Effects of Dietary Sodium Butyrate on Growth Performance, Digestive Ability, Blood Biochemistry, and Ammonia Tolerance of Largemouth Bass (Micropterus salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Animals and Experimental Conditions
2.3. Sampling
2.4. Ammonia Challenge
2.5. Biochemical Analysis
2.6. Histological Analysis
2.7. Q-PCR Analysis
3. Results
3.1. Dietary SB Supplementation on Growth
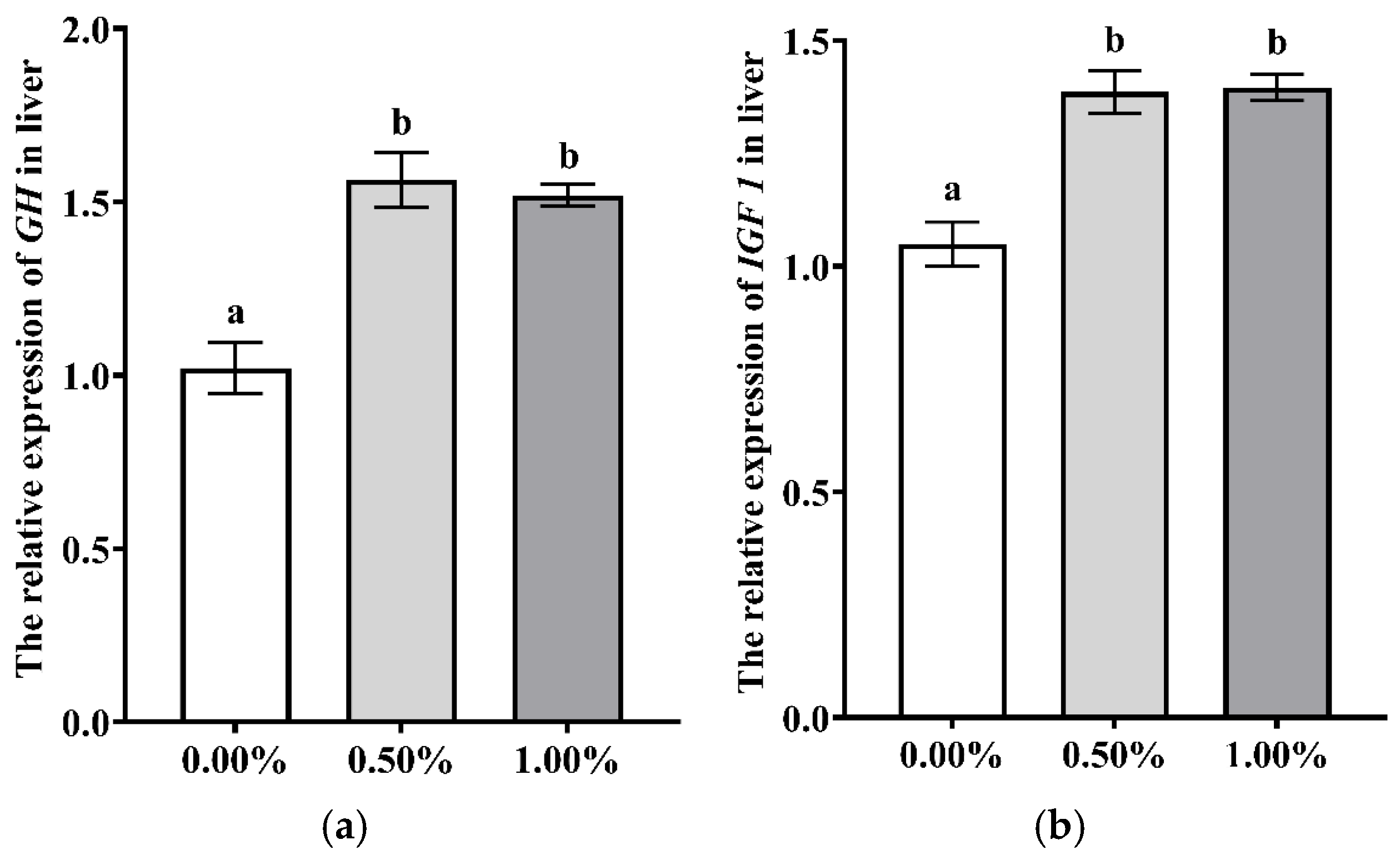
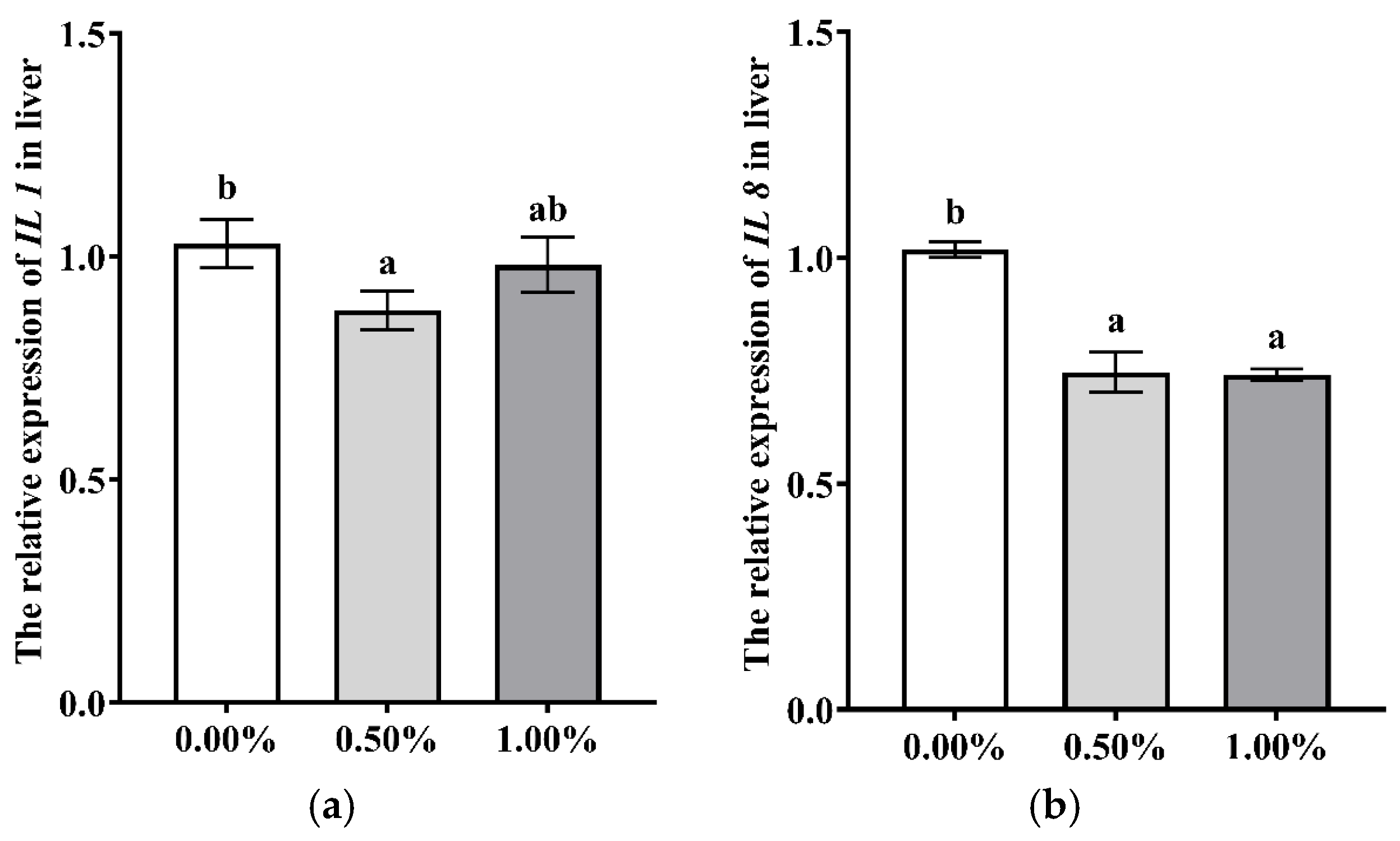
3.2. Dietary SB Supplementation on Health
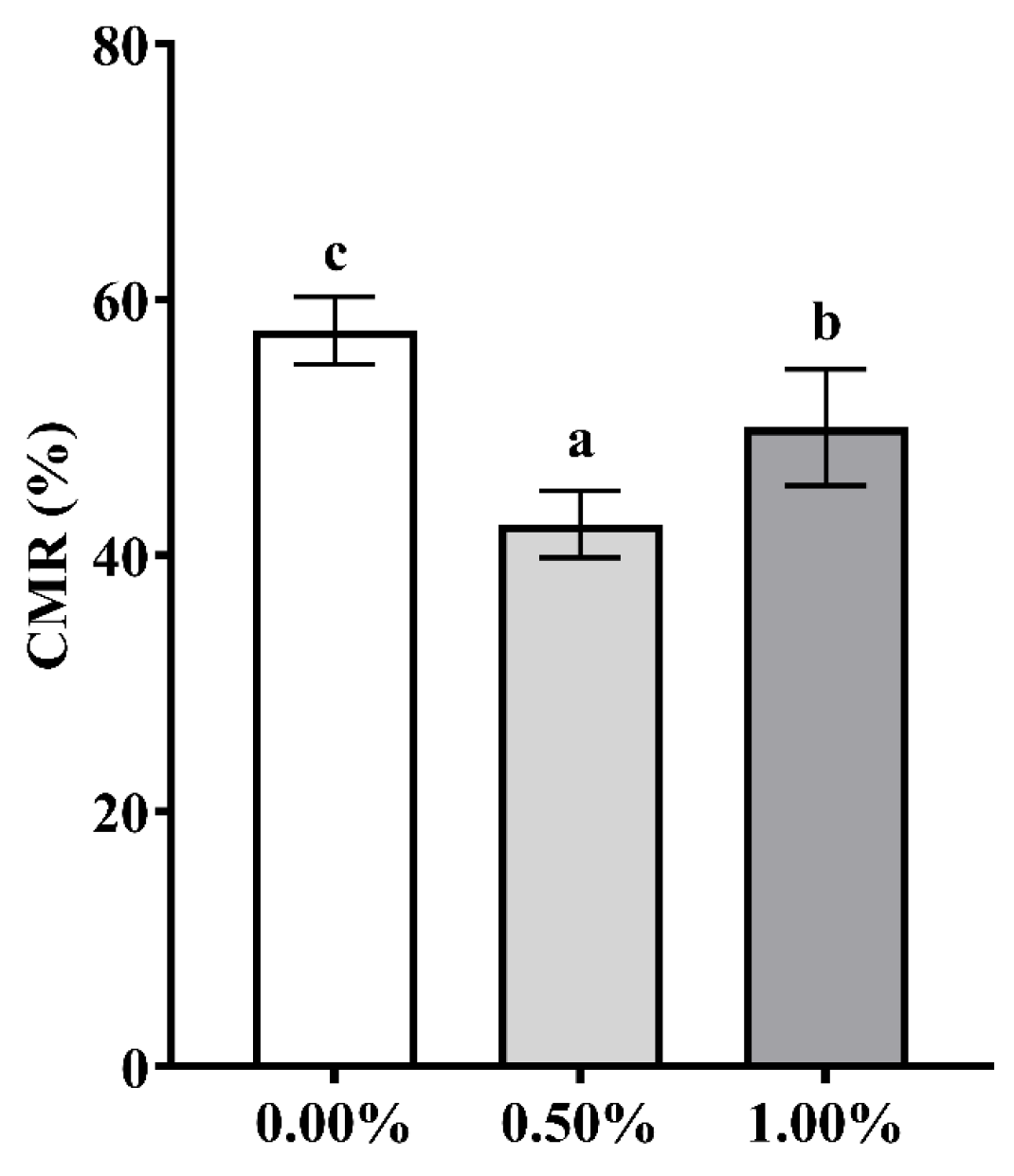
3.3. Dietary SB Supplementation on Ammonia Tolerance
4. Discussion
4.1. Dietary SB Supplementation on Growth
4.2. Dietary SB Supplementation on Health
4.3. Dietary SB Supplementation on Ammonia Tolerance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Heng, X.; Guo, L.; Lessing, D.J.; Chu, W. SCFAs improve disease resistance via modulate gut microbiota, enhance immune response and increase antioxidative capacity in the host. Fish Shellfish Immunol. 2022, 120, 560–568. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, Q.; Wang, A.; Liu, Y.; Teame, T.; Ran, C.; Yang, Y.; He, S.; Zhou, W.; Olsen, R.E.; et al. Effects of dietary sodium acetate on food intake, weight gain, intestinal digestive enzyme activities, energy metabolism and gut microbiota in cultured fish: Zebrafish as a model. Aquaculture 2020, 523, 735188. [Google Scholar] [CrossRef]
- Zhou, W.-H.; Limbu, S.M.; Luo, Y.; Li, R.-X.; Ren, J.; Qiao, F.; Zhang, M.-L.; Du, Z.-Y. Dietary acetate promotes growth and nutrients deposition in Nile tilapia (Oreochromis niloticus) through increasing acetyl-CoA-triggered energy production. Aquaculture 2023, 575, 739750. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. Benefits of Dietary Butyric Acid, Sodium Butyrate, and Their Protected Forms in Aquafeeds: A Review. Rev. Fish. Sci. Aquac. 2020, 28, 421–448. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.-Z.; Caipang, C.M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquac. Res. 2017, 48, 1380–1391. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Sodium butyrate improved intestinal immune function associated with NF-kappaB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 66, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Mirghaed, A.T.; Yarahmadi, P.; Soltani, M.; Paknejad, H.; Hoseini, S.M. Dietary sodium butyrate (Butirex® C4) supplementation modulates intestinal transcriptomic responses and augments disease resistance of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 92, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Ou, W.; Ai, Q.; Zhang, W.; Mai, K.; Zhang, Y. In vitro study of sodium butyrate on soyasaponin challenged intestinal epithelial cells of turbot (Scophthalmus maximus L.) refer to inflammation, apoptosis and antioxidant enzymes. Fish Shellfish Immunol. Rep. