Fick’s Diffusion Laws and Scaling of the Gill Surface Area and Oxygen Uptake in Fish
Abstract
1. Introduction
2. Materials and Methods
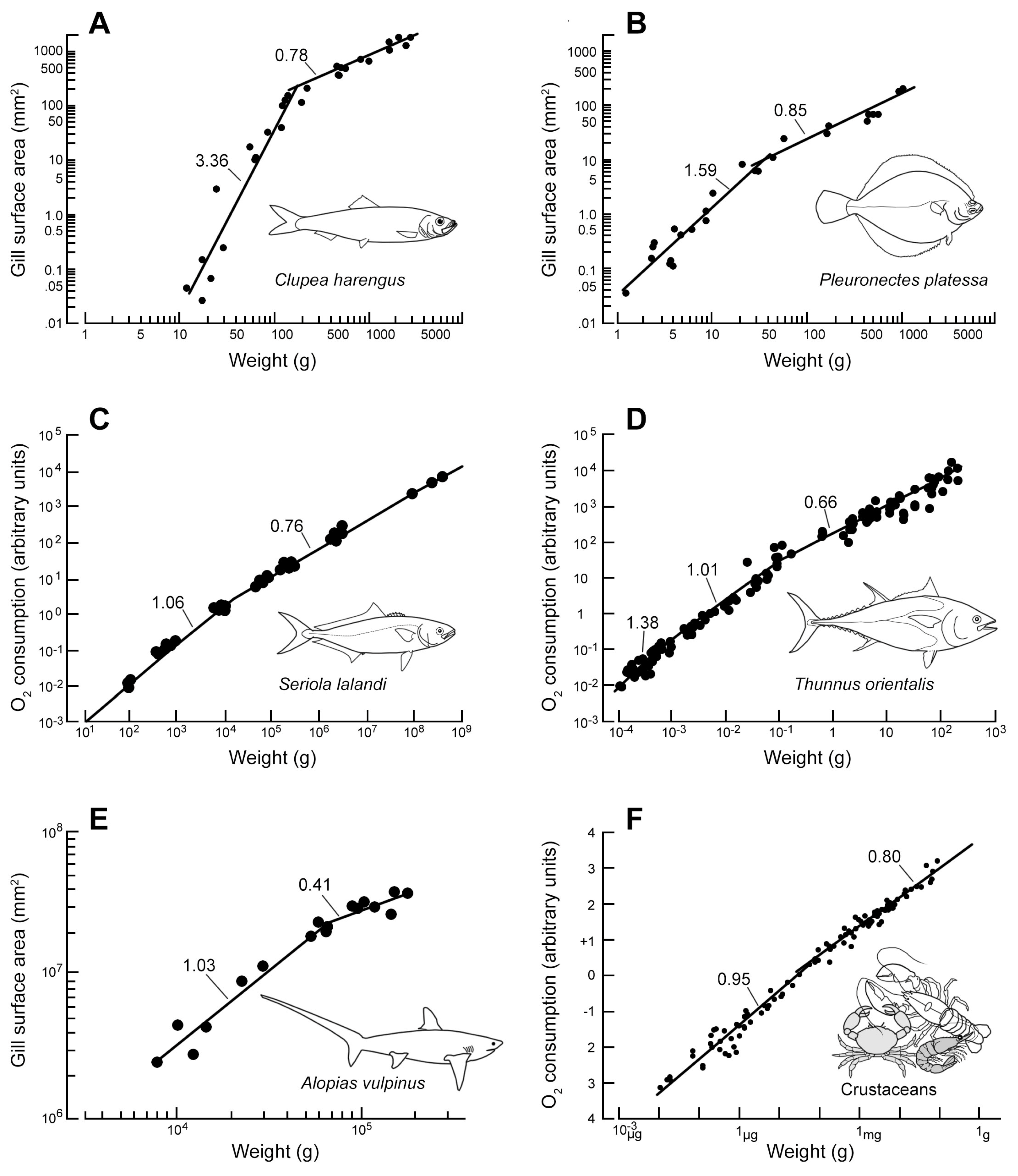
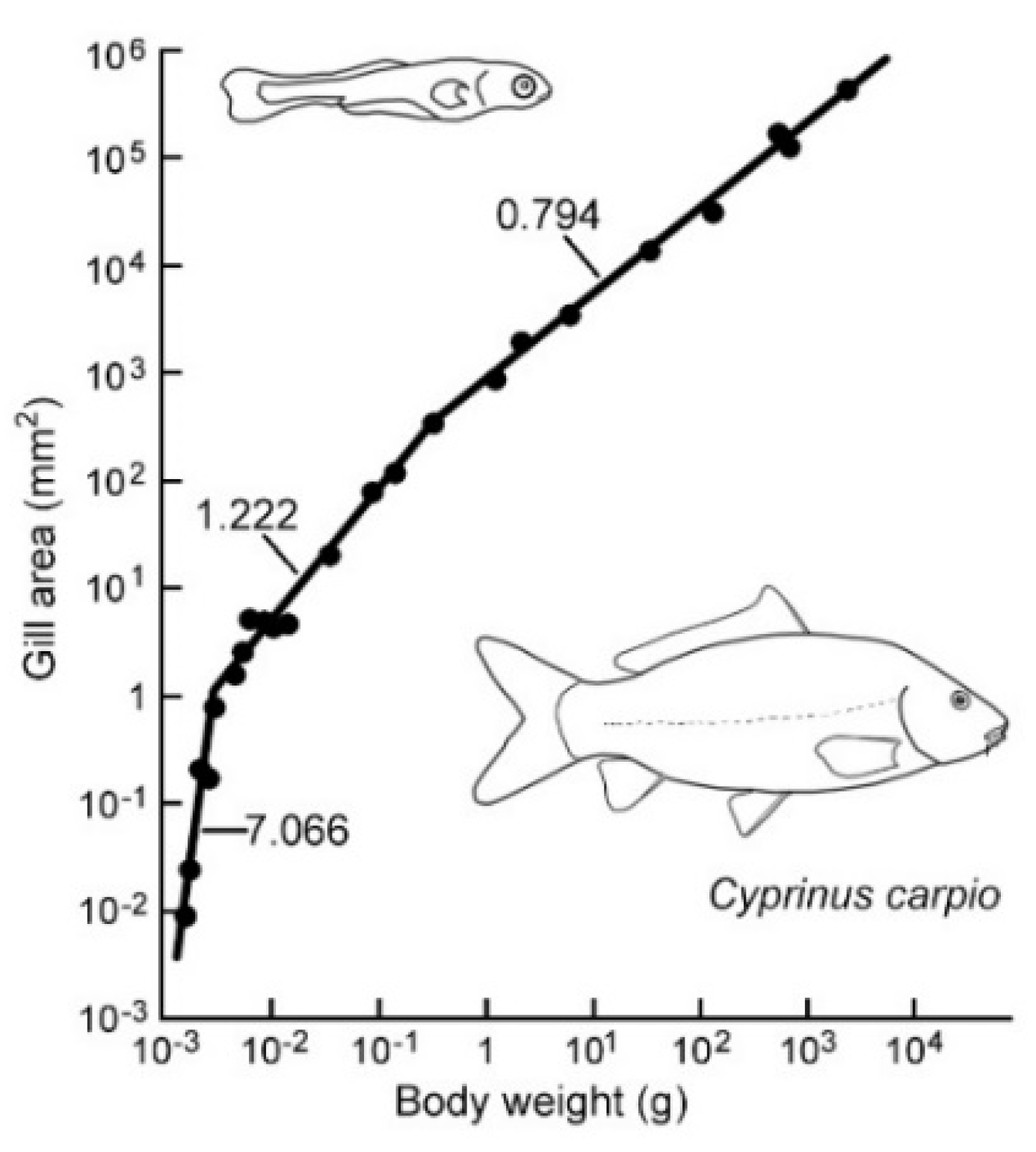
3. Results
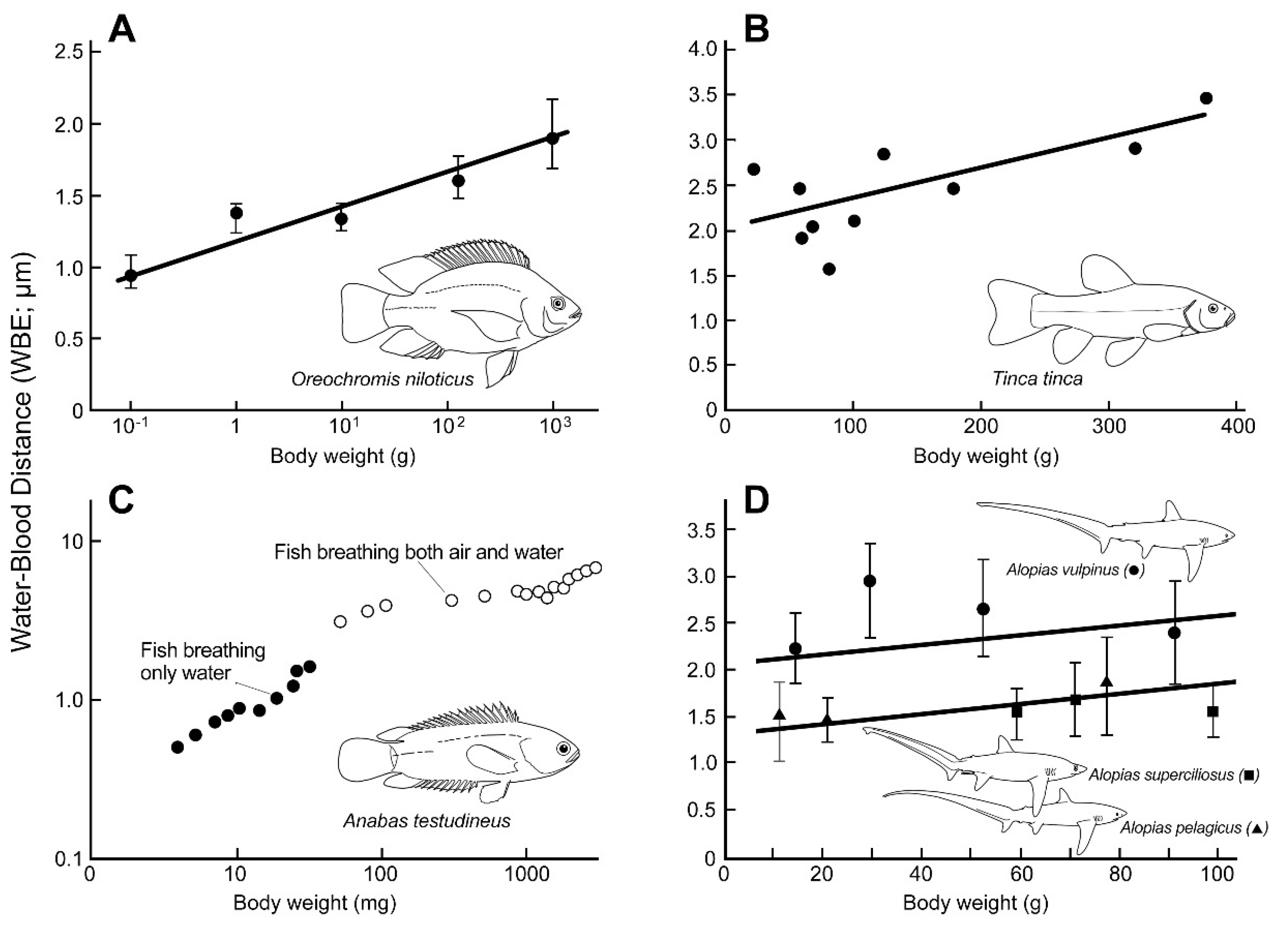
Ontogenetic Changes in Scaling and Diffusion Distance
4. Discussion
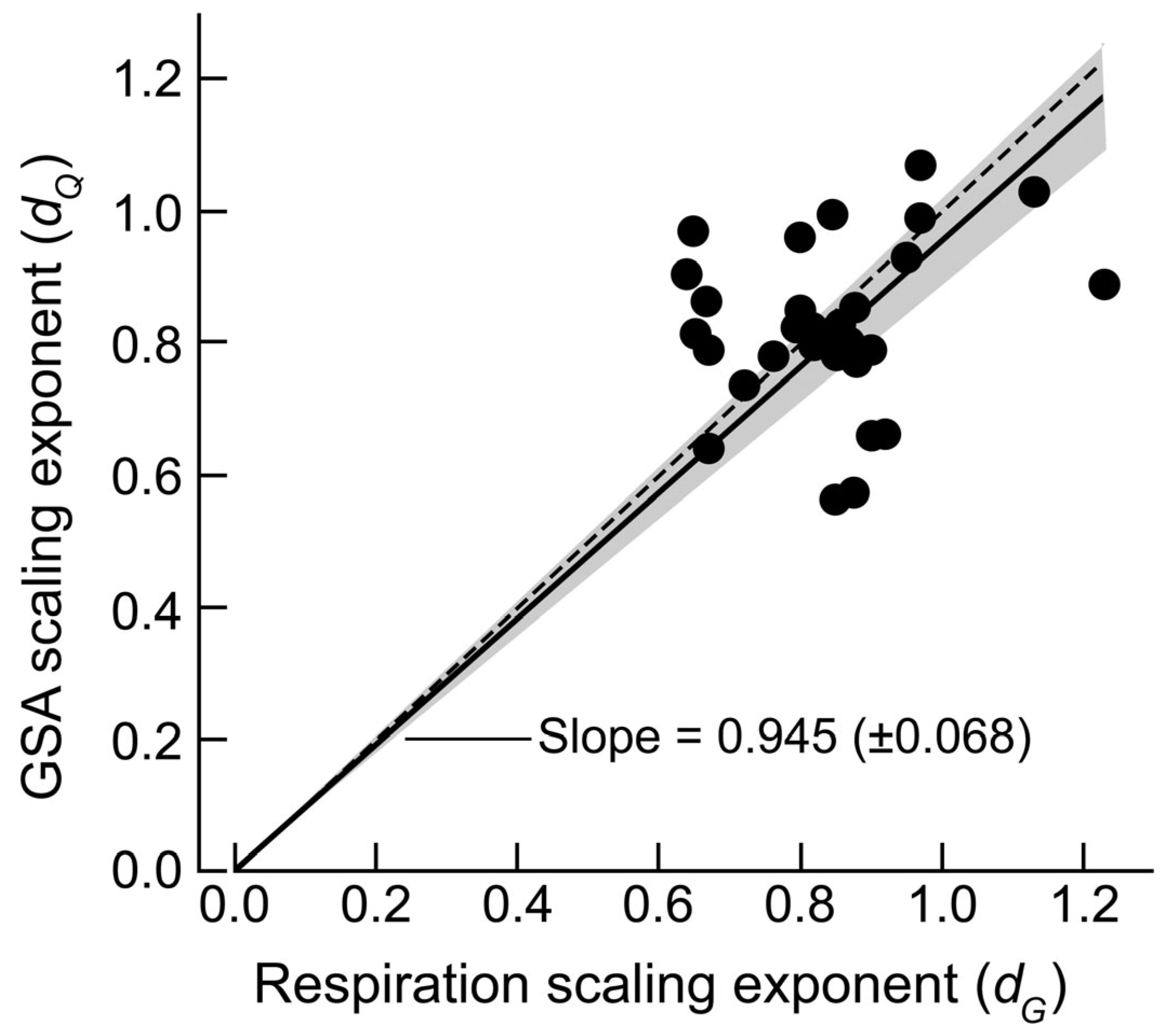
4.1. Ontogenetic Changes in Scaling (OCS) of dG and dG and Their Effect
4.2. Ontogenetic Changes in Scaling (OCS) of WBD
4.3. Surface Area, Diffusion, and Ventilation Rates
| Species | R |
|---|---|
| Coregonus pollan | 57 |
| Gadus morhua (9 years old) | <49 |
| Coregonus albula (different seasons) | 41–59 |
| Micropterus salmoides | 40 |
| Gadus morhua (3 years old) | <37 |
| Gasterosteus aculeatus (diff. seasons) | 36–52 |
| Esox lucius (diff. seasons) | 16–50 |
| Anguilla anguilla | 7 |
| Mean | ~30 |
4.4. Active and Standard Metabolic Rates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GOLT | Gill-Oxygen Limitation Theory |
| OCS | Ontogenetic changes in scaling |
| WBEs | Water-breathing ectotherms |
| GSA | Gill surface area (of adult WBE) |
| WBD | Water–Blood Distance, or diffusion distance |
| AMR | Active metabolic rate |
| SMR | Standard metabolic rate |
References
- Cheung, W.W.L.; Sarmiento, J.L.; Dunne, J.; Fröhlicher, T.L.; Lam, V.W.Y.; Palomares, M.L.D.; Watson, R.; Pauly, D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Rubalcaba, J.G.; Verberk, W.C.; Hendriks, A.J.; Saris, B.; Woods, H.A. Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proc. Natl. Acad. Sci. USA 2020, 117, 31963–31968. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.C.; Atkinson, D.; Hoefnagel, K.N.; Hirst, H.G.; Horne, C.R.; Siepel, H. Shrinking body sizes in response to warming: Explanations for the temperature–size rule with special emphasis on the role of oxygen. Biol. Rev. 2021, 96, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Houben, N.; Pauly, D. On being the wrong size: What is the role of body size in fish kills and hypoxia exposure? Environ. Biol. Fishes 2023, 106, 1651–1667. [Google Scholar] [CrossRef]
- Pauly, D. The relationships between gill surface area and growth performance in fish: A generalization of von Bertalanffy’s theory of growth. Berichte Dtsch. Wiss. Komm. Meeresforsch. 1981, 28, 251–282. [Google Scholar]
- Pauly, D. A mechanism for the juvenile-to-adult transition in fishes. J. Cons. Int. l’Explor. Mer 1984, 41, 280–284. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size—A biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar] [CrossRef]
- Glazier, D.S. Multiphasic allometry: The reality and significance of ontogenetic shifts in the body-mass scaling of metabolic rate. Acad. Biol. 2024, 2, AcadBiol7411. [Google Scholar] [CrossRef]
- Glennan, S. Rethinking mechanistic explanation. Philos. Sci. 2002, 69, S342–S353. [Google Scholar] [CrossRef]
- Bechtel, W.; Bich, L. Organisms Need Mechanisms; Mechanisms Need Organisms. New Mechanism. Explan. Emerg. Reduct. 2024, 35, 85–108. [Google Scholar] [CrossRef]
