L-Arginine Effect as an Additive on Overall Performance, Health Status, and Expression of Stress Molecular Markers in Nile Tilapia (Oreochromis niloticus) Under Chronic Salinity Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Diet Design
2.3. Proximal Composition
2.4. Sampling
2.5. Hematology Assay
2.6. Serum Biochemical Parameters
2.7. RNA Extraction and Quantitative PCR
2.8. Statistical Analysis
3. Results
3.1. Performance and Biological Index
3.2. Hematology
3.3. Differential Leukocyte Count
3.4. Micronucleus and Nuclear Aberrations Assay
3.5. Serum Biochemistry Parameters
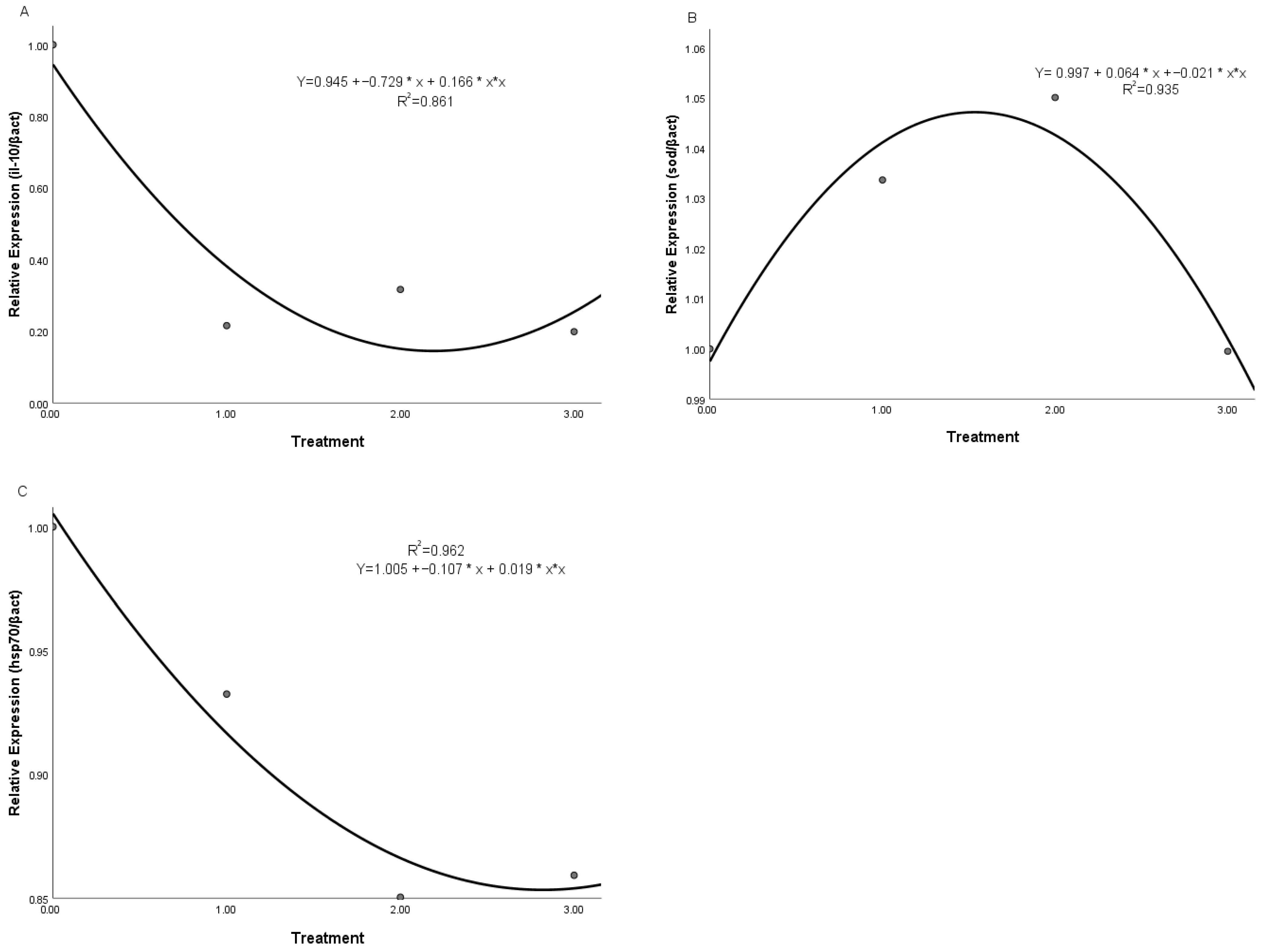
3.6. Gene Relative Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ‰ | Parts per tdousand |
| RAS | Recirculation system |
| GLY | Glycine |
| Arg | Arginine |
| CP | Crude protein |
| NFE | Nitrogen-free extract |
| SGR | Specific growtd rate |
| FCR | Feed conversion ratio |
| CF | Condition factor |
| SR | Survival rate |
| HIS | Hepatosomatic index |
| VSI | Viscerosomatic index |
| Hb | Hemoglobin |
| Hct | Hematocrit |
| RBC | Red blood cell |
| WBC | White blood cell |
| MVC | Mean corpuscular volume |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| hsp 70 | Heat shock protein 70 |
| Sod | Superoxide dismutase |
| il-10 | Interleukin-10 |
References
- Food and Agriculture Organization of the United Nations (FAO). El Estado Mundial de La Pesca y La Acuicultura 2024; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. Climate Change 2023: Synthesis Report. In Contribution of Working Groups I, II, and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). El Estado Mundial de La Pesca y La Acuicultura 2020; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Zhang, X.; Zou, C.; Lin, L. Improvement of Muscle Quality in Tilapia (Oreochromis niloticus) with Dietary Faba Bean (Vicia faba L.). Front. Nutr. 2023, 10, 1153323. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Evaluación del Estado de Avance de Políticas y Planes de Adaptación al Cambio Climático en la Acuicultura en América Latina y el Caribe; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Kombat, E.O.; Zhao, J.; Godwin, A.; Owusu-Afriyie, G.; Birteeb, P.T.; Alhassan, E.H. Metabolic Cost of Acute and Chronic Exposure of Nile Tilapia (Oreochromis niloticus) to Different Levels of Salinity. Aquac. Res. 2021, 52, 6152–6163. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Tilapia del Nilo; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2024; Available online: https://www.fao.org/fishery/affris/perfiles-de-las-especies/nile-tilapia/tilapia-del-nilo-pagina-principal/es/ (accessed on 15 April 2025).
- Fitzsimmons, K. Oreochromis mossambicus (Mozambique tilapia); CABI Compendium: Wallingford, UK, 2019. [Google Scholar] [CrossRef]
- Munguti, J.M.; Nairuti, R.; Iteba, J.O.; Obiero, K.O.; Kyule, D.; Opiyo, M.A.; Abwao, J.; Kirimi, J.G.; Outa, N.; Muthoka, M.; et al. Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) Culture in Kenya: Emerging Production Technologies and Socio-Economic Impacts on Local Livelihoods. Aquac. Fish Fish. 2022, 2, 265–276. [Google Scholar] [CrossRef]