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, S.; Li, X.; Zhang, M.; Li, M. Effects of ammonia and roxithromycin exposure on skin mucus microbiota composition and immune response of juvenile yellow catfish Pelteobagrus fulvidraco. Fish Shellfish Immunol. 2023, 141, 109048. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, D.M.; Li, B.; Zhao, Z.G.; Fang, W.; Yang, K.; Fan, Q.X. Toxicity of ammonia and nitrite to yellow catfish (Pelteobagrus fulvidraco). J. Appl. Ichthyol. 2012, 28, 82–86. [Google Scholar] [CrossRef]
- Yousefi, M.; Vatnikov, Y.A.; Kulikov, E.V.; Plushikov, V.G.; Drukovsky, S.G.; Hoseinifar, S.H.; Van Doan, H. The protective effects of dietary garlic on common carp (Cyprinus carpio) exposed to ambient ammonia toxicity. Aquaculture 2020, 526, 735400. [Google Scholar] [CrossRef]
- Egnew, N.; Renukdas, N.; Ramena, Y.; Yadav, A.K.; Kelly, A.M.; Lochmann, R.T.; Sinha, A.K. Physiological insights into largemouth bass (Micropterus salmoides) survival during long-term exposure to high environmental ammonia. Aquat. Toxicol. 2019, 207, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, M.; Li, M.; Zhang, Q.; Qian, Y.; Wang, R. Effect of dietary alanyl-glutamine dipeptide against chronic ammonia stress induced hyperammonemia in the juvenile yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 213, 55–61. [Google Scholar] [CrossRef]
- Li, M.; Lai, H.; Li, Q.; Gong, S.; Wang, R. Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish Pelteobagrus fulvidraco fed all-plant protein diets. Aquaculture 2016, 450, 349–355. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Jiang, H.; Qin, C. Comparison of dietary arginine or/and inulin supplementation on growth, digestive ability and ammonia tolerance of juvenile yellow catfish Pelteobagrus fulvidraco. Aquac. Rep. 2023, 30, 101543. [Google Scholar] [CrossRef]
- Pan, C.H.; Chien, Y.H.; Wang, Y.J. Antioxidant defence to ammonia stress of characins (Hyphessobrycon eques Steindachner) fed diets supplemented with carotenoids. Aquac. Nutr. 2011, 17, 258–266. [Google Scholar] [CrossRef]
- Jiang, X.; Zu, L.; Wang, Z.; Cheng, Y.; Yang, Y.; Wu, X. Micro-algal astaxanthin could improve the antioxidant capability, immunity and ammonia resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2020, 102, 499–510. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, K.; Wang, G.; Mo, W.; Huang, Y.; Cao, J. Metabolic changes, antioxidant status, immune response and resistance to ammonia stress in juvenile yellow catfish (Pelteobagrus fulvidraco) fed diet supplemented with sodium butyrate. Aquaculture 2021, 536, 736441. [Google Scholar] [CrossRef]
