Effects of Social Enrichment Induced by Different-Sized Groups and Live Bait on Growth, Aggressive Behavior, Physiology, and Neurogenesis in Juvenile Sebastes schlegelii
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Behavioral Observations
2.3. Taking Samples
2.4. Cortisol, MDA, CAT, GSH-Px, and SOD Level Measurement
2.5. Genetic Examination
2.6. Calculations and Data Analysis
3. Results
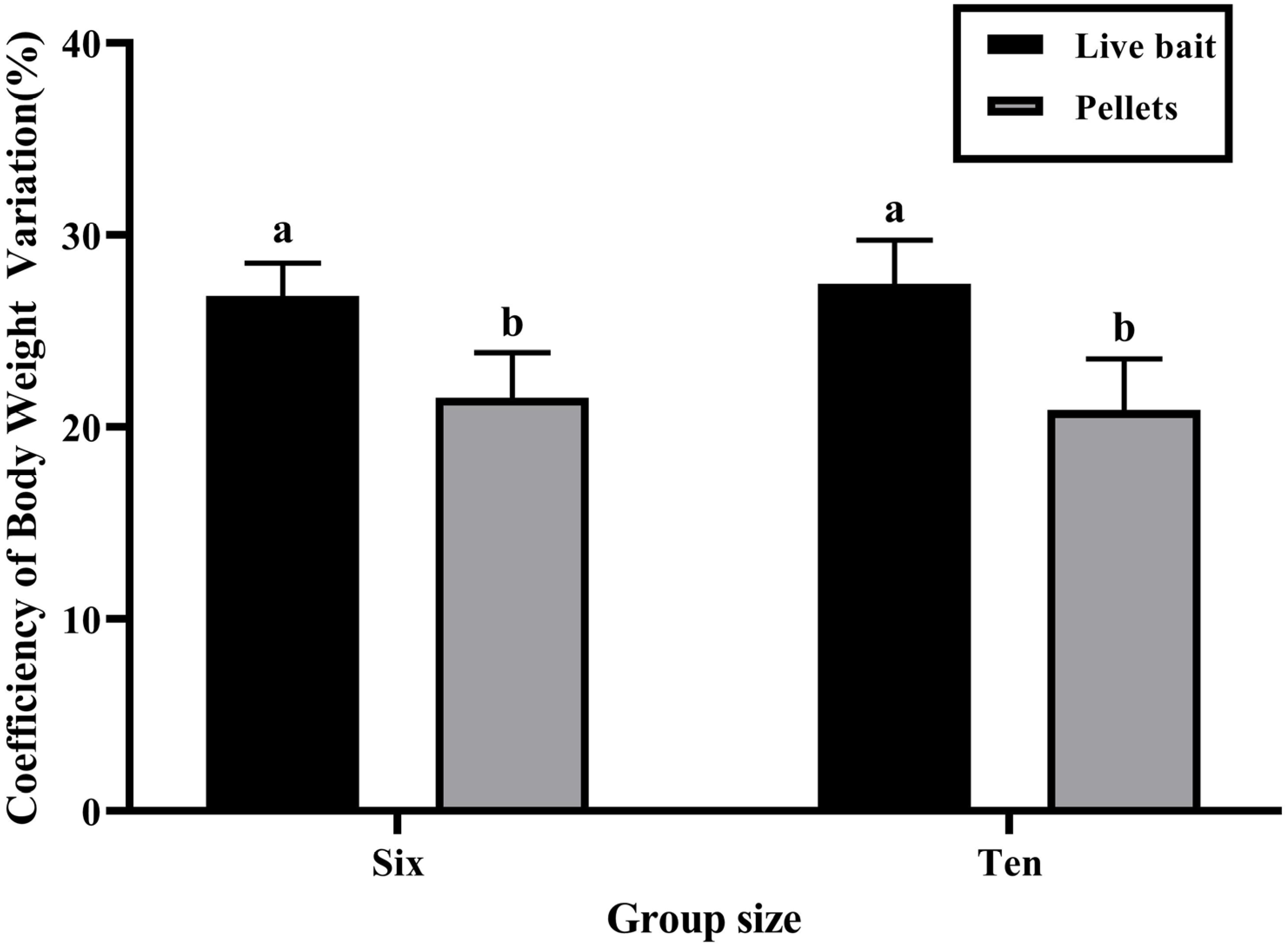
3.1. Growth Performance
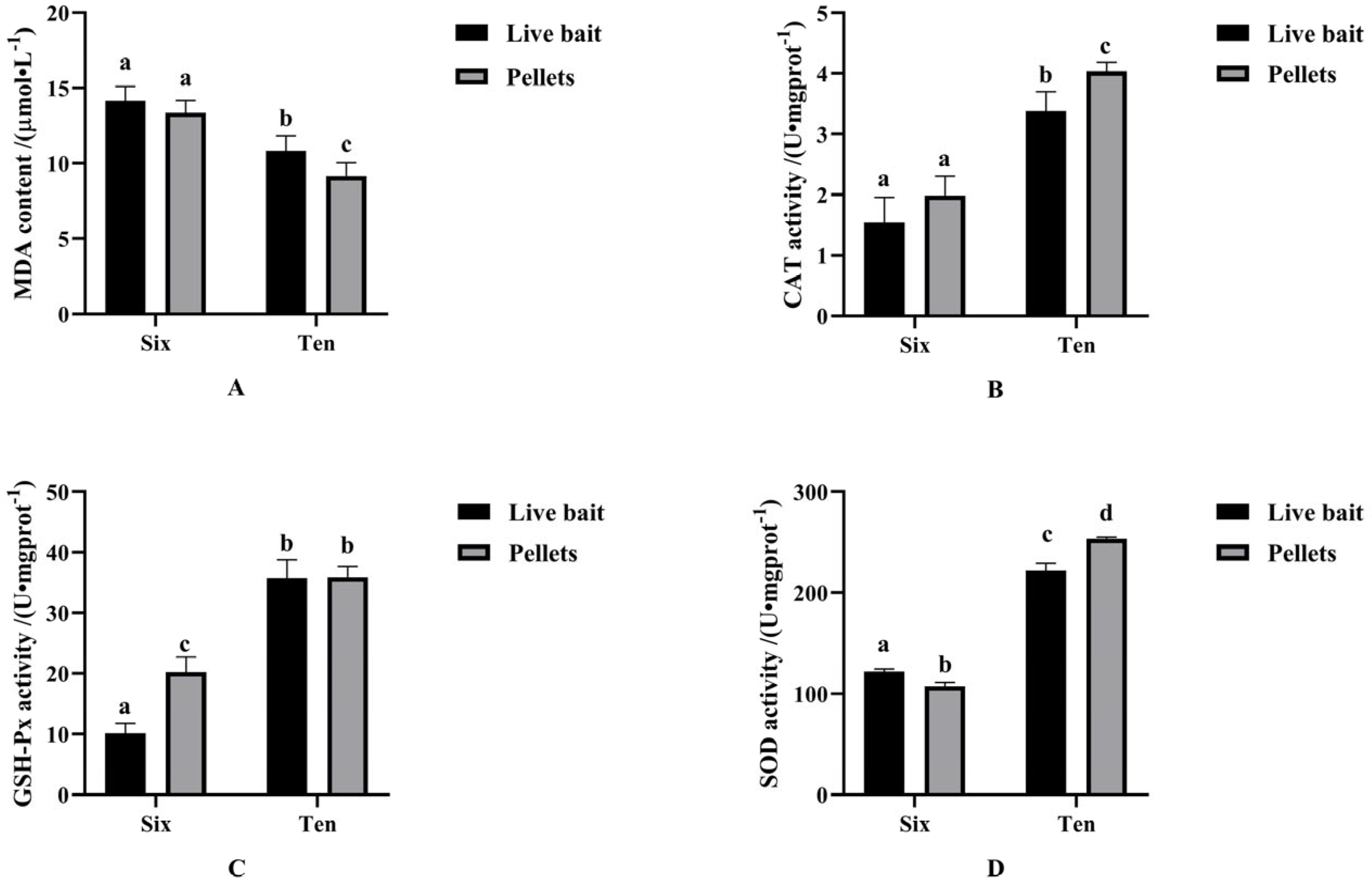
3.2. Antioxidant Indicators
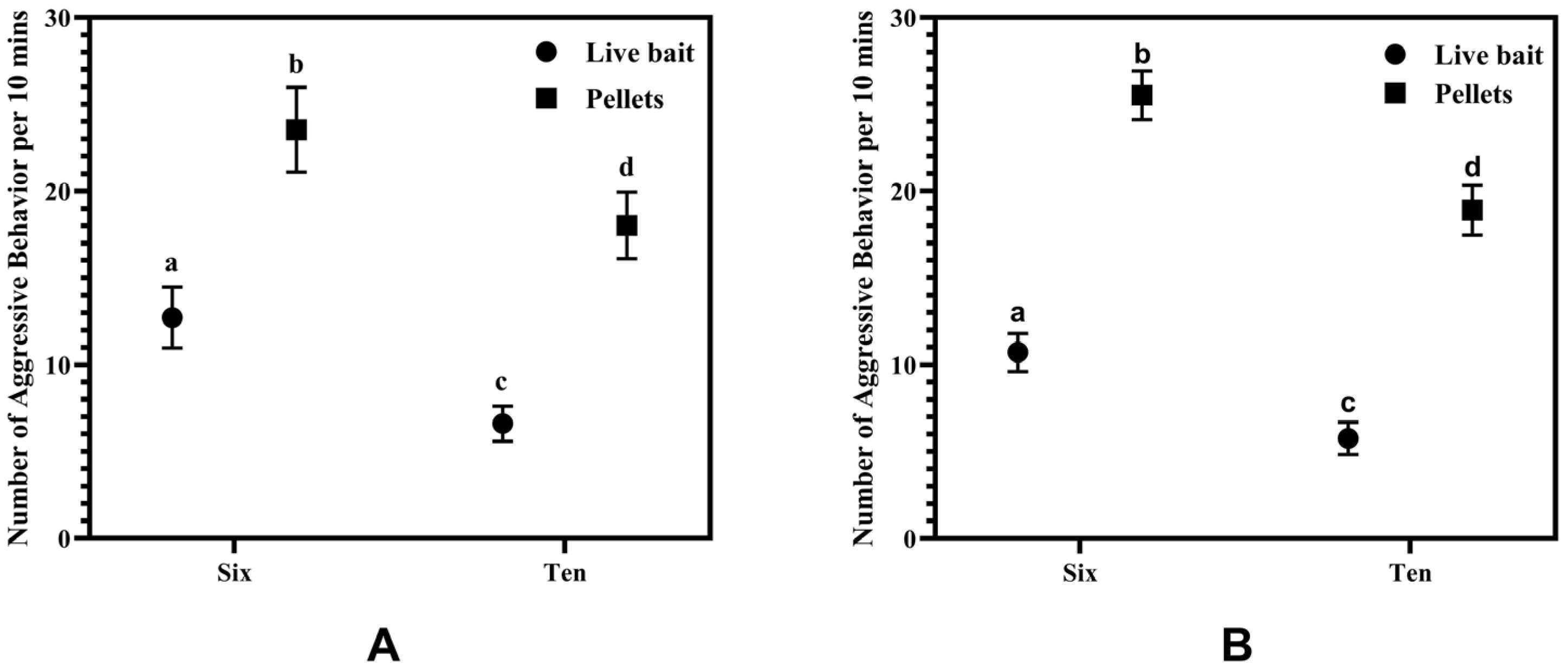
3.3. Attack Behavior
3.4. Cortisol
3.5. Expression of Genes Linked to Neurogenesis
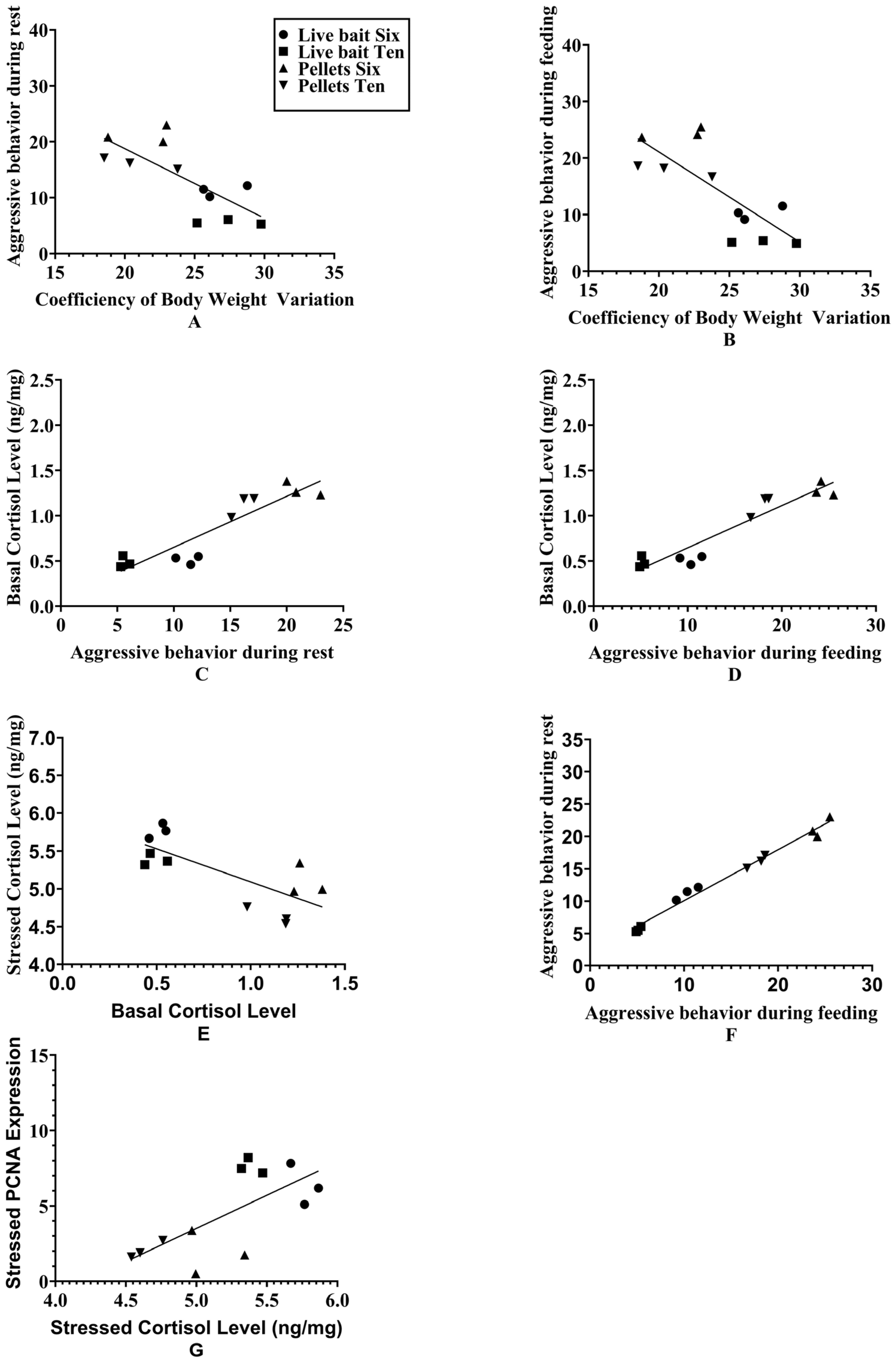
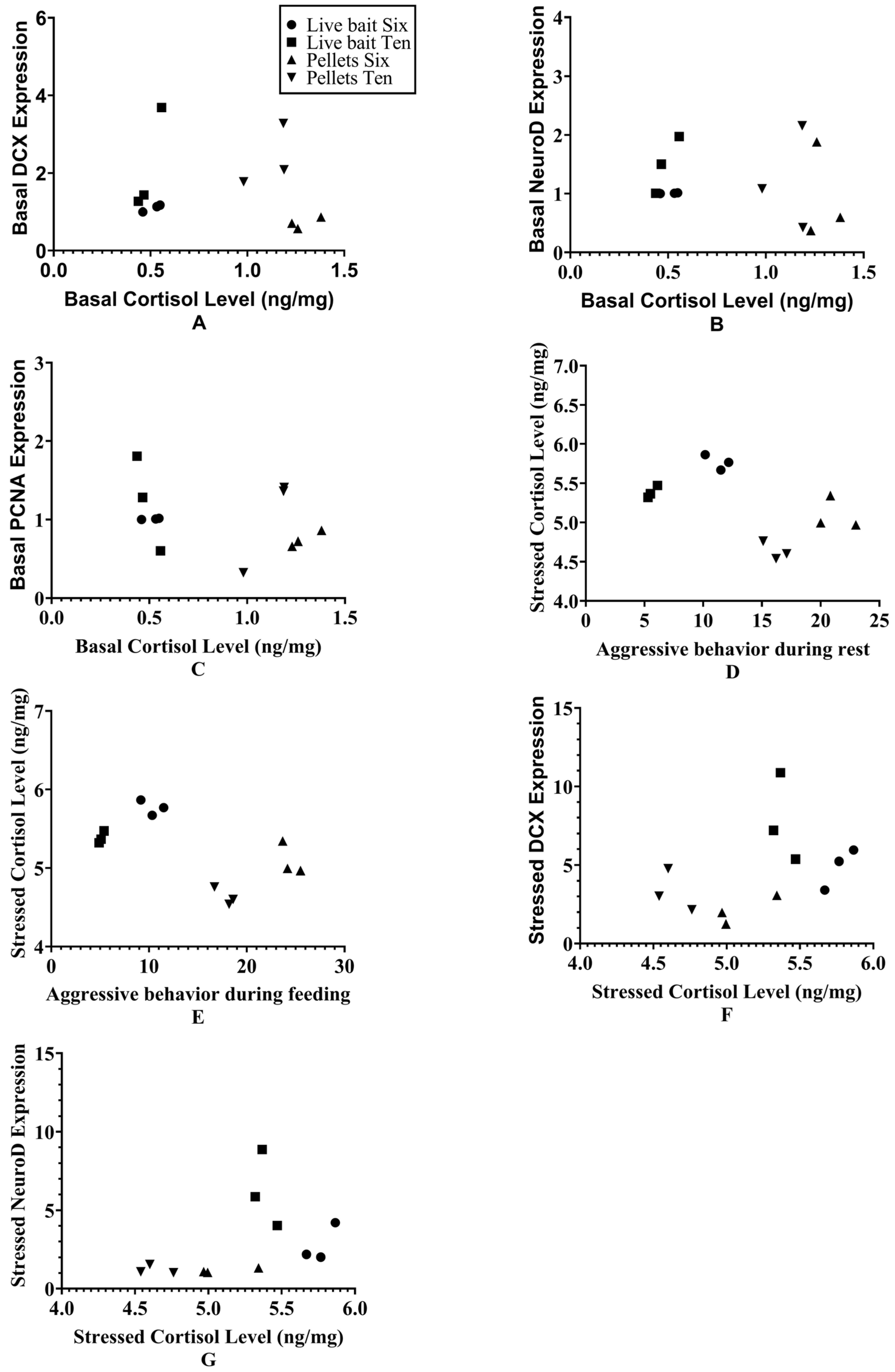
3.6. Relationships Among Body-Weight Coefficients of Variation, Aggressive Behavior, Cortisol, and Neurogenic Gene Markers
4. Discussion
4.1. The Importance of Growth Performance and Antioxidant Capacity to the Aquaculture Sector
4.2. The Impact of the Environment on Cortisol Levels and Aggressive Behavior
4.3. Effects of Stress and Social Enrichment on Brain Cell Proliferation and Neural Transmission
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oladottir, A.D.; Einarsson, G. Fisheries and Aquaculture: The Food Security of the Future, 1st ed.; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128231920. [Google Scholar]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandøe, P.; Turnbull, J.F. Current issues in fish welfare. J. Fish. Biol. 2006, 70, 1311–1316. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Kadri, S. Defining, assessing and promoting the welfare of farmed fish. Rev. Sci. Et Tech. Int. Off. Epizoot. 2014, 33, 233–244. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Cabrera-Lvarez, M.J.; Maia, C.M.; Saraiva, J.L. Environmental enrichment in fish aquaculture: A review of fundamental and practical aspects. Rev. Aquac. 2022, 14, 704–728. [Google Scholar] [CrossRef]
- Brydges, N.M.; Braithwaite, V.A. Does environmental enrichment affect the behaviour of fish commonly used in laboratory work? Appl. Anim. Behav. Sci. 2009, 118, 137–143. [Google Scholar] [CrossRef]
- Martins, C.I.; Galhardo, L.; Noble, C.; Damsgard, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish. Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.R.; Webster, M.M.; Salvanes, A.G.V. Physical enrichment research for captive fish: Time to focus on the DETAILS. J. Fish. Biol. 2021, 99, 704–725. [Google Scholar] [CrossRef]
- NäSlund, J.; Johnsson, J.R.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish Fish. 2016, 17, 1–30. [Google Scholar] [CrossRef]