- Tesfahun, A.; Alebachew, S. Food and Feeding Habits of the Nile Tilapia Oreochromis niloticus (Linnaeus, 1758) from Ribb Reservoir, Lake Tana Sub-Basin, Ethiopia. Cogent Food Agric. 2023, 9, 2212457. [Google Scholar] [CrossRef]
- Foroutan, B.; Pongtippatee, P.; Kerdmusic, C.; Sirimanapong, W.; Vanichviriyakit, R.; Withyachumnarnkul, B. Myo-Inositol Supplement Helps the Performance of Seawater-Acclimated Nile Tilapia, Oreochromis niloticus. Aquac. Fish. 2024, 9, 597–602. [Google Scholar] [CrossRef]
- Rairat, T.; Liu, Y.-K.; Hsu, J.C.-N.; Hsieh, C.-Y.; Chuchird, N.; Chou, C.-C. Combined effects of temperature and salinity on the pharmacokinetics of florfenicol in Nile Tilapia (Oreochromis niloticus) reared in brackish water. Front. Vet. Sci. 2022, 9, 826586. [Google Scholar] [CrossRef]
- Larumbe-Morán, E.; Hernández-Vergara, M.P.; Olvera-Novoa, M.A.; Pérez Rostro, C.I. Protein Requirements of Nile Tilapia (Oreochromis niloticus) Fry Cultured at Different Salinities. Aquac. Res. 2010, 41, 1150–1157. [Google Scholar] [CrossRef]
- Meurer, F.; Novodworski, J.; Bombardelli, R.A. Protein Requirements in Nile Tilapia (Oreochromis niloticus) during Production and Reproduction Phases. Aquac. Fish. 2024, 10, 171–182. [Google Scholar] [CrossRef]
- Wu, L.; Liang, H.; Chama; Ge, X.; Ji, K.; Yu, H.; Huang, D.; Xu, H.; Ren, M. Culture Salinity Alters Dietary Protein Requirement, Whole Body Composition and Nutrients Metabolism Related Genes Expression in Juvenile Genetically Improved Farmed Tilapia (GIFT) (Oreochromis niloticus). Aquaculture 2020, 531, 735961. [Google Scholar] [CrossRef]
- Martins, A.W.S.; Dellagostin, E.N.; Blödorn, E.B.; Silveira, T.L.R.; Sampaio, L.A.; Komninou, E.R.; Varela Junior, A.S.; Corcini, C.D.; Nunes, L.S.; Remião, M.H.; et al. Exposure to salinity induces oxidative damage and changes in the expression of genes related to appetite regulation in Nile tilapia (Oreochromis niloticus). Front. Genet. 2022, 13, 948228. [Google Scholar] [CrossRef]
- Kombat, E.O.; Abakari, G.; Alhassan, E.H.; Zhao, J.-L. Effect of Acute and Chronic Salinity Exposure on the Amino Acid Composition in Muscle, Intestine and Gill Tissues of Nile Tilapia (Oreochromis niloticus). Aquaculture 2023, 570, 739406. [Google Scholar] [CrossRef]
- Balasch, J.C.; Tort, L. Netting the Stress Responses in Fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, T.; Zhang, T.; Sun, M.; Ning, Z.; Chen, Y.; Mu, W. Effects of Chronic Saline-Alkaline Stress on Gill, Liver and Intestinal Histology, Biochemical, and Immune Indexes in Amur Minnow (Phoxinus lagowskii). Aquaculture 2024, 579, 740153. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish. Fish Physiol. 2016, 35, 1–34. [Google Scholar] [CrossRef]
- Sun, S.; Gong, C.; Deng, C.; Yu, H.; Zheng, D.; Wang, L.; Sun, J.; Song, F.; Luo, J. Effects of Salinity Stress on the Growth Performance, Health Status, and Intestinal Microbiota of Juvenile Micropterus Salmoides. Aquaculture 2023, 576, 739888. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, J.; Peña-Herrejón, G.A.; Aguirre-Becerra, H. Fish Responses to Alternative Feeding Ingredients under Abiotic Chronic Stress. Animals 2024, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Kunzmann, A.; Henjes, J.; Slater, M.J. Can Dietary Manipulation Mitigate Extreme Warm Stress in Fish? The Case of European Seabass, Dicentrarchus labrax. Aquaculture 2021, 545, 737153. [Google Scholar] [CrossRef]