- Bai, J.; Lutz-Carrillo, D.J.; Quan, Y.; Liang, S. Taxonomic status and genetic diversity of cultured largemouth bass Micropterus salmoides in China. Aquaculture 2008, 278, 27–30. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yang, F.-F.; Ling, R.-Z.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Zhang, M.; Li, M. Sodium butyrate improves the growth performance, intestinal immunity, and ammonia tolerance of yellow catfish Pelteobagrus fulvidraco by increasing the relative abundance of Candidatus_Arthromitus. Aquaculture 2024, 580, 740359. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; Khattaby, A.E.-R.A.; Samir, F.; Awad, S.M.M.; Abdel-Tawwab, M. Stimulatory effect of dietary butyrate on growth, immune response, and resistance of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Anim. Feed Sci. Technol. 2019, 254, 114212. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Mu, J.; Song, J.; Li, R.; Xue, T.; Wang, D.; Yu, J. Isovitexin prevents DSS-induced colitis through inhibiting inflammation and preserving intestinal barrier integrity through activating AhR. Chem.-Biol. Interact. 2023, 382, 110583. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.P.; Brennan, T.A.; Pignolo, R.J. Bone histomorphometry using free and commonly available software. Histopathology 2012, 61, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Wu, F.; Qu, Q.; Tan, Q.; Gong, W. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 4102–4111. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Effect of dietary sodium butyrate on growth performance, enzyme activities and intestinal proliferation-related gene expression of juvenile golden pompano Trachinotus ovatus. Aquac. Nutr. 2019, 25, 1261–1271. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, G.; Wang, H.; Mo, W.; Huang, Y.; Cao, J.; Li, P. Effects of dietary sodium butyrate on growth, digestive enzymes, body composition and nutrient retention-related gene expression of juvenile yellow catfish (Pelteobagrus fulvidraco). Anim. Nutr. 2021, 7, 539–547. [Google Scholar] [CrossRef]
- Monier, M.N.; Abd El-Naby, A.S.; Samir, F.; Abdel-Tawwab, M. Positive effects of dietary nanosized sodium butyrate on growth performance, immune, antioxidant indices, and resistance of Nile tilapia to waterborne copper toxicity. Aquac. Rep. 2022, 26, 101323. [Google Scholar] [CrossRef]
- Piva, A.; Morlacchini, M.; Casadei, G.; Gatta, P.P.; Biagi, G.; Prandini, A. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Ital. J. Anim. Sci. 2016, 1, 35–41. [Google Scholar] [CrossRef]
- Luz, J.R.; Ramos, A.P.S.; Melo, J.F.B.; Braga, L.G.T. Use of sodium butyrate in the feeding of Arapaima gigas (Schinz, 1822) juvenile. Aquaculture 2019, 510, 248–255. [Google Scholar] [CrossRef]