- Baldwin, L. The effects of stocking density on fish welfare. Plymouth Stud. Sci. 2011, 4, 372–383. [Google Scholar] [CrossRef]
- Turnbull, J.F.; North, B.P.; Ellis, T.; Adams, C.E.; Huntingford, F.A. Stocking Density and the Welfare of Farmed Salmonids. In Fish Welfare; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 111–120. [Google Scholar] [CrossRef]
- Adams, C.E.; Turnbull, J.F.; Bell, A.; Bron, J.E.; Huntingford, F.A. Multiple determinants of welfare in farmed fish: Stocking density, disturbance, and aggression in Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2007, 64, 336–344. [Google Scholar] [CrossRef]
- Ellis, T.; North, B.; Scott, A.P.; Bromage, N.R.; Porter, M.; Gadd, D. The relationships between stocking density and welfare in farmed rainbow trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Ma, Z.; Li, H.; Hu, Y.; Fan, J.; Liu, Y. Growth performance, physiological, and feeding behavior effect of Dicentrarchus labrax under different culture scales. Aquaculture 2021, 534, 736291. [Google Scholar] [CrossRef]
- Haugland, G.T.; Imsland, A.K.D.; Reynolds, P.; Treasurer, J. Application of biological control: Use of cleaner fish. In Aquaculture Health Management; Academic Press: Cambridge, MA, USA, 2020; pp. 319–369. [Google Scholar] [CrossRef]
- Thomas, M.; Lecocq, T.; Abregal, C.; Nahon, S.; Pasquet, A. The effects of polyculture on behaviour and production of pikeperch in recirculation systems. Aquac. Rep. 2020, 17, 100333. [Google Scholar] [CrossRef]
- Anras, L.; Boglione, C.; Cataudella, S.; Dinis, M.; Makridis, P.; Marino, G.; Ramalho Ribeiro, A.; Yúfera, M. The current status of extensive and semi-intensive aquaculture practices in Southern Europe. Aquac. Eur. 2010, 35, 12–16. [Google Scholar]
- Yúfera, M.; Arias, A.M. Traditional polyculture in “Esteros” in the Bay of Cádiz (Spain). Hopes and expectancies for the prevalence of a unique activity in Europe. Aquac. Eur. 2010, 35, 22–25. [Google Scholar]
- Zhang, Z.; Fu, Y.; Zhao, H.; Zhang, X. Social enrichment affects fish growth and aggression depending on fish species: Applications for aquaculture. Front. Mar. Sci. 2022, 9, 1011780. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Q.; Cai, Z.; Ye, J.; Tong, W.; Ren, S.; Wu, X.; Chen, H.; Lü, M.; Zheng, S. Effects of Environmental Enrichment on the Growth, Gonadal Development, and Welfare of the Chinese Hooksnout Carp (Opsariichthys bidens). Fishes 2024, 9, 339. [Google Scholar] [CrossRef]
- Grossman, G.D. Food, fights, and burrows: The adaptive significance of intraspecific aggression in the bay goby (Pisces: Gobiidae). Oecologia 1980, 45, 261–266. [Google Scholar] [CrossRef]