- Herrera, M.; Mancera, J.M.; Costas, B. The Use of Dietary Additives in Fish Stress Mitigation: Comparative Endocrine and Physiological Responses. Front. Endocrinol. 2019, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Prakash, S.; Kumar, S. Role of Dietary Supplements in Stress Amelioration of Teleost Fishes. In Outlook of Climate Change and Fish Nutrition; Springer: Singapore, 2022; pp. 287–310. [Google Scholar] [CrossRef]
- Salamanca, N.; Moreno, O.; Giráldez, I.; Morales, E.; de la Rosa, I.; Herrera, M. Effects of Dietary Phenylalanine and Tyrosine Supplements on the Chronic Stress Response in the Seabream (Sparus aurata). Front. Physiol. 2022, 12, 775771. [Google Scholar] [CrossRef]
- Herrera, M.; Matias, A.C.; Soares, F.; Ribeiro, L.; Moreira, M.; Salamanca, N.; Jerez-Cepa, I.; Mancera, J.M.; Astola, A. Effect of Amino Acid Supplementation and Stress on Expression of Molecular Markers in Meagre (Argyrosomus regius). Aquaculture 2021, 534, 736238. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress Management in Aquaculture: A Review of Dietary Interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Ding, L.; Chen, J.; He, F.; Chen, Q.; Li, Y.; Chen, W. Effects of Dietary Arginine Supplementation on Growth Performance, Antioxidant Capacity, Intestinal Digestive Enzyme Activity, Muscle Transcriptome, and Gut Health of Siniperca chuatsi. Front. Mar. Sci. 2024, 10, 1305192. [Google Scholar] [CrossRef]
- Vijayaram, S.; Ringø, E.; Zuorro, A.; van Doan, H.; Sun, Y. Beneficial Roles of Nutrients as Immunostimulants in Aquaculture: A Review. Aquac. Fish. 2024, 9, 707–720. [Google Scholar] [CrossRef]
- Varghese, T.; Rejish Kumar, V.; Gopan, A.; Valappil, R.K.; Sajina, K.A.; Mishal, P.; Pal, A.K. Dietary Arginine Modulates Nonspecific Immune Responses in Indian Major Carp, Cirrhinus mrigala Exposed to Hypoxia. Aquaculture 2020, 529, 735613. [Google Scholar] [CrossRef]
- Qi, C.; Wang, X.; Han, F.; Chen, X.; Li, E.; Zhang, M.; Qin, J.G.; Chen, L. Dietary Arginine Alleviates the Oxidative Stress, Inflammation and Immunosuppression of Juvenile Chinese Mitten Crab Eriocheir sinensis under High PH Stress. Aquac. Rep. 2021, 19, 100619. [Google Scholar] [CrossRef]
- Costas, B.; Conceição, L.E.C.; Dias, J.; Novoa, B.; Figueras, A.; Afonso, A. Dietary Arginine and Repeated Handling Increase Disease Resistance and Modulate Innate Immune Mechanisms of Senegalese Sole (Solea senegalensis Kaup, 1858). Fish Shellfish. Immunol. 2011, 31, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Z.; Ai, Q. Arginine Metabolism and Its Functions in Growth, Nutrient Utilization, and Immunonutrition of Fish. Anim. Nutr. 2021, 7, 716–727. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khan, M.A.; Yousefi, M.; Costas, B. Roles of Arginine in Fish Nutrition and Health: Insights for Future Researches. Rev. Aquac. 2020, 12, 2091–2108. [Google Scholar] [CrossRef]