- Jesus, G.F.A.; Pereira, S.A.; Owatari, M.S.; Addam, K.; Silva, B.C.; Sterzelecki, F.C.; Sugai, J.K.; Cardoso, L.; Jatobá, A.; Mouriño, J.L.P.; et al. Use of protected forms of sodium butyrate benefit the development and intestinal health of Nile tilapia during the sexual reversion period. Aquaculture 2019, 504, 326–333. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Li, X.; Zhang, M.; Qian, Y.; Li, M. Effects of three short-chain fatty acids on growth, intestinal microbiota composition, and ammonia tolerance of juvenile yellow catfish Pelteobagrus fulvidraco. Aquac. Rep. 2024, 36, 102066. [Google Scholar] [CrossRef]
- Krogdahl, A.; Bakke-McKellep, A.M. Fasting and refeeding cause rapid changes in intestinal tissue mass and digestive enzyme capacities of Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 450–460. [Google Scholar] [CrossRef]
- Tibaldi, E.; Hakim, Y.; Uni, Z.; Tulli, F.; de Francesco, M.; Luzzana, U.; Harpaz, S. Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax). Aquaculture 2006, 261, 182–193. [Google Scholar] [CrossRef]
- El-Sharkawy, E.A.; El-Razek, I.M.A.; Amer, A.A.; Soliman, A.A.; Shukry, M.; Gewaily, M.S.; Téllez-Isaías, G.; Kari, Z.A.; Dawood, M.A.O. Effects of sodium butyrate on the growth performance, digestive enzyme activity, intestinal health, and immune responses of Thinlip grey mullet (Liza ramada) juveniles. Aquac. Rep. 2023, 30, 101530. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Fayaz, S.; Hoseini, S.M. Effects of dietary 1,8-cineole supplementation on serum stress and antioxidant markers of common carp (Cyprinus carpio) acutely exposed to ambient ammonia. Aquaculture 2019, 509, 8–15. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Zhang, J.; Gatlin, D.M.; Ringo, E.; Zhou, Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014, 112, 15–29. [Google Scholar] [CrossRef]
- Li, P.; Hou, D.; Zhao, H.; Peng, K.; Chen, B.; Guo, H.; Cao, J. Effects of dietary arginine levels on intestinal morphology, digestive enzyme activity, antioxidant capacity and intestinal flora of hybrid snakehead(Channa maculata ♀×Channa argus ♂). Aquac. Rep. 2022, 25, 101244. [Google Scholar] [CrossRef]
- Zhou, J.S.; Guo, P.; Yu, H.B.; Ji, H.; Lai, Z.W.; Chen, Y.A. Growth performance, lipid metabolism, and health status of grass carp (Ctenopharyngodon idella) fed three different forms of sodium butyrate. Fish Physiol. Biochem. 2019, 45, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, Q.; Guo, X.; Pan, X.; Li, X. Effects of dietary sodium butyrate on growth performance, antioxidant capacity, intestinal histomorphology and immune response in juvenile Pengze crucian carp (Carassius auratus Pengze). Aquac. Rep. 2021, 21, 100828. [Google Scholar] [CrossRef]
