (RAS). Aquaculture 2018, 492, 265–272. [Google Scholar] [CrossRef]
- Timmerhaus, G.; Lazado, C.C.; Cabillon, N.A.R.; Reiten, B.K.M.; Johansen, L.H. The optimum velocity for Atlantic salmon post-smolts in RAS is a compromise between muscle growth and fish welfare. Aquaculture 2021, 532, 736076. [Google Scholar] [CrossRef]
- Murray, F.; Bostock, J.; Fletcher, D. Review of Recirculation Aquaculture System Technologies and Their Commercial Application; Highlands and Islands Enterprise: Inverness, UK, 2014. [Google Scholar]
- Karachle, P.K.; Stergiou, K.I. Morphometrics and allometry in fishes. In Morphometrics; InTechOpen: London, UK, 2012; Volume 2, pp. 65–86. [Google Scholar] [CrossRef]
- Mojekwu, T.O.; Anumudu, C.I. Advanced Techniques for Morphometric Analysis in Fish. J. Aquac. Res. Dev. 2015, 6, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Cao, D.; Yang, J.; Mao, H.; Sun, L.; Wang, C. Integrated Multi-Omics Reveals the Relationship between Growth Performance, Rumen Microbes and Metabolic Status of Hu Sheep with Different Residual Feed Intakes. Anim. Nutr. 2024, 18, 284–295. [Google Scholar] [CrossRef]
- Abdelmonaim, M.; Radouane, E.M.; Abdelkader, C.; Mourad, D.; Youssef, O.; Abderrazzaq, B.; Mhamed, K.; Taouraout, A. Evaluating the Quality of Groundwater in the Zagora Region, Southeast Morocco, Using GIS and the Water Quality Index (WQI). Geol. Ecol. Landsc. 2024, 1–17. [Google Scholar] [CrossRef]
- Péter, G.; Lukić, J.; Alvestad, R.; Horváth, Z.; Nagy, Z.; Rónyai, A.; Bársony, P.; Ljubobratović, U. Nursing of pike-perch (Sander lucioperca) in Recirculating Aquaculture System (RAS) provides growth advantage in juvenile growth phase. Animals 2023, 13, 347. [Google Scholar] [CrossRef]
- Abanikannda, O.T.F.; Jimoh, A.A.; Giwa, A.O.; Awosanya, L.A. Sexual dimorphism in body weight, morphometric measures and indices of African Catfish (Clarias gariepinus). Aquaculture 2019, 502, 148–152. [Google Scholar] [CrossRef]
- Jargal, N.; Atique, U.; Kim, J.Y.; Mamun, M.; An, K.G. Functional Trait Analysis and the Multi-Metric Integrity Model, Based on Stream Fish Indicators, and Their Relations to Chemical Water Quality. Water Air Soil Pollut. 2022, 233, 511. [Google Scholar] [CrossRef]
- Moustaine Radouane, E.; Abdelkader, C.; Mhamed, K.; El Habib, R.; Mokhtar, B. Groundwater Quality and Aquatic Fauna of Some Wells and Springs from Meknes Area (Morocco). Geol. Ecol. Landsc. 2024, 8, 327–338. [Google Scholar] [CrossRef]
- Dawood, M.A.; Metwally, A.E.S.; El-Sharawy, M.E.; Atta, A.M.; Elbialy, Z.I.; Abdel-Latif, H.M.; Paray, B.A. The Role of β-Glucan in the Growth, Intestinal Morphometry, and Immune-Related Gene and Heat Shock Protein Expressions of Nile Tilapia (Oreochromis niloticus) under Different Stocking Densities. Aquaculture 2020, 523, 735205. [Google Scholar] [CrossRef]
- Vallecillos, A.; María-Dolores, E.; Villa, J.; Rueda, F.M.; Carrillo, J.; Ramis, G.; Soula, M.; Afonso, J.M.; Armero, E. Phenotypic and Genetic Components for Growth, Morphology, and Flesh-Quality Traits of Meagre (Argyrosomus regius) Reared in Tank and Sea Cage. Animals 2021, 11, 3285. [Google Scholar] [CrossRef]
- Badran, M.F.; Ali, M.A.; Yusuf, M.S. The growth performance, haematological parameters and serum biochemistry of the flathead grey mullet Mugil cephalus under recirculating aquaculture system and traditional culture systems. J. Anim. Physiol. Anim. Nutr. 2024, 109, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Hisano, H.; Barbosa, P.T.L.; de Arruda Hayd, L.; Mattioli, C.C. Comparative study of growth, feed efficiency, and hematological profile of Nile tilapia fingerlings in biofloc technology and recirculating aquaculture system. Trop. Anim. Health Prod. 2021, 53, 60. [Google Scholar] [CrossRef] [PubMed]
- Rind, K.H.; Arshad, M.; Majeed, S.; Habib, S.S.; Al-Rejaie, S.S.; Mohany, M.; Aragona, F.; Fazio, F. Impact of heavy metals on health and quality of Oreochromis niloticus cultured in biofloc and earthen pond systems. J. Environ. Sci. Health Part B 2025, 60, 129–137. [Google Scholar] [CrossRef]