- Costas, B.; Rêgo, P.C.N.P.; Conceição, L.E.C.; Dias, J.; Afonso, A. Dietary Arginine Supplementation Decreases Plasma Cortisol Levels and Modulates Immune Mechanisms in Chronically Stressed Turbot (Scophthalmus maximus). Aquac. Nutr. 2013, 19, 25–38. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Wu, Z. Dietary L-Arginine Supplementation of Tilapia (Oreochromis niloticus) Alters the Microbial Population and Activates Intestinal Fatty Acid Oxidation. Amino Acids 2021, 54, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Liu, N.; Chen, J.; Guo, L.; Dai, Z.; Wang, C.; Wu, Z.; Wu, G. Dietary L-Arginine Supplementation Reduces Lipid Accretion by Regulating Fatty Acid Metabolism in Nile Tilapia (Oreochromis niloticus). J. Anim. Sci. Biotechnol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Vianna, R.A.; Chideroli, R.T.; Costa, A.R.; Filho, O.P.R.; Oliveira, L.L.; Donzele, J.L.; Lanna, E.A.T.; Gonçalves, D.D.; Pereira, U.d.P. Effect of Experimental Arginine Supplementation on the Growth, Immunity and Resistance of Tilapia Fingerlings to Streptococcus agalactiae. Aquac. Res. 2020, 51, 1276–1283. [Google Scholar] [CrossRef]
- Yousefi, M.; Abtahi, B.; Adineh, H.; Hoseinifar, S.H.; Taheri Mirghaed, A.; Paolucci, M.; Van Doan, H. Effects of Dietary Arginine Supplementation on Cytokine- and Antioxidant-Related Gene Expressions in Common Carp (Cyprinus carpio) Fingerling during Ammonia Toxicity. Aquac. Res. 2021, 52, 2751–2758. [Google Scholar] [CrossRef]
- Neu, D.; Boscolo, W.; Zaminhan, M.; Almeida, F.; Sary, C.; Furuya, W. Growth Performance, Biochemical Responses, and Skeletal Muscle Development of Juvenile Nile Tilapia, Oreochromis niloticus, Fed with Increasing Levels of Arginine. J. World Aquac. Soc. 2016, 47, 248–259. [Google Scholar] [CrossRef]
- Yue, Y.; Zou, Z.; Zhu, J.; Li, D.; Xiao, W.; Han, J.; Yang, H. Effects of Dietary Arginine on Growth Performance, Feed Utilization, Haematological Parameters and Non-Specific Immune Responses of Juvenile Nile Tilapia (Oreochromis niloticus L.). Aquac. Res. 2013, 46, 1801–1809. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Analytical Chemists: Arlington, VA, USA, 2015. [Google Scholar]
- Del Rio-Zaragoza, O.B.; Hernández-Rodríguez, M.; Bücle-Ramirez, L.F. Thermal Stress Effect on Tilapia Oreochromis mossambicus (Pisces: Cichlidae) Blood Parameters. Mar. Freshw. Behav. Physiol. 2008, 41, 79–89. [Google Scholar] [CrossRef]
- Natt, M.P.; Herrick, C.A. A New Blood Diluent for Counting the Erythrocytes and Leucocytes of the Chicken. Poult. Sci. 1952, 31, 735–738. [Google Scholar] [CrossRef]
- Melo, K.M.; Grisolia, C.K.; Pieczarka, J.C.; de Souza, L.R.; de Souza Filho, J.; Nagamachi, C.Y. FISH in Micronucleus Test Demonstrates Aneugenic Action of Rotenone in a Common Freshwater Fish Species, Nile Tilapia (Oreochromis niloticus). Mutagenesis 2014, 29, 215–219. [Google Scholar] [CrossRef]
- Hussain, B.; Sultana, T.; Sultana, S.; Masoud, M.S.; Ahmed, Z.; Mahboob, S. Fish Eco-Genotoxicology: Comet and Micronucleus Assay in Fish Erythrocytes as in Situ Biomarker of Freshwater Pollution. Saudi J. Biol. Sci. 2017, 25, 393–398. [Google Scholar] [CrossRef]