- Duan, C.; Hirano, T. Effects of insulin-like growth factor-I and insulin on the in-vitro uptake of sulphate by eel branchial cartilage: Evidence for the presence of independent hepatic and pancreatic sulphation factors. J. Endocrinol. 1992, 133, 211–219. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Shukry, M.; Farrag, F.A.; El-Shafai, N.M.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture 2021, 536, 736467. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Lionetti, L.; Faggio, C. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total. Environ. 2019, 670, 1170–1183. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.; Pal, A.; Choudhury, D.; Yengkokpam, S.; Mukherjee, S. Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in juveniles. Fish Shellfish Immunol. 2005, 19, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tan, X.; Zhou, C.; Niu, J.; Xia, D.; Huang, Z.; Wang, J.; Wang, Y. Effect of dietary arginine levels on the growth performance, feed utilization, non-specific immune response and disease resistance of juvenile golden pompano Trachinotus ovatus. Aquaculture 2015, 437, 382–389. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lee, K.-J. Dietary valine requirement of juvenile red sea bream Pagrus major. Aquaculture 2013, 416–417, 212–218. [Google Scholar] [CrossRef]
- Liu, B.; Xie, J.; Ge, X.-p.; Miao, L.-h.; Wang, G. Effect of high dietary carbohydrate on growth, serum physiological response, and hepatic heat shock cognate protein 70 expression of the top-mouth culter Erythroculter ilishaeformis Bleeker. Fish. Sci. 2012, 78, 613–623. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Chen, S.; Chen, S.; Huang, Z.; Zhou, C.; Zou, C.; Liu, Q.; Ye, H.; Lin, H.; et al. Effects of dietary dandelion extracts on growth performance, body composition, plasma biochemical parameters, immune responses and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2017, 66, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Yaqub, A.; Ayub, M. Partial and full substitution of fish meal and soybean meal by canola meal in diets for genetically improved farmed tilapia (O. niloticus): Growth performance, carcass composition, serum biochemistry, immune response, and intestine histology. J. Appl. Aquac. 2021, 34, 829–854. [Google Scholar] [CrossRef]