- SøRensen, C.; Bohlin, L.C.; Verli, Y.; Nilsson, G.R.E. Cortisol reduces cell proliferation in the telencephalon of rainbow trout (Oncorhynchus mykiss). Physiol. Behav. 2011, 102, 518–523. [Google Scholar] [CrossRef]
- SøRensen, C.; Nilsson, G.R.E.; Summers, C.H.; Verli, Y. Social stress reduces forebrain cell proliferation in rainbow trout (Oncorhynchus mykiss). Behav. Brain Res. 2012, 227, 311–318. [Google Scholar] [CrossRef]
- Marcon, M.; Mocelin, R.; Benvenutti, R.; Costa, T.; Piato, A. Environmental enrichment modulates the response to chronic stress in zebrafish. J. Exp. Biol. 2018, 221, jeb176735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Wang, Y.; Zhang, X. Effects of environmental enrichment on growth performance, aggressive behavior and stress-induced changes in cortisol release and neurogenesis of black rockfish Sebastes schlegelii—ScienceDirect. Aquaculture 2020, 528, 735483. [Google Scholar] [CrossRef]
- Braithwaite, V.A.; Salvanes, A.G.V. Environmental variability in the early rearing environment generates behaviourally flexible cod: Implications for rehabilitating wild populations. Proc. R. Soc. B Biol. Sci. 2005, 272, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Salvanes, A.G.V.; Moberg, O.; Ebbesson, L.O.E.; Nilsen, T.O.; Jensen, K.H.; Braithwaite, V.A. Environmental enrichment promotes neural plasticity and cognitive ability in fish. Proc. Biol. Sci. 2013, 280, 20131331. [Google Scholar] [CrossRef] [PubMed]
- Bergendahl, I.A.; Gro, V.S.A.; Victoria, A.B. Determining the effects of duration and recency of exposure to environmental enrichment. Appl. Anim. Behav. Sci. 2016, 176, 163–169. [Google Scholar] [CrossRef]
- Roy, T.; Bhat, A.; Herberstein, M. Learning and Memory in Juvenile Zebrafish: What makes the Difference—Population or Rearing Environment? Ethology 2016, 122, 308–318. [Google Scholar] [CrossRef]
- von Krogh, K.; Sørensen, C.; Nilsson, G.E.; Øverli, Ø. Forebrain cell proliferation, behavior, and physiology of zebrafish, Danio rerio, kept in enriched or barren environments. Physiol. Behav. 2010, 101, 32–39. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Johnsson, J.R.I. Effects of size distribution on social interactions and growth of juvenile black rockfish (Sebastes schlegelii). Appl. Anim. Behav. Sci. 2017, 194, 135–142. [Google Scholar] [CrossRef]
- Xi, D.; Zhang, X.; Lü, H.; Zhang, Z. Cannibalism in juvenile black rockfish, Sebastes schlegelii (Hilgendorf, 1880), reared under controlled conditions. Aquaculture 2017, 479, 682–689. [Google Scholar] [CrossRef]