- Ursin, E. A mathematical model of some aspects of fish growth, respiration, and mortality. J. Fish. Res. Bd. Can. 1967, 24, 2355–2453. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. Theoretische Biologie. Zweiter Band: Stoffwechsel, Wachstum; A. Francke Verlag: Bern, Switzerland, 1951. [Google Scholar]
- Hemmingsen, A.M. Energy metabolism as related to body size and respiratory surfaces, and its evolution. Rep. Steno Mem. Hosp. Nord. Insul. Lab. 1960, 9, 1–110. [Google Scholar]
- Pauly, D. The Gill-Oxygen Limitation Theory (GOLT) and its critics. Sci. Adv. 2021, 7, eabc6050. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Pauly, D. Fishes in Warming Waters, the Gill-Oxygen Limitation Theory and the Debate around Mechanistic Growth Models. Fishes 2024, 9, 430. [Google Scholar] [CrossRef]
- Pauly, D.; Lam, M.E. Too hot or too cold: The biochemical basis of temperature-size rules for fish and other ectotherms. Environ. Biol. Fishes 2023, 106, 1519–1527. [Google Scholar] [CrossRef]
- Scheuffele, H.; Jutfelt, F.; Clark, T.D. Investigating the gill-oxygen limitation hypothesis in fishes: Intraspecific scaling relationships of metabolic rate and gill surface area. Conserv. Physiol. 2021, 9, coab040. [Google Scholar] [CrossRef]
- Jutfelt, F.; Ern, R.; Leeuwis, R.H.J.; Clark, T.D. Effects of climate warming. In Encyclopedia of Fish Physiology, 2nd ed.; Aldemann, S.L., Gillis, T.E., Eds.; Academic Press: New York, NY, USA, 2024; Volume 3, pp. 14–31. [Google Scholar] [CrossRef]
- Lonthair, J.K.; Wegner, N.C.; Cheng, B.S.; Fangue, N.A.; O’Donnell, M.J.; Regish, A.M.; Swenson, J.D.; Argueta, E.; McCormick, S.D.; Letcher, B.H.; et al. Smaller body size under warming is not due to gill-oxygen limitation in a cold-water salmonid. J. Exp. Biol. 2024, 227, jeb246477. [Google Scholar] [CrossRef]
- Fick, A. On liquid diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 10, 30–39. [Google Scholar] [CrossRef]
- De Jager, S.; Dekkers, W.J. Relations between gill structure and activity in fish. Netherl. J. Zool. 1974, 25, 276–308. [Google Scholar] [CrossRef]
- Palzenberger, M.; Pohla, H. Gill surface area of water-breathing freshwater fish. Rev. Fish Biol. Fish. 1992, 2, 187–216. [Google Scholar] [CrossRef]
- Hughes, G.M. Morphometrics of fish gills. Respir. Physiol. 1972, 14, 1–25. [Google Scholar] [CrossRef]
- Prasad, M.S.; Prasad, P. Blood-water diffusion barrier at the secondary gill lamellae in Anabas testudineus (Bloch) during early ontogenesis. Curr. Sci. 1984, 53, 156–158. [Google Scholar]
- Kisia, S.; Hughes, G.M. Estimation of oxygen-diffusing capacity in the gills of different sizes of a tilapia, Oreochromis niloticus. J. Zool. 1992, 227, 405–415. [Google Scholar] [CrossRef]
- Wootton, T.P.; Sepulveda, C.A.; Wegner, N.C. Gill morphometrics of the thresher sharks (Genus Alopias): Correlation of gill dimensions with aerobic demand and environmental oxygen. J. Morph. 2015, 276, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Frommel, A.Y.; Kwan, G.T.; Prime, K.J.; Tresguerres, M.; Lauridsen, H.; Val, A.L.; Gonçalves, L.U.; Brauner, C.J. Changes in gill and air-breathing organ characteristics during the transition from water-to air-breathing in juvenile Arapaima gigas. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Pütter, A. Vergleichende Physiologie; Georg Fischer: Jena, Germany, 1911. [Google Scholar]