- Li, M.Y.; Gao, C.S.; Du, X.Y.; Zhao, L.; Niu, X.T.; Wang, G.Q.; Zhang, D.M. Effect of sub-chronic exposure to selenium and astaxanthin on Channa argus: Bioaccumulation, oxidative stress and inflammatory response. Chemosphere 2020, 244, 125546. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Qian, Y.; Shi, G.; Wang, R. Ammonia toxicity in the yellow catfish (Pelteobagrus fulvidraco): The mechanistic insight from physiological detoxification to poisoning. Fish Shellfish Immunol. 2020, 102, 195–202. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, J.; Chen, F.; Tang, X.; Liao, L.; Liu, Q.; Luo, J.; Du, Z.; Li, Z.; Luo, W.; et al. High carbohydrate diet induced endoplasmic reticulum stress and oxidative stress, promoted inflammation and apoptosis, impaired intestinal barrier of juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 119, 308–317. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.N.; Capettini, L.S.A.; Lemos, V.S.; Santos, R.A.S.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Dai, J.; Yang, P.; Xu, W.; Ai, Q.; Zhang, W.; Zhang, Y.; Zhang, Y.; Mai, K. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): Effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immunol. 2019, 88, 65–75. [Google Scholar] [CrossRef]
- Wu, P.; Tian, L.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Jiang, J.; Xie, F.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Sodium butyrate enhanced physical barrier function referring to Nrf2, JNK and MLCK signaling pathways in the intestine of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 73, 121–132. [Google Scholar] [CrossRef]
- Song, P.; Li, M.; Zhang, M.; Jiang, H.; Shao, J.; Wang, R.; Qian, Y.; Feng, D. Inhibition of argininosuccinate synthase (ASS) affected ammonia excretion in yellow catfish Pelteobagrus fulvidraco during acute ammonia poisoning. Aquac. Rep. 2022, 22, 100931. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Jiang, H.; Fan, Y.; Li, X.; Wang, R.; Qian, Y.; Li, M. Arginase plays an important role in ammonia detoxification of yellow catfish Pelteobagrus fulvidraco. Fish Shellfish Immunol. 2021, 115, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Itoh, K.; Kawabuchi, M. NADPH-diaphorase and cytosolic urea cycle enzymes in the rat accessory olfactory bulb. J. Chem. Neuroanat. 1999, 17, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lepiller, S.; Franche, N.; Solary, E.; Chluba, J.; Laurens, V. Comparative analysis of zebrafish nos2a and nos2b genes. Gene 2009, 445, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Achike, F.I. The l-arginine–nitric oxide pathway: A potential therapeutic target in dengue haemorhagic fever. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1135–1136. [Google Scholar] [CrossRef]
- Ayata, C.; Ayata, G.; Hara, H.; Matthews, R.T.; Beal, M.F.; Ferrante, R.J.; Endres, M.; Kim, A.; Christie, R.H.; Waeber, C.; et al. Mechanisms of Reduced Striatal NMDA Excitotoxicity in Type I Nitric Oxide Synthase Knock-Out Mice. J. Neurosci. 1997, 17, 6908–6917. [Google Scholar] [CrossRef]