- Lü, H.; Zhang, X.; Fu, M.; Xi, D.; Gao, T. Use of tetracycline hydrochloride and alizarin complexone for immersion marking black rockfish Sebastes schlegelii. J. Oceanol. Limnol. 2014, 32, 810–820. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Q.; Xu, X.; Guo, H.; Zhang, X. Effects of environmental enrichment on the welfare of juvenile black rockfish Sebastes schlegelii: Growth, behavior and physiology. Aquaculture 2020, 518, 734782. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Li, Z.; Zhang, X. Effects of different levels of environmental enrichment on the sheltering behaviors, brain development and cortisol levels of black rockfish Sebastes schlegelii. Appl. Anim. Behav. Sci. 2019, 218, 104825. [Google Scholar] [CrossRef]
- Liu, S.; Cai, H.; Liu, Y.; Zhang, Y.; Fang, Y.; Sun, F.; Wu, Y.; Li, X.; Lv, L.; Zhang, Q. Effects of LED spectra on the growth and physiological mechanism of juvenile Sebastes schlegelii. Part I: Growth, feeding and digestion and metabolism. Aquaculture 2024, 580, 740295. [Google Scholar] [CrossRef]
- Oh, S.; Kim, C.; Jang, Y.; Choi, H.; Myoung, J. Effect of Salinity on Survival, Oxygen Consumption and Blood Physiology of Korean Rockfish Sebastes schlegelii. Ocean Polar Res. 2014, 36, 135–143. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Zhang, J.; Ren, X.; Zhang, X.; Liu, Y.; Shi, X. Effect of water flow on growth and metabolism in Sebastes schlegelii. Aquaculture 2023, 571, 739485. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Ge, X.; Chen, S.; Ma, Z. The Effects of Short-Term Exposure to pH Reduction on the Behavioral and Physiological Parameters of Juvenile Black Rockfish (Sebastes schlegelii). Biology 2023, 12, 876. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Wei, S.; Xiao, W.; Zhao, C.; Wang, Y.; Yang, L. Transcriptome Analysis of Juvenile Black Rockfish Sebastes schlegelii under Air Exposure Stress. Fishes 2024, 9, 239. [Google Scholar] [CrossRef]
- Lee, J.W.; Min, B.H.; Lee, B.; Kim, K.; Yoon, M. Effects of Stocking Density on Stress, Hematological Responses, and Growth of Black Rockfish Sebastes schlegelii. J. Aquat. Anim. Health 2022, 34, 82–91. [Google Scholar] [CrossRef]
- Balshine, S.; Leach, B.; Neat, F.; Reid, H.; Taborsky, M.; Werner, N. Correlates of Group Size in a Cooperatively Breeding Cichlid Fish (Neolamprologus pulcher). Behav. Ecol. Sociobiol. 2001, 50, 134–140. [Google Scholar] [CrossRef]
- Dierkes, P.; Heg, D.; Taborsky, M.; Skubic, E.; Achmann, R. Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 2005, 8, 968–975. [Google Scholar] [CrossRef]