- Mata-Sotres, J.A.; Flores-Salas, C.; Skrzynska, A.K.; Tinajero, A.; Araújo, B.C.; Viana, M.T. Lipid Metabolism in Juvenile of Yellowtail, Seriola dorsalis Fed Diets Containing Different Lipid Levels. Aquaculture 2022, 550, 737870. [Google Scholar] [CrossRef]
- Metwaly, S.; Nasr, H.; Ahmed, K.; Fathi, M. Multifaceted Stress Response in Nile Tilapia (Oreochromis niloticus) Fingerlings: Integrative Analysis of Salinity, Ammonia, and Stocking Density Effects on Growth, Physiology, and Gene Expression. Fish Physiol. Biochem. 2025, 51, 48. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Saad, M.F.; Shukry, M.; El-Keredy, A.M.; Nasif, O.; Van Doan, H.; Dawood, M.A. Physiological and Ion Changes of Nile Tilapia (Oreochromis niloticus) under the Effect of Salinity Stress. Aquac. Rep. 2020, 19, 100567. [Google Scholar] [CrossRef]
- Furuya, W.M.; da Cruz, T.P.; Gatlin, D.M. Amino Acid Requirements for Nile Tilapia: An Update. Animals 2023, 13, 900. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Jiang, J.; Jiang, W.; Kuang, S.; Tang, L.; Tang, W.; Zhang, Y.-A.; Zhou, X.; Feng, L. Effect of Dietary Arginine on the Immune Response and Gene Expression in Head Kidney and Spleen Following Infection of Jian Carp with Aeromonas hydrophila. Fish. Shellfish. Immunol. 2015, 44, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, R.Y.; Santos, R.F.B.; Pala, G.; Gallani, S.U.; Valladão, G.M.R.; Morais, G.C.; Lee, J.T.; Sousa, N.d.C.; Cunha, F.d.S.; Maria, A.N.; et al. Supplementation with Arginine in the Diet of Nile Tilapia Reared in Net Cages. Pesqui. Agropecuária Bras. 2019, 54, e01099. [Google Scholar] [CrossRef]
- Pohlenz, C.; Buentello, A.; Helland, S.l.J.; Gatlin, D.M. Effects of Dietary Arginine Supplementation on Growth, Protein Optimization and Innate Immune Response of Channel Catfish Ictalurus punctatus (Rafinesque 1818). Aquac. Res. 2012, 45, 491–500. [Google Scholar] [CrossRef]
- Khan, M.M.; Moniruzzaman; Mostakim, G.M.; Khan, M.S.R.; Rahman, K.; Islam, M.S. Aberrations of the Peripheral Erythrocytes and Its Recovery Patterns in a Freshwater Teleost, Silver Barb Exposed to Profenofos. Environ. Pollut. 2017, 234, 830–837. [Google Scholar] [CrossRef]
- Prabu, E.; Felix, N.; Uma, A. Dietary Arginine Requirement in Diets of GIFT Strain of Nile Tilapia, Oreochromis niloticus: Effects on Growth Performance, Whole-Body Composition, Growth-Related Gene Expression and Haemato-Biochemical Responses. Aquac. Res. 2021, 52, 4816–4828. [Google Scholar] [CrossRef]
- Caruso, M.A.; Sheridan, M.A. New Insights into the Signaling System and Function of Insulin in Fish. Gen. Comp. Endocrinol. 2011, 173, 227–247. [Google Scholar] [CrossRef]
- Sink, T.D.; Lochmann, R.T. Insulin Response of Largemouth Bass to Glucose, Amino Acid, and Diet Stimulation. North. Am. J. Aquac. 2007, 69, 429–434. [Google Scholar] [CrossRef]
- Andersen, S.M.; Taylor, R.; Holen, E.; Aksnes, A.; Espe, M. Arginine Supplementation and Exposure Time Affects Polyamine and Glucose Metabolism in Primary Liver Cells Isolated from Atlantic Salmon. Amino Acids 2014, 46, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Lv, Y.; Hou, L.; Jia, Z.; Lin, S.; Wang, S.; He, X.; Hou, J. Effect of Acute Temperature Stress on Energy Metabolism, Immune Performance and Gut Microbiome of Largemouth Bass (Micropterus Salmoides). Aquac. Fish. 2023, 10, 260–270. [Google Scholar] [CrossRef]