- Pütter, A. Studien über physiologische Ähnlichkeit VI. Wachstumsähnlichkeiten. Pflügers Arch. Gesamte Physiol. Menschen Tiere 1920, 180, 298–340. [Google Scholar] [CrossRef]
- Wegner, N.C.; Sepulveda, C.A.; Bull, K.B.; Graham, J.B. Gill morphometrics in relation to gas transfer and ram ventilation in high-energy demand teleosts: Scombrids and billfishes. J. Morph. 2010, 271, 36–49. [Google Scholar] [CrossRef]
- Rombough, P.; Moroz, B. The scaling and potential importance of cutaneous and branchial surfaces in respiratory gas exchange in young chinook salmon (Oncorhynchus tshawytscha). J. Exp. Biol. 1990, 154, 1–12. [Google Scholar] [CrossRef]
- Muir, B.S.; Hughes, G.M. Dimensions for three species of tunny. J. Exp. Biol. 1969, 51, 271–285. [Google Scholar] [CrossRef]
- Holeton, G.F. Respiratory morphometrics of white and red-blooded Antarctic fish. Comp. Biochem. Physiol. 1976, 54, 215–220. [Google Scholar] [CrossRef]
- Hughes, G.M. Morphometry of fish gas exchange in relation to their respiratory function. In Environmental Physiology of Fishes; Ali, M.E., Ed.; Plenum: New York, NY, USA, 1981; pp. 33–56. [Google Scholar]
- Hughes, G.M. Measurement of gill area in fishes: Practices and problems. J. Mar. Biol. Assoc. UK 1984, 64, 637–655. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Scaling: Why is Animal Size So Important; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Duthie, G.G.; Hughes, G.M. The effects of reduced gill area and hyperoxia on the oxygen consumption and swimming speed of rainbow trout. J. Exp. Biol. 1987, 127, 349–354. [Google Scholar] [CrossRef]
- Brill, R.W. On the standard metabolic rates of tropical tunas, including the effect of body size and acute temperature change. Fish. Bull. 1987, 85, 25–35. [Google Scholar]
- Dewar, H.; Graham, J.B. Studies of tropical tuna swimming performance in a large water tunnel: I. Energetics. J. Exp. Biol. 1994, 192, 13–31. [Google Scholar] [CrossRef]
- Benetti, D.D.; Brill, R.W.; Kraul, S.A. The standard metabolic rate of dolphin fish. J. Fish Biol. 1995, 46, 987–996. [Google Scholar] [CrossRef]
- Rosenfeld, J.; Van Leeuwen, T.; Richards, J.; Allen, D. Relationship between growth and standard metabolic rate: Measurement artifacts and implications for habitat use and life-history adaptation in salmonids. J. Anim. Ecol. 2015, 84, 4–20. [Google Scholar] [CrossRef]
- Oikawa, S.; Itazawa, Y. Gill and body surface areas of the carp in relation to body mass, with special reference to the metabolism-size relationship. J. Exp. Biol. 1985, 117, 1–14. [Google Scholar] [CrossRef]
- Bochdansky, A.B.; Leggett, W.C. Winberg revisited: Convergence of routine metabolism in larval and juvenile fish. Can. J. Fish. Aquat. Sci. 2001, 58, 220–230. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Q.; Zhu, X.; Zhou, J.; Shen, C.; Xia, D.; Djiba, P.K.; Xie, H.; Lv, X.; Jia, J.; et al. Ventilation frequency reveals the roles of exchange surface areas in metabolic scaling. Physiol. Biochem. Zool. 2020, 93, 13–22. [Google Scholar] [CrossRef]