- Näslund, J.; Rosengren, M.; Del Villar, D.; Gansel, L.; Norrgard, J.R.; Persson, L.; Winkowski, J.J.; Kvingedal, E.; Sveriges, L. Hatchery tank enrichment affects cortisol levels and shelter-seeking in Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2013, 70, 585–590. [Google Scholar] [CrossRef]
- Rosengren, M.; Kvingedal, E.; Näslund, J.; Johnsson, J.I.; Sundell, K. Born to be wild: Effects of rearing density and environmental enrichment on stress, welfare, and smolt migration in hatchery-reared Atlantic salmon. Can. J. Fish Aquat. Sci. 2017, 74, 396–405. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Shetty, A.K. Efficacy of doublecortin as a marker to analyse the absolute number anddendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 2004, 19, 234–246. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Q.; Huang, Z.; Hong, Y. Effects of Formulated Pellet Feed or Live Fish Food on the Intestinal and Aquaculture Water Microbial Communities in Goldfish, Carassius auratus. Water 2024, 16, 2486. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, X.; Wu, H.; Gao, J.; Wu, J.; Xiong, Z.; Feng, Z.; Xie, M.; Li, S.; Xie, Z.; et al. The Effect of Short-Term Artificial Feed Domestication on the Expression of Oxidative-Stress-Related Genes and Antioxidant Capacity in the Liver and Gill Tissues of Mandarin Fish (Siniperca chuatsi). Genes 2024, 15, 487. [Google Scholar] [CrossRef]
- Turnbull, J.; Bell, A.; Adams, C.; Bron, J.; Huntingford, F. Stocking density and welfare of cage farmed Atlantic salmon: Application of a multivariate analysis. Aquaculture 2005, 243, 121–132. [Google Scholar] [CrossRef]
- Roy, J.; Terrier, F.; Marchand, M.; Herman, A.; Heraud, C.; Surget, A.; Lanuque, A.; Sandres, F.; Marandel, L. Effects of Low Stocking Densities on Zootechnical Parameters and Physiological Responses of Rainbow Trout (Oncorhynchus mykiss) Juveniles. Biology 2021, 10, 1040. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Yu, Y.; Sun, M.; Xiang, J.; Li, F. Effects of ammonia stress on the hemocytes of the Pacific white shrimp Litopenaeus vannamei. Chemosphere 2020, 239, 124759. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.C.N.; Lee, T.H.C.N. Antioxidant enzymes as redox-based biomarkers: A brief review. BMB Rep. 2015, 48, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, L.; Du, X.; Zhang, P.; Li, W.; Guo, X.; Li, Y. Effect of Lead on Antioxidant Ability and Immune Responses of Crucian Carp. Biol. Trace Elem. Res. 2018, 186, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ran, C.; He, S.; Zhang, J.; Hu, J.; Yang, Y.; Du, Z.; Yang, Y.; Zhou, Z. Effects of dietary yeast nucleotides on growth, non-specific immunity, intestine growth and intestinal microbiota of juvenile hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus. Anim. Nutr. 2015, 1, 244–251. [Google Scholar] [CrossRef]
