Core–Shell Magnetic Gold Nanoparticles with Chitosan Coating as a SERS Substrate: A Rapid Detection Strategy for Malachite Green Contamination in Aquatic Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
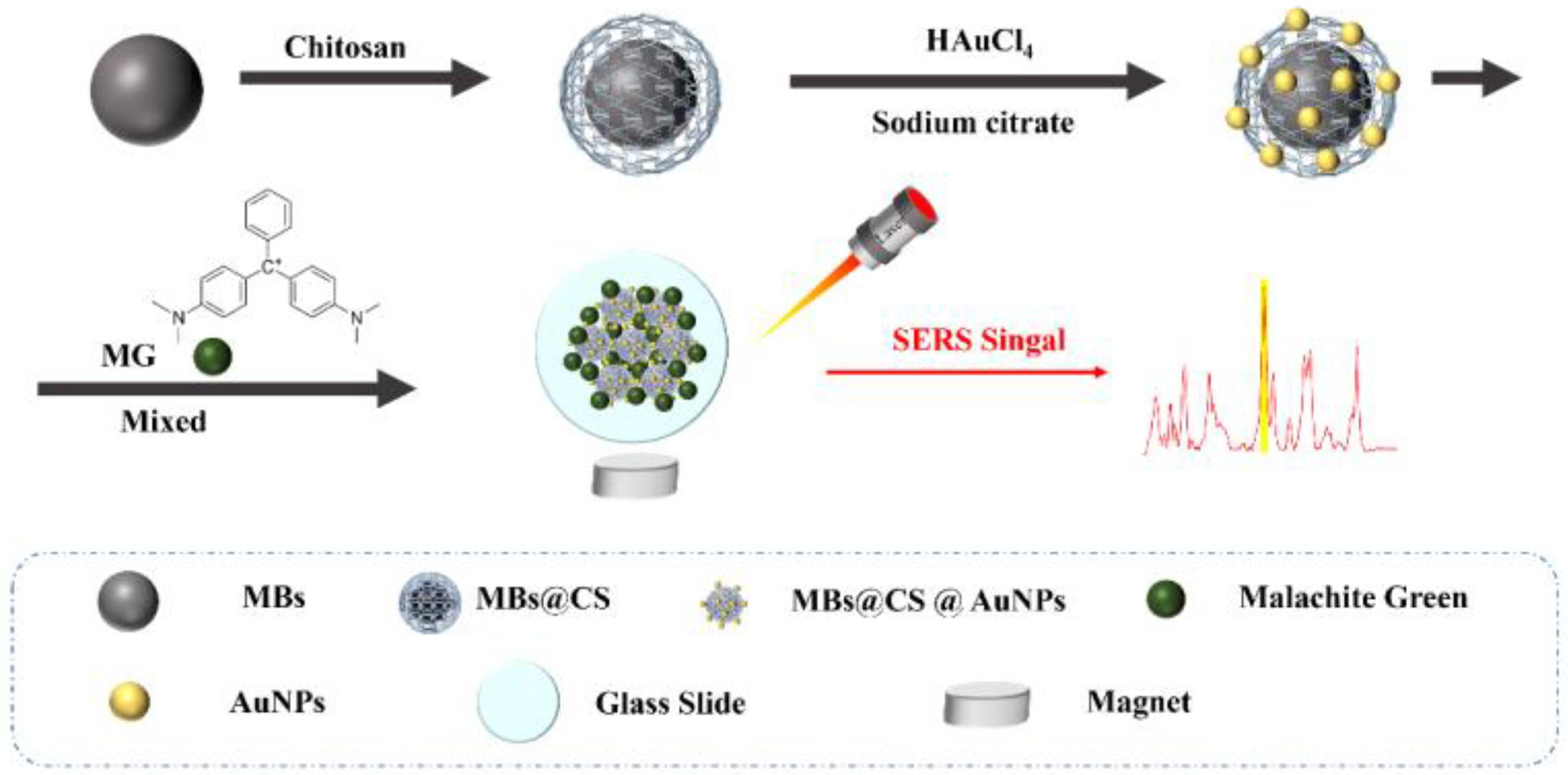
2.3. Raman Substrate Preparation
2.3.1. Activation of Carboxylated Beads
2.3.2. Preparation of MBs@CS
2.3.3. Preparation of MBs@CS@AuNPs
2.4. Samples Pretreatment
2.5. SERS Signal Collection
3. Results and Discussion
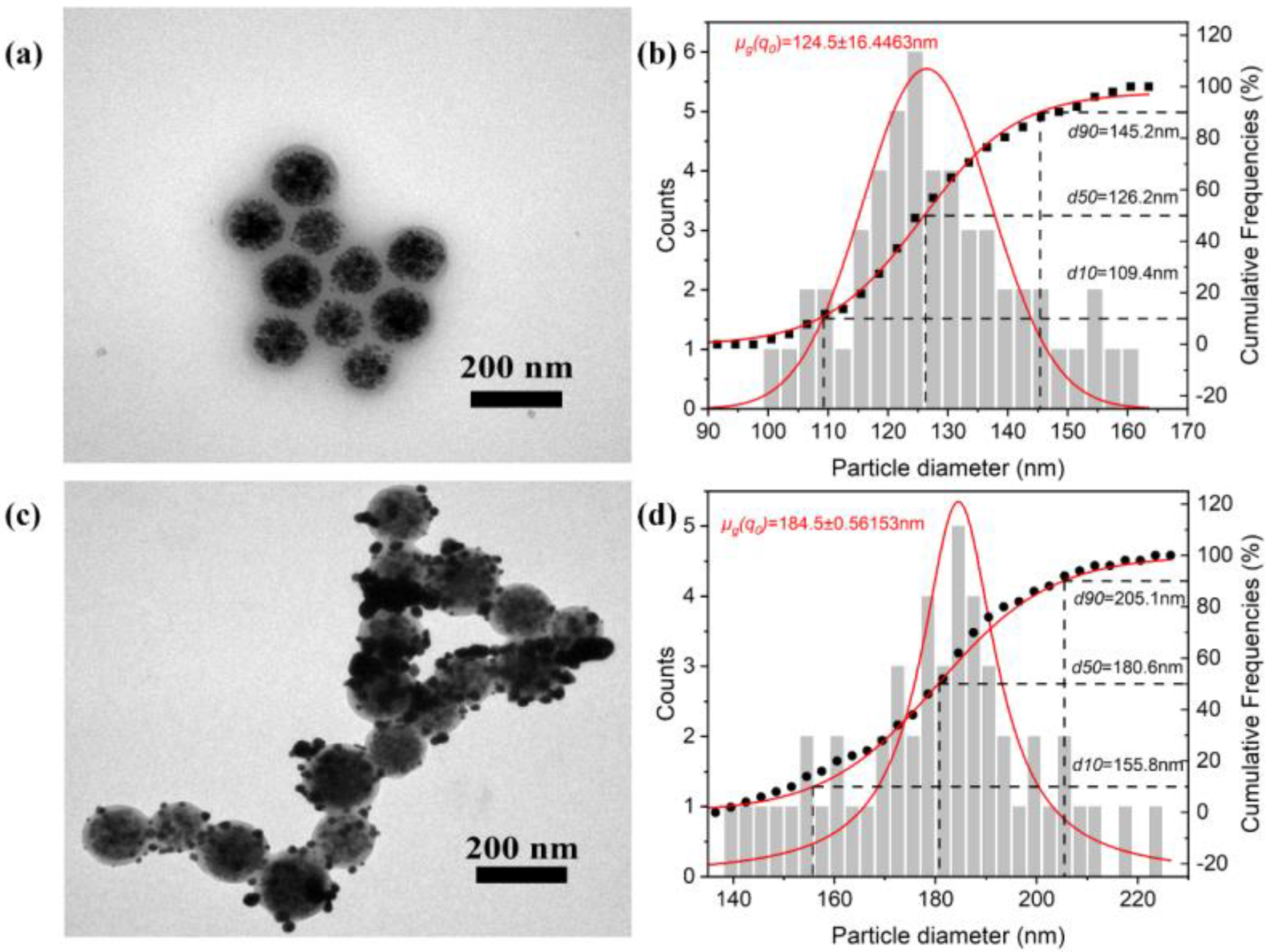
3.1. Morphological and Structural Characterization
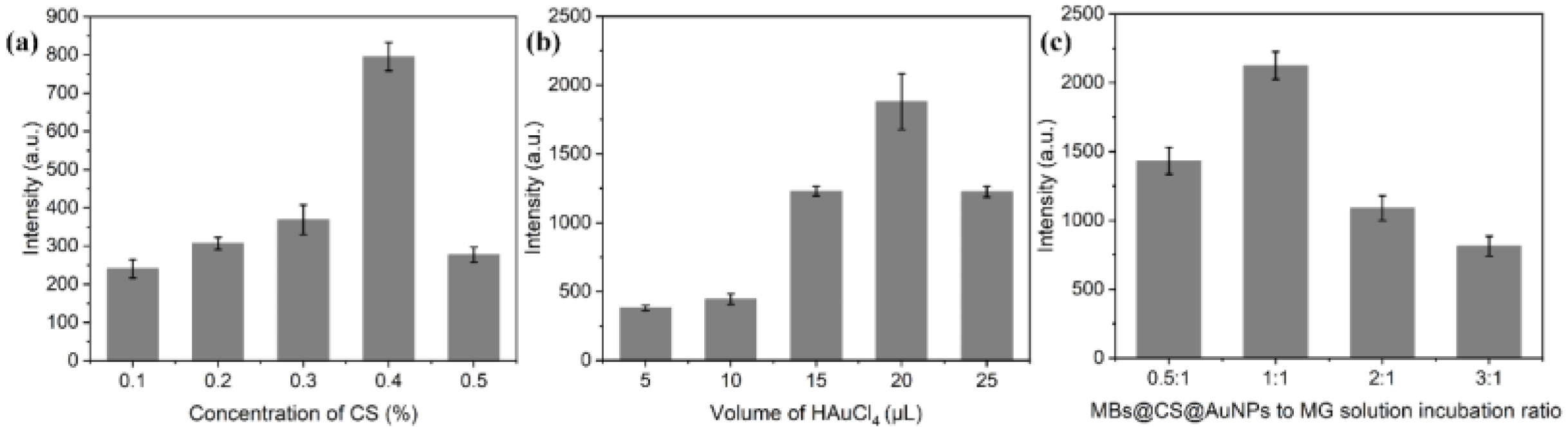
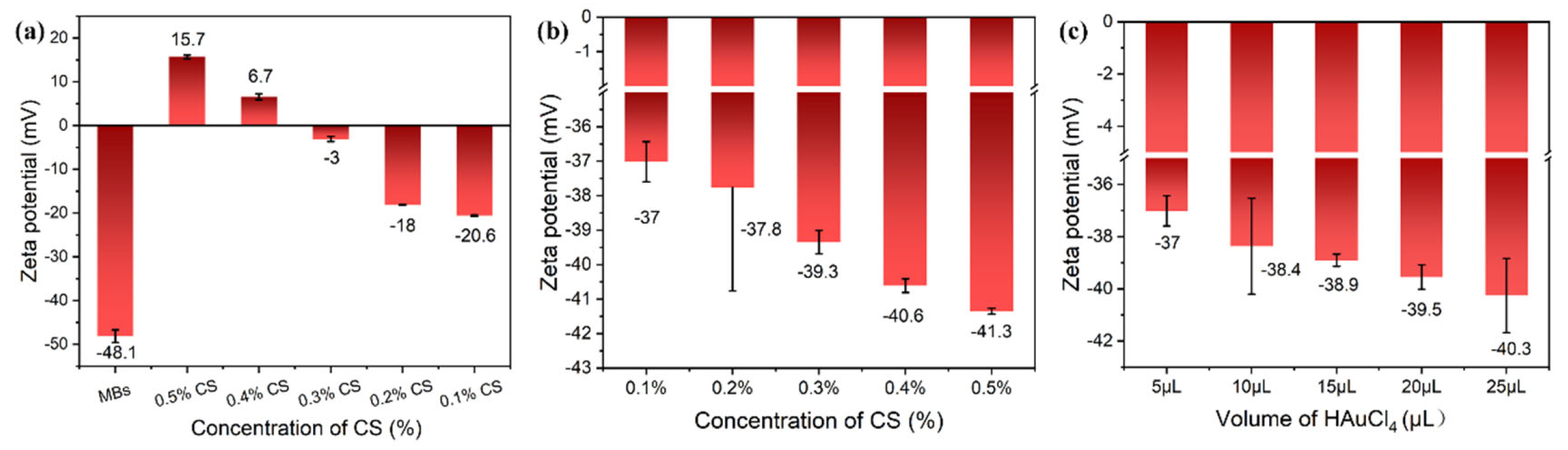
3.2. Factors Influencing the Ultrasensitive SERS Detection of MG
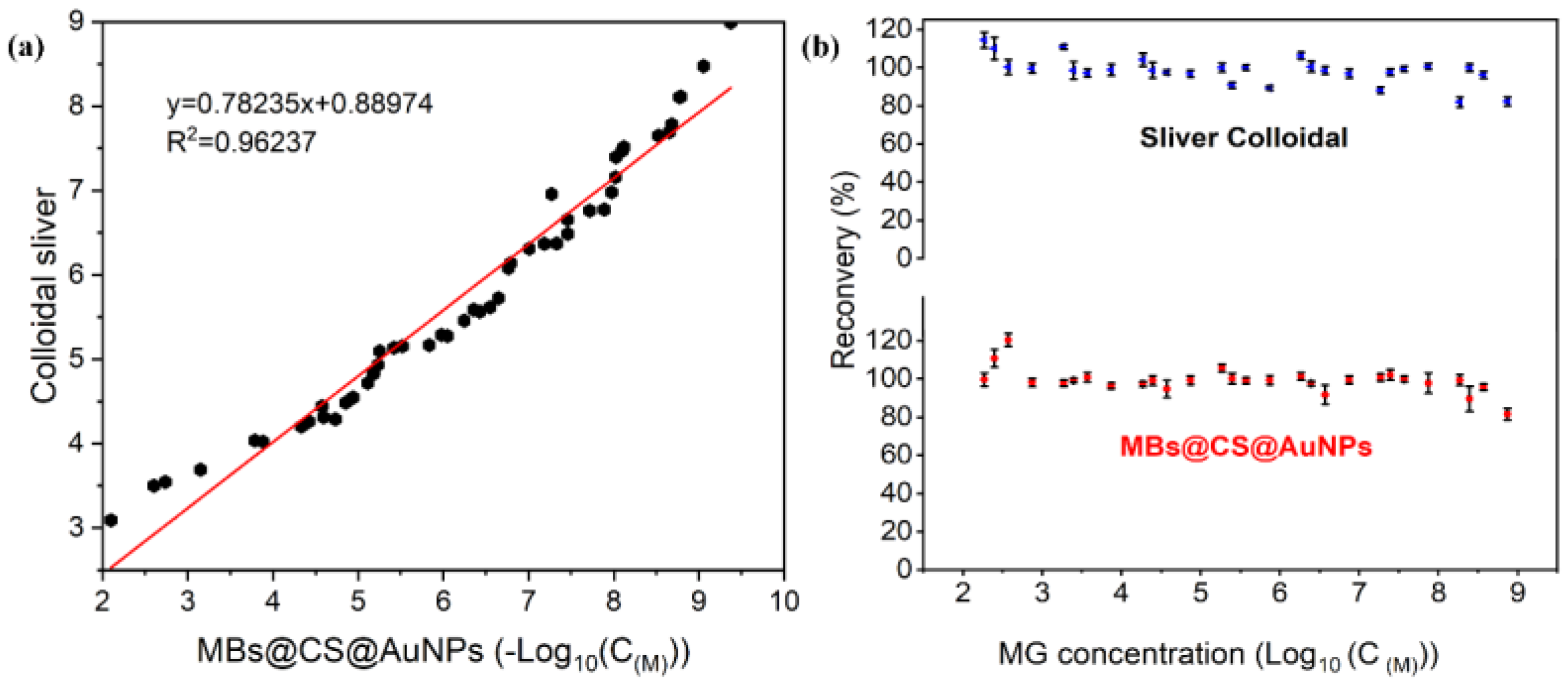
3.3. SERS Determination of MG Based on MBs@CS@AuNPs Substrate
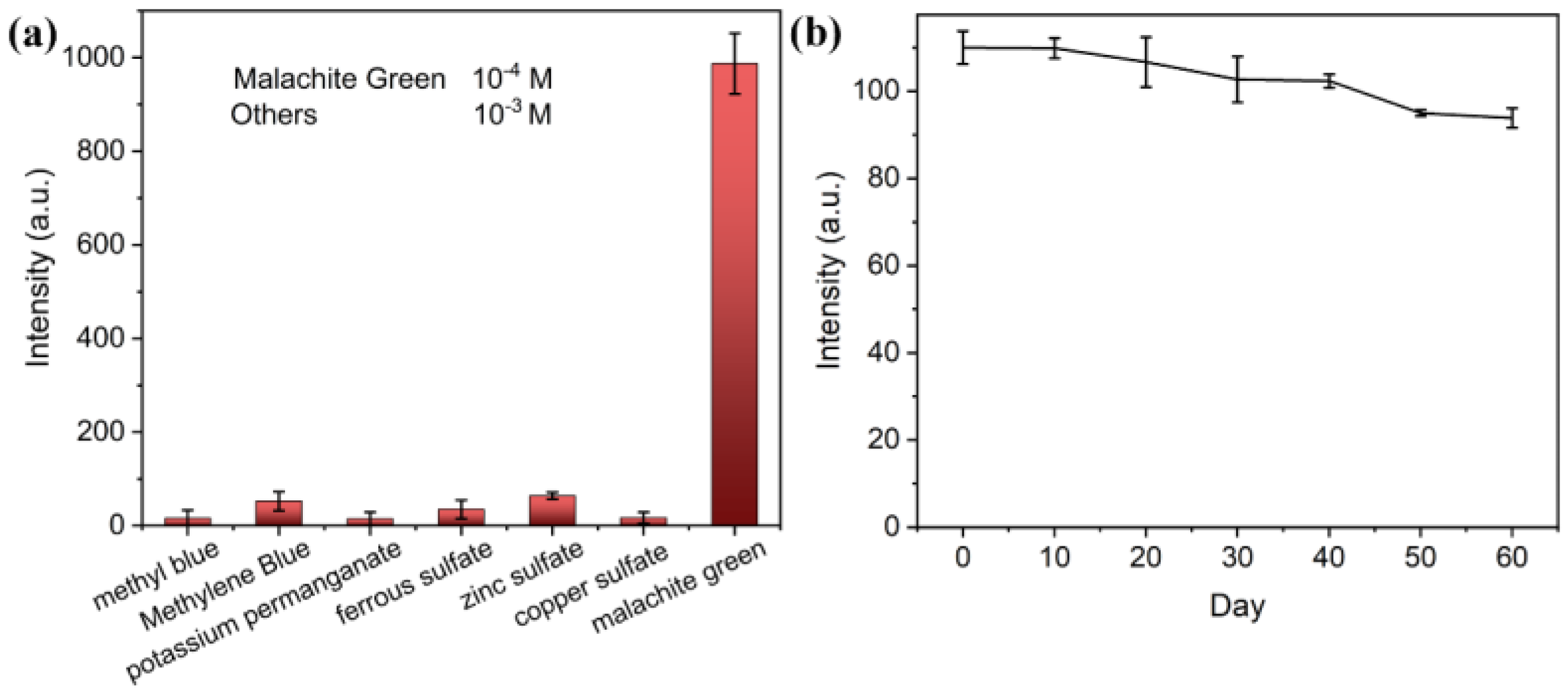