- Fieller, E.C. The biological standardization of insulin. Suppl. J. R. Stat. Soc. 1940, 7, 1–64. [Google Scholar] [CrossRef]
- Pauly, D.; Amarasinghe, U.S.; Chu, E.; Freire, K.M.F.; Vasquez, E.; Butler, M.J. The growth, respiration, and reproduction of crustaceans: A synthesis through the Gill- Oxygen Limitation Theory (GOLT). J. Crustac. Biol. 2022, 42, 1–13. [Google Scholar] [CrossRef]
- De Silva, D.P. Development of the Respiratory System in Herring and Plaice Larvae. In The Early Life History of Fishes; Blaxter, J.H., Ed.; Springer: Berlin, Germany; New York, NY, USA, 1974; pp. 465–485. [Google Scholar]
- Moran, D.; Wells, R.M. Ontogenetic scaling of fish metabolism in the mouse-to-elephant mass magnitude range. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, S.; Hattori, N.; Sawada, Y.; Ishibashi, Y.; Nakatsukasa, H.; Okada, T.; Murata, O.; Kumai, H. Ontogenetic change in oxygen consumption of bluefin tuna, Thunnus thynnus. Aquacult. Sci. 1999, 47, 269–275. [Google Scholar]
- Pauly, D.; Cheung, W.W.L. Sound physiological knowledge and principles in modelling shrinking of fishes under climate change. Glob. Change Biol. 2018, 24, e15–e26. [Google Scholar] [CrossRef]
- Wegner, N.C. Elasmobranch gill structure. In Physiology of Elasmobranch Fishes: Structure and Interaction with Environment; Shadwick, R.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: London, UK, 2016; pp. 102–153. [Google Scholar]
- Zeuthen, E. Oxygen Uptake as Related to Body Size in Organisms. Q. Rev. Biol. 1953, 28, 1–12. [Google Scholar] [CrossRef]
- Wootton, T.P. Gill Morphometrics of Thresher Sharks (Genus Alopias): An Investigation of the Evolutionary Pressures Influencing Gill Morphology. Ph.D. Thesis, University of California, San Diego, CA, USA, 2011. [Google Scholar]
- Satora, L.; Wegner, N.C. Reexamination of the Byczkowska-Smyk gill surface area data for European teleosts, with new measurements on the pikeperch, Sander lucioperca. Rev. Fish Biol. Fish. 2012, 22, 1–9. [Google Scholar] [CrossRef]
- Bennett, M. Morphometric analysis of the gills of the European eel, Anguilla anguilla. J. Zool. 1988, 215, 549–560. [Google Scholar] [CrossRef]
- Degani, G.; Gallagher, M.L.; Meltzer, A. The influence of body size and temperature on oxygen consumption of the European eel, Anguilla anguilla. J. Fish Biol. 1989, 34, 19–24. [Google Scholar] [CrossRef]
- Li, G.; Lv, X.; Zhou, J.; Shen, C.; Xia, D.; Xie, H.; Luo, Y. Are the surface areas of the gills and body involved with changing metabolic scaling with temperature? J. Exp. Biol. 2018, 221, jeb174474. [Google Scholar] [CrossRef]
- Killen, S.S.; Atkinson, D.S.; Glazier, D.S. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 2010, 13, 184–193. [Google Scholar] [CrossRef]
- Beamish, F.W.H. Respiration of fishes with special emphasis on standard oxygen consumption. II. Influence of weight and temperature on respiration of several species. Can. J. Zool. 1964, 42, 177–188. [Google Scholar] [CrossRef]