- Xiong, J.; Jin, M.; Yuan, Y.; Luo, J.X.; Lu, Y.; Zhou, Q.C.; Liang, C.; Tan, Z.L. Dietary nucleotide-rich yeast supplementation improves growth, innate immunity and intestinal morphology of Pacific white shrimp (Litopenaeus vannamei). Aquacult Nutr. 2018, 24, 1425–1435. [Google Scholar] [CrossRef]
- de Oliveira, E.G.; Pinheiro, A.B.; de Oliveira, V.Q.; Da Silva, A.R.M.; de Moraes, M.G.; Rocha, Í.R.C.B.; de Sousa, R.R.; Costa, F.H.F. Effects of stocking density on the performance of juvenile pirarucu (Arapaima gigas) in cages. Aquaculture 2012, 370–371, 96–101. [Google Scholar] [CrossRef]
- Farias, C.F.H. Preliminary Studies on the Optimum Feeding Rate for Pirarucu Arapaima gigas Juveniles Reared in Floating Cages. Int. J. Aquac. 2013, 3, 147–151. [Google Scholar] [CrossRef]
- Taheri, M.; Mousavi, S.M.; Zanguee, N.; Mozanzadeh, M.T. The interactive effects of stocking density and salinity on growth, immune response, antioxidant capacity, digestive enzyme activity, and plasma biochemical parameters in Asian seabass (Lates calcarifer) juveniles. Aquac. Int. 2025, 33, 146. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. A review on recirculating aquaculture system: Influence of stocking density on fish and crustacean behavior, growth performance, and immunity. Ann. Anim. Sci. 2022, 22, 873. [Google Scholar] [CrossRef]
- Anderson, H.M.; Little, A.G.; Fisher, D.N.; McEwen, B.L.; Culbert, B.M.; Balshine, S.; Pruitt, J.N. Correction: Behavioral and physiological evidence that increasing group size ameliorates the impacts of social disturbance. J. Exp. Biol. 2024, 223, jeb217075. [Google Scholar] [CrossRef]
- Jordan, L.A.; Wong, M.Y.L.; Balshine, S.S. The effects of familiarity and social hierarchy on group membership decisions in a social fish. Biol. Lett. 2010, 6, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A.; Ribas, L.; Acerete, L.; Tort, L. Effects of chronic confinement on physiological responses of juvenile gilthead sea bream, Sparus aurata L., to acute handling. Aquac. Res. 2005, 36, 172–179. [Google Scholar] [CrossRef]
- Johansen, I.B.; Sørensen, C.; Sandvik, G.K.; Nilsson, G.E.; Höglund, E.; Bakken, M.; Øverli, Ø. Neural plasticity is affected by stress and heritable variation in stress coping style. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 161–171. [Google Scholar] [CrossRef]