3.4. Specificity
3.5. Stability
3.6. Real Sample Detection
3.7. Methodological Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zhao, C.; Hong, C.; Lin, Z.; Huang, Z. Rapid detection of malachite green in fish and water based on the peroxidase-like activity of Fe3O4NPs enhanced with aptamer. J. Food Compos. Anal. 2021, 104, 104162. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Z.; Du, X.; Xie, J.; Ding, Z. Novel aptamer fluorescence assays for malachite green and leucomalachite green detection. Microchem. J. 2024, 205, 111391. [Google Scholar] [CrossRef]
- Nanjappa, M.D.; Jayaprakash, G.K. Some progress in voltammetric methods to detect malachite green in real samples using carbon electrodes. J. Electrochem. Sci. Eng. 2023, 13, 437–449. [Google Scholar] [CrossRef]
- Teepoo, S.; Jantra, J.; Panapong, K.; Ajayi, D.T. A highly sensitive hyperbranched Au plasmonic blackbody immunochromatographic assay for detection of leucomalachite green in fish and shrimp. Anal. Chim. Acta 2024, 1285, 342031. [Google Scholar] [CrossRef]
- Karthik, P.; Jose, P.A.; Chellakannu, A.; Gurusamy, S.; Ananthappan, P.; Karuppathevan, R.; Vasantha, V.S.; Rajesh, J.; Ravichandran, S.; Sankarganesh, M. Green synthesis of MnO2 nanoparticles from Psidium guajava leaf extract: Morphological characterization, photocatalytic and DNA/BSA interaction studies. Int. J. Biol. Macromol. 2024, 258, 128869. [Google Scholar] [CrossRef]
- Meng, E.-Q.; Nian, Q.-X.; Li, F.; Zhang, Q.-P.; Xu, Q.; Wang, C.-M. Sulfonated magnetic graphite carbon nitride solid-phase extraction-ultra performance liquid chromatography-tandem mass spectrometry for screening malachite green and leucomalachite green in freshwater fish. Se Pu Chin. J. Chromatogr. 2023, 41, 673–682. [Google Scholar] [CrossRef]