- Roy, P.K.; Munshi, J.S.D. Oxygen uptake in relation to body weight and respiratory surface area in Cirrhinus mrigala (Ham.) at two different seasonal temperatures. Proc. Indian Natl. Sci. Acad. 1984, B50, 387–394. [Google Scholar]
- Hughes, G.M. Morphological measurements on the gills of fishes in relation to their respiratory function. Folia Morphol. 1970, 18, 78–95. [Google Scholar]
- Winberg, G.G. Rate of Metabolism and Food Requirements of Fishes; Minsk, USSR. Fisheries Research Board of Canada, Translation Series No. 194; Fisheries Research Board of Canada, Biological Station: Nanaimo, BC, Canada, 1960. [Google Scholar]
- Jakubowski, M. Reexamination of the gill respiratory surface area in the pike, Esox lucius, and remarks on the other fish species. Acta Biol. Cracov. Ser. Zool. 1992, 34, 25–32. [Google Scholar]
- Diana, J.S. An experimental analysis of the metabolic rate and food utilization of northern pike. Comp. Biochem. Physiol. 1982, 71A, 395–399. [Google Scholar] [CrossRef]
- Armstrong, J.D.; Priede, I.G.; Lucas, M.C. The link between respiratory capacity and changing metabolic demands during growth of northern pike, Esox lucius L. J. Fish Biol. 1992, 41 (Suppl. B), 65–75. [Google Scholar] [CrossRef]
- Skeeles, M.R.; Clark, T.D. Fish gill surface area can keep pace with metabolic oxygen requirements across body mass and temperature. Funct. Ecol. 2024, 38, 755–764. [Google Scholar] [CrossRef]
- Hölker, F.; Thiel, R. Biology of ruffe (Gymnocephalus cernuus (L.))—A review of selected aspects from European literature. J. Great Lakes Res. 1998, 24, 186–204. [Google Scholar] [CrossRef]
- Tátrai, I. Feeding Habits and Energy Utilization by Ruffe (Acerina cernua L.) in Lake Balaton. Ph.D. Thesis, Balaton Limnological Research Institute, Tihany, Hungary, 1977. [Google Scholar]
- Xiong, W.; Zhu, Y.; Zhang, P.; Xu, Y.; Zhou, J.; Zhang, J.; Luo, Y. Effects of temperature on metabolic scaling in silver carp. J. Exp. Zool. Part A 2022, 337, 141–149. [Google Scholar] [CrossRef]
- Brill, R.W. The effect of body size on the standard metabolic rate of skipjack tuna, Katsuwonus pelamis (L.). Fish Bull. US 1979, 77, 494–498. [Google Scholar]
- Davies, R.; Moyes, C.D. Allometric scaling in centrarchid fish: Origins of intra-and inter-specific variation in oxidative and glycolytic enzyme levels in muscle. J. Exp. Biol. 2007, 210, 3798–3804. [Google Scholar] [CrossRef] [PubMed]
- Ojha, J.; Singh, R. Oxygen uptake in relation to body weight of the freshwater catfish, Mystus cavasius (Ham.). Indian J. Exp. Biol. 1981, 19, 126–129. [Google Scholar]
- Somo, D.A.; Chu, K.; Richards, J.G. Gill surface area allometry does not constrain the body mass scaling of maximum oxygen uptake rate in the tidepool sculpin, Oligocottus maculosus. J. Comp. Physiol. B 2023, 193, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Killen, S.S.; Costa, I.; Brown, J.A.; Gamperl, A.K. Little left in the tank: Metabolic scaling in marine teleosts and its implications for aerobic scope. Proc. R. Soc. B. 2007, 274, 431–438. [Google Scholar] [CrossRef]