- Dunlap, K.D. Fish Neurogenesis in Context: Assessing Environmental Influences on Brain Plasticity within a Highly Labile Physiology and Morphology. Brain Behav. Evol. 2016, 87, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Boerrigter, J.G.J.; van den Bos, R.; van de Vis, H.; Spanings, T.; Flik, G. Effects of density, PVC-tubes and feeding time on growth, stress and aggression in African catfish (Clarias gariepinus). Aquac. Res. 2016, 47, 2553–2568. [Google Scholar] [CrossRef]
- Madison, B.N.; Heath, J.W.; Heath, D.D.; Bernier, N.J. Effects of early rearing environment and breeding strategy on social interactions and the hormonal response to stressors in juvenile Chinook salmon. Can. J. Fish. Aquat. Sci. 2015, 72, 673–683. [Google Scholar] [CrossRef]
- Manuel, R.; Boerrigter, J.G.J.; Cloosterman, M.; Gorissen, M.; Flik, G.; van den Bos, R.; van de Vis, H. Effects of acute stress on aggression and the cortisol response in the African sharptooth catfish Clarias gariepinus: Differences between day and night. J. Fish. Biol. 2016, 88, 2175–2187. [Google Scholar] [CrossRef]
- Pounder, K.C.; Mitchell, J.L.; Thomson, J.S.; Pottinger, T.G.; Buckley, J.; Sneddon, L.U. Does environmental enrichment promote recovery from stress in rainbow trout? Appl. Anim. Behav. Sci. 2016, 176, 136–142. [Google Scholar] [CrossRef]
- Nishi, M.; Horii-Hayashi, N.; Sasagawa, T.; Matsunaga, W. Effects of early life stress on brain activity: Implications from maternal separation model in rodents. Gen. Comp. Endocr. 2013, 181, 306–309. [Google Scholar] [CrossRef]
- Øverli, Ø.; Sørensen, C. On the Role of Neurogenesis and Neural Plasticity in the Evolution of Animal Personalities and Stress Coping Styles. Brain Behav. Evol. 2016, 87, 167–174. [Google Scholar] [CrossRef]
- Van Weerd, J.H.; Komen, J. The effects of chronic stress on growth in fish: A critical appraisal. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 107–112. [Google Scholar] [CrossRef]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Hollenberg, S.M.; Snider, L.; Turner, D.L.; Lipnick, N.; Weintraub, H. Conversion of Xenopus Ectoderm into Neurons by NeuroD, a Basic Helix-Loop- Helix Protein. Sci. Am. Assoc. Adv. Sci. 1995, 268, 836–844. [Google Scholar] [CrossRef]
- Mes, D.; Palstra, A.P.; Henkel, C.V.; Mayer, I.; Vindas, M.A. Swimming exercise enhances brain plasticity in fish. R. Soc. Open Sci. 2020, 7, 191640. [Google Scholar] [CrossRef]
- Brenes, J.C.; Lackinger, M.; Höglinger, G.U.; Schratt, G.; Schwarting, R.K.W.; Wöhr, M. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J. Comp. Neurol. 2016, 524, 1586–1607. [Google Scholar] [CrossRef]