- Chuphal, N.; Sardar, P.; Sahu, N.P.; Shamna, N.; Krishnan, S.; Varghese, T.; Malik, M.A.; Maiti, M.K.; Phulia, V. Optimal Level of Dietary Arginine Enhances Growth Performance, Haemato-Biochemical Status, Metabolic Responses and Growth-Related Gene Expression of GIFT Juveniles Reared in Inland Saline Water. Aquaculture 2024, 592, 741219. [Google Scholar] [CrossRef]
- Huo, H.J.; Chen, S.N.; Li, L.; Nie, P. Functional Characterization of IL-10 and Its Receptor Subunits in a Perciform Fish, the Mandarin Fish, Siniperca chuatsi. Dev. Comp. Immunol. 2019, 97, 64–75. [Google Scholar] [CrossRef] [PubMed]

| Treatments | ||||
|---|---|---|---|---|
| Ingredients | T0 | T1 | T2 | T3 |
| Poultry byproducts meal (68% CP) a | 10 | 10 | 10 | 10 |
| Bovine byproducts meal (50% CP) a | 10 | 10 | 10 | 10 |
| Fish meal (70% CP) b | 24 | 24 | 24 | 24 |
| Corn gluten meal (65% CP) c | 10 | 10 | 10 | 10 |
| Corn starch d | 28.3 | 28.3 | 28.3 | 28.3 |
| Glycine e | 3 | 2 | 1 | 0 |
| Arginine e | 0 | 1 | 2 | 3 |
| Beef tallow f | 2.5 | 2.5 | 2.5 | 2.5 |
| DHA Nature TM (24% DHA) g | 5 | 5 | 5 | 5 |
| Gelatin h | 2.5 | 2.5 | 2.5 | 2.5 |
| Methionine e | 1 | 1 | 1 | 1 |
| Rovimix i | 3 | 3 | 3 | 3 |
| Taurine j | 0.1 | 0.1 | 0.1 | 0.1 |
| Stay c k | 0.5 | 0.5 | 0.5 | 0.5 |
| TOTAL | 100 | 100 | 100 | 100 |
| Arginine on diet (%) | 2.6 | 3.6 | 4.6 | 5.6 |
| Arginine on crude protein (%) | 4.3 | 5.91 | 7.54 | 9.16 |
| Proximal composition (%) | ||||
| Crude protein | 45.72 | 45.52 | 44.92 | 45.83 |
| Crude fat | 10.40 | 10.57 | 10.35 | 10.46 |
| Ash | 10.58 | 10.06 | 10.06 | 10.23 |
| NFE | 34.95 | 33.85 | 35.16 | 33.46 |
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | e | NCBI Reference |
|---|---|---|---|---|
| hsp 70 | CCGGTTTGATGACACAGTTG | CGAGGTAGGCTTCAGCAATC | 0.94 | XM_023404852 |
| sod | GACGTGACAACACAGGTTGC | TACAGCCACCGTAACAGCAG | 0.95 | XM_003449940.5 |
| il-10 | CTGCTAGATCAGTCCGTCGAA | GCAGAACCGTGTCCAGGTAA | 0.99 | XM_013269189.3 |
| Β-actin | TGGTGGGTATGGGTCAGAAAG | CTGTTGGCTTTGGGGTTCA | 0.96 | ENSONIG00000008505 |
| Treatments | Initial Weight (g) | Final Weight (g) | Final Length (cm) | Weight Gain (g) | Specific Growth Rate (%/d) | Feed Conversion Ratio | Survival Rate (%) |
|---|---|---|---|---|---|---|---|
| T0 | 4.06 ± 0.76 a | 48.03 ± 19.17 a | 13.43 ± 1.82 a | 42.53 ± 4.38 a | 4.27 ± 0.15 a | 1.23 ± 0.08 a | 76.0 ± 4.00 a |
| T1 | 4.05 ± 0.76 a | 56.14 ± 23.09 a | 13.96 ± 1.95 a | 44.4 ± 3.23 ab | 4.35 ± 0.13 ab | 1.25 ± 0.02 a | 83.7 ± 0.38 ab |
| T2 | 4.05 ± 0.73 a | 45.87 ± 9.25 a | 13.43 ± 1.11 a | 48.34 ± 0.43 ab | 4.49 ± 0.02 ab | 1.16 ± 0.04 a | 94.0 ± 2.82 b |
| T3 | 4.01 ± 0.66 a | 68.16 ± 18.98 a | 14.91 ± 1.39 a | 52.44 ± 3.84 b | 4.63± 0.11 b | 1.05 ± 0.09 a | 76.0 ± 8.00 a |
| p-value | 0.44 A | 0.09 A | 0.24 K | 0.04 A | 0.04 A | 0.24 K | 0.01 A |
| Regressions | |||||||
| Orthogonal contrast | |||||||
| Lineal (p-value) | 0.01 | 0.007 | 0.10 | 0.84 | |||
| Quadratic (p-value) | 0.05 | 0.004 | 0.16 | 0.46 | |||
| Lineal (R2) | 0.97 | 0.98 | 0.79 | 0.02 | |||