- Blasco, F.R.; Taylor, E.W.; Leite, C.A.C.; Monteiro, D.A.; Rantin, F.T.; McKenzie, D.J. Tolerance of an acute warming challenge declines with body mass in Nile tilapia: Evidence of a link to capacity for oxygen uptake. J. Exp. Biol. 2022, 225, jeb244287. [Google Scholar] [CrossRef]
- Ken-Ichi, Y. Relationship of respiration to body weight in the tilapia Oreochromis niloticus under resting and normoxic conditions. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1992, 103, 81–83. [Google Scholar] [CrossRef]
- Christensen, E.A.F.; Svendsen, M.B.S.; Steffensen, J.F. The combined effect of body size and temperature on oxygen consumption rates and the size-dependency of preferred temperature in European perch Perca fluviatilis. J. Fish Biol. 2020, 97, 794–803. [Google Scholar] [CrossRef]
- Al-Kadhomiy, N.K. Gill Development, Growth and Respiration of the Flounder, Platichthys flesus (L.). Ph.D. Thesis, Bristol University, Bristol, UK, 1985. [Google Scholar]
- Fonds, M.; Cronie, R.; Vethaak, A.D.; Van der Puyl, P. Metabolism, food consumption and growth of plaice (Pleuronectes platessa) and flounder (Platichthys flesus) in relation to fish size and temperature. Neth. J. Sea Res. 1992, 29, 127–143. [Google Scholar] [CrossRef]
- Koch, F.; Wieser, W.; Niederstätter, H. Interactive effects of season and temperature on enzyme activities, tissue and whole animal respiration in roach, Rutilus rutilus. Environ. Biol. Fishes 1992, 33, 73–85. [Google Scholar] [CrossRef]
- Hölker, F. The metabolic rate of roach in relation to body size and temperature. J. Fish Biol. 2003, 62, 565–579. [Google Scholar] [CrossRef]
- Mel’nichuk, G.L. Metabolic rate in young commercial fishes of Kremenchug reservoir. Hydrobiol. J. 1969, 5, 41–45. [Google Scholar]
- Wells, P.; Pinder, A. The respiratory development of Atlantic salmon. I. Morphometry of gills, yolk sac and body surface. J. Exp. Biol. 1996, 199, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Bihun, C.J.; Murphy, M.K.; Johnson, T.B.; Fisk, A.T.; Guzzo, M.M.; Madenjian, C.P.; Raby, G.D. Effects of temperature, body size, and sex on the standard metabolic rates of a sexually dimorphic freshwater piscivore, walleye (Sander vitreus). Can. J. Fish. Aquat. Sci. 2024, 81, 879–886. [Google Scholar] [CrossRef]
- Hughes, G.M. On the respiration of Torpedo marmorata. J. Exp. Biol. 1978, 73, 85–105. [Google Scholar] [CrossRef]
- Hughes, G.M. The dimensions of fish gills in relation to their function. J. Exp. Biol. 1966, 45, 177–195. [Google Scholar] [CrossRef]
- Herrmann, J.P.; Enders, E. Effect of body size on the standard metabolism of horse mackerel. J. Fish Biol. 2000, 57, 746–760. [Google Scholar] [CrossRef]
- Fonds, M.; Jaworski, A.; Iedema, A.; Puyl, P.V.D. Metabolism, Food Consumption, Growth, and Food Conversion of Shorthorn Sculpin (Myoxocephalus scorpius) and Eelpout (Zoarces viviparus). International Council for the Exploration of the Seas, Copenhagen, I.C.E.S. Demersal Fish Committee, C.M. 1989, 31, 19 p. Available online: https://www.ices.dk/sites/pub/CM%20Doccuments/1989/G/1989_G31.pdf (accessed on 5 January 2025).