- Vindas, M.A.; Gorissen, M.; Höglund, E.; Flik, G.; Tronci, V.; Damsgård, B.; Thörnqvist, P.; Nilsen, T.O.; Winberg, S.; Øverli, Ø.; et al. How do individuals cope with stress? Behavioural, physiological and neuronal differences between proactive and reactive coping styles in fish. J. Exp. Biol. 2017, 220, 1524–1532. [Google Scholar] [CrossRef]


| Treatment | Indicator | Group Means ± SD | Factor | F Value | p Value | |
|---|---|---|---|---|---|---|
| Bait Type | Group Size | |||||
| Live Bait | Six | WG | 1.862 ± 0.826 | Bait Type | 1.244 | 0.297 |
| Live Bait | Ten | 2.145 ± 1.136 | ||||
| Pellet | Six | 2.286 ± 0.363 | Group Size | 0.616 | 0.455 | |
| Pellet | Ten | 2.669 ± 0.246 | ||||
| Interaction | 0.014 | 0.909 | ||||
| Live Bait | Six | FCR | 1.240 ± 0.482 | Bait Type | 5.164 | 0.053 |
| Live Bait | Ten | 1.174 ± 0.594 | ||||
| Pellet | Six | 1.578 ± 0.326 | Group Size | 0.767 | 0.407 | |
| Pellet | Ten | 2.149 ± 0.553 | ||||
| Interaction | 1.217 | 0.302 | ||||
| Live Bait | Six | CF(%) | 2.297 ± 0.710 | Bait Type | 0.139 | 0.719 |
| Live Bait | Ten | 2.001 ± 0.594 | ||||
| Pellet | Six | 2.364 ± 0.277 | Group Size | 0.626 | 0.452 | |
| Pellet | Ten | 2.166 ± 0.483 | ||||
| Interaction | 0.026 | 0.877 | ||||
| Live Bait | Six | SGR(%) | 2.427 ± 0.768 | Bait Type | 1.814 | 0.215 |
| Live Bait | Ten | 2.393 ± 0.725 | ||||
| Pellet | Six | 2.572 ± 0.198 | Group Size | 0.599 | 0.461 | |
| Pellet | Ten | 3.091 ± 0.158 | ||||
| Interaction | 0.778 | 0.403 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yu, X.; Wu, Z.; Tian, T. Effects of Social Enrichment Induced by Different-Sized Groups and Live Bait on Growth, Aggressive Behavior, Physiology, and Neurogenesis in Juvenile Sebastes schlegelii. Fishes 2025, 10, 242. https://doi.org/10.3390/fishes10050242
Zhang Z, Yu X, Wu Z, Tian T. Effects of Social Enrichment Induced by Different-Sized Groups and Live Bait on Growth, Aggressive Behavior, Physiology, and Neurogenesis in Juvenile Sebastes schlegelii. Fishes. 2025; 10(5):242. https://doi.org/10.3390/fishes10050242
Chicago/Turabian StyleZhang, Zhen, Xiaoming Yu, Zhongxin Wu, and Tao Tian. 2025. "Effects of Social Enrichment Induced by Different-Sized Groups and Live Bait on Growth, Aggressive Behavior, Physiology, and Neurogenesis in Juvenile Sebastes schlegelii" Fishes 10, no. 5: 242. https://doi.org/10.3390/fishes10050242
APA StyleZhang, Z., Yu, X., Wu, Z., & Tian, T. (2025). Effects of Social Enrichment Induced by Different-Sized Groups and Live Bait on Growth, Aggressive Behavior, Physiology, and Neurogenesis in Juvenile Sebastes schlegelii. Fishes, 10(5), 242. https://doi.org/10.3390/fishes10050242