| Quadratic (R2) | 0.99 | 0.99 | 0.98 | 0.78 | |||
| Best model | Lineal p | Lineal t | Quadratic r | Quadratic r | |||
| Treatments | Hepatosomatic Index | Visceral Index | Condition Index |
|---|---|---|---|
| T0 | 2.16 ± 0.47 a | 12.65 ± 1.60 a | 1.86 ± 0.15 a |
| T1 | 2.18 ± 0.87 a | 13.55 ± 1.05 a | 1.99 ± 0.39 a |
| T2 | 1.8 ± 0.43 a | 13.72 ± 1.92 a | 1.88 ± 0.15 a |
| T3 | 2.29 ± 0.34 a | 13.30 ± 2.36 a | 2.01 ± 0.22 a |
| p-value | 0.25 K | 0.63 K | 0.54 K |
| Regressions | |||
| Orthogonal contrast | |||
| Lineal (p-value) | 0.99 | 0.41 | 0.42 |
| Quadratic (p-value) | 0.77 | 0.03 | 0.81 |
| Lineal (R2) | 0.00 | 0.33 | 0.33 |
| Quadratic (R2) | 0.77 | 0.99 | 0.33 |
| Best model | Quadratic r | Quadratic t | None |
| Treatments | Hb (g/dL) | Hct (%) | RBC (×106/mm3) | WBC (×103/mm3) | MCV (fL) | MCH (pg) | MCHC (g/dL) |
|---|---|---|---|---|---|---|---|
| T0 | 6.76 ± 1.37 a | 23.88 ± 5.79 a | 1.39 ± 0.55 a | 11.20 ± 2.11 a | 183.21 ± 37.82 b | 53.41 ± 17.11 a | 28.88 ± 4.80 a |
| T1 | 8.15 ± 0.92 b | 28.10 ± 4.13 a | 1.59 ± 0.24 a | 12.40 ± 1.13 ab | 179.91 ± 39.41 b | 52.22 ± 10.62 a | 29.30 ± 3.10 a |
| T2 | 8.16 ± 0.53 b | 23.50 ± 2.66 a | 1.77 ± 0.22 a | 13.00 ± 0.58 ab | 134.18 ± 20.05 a | 46.69 ± 6.18 a | 34.90 ± 1.60 b |
| T3 | 8.25 ± 0.56 b | 25.00 ± 4.20 a | 1.80 ± 0.27 a | 13.60 ± 0.99 b | 140.87 ± 32.25 a | 46.55 ± 7.82 a | 34.20 ± 8.80 ab |
| p-value | 0.01 A | 0.14 K | 0.16 K | 0.02 K | 0.02 K | 0.67 K | 0.02 K |
| Regressions | |||||||
| Orthogonal contrast | |||||||
| Lineal (p-value) | 0.19 | 0.92 | 0.03 | 0.01 | 0.12 | 0.06 | 0.12 |
| Quadratic (p-value) | 0.26 | 0.92 | 0.08 | 0.07 | 0.47 | 0.34 | 0.46 |
| Lineal (R2) | 0.65 | 0.01 | 0.92 | 0.96 | 0.75 | 0.87 | 0.77 |
| Quadratic (R2) | 0.93 | 0.14 | 0.99 | 0.99 | 0.77 | 0.88 | 0.78 |
| Best model | Quadratic r | None | Lineal p | Lineal p | Quadratic r | Quadratic r | Quadratic r |
| Treatments | Lymphocytes (%) | Monocytes (%) | Neutrophils (%) | Basophil (%) | Eosinophils (%) | Thrombocytes (%) |
|---|---|---|---|---|---|---|
| T0 | 64.95 ± 13.73 a | 27.25 ± 9.19 b | 7.42 ± 5.91 a | 0.00 ± 0.00 a | 1.16 ± 0.55 a | 75.78 ± 6.14 b |
| T1 | 57.54 ± 14.18 a | 29.23 ± 11.62 b | 11.43 ± 5.18 a | 0.19 ± 0.40 a | 2.55 ± 1.05 a | 66.25 ± 6.14 a |
| T2 | 75.50 ± 10.69 b | 16.79 ± 4.51 a | 7.70 ± 7.002 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 66.36 ± 5.77 a |
| T3 | 61.57 ± 19.49 a | 27.77 ± 12.29 b | 8.92 ± 7.13 a | 0.29 ± 0.47 a | 2.69 ± 1.62 a | 59.71 ± 5.86 a |
| p-value | 0.037 K | 0.01 K | 0.166 K | 0.07 K | 1.00 K | 0.00 A |
| Regressions | ||||||
| Orthogonal contrast | ||||||
| Lineal (p-value) | 0.86 | 0.75 | 0.94 | 0.39 | 0.79 | 0.06 |
| Quadratic (p-value) | 0.96 | 0.85 | 0.86 | 0.76 | 0.93 | 0.32 |
| Lineal (R2) | 0.01 | 0.06 | 0.00 | 0.36 | 0.04 | 0.88 |
| Quadratic (R2) | 0.07 | 0.26 | 0.19 | 0.40 | 0.13 | 0.89 |
| Best model | None | None | None | None | None | Quadratic r |
| Treatments | Micronucleus (%) | Binucleated (%) | Blebbel (%) | Notched (%) |
|---|---|---|---|---|
| T0 | 0.488 ± 0.25 a | 0.33 ± 0.14 b | 0.60 ± 0.47 a | 2.95 ± 2.01 a |
| T1 | 0.190 ± 0.01 a | 0.20 ± 0.00 a | 0.31 ± 0.14 a | 2.23 ± 2.19 a |