| Ingredients | SB Levels (%) | ||
|---|---|---|---|
| 0.00 | 0.50 | 1.00 | |
| Fish meal | 15.00 | 15.00 | 15.00 |
| Soybean meal | 25.00 | 25.00 | 25.00 |
| Soy protein concentrate | 16.00 | 16.00 | 16.00 |
| Corn gluten meal | 9.00 | 9.00 | 9.00 |
| Rapeseed meal | 3.00 | 3.00 | 3.00 |
| Cottonseed meal | 2.00 | 2.00 | 2.00 |
| Fish oil | 2.50 | 2.50 | 2.50 |
| Soyabean oil | 2.50 | 2.50 | 2.50 |
| Starch | 19.00 | 19.00 | 19.00 |
| Vitamin premix a | 0.50 | 0.50 | 0.50 |
| Mineral premix b | 0.50 | 0.50 | 0.50 |
| Monocalcium phosphate | 1.00 | 1.00 | 1.00 |
| Sodium chloride | 2.00 | 2.00 | 2.00 |
| Zeolite powder | 2.00 | 1.50 | 1.00 |
| Sodium butyrate | 0.00 | 0.50 | 1.00 |
| Proximate composition | |||
| Protein | 39.48 | 39.12 | 39.01 |
| Lipid | 7.58 | 7.93 | 7.41 |
| Target Gene | Primer Sequence (5′–3′) | Size (bp) |
|---|---|---|
| GH | F: ATCAGAGCCAATCAGGACGG | 112 |
| R: TACGTTCGTCTCAGCGACTC | ||
| IGF 1 | F: AGTGCGATGTGCTGTATCTC | 281 |
| R: TTGCTAGTCTTGGCAGGTG | ||
| IL 1 | F: ACCTCTAACCCAGGGCTGAT | 102 |
| R: GGTTCCATTCAGTGGCGAGA | ||
| IL 8 | F: CAGTGGTGTCGCTGCATACG | 129 |
| R: AGAGCCGTTTCTCCTGGTGA | ||
| β-actin | F: CTCTGCATACATGCCTACAC | 117 |
| R: GTAGAGTTTCTCCCCATCAGG | ||
| GAPDH | F: GTGCCAGCCAGAACATCATCC | 141 |
| R: GGACCGTCAAGTCAACCACTGA |
| SB Levels (%) | |||
|---|---|---|---|
| 0.00 | 0.50 | 1.00 | |
| FBW (g) | 34.73 ± 0.37 a | 39.47 ± 0.69 b | 39.01 ± 0.36 b |
| WGR (%) | 369.32 ± 5.05 a | 433.38 ± 9.26 b | 427.12 ± 4.85 b |
| SGR (%/d) | 2.76 ± 0.02 a | 2.99 ± 0.03 b | 2.96 ± 0.02 b |
| FCR | 1.47 ± 0.04 | 1.46 ± 0.02 | 1.47 ± 0.02 |
| FI (g/fish) | 37.88 ± 0.23 a | 47.94 ± 0.83 b | 47.70 ± 0.79 b |
| CF (g/cm3) | 1.57 ± 0.02 a | 1.75 ± 0.04 b | 1.67 ± 0.06 b |
| HSI (%) | 1.74 ± 0.02 | 1.77 ± 0.05 | 1.75 ± 0.02 |
| SR (%) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| SB Levels (%) | |||
|---|---|---|---|
| 0.00 | 0.50 | 1.00 | |
| Protein (% dry matter) | 57.70 ± 0.28 a | 59.68 ± 0.42 b | 58.98 ± 0.45 b |
| Lipid (% dry matter) | 27.13 ± 0.63 | 27.49 ± 0.37 | 27.75 ± 0.24 |
| Ash (% dry matter) | 11.22 ± 0.44 a | 12.48 ± 0.20 b | 11.97 ± 0.28 b |
| Moisture (%) | 69.83 ± 0.57 | 69.71 ± 0.40 | 69.40 ± 0.26 |
| SB Levels (%) | |||
|---|---|---|---|
| 0.00 | 0.50 | 1.00 | |
| Liver | |||
| Pepsin (U/mg prot) | 6.69 ± 0.29 a | 9.02 ± 0.53 b | 8.80 ± 0.50 b |
| Lipase (U/g prot) | 3.78 ± 0.05 a | 3.95 ± 0.01 b | 3.91 ± 0.03 b |
| Amylase (U/mg prot) | 0.48 ± 0.02 | 0.46 ± 0.043 | 0.44 ± 0.04 |
| Intestine | |||
| Pepsin (U/mg prot) | 0.75 ± 0.06 a | 0.93 ± 0.05 b | 0.91 ± 0.01 b |
| Lipase (U/g prot) | 1.20 ± 0.01 a | 1.46 ± 0.05 b | 1.47 ± 0.04 b |
| Amylase (U/mg prot) | 0.21 ± 0.01 a | 0.24 ± 0.01 b | 0.24 ± 0.03 b |
| Intestinal content | |||