- Tabarrok, M.; Seyfabadi, J.; Salehi Jouzani, G.; Younesi, H. Comparison between recirculating aquaculture and biofloc systems for rearing juvenile common carp (Cyprinus carpio): Growth performance, haemato-immunological indices, water quality and microbial communities. Aquacult. Res. 2020, 51, 4881–4892. [Google Scholar] [CrossRef]
- Zafar, A.; Roni, M.A.; Rana, M.; Akter, N. Growth, digestive enzyme activities, proximate composition and hemato-biochemical responses of juvenile Nile tilapia (Oreochromis niloticus) reared at various stocking densities in a recirculatory aquaculture system. J. Appl. Aquacult. 2023, 35, 1179–1201. [Google Scholar] [CrossRef]
- Barbacariu, C.A.; Rimbu, C.M.; Burducea, M.; Dirvariu, L.; Miron, L.D.; Boiangiu, R.S.; Dumitru, G.; Todirascu-Ciornea, E. Comparative study of flesh quality, blood profile, antioxidant status, and intestinal microbiota of European catfish (Silurus glanis) cultivated in a recirculating aquaculture system (RAS) and earthen pond system. Life 2023, 13, 1282. [Google Scholar] [CrossRef]
| Parameters | Mean ± SD | Min–Max |
|---|---|---|
| T (°C) | 23.5 ± 1.2 | 21.0–25.0 |
| DO (mg/L) | 6.2 ± 0.4 | 5.5–7.0 |
| pH | 7.1 ± 0.2 | 6.8–7.4 |
| NH3 (mg/L) | 0.07 ± 0.02 | 0.03–0.10 |
| NO2 (mg/L) | 0.18 ± 0.04 | 0.10–0.22 |
| NO3 (mg/L) | 190 ± 20 | 160–210 |
| CTD (μS/cm) | 1450 ± 300 | 400–2000 |
| Parameters | Mean ± SD | Min | Max |
|---|---|---|---|
| Total length (cm) | 37.50 ± 2.57 | 33 | 42.5 |
| Standard length (cm) | 28.33 ± 1.04 | 27.5 | 36.5 |
| Head length (cm) | 9.86 ± 0.87 | 8 | 11.5 |
| Max. height (cm) | 8.62 ± 0.82 | 7 | 10 |
| Total weight (g) | 571.33 ± 129.32 | 300 | 802 |
| Eviscerated weight (g) | 495.05 ± 109.94 | 268 | 692 |
| Liver weight (g) | 10.76 ± 3.87 | 6 | 18 |
| Condition factor (K) | 0.149 ± 0.027 | 0.090 | 0.190 |
| Cranial index (CI) | 0.261 ± 0.011 | 0.240 | 0.280 |
| Relative profile (RP) | 0.228 ± 0.012 | 0.210 | 0.260 |
| Hepatosomatic index (HSI) | 1.845 ± 0.433 | 1.100 | 2.900 |
| Carcass yield (CY) | 86.680 ± 1.461 | 83.600 | 89.300 |
| Parameters | Mean ± SD | Min | Max |
|---|---|---|---|
| WBC (×103/µL) | 28.82 ± 4.93 | 20.5 | 36.4 |
| RBC (×106/µL) | 3.16 ± 0.28 | 2.46 | 3.8 |
| Hb (g/dL) | 7.54 ± 0.85 | 5.7 | 9 |
| Hct (%) | 45.40 ± 4.81 | 34.1 | 52.3 |
| MCV (fL) | 145.38 ± 12.44 | 109 | 160 |
| MCH (Pg) | 24.03 ± 2.25 | 20.6 | 28 |
| MCHC (%) | 17.22 ± 3.22 | 13.9 | 28 |
| Parameters | Mean ± SD | Min | Max |
|---|---|---|---|
| Moisture content (%) | 75.55 ± 1.49 | 73.31 | 77.44 |
| Crude protein (%) | 20.29 ± 0.26 | 19.75 | 20.78 |
| Total lipid (%) | 4.24 ± 0.97 | 2.73 | 5.66 |
| Ash content (%) | 1.69 ± 0.17 | 1.33 | 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragona, F.; Habib, S.S.; Fazio, F.; Zumbo, A.; Costa, A.; Riolo, K.; Giannetto, A.; Parrino, V. Morphometric, Nutritional, and Blood Analyses in Hybrid Striped Bass (Morone chrysops x Morone saxatilis, Walbaum 1972) Reared in a Recirculating Aquaculture System (RAS) Implant in Sicily, Italy. Fishes 2025, 10, 278. https://doi.org/10.3390/fishes10060278
Aragona F, Habib SS, Fazio F, Zumbo A, Costa A, Riolo K, Giannetto A, Parrino V. Morphometric, Nutritional, and Blood Analyses in Hybrid Striped Bass (Morone chrysops x Morone saxatilis, Walbaum 1972) Reared in a Recirculating Aquaculture System (RAS) Implant in Sicily, Italy. Fishes. 2025; 10(6):278. https://doi.org/10.3390/fishes10060278
Chicago/Turabian StyleAragona, Francesca, Syed Sikandar Habib, Francesco Fazio, Alessandro Zumbo, Antonino Costa, Kristian Riolo, Alessia Giannetto, and Vincenzo Parrino. 2025. "Morphometric, Nutritional, and Blood Analyses in Hybrid Striped Bass (Morone chrysops x Morone saxatilis, Walbaum 1972) Reared in a Recirculating Aquaculture System (RAS) Implant in Sicily, Italy" Fishes 10, no. 6: 278. https://doi.org/10.3390/fishes10060278
APA StyleAragona, F., Habib, S. S., Fazio, F., Zumbo, A., Costa, A., Riolo, K., Giannetto, A., & Parrino, V. (2025). Morphometric, Nutritional, and Blood Analyses in Hybrid Striped Bass (Morone chrysops x Morone saxatilis, Walbaum 1972) Reared in a Recirculating Aquaculture System (RAS) Implant in Sicily, Italy. Fishes, 10(6), 278. https://doi.org/10.3390/fishes10060278