| T2 | 0.197 ± 0.01 a | 0.50 ± 0.50 ab | 0.28 ± 0.22 a | 2.71 ± 1.32 a |
| T3 | 0.177 ± 0.04 a | 0.42 ± 0.26 b | 0.35 ± 0.27 a | 2.47 ± 1.76 a |
| p-value | 0.13 K | 0.03 K | 0.09 K | 0.22 A |
| Regressions | ||||
| Orthogonal contrast | ||||
| Lineal (p-value) | 0.52 | 0.42 | 0.31 | 0.6 |
| Quadratic (p-value) | 0.57 | 0.81 | 0.14 | 0.8 |
| Lineal (R2) | 0.22 | 0.32 | 0.47 | 0.16 |
| Quadratic (R2) | 0.66 | 0.33 | 0.98 | 0.36 |
| Best model | None | None | Quadratic r | None |
| Treatments | Cholesterol (mg/dL) | Triglycerides (mg/dL) | Glucose (mg/dL) | Total Protein (g/dL) | Globulin (g/dL) | albumin (g/dL) |
|---|---|---|---|---|---|---|
| T0 | 106.99 ± 12.72 a | 138.26 ± 55.86 ab | 105.85 ± 24.65 a | 2.68 ± 0.37 bc | 1.39 ± 0.39 ab | 1.26 ± 0.26 ab |
| T1 | 100.83 ± 14.31 a | 115.93 ± 39.98 a | 92.17 ± 20.78 a | 3.17 ± 0.59 b | 1.78 ± 0.48 b | 1.38 ± 0.17 a |
| T2 | 94.89 ± 11.61 a | 120.87 ± 45.25 ab | 93.59 ± 18.63 a | 2.22 ± 0.49 a | 1.13 ± 0.54 a | 1.08 ± 0.13 b |
| T3 | 97.63 ± 17.61 a | 182.75 ± 99.97 b | 93.72 ± 27.76 a | 2.74 ± 0.56 bc | 1.41 ± 0.36 ab | 1.32 ± 0.24 a |
| p-value | 0.10 K | 0.04 K | 0.48 K | 0.00 K | 0.00 A | 0.00 K |
| Regressions | ||||||
| Orthogonal contrast | ||||||
| Lineal (p-value) | 0.15 | 0.41 | 0.29 | 0.74 | 0.71 | 0.88 |
| Quadratic (p-value) | 0.20 | 0.12 | 0.33 | 0.966 | 0.95 | 0.95 |
| Lineal (R2) | 0.71 | 0.34 | 0.50 | 0.06 | 0.08 | 0.01 |
| Quadratic (R2) | 0.95 | 0.98 | 0.89 | 0.06 | 0.09 | 0.08 |
| Best model | Quadratic r | Quadratic r | Quadratic r | None | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguía-Casillas, A.I.; Viana, M.T.; Vivanco-Aranda, M.; Ruiz-González, L.E.; Peña-Marín, E.S.; Del Rio-Zaragoza, O.B. L-Arginine Effect as an Additive on Overall Performance, Health Status, and Expression of Stress Molecular Markers in Nile Tilapia (Oreochromis niloticus) Under Chronic Salinity Exposure. Fishes 2025, 10, 387. https://doi.org/10.3390/fishes10080387
Munguía-Casillas AI, Viana MT, Vivanco-Aranda M, Ruiz-González LE, Peña-Marín ES, Del Rio-Zaragoza OB. L-Arginine Effect as an Additive on Overall Performance, Health Status, and Expression of Stress Molecular Markers in Nile Tilapia (Oreochromis niloticus) Under Chronic Salinity Exposure. Fishes. 2025; 10(8):387. https://doi.org/10.3390/fishes10080387
Chicago/Turabian StyleMunguía-Casillas, Andrea Itzel, María Teresa Viana, Miroslava Vivanco-Aranda, Luis Eduardo Ruiz-González, Emyr Saul Peña-Marín, and Oscar Basilio Del Rio-Zaragoza. 2025. "L-Arginine Effect as an Additive on Overall Performance, Health Status, and Expression of Stress Molecular Markers in Nile Tilapia (Oreochromis niloticus) Under Chronic Salinity Exposure" Fishes 10, no. 8: 387. https://doi.org/10.3390/fishes10080387
APA StyleMunguía-Casillas, A. I., Viana, M. T., Vivanco-Aranda, M., Ruiz-González, L. E., Peña-Marín, E. S., & Del Rio-Zaragoza, O. B. (2025). L-Arginine Effect as an Additive on Overall Performance, Health Status, and Expression of Stress Molecular Markers in Nile Tilapia (Oreochromis niloticus) Under Chronic Salinity Exposure. Fishes, 10(8), 387. https://doi.org/10.3390/fishes10080387