| Pepsin (U/mg prot) | 6.68 ± 0.45 a | 8.68 ± 0.36 b | 8.92 ± 0.65 b |
| Lipase (U/g prot) | 2.98 ± 0.05 a | 3.12 ± 0.04 b | 3.19 ± 0.01 b |
| Amylase (U/mg prot) | 0.71 ± 0.01 a | 0.81 ± 0.01 b | 0.79 ± 0.03 b |
| SB Levels (%) | |||
|---|---|---|---|
| 0.00 | 0.50 | 1.00 | |
| AKP (U/mg prot) | 37.12 ± 0.33 a | 46.06 ± 0.70 c | 45.44 ± 0.80 b |
| Na+/K+-ATPase (U/mg prot) | 3.83 ± 0.37 a | 6.86 ± 0.44 c | 4.06 ± 0.35 b |
| GGT (U/mg prot) | 8.75 ± 0.05 a | 11.12 ± 0.09 c | 10.01 ± 0.11 b |
| CK (U/mg prot) | 19.88 ± 0.06 a | 25.33 ± 0.15 c | 21.12 ± 0.11 b |
| SB Levels (%) | |||
|---|---|---|---|
| 0.00 | 0.50 | 1.00 | |
| TP (g/L) | 33.63 ± 1.13 a | 36.80 ± 1.30 b | 36.83 ± 0.68 b |
| ALB (g/L) | 11.53 ± 0.38 a | 12.25 ± 0.15 b | 12.65 ± 0.15 b |
| GLO (g/L) | 21.04 ± 0.44 a | 23.28 ± 0.74 b | 23.05 ± 0.75 b |
| TG (mmol/L) | 10.98 ± 0.52 b | 8.60 ± 0.37 a | 8.50 ± 0.08 a |
| CHOL (mmol/L) | 0.13 ± 0.03 b | 0.05 ± 0.01 a | 0.06 ± 0.01 a |
| ALT (U/L) | 2.33 ± 0.58 | 2.25 ± 0.25 | 2.50 ± 0.50 |
| AST (U/L) | 77.63 ± 4.13 b | 62.88 ± 4.88 a | 64.33 ± 2.31 a |
| SB Levels (%) | |||
|---|---|---|---|
| 0.00 | 0.50 | 1.00 | |
| ASS (U/g prot) | 9.33 ± 0.09 a | 11.12 ± 0.05 b | 11.08 ± 1.01 b |
| ASL (U/g prot) | 15.35 ± 1.01 a | 17.66 ± 0.05 b | 17.35 ± 0.07 b |
| ARG (U/g prot) | 6.58 ± 0.01 a | 7.15 ± 0.26 b | 7.09 ± 0.05 b |
| OTC (U/g prot) | 15.35 ± 0.15 a | 16.47 ± 0.09 b | 16.89 ± 0.12 b |
| nNOS (U/g prot) | 0.43 ± 0.04 b | 0.35 ± 0.01 a | 0.31 ± 0.03 a |
| iNOS (U/g prot) | 0.66 ± 0.01 a | 1.12 ± 0.01 b | 1.15 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, W.; Tang, Y.; Zhang, M.; Li, M. Effects of Dietary Sodium Butyrate on Growth Performance, Digestive Ability, Blood Biochemistry, and Ammonia Tolerance of Largemouth Bass (Micropterus salmoides). Fishes 2025, 10, 259. https://doi.org/10.3390/fishes10060259
Chen X, Chen W, Tang Y, Zhang M, Li M. Effects of Dietary Sodium Butyrate on Growth Performance, Digestive Ability, Blood Biochemistry, and Ammonia Tolerance of Largemouth Bass (Micropterus salmoides). Fishes. 2025; 10(6):259. https://doi.org/10.3390/fishes10060259
Chicago/Turabian StyleChen, Xuan, Wu Chen, Yanjie Tang, Muzi Zhang, and Ming Li. 2025. "Effects of Dietary Sodium Butyrate on Growth Performance, Digestive Ability, Blood Biochemistry, and Ammonia Tolerance of Largemouth Bass (Micropterus salmoides)" Fishes 10, no. 6: 259. https://doi.org/10.3390/fishes10060259
APA StyleChen, X., Chen, W., Tang, Y., Zhang, M., & Li, M. (2025). Effects of Dietary Sodium Butyrate on Growth Performance, Digestive Ability, Blood Biochemistry, and Ammonia Tolerance of Largemouth Bass (Micropterus salmoides). Fishes, 10(6), 259. https://doi.org/10.3390/fishes10060259