- Dekking, M.; Kraaikamp, C.; Lopuhaä, H.P.; Meester, L.E. A Modern Introduction to Probability and Statistics: Understanding Why and How; Springer: London, UK, 2005. [Google Scholar]
- Clark, T.D.; Brandt, W.T.; Nogueira, J.; Rodriguez, L.E.; Price, M.; Farwell, C.J.; Block, B.A. Postprandial metabolism of Pacific bluefin tuna (Thunnus orientalis). J. Exp. Biol. 2010, 213, 2379–2385. [Google Scholar] [CrossRef]
- Clark, T.D.; Farwell, C.J.; Rodriguez, L.E.; Brandt, W.T.; Block, B.A. Heart rate responses to temperature in free-swimming Pacific bluefin tuna (Thunnus orientalis). J. Exp. Biol. 2013, 216, 3208–3214. [Google Scholar] [CrossRef]
- Jerde, C.L.; Kraskura, K.; Eliason, E.J.; Csik, S.R.; Stier, A.C.; Taper, M.L. Strong evidence for an intraspecific metabolic scaling coefficient near 0.89 in fish. Front. Physiol. 2019, 10, 1166. [Google Scholar] [CrossRef]
- Niimi, A.J.; Morgan, S.L. Morphometric examination of the gills of walleye, Stizostedion vitreum vitreum (Mitchill) and rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 1980, 16, 685–692. [Google Scholar] [CrossRef]
- Morgan, M. Fill Development, Growth, and Respiration in the Trout, Salmo gairdneri (Richardson). Ph.D. Thesis, Bristol University, Bristol, UK, 1971. [Google Scholar]
- Teletchea, F.; Pauly, D. Why do fish larvae hatch when they do? Environ. Biol. Fishes 2024, 107, 583–591. [Google Scholar] [CrossRef]
- Hughes, G.M. A comparative approach to fish respiration. Experientia 1970, 26, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, P.C. Cardio-respiratory morphometrics of spangled perch, Leiopotherapon unicolor (Günther, 1859), (Percoidei, Teraponidae). J. Fish Biol. 1987, 31, 617–623. [Google Scholar] [CrossRef]
- Lefevre, S.; McKenzie, D.J.; Nilsson, G.E. Models projecting the fate of fish populations under climate change need to be based on valid physiological mechanisms. Global Change Biol. 2017, 23, 3449–3459. [Google Scholar] [CrossRef]
- Allen, G.R. Freshwater Fishes of Australia; T.F.H. Publications: Neptune City, NJ, USA, 1989. [Google Scholar]
- Sternberg, D.; Cockayne, B. Length–length and length–weight relationships of fish species from the Lake Eyre Basin, Australia. J. Appl. Ichthyol. 2015, 31, 1168–1170. [Google Scholar] [CrossRef]
- Singh, O.N.; Munshi, J.S.D. Oxygen uptake in relation to body weight, respiratory surface area and group size in a freshwater goby, Glossogobiu giuris (Ham.). Proc. Indian Natl. Sci. Acad. 1985, B51, 33–40. [Google Scholar]
- Prasad, M.S. Oxygen uptake during early life in the freshwater fish, Esomus danricus (Ham) (Pisces, Cypriniformes). Acta Physiol. Hung. 1984, 67, 367–376. [Google Scholar]
- Kobayashi, H.; Murata, O.; Harada, T. Some aspects of gill measurements in relation to growth of the yellowtail Seriola quinqueradiata. Nippon Suisan Gakkaishi 1988, 54, 49–54. [Google Scholar] [CrossRef]
- Mallatt, J. Fish gill structural changes induced by toxicants and other irritants: A statistical review. Can. J. Fish. Aquat. Sci. 1985, 42, 630–648. [Google Scholar] [CrossRef]
- Cumming, H.; Herbert, N.A. Gill structural change in response to turbidity has no effect on the oxygen uptake of a juvenile sparid fish. Conserv. Physiol. 2016, 4, cow033. [Google Scholar] [CrossRef]
- Greco, A.M.; Fenwick, J.C.; Perry, S.F. The effects of soft-water acclimation on gill structure in the rainbow trout Oncorhynchus mykiss. Cell Tissue Res. 1996, 285, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Waser, W.; Bausheva, O.; Nikinmaa, N. The copper-induced reduction of critical swimming speed in rainbow trout (Oncorhynchus mykiss) is not caused by changes in gill structure. Aquat. Toxicol. 2009, 94, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; Eom, J. The osmorespiratory compromise in the fish gill. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 254, 110895. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, V.N.; Pasternak, A.F.; Valtonen, E.T.; Taskinen, J. Increased ventilation by fish leads to a higher risk of parasitism. Parasit. Vectors 2014, 7, 281. [Google Scholar] [CrossRef]
- McKim, J.M.; Erickson, R.J. Environmental impacts on the physiological mechanisms controlling xenobiotic transfer across fish gills. Physiol. Zool. 1991, 64, 39–67. [Google Scholar] [CrossRef]
- Dabrowski, K.R. Energy budget of coregonid (Coregonus spp.) fish growth, metabolism and reproduction. Oikos 1985, 45, 358–364. [Google Scholar] [CrossRef]
- Brett, J.R. The relation of size to rate of oxygen consumption and sustained swimming speed of sockeye salmon (Oncorhynchus nerka). J. Fish. Res. Bd. Can. 1965, 22, 1491–1501. [Google Scholar] [CrossRef]
- Gilmour, K.M.; Turko, A.J. Effects of structural remodelling on gill physiology. J. Comp. Physiol. B 2024, 194, 595–609. [Google Scholar] [CrossRef]
- Priede, I.G. Metabolic scope in fishes. In Fish Energetics: New Perspectives; Tyler, P., Calow, P., Eds.; Croom: London, UK, 1985; pp. 33–64. [Google Scholar]
| Species | dG | dQ | Remarks and Reference(s) |
|---|---|---|---|
| Anguilla anguilla | 0.81 | 0.83 | dG: mean of 0.715 [54] and 0.9 [17], based on [55]; dQ: [17] based on [56] |
| Carassius auratus | 0.8 | 0.85 | dG: mean of 0.74 (15 °C) and 0.87 (25 °C) [57]; dQ: mean of 0.87 (15 °C) [57] and 0.83 (25 °C) [17] |
| Catostomus commersoni | 0.639 | 0.903 | dG: [21]; dQ: [58] based on [59] |
| Cirrhinus mrigala | 0.82 | 0.796 | dG: [22]: dQ: [58] based on [60] |
| Coryphaena hippurus | 0.713 | 0.384 | dG: [61]; dQ: [58] based on [40] (a) |
| Ctenopharyngodon idella | 0.848 | 0.823 | Both: [44], but not accounting for ventilation frequency |
| Cyprinus carpio | 0.794 | 0.825 | dG: [42]; dQ: mean of 0.85 [62] and 0.8 [57] |
| Esox lucius | 0.651 | 0.815 | dG: [54] based on [63]; dQ: mean of 0.81 [64] and 0.82 [65] |
| Galaxias maculatus | 0.88 | 0.77 | Both: [66]; ‘warm normoxia’ and SMR |
| Gymnocephalus cernua | 0.721 | 0.735 | dG: [54]; dQ: mean of 0.72 [67] and 0.75 [68] |
| Hypophthalmichthys molitrix | 0.856 | 0.782 | dG: mean of 0.801 [44] and 0.921 [69]; dQ: mean of 0.792 [44] and 0.772 [69] |
| Hypophthalmichthys nobilis | 0.857 | 0.829 | Both: [44]; not accounting for ventilation frequency |
| Ictalurus nebulosus | 0.845 | 0.994 | dG: [21]; dQ: [58] based on [59] |
| Katsuwonus pelamis | 0.85 | 0.563 | dG: [32]; dQ: [58] based on [70] (a) |
| Micropterus dolomieu | 0.8 | 0.96 | dG: mean of 0.79 [22] and 0.82 [21]; dQ: [71] |
| Mystus cavasius | 0.92 | 0.662 | dG: [22]; dQ: [58] based on [72] |
| Mylopharyngodon piceus | 0.877 | 0.854 | Both: [44], not accounting for ventilation frequency |
| Oligocottus maculosus | 0.95 | 0.93 | Both: [73]; dQ: mean of SMR (0.68) and AMR (1.18) |
| Oncorhynchus mykiss | 0.900 | 0.789 | dG: [21]; dQ: [58] based on [74] |
| Oreochromis niloticus | 0.67 | 0.64 | dG: [17] based on [25]; dQ: [17] based on [75,76] |
| Perca fluviatilis | 0.667 | 0.863 | dG: [54]; dQ: [77] |
| Platichthys flesus | 0.762 | 0.781 | dG: mean of 0.7 [78] and 0.824 [54]; dQ: mean of ‘fasting’ (0.739) and ‘well-fed’ (0.831) [79] |
| Pleuronectes platessa | 0.85 | 0.782 | dG: [47]; dQ: [58] based on [79] |
| Rutilus rutilus | 0.87 | 0.80 | dG: mean of 0.85 and 0.90 [22]; dQ: mean of 0.76 [80], 0.87 [81], and 0.72 [82] |
| Salmo salar | 0.97 | 1.07 | dG: [17] based on [83]; dQ: mean SMR, 4 different sources |
| Salvelinus fontinalis | 0.969 | 0.99 | dG: [19]; dQ: mean of 0.943 [19] and 1.036 [58] based on [59] |
| Sander vitreus | 1.13 | 1.03 | dG: [22]; dQ: [84] |
| Tinca tinca | 0.67 | 0.79 | dG: [21]; dQ: [62] |
| Thunnus albacares | 0.875 | 0.573 | dG [32], but combined w/T. thynnus; dQ: [58] based on [70] (a) |
| Thunnus thynnus | 0.901 | 0.66 | dG: [32]; dQ: [49], with T. thynnus = T. orientalis |
| Torpedo marmorata | 0.65 | 0.97 | Both: [17] based on [85] |
| Trachurus trachurus | 1.23 | 0.89 | dG: [17] based on [86]; dQ: based on [87] |
| Zoarces viviparus | 0.83 | 0.8 | dG: [11]; dQ: [88] |
| Mean of the 33 species | 0.836 | 0.810 | dG: standard deviation = 0.130; dQ: 0.143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauly, D.; Müller, J. Fick’s Diffusion Laws and Scaling of the Gill Surface Area and Oxygen Uptake in Fish. Fishes 2025, 10, 233. https://doi.org/10.3390/fishes10050233
Pauly D, Müller J. Fick’s Diffusion Laws and Scaling of the Gill Surface Area and Oxygen Uptake in Fish. Fishes. 2025; 10(5):233. https://doi.org/10.3390/fishes10050233
Chicago/Turabian StylePauly, Daniel, and Johannes Müller. 2025. "Fick’s Diffusion Laws and Scaling of the Gill Surface Area and Oxygen Uptake in Fish" Fishes 10, no. 5: 233. https://doi.org/10.3390/fishes10050233
APA StylePauly, D., & Müller, J. (2025). Fick’s Diffusion Laws and Scaling of the Gill Surface Area and Oxygen Uptake in Fish. Fishes, 10(5), 233. https://doi.org/10.3390/fishes10050233