- Yu, Z.; Gong, H.; Li, Y.; Xu, J.; Zhang, J.; Zeng, Y.; Liu, X.; Tang, D. Chemiluminescence-derived self-powered photoelectrochemical immunoassay for detecting a low-abundance disease-related protein. Anal. Chem. 2021, 93, 13389–13397. [Google Scholar] [CrossRef]
- Mikac, L.; Rigó, I.; Himics, L.; Tolić, A.; Ivanda, M.; Veres, M. Surface-enhanced Raman spectroscopy for the detection of microplastics. Appl. Surf. Sci. 2023, 608, 155239. [Google Scholar] [CrossRef]
- Almaviva, S.; Artuso, F.; Giardina, I.; Lai, A.; Pasquo, A. Fast detection of different water contaminants by Raman spectroscopy and surface-enhanced Raman spectroscopy. Sensors 2022, 22, 8338. [Google Scholar] [CrossRef]
- Darienzo, R.E.; Chen, O.; Sullivan, M.; Mironava, T.; Tannenbaum, R. Au nanoparticles for SERS: Temperature-controlled nanoparticle morphologies and their Raman enhancing properties. Mater. Chem. Phys. 2020, 240, 122143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, L.; Liu, H.; Ding, Q.; Jia, C.; Liao, S.; Cheng, N.; Yue, M.; Yang, S. Nanoporous silver nanorods as surface-enhanced Raman scattering substrates. Biosens. Bioelectron. 2022, 202, 114004. [Google Scholar] [CrossRef] [PubMed]
- Gebavi, H.; Ristić, D.; Baran, N.; Mikac, L.; Mohaček-Grošev, V.; Gotić, M.; Ivanda, M. Silicon nanowires as sensory material for surface-enhanced Raman spectroscopy. Silicon 2019, 11, 1151–1157. [Google Scholar] [CrossRef]
- Parmigiani, M.; Albini, B.; Pellegrini, G.; Genovesi, M.; De Vita, L.; Pallavicini, P.; Dacarro, G.; Galinetto, P.; Taglietti, A. Surface-enhanced raman spectroscopy chips based on silver coated gold nanostars. Nanomaterials 2022, 12, 3609. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, L.; Liu, Y.; Chen, L. Photocatalytic deposition of Au nanoparticles on Ti3C2Tx MXene substrates for surface-enhanced Raman scattering. Molecules 2024, 29, 2383. [Google Scholar] [CrossRef]
- Michałowska, A.; Kudelski, A. Plasmonic substrates for biochemical applications of surface-enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 308, 123786. [Google Scholar] [CrossRef]
- Berganza, L.B.; Litti, L.; Meneghetti, M.; Lanceros-Méndez, S.; Reguera, J. Enhancement of magnetic surface-enhanced Raman scattering detection by tailoring Fe3O4@ Au nanorod shell thickness and its application in the on-site detection of antibiotics in water. ACS Omega 2022, 7, 45493–45503. [Google Scholar] [CrossRef]
- Juang, R.-S.; Chen, W.-T.; Cheng, Y.-W.; Wang, K.-S.; Jeng, R.-J.; Zeng, Z.-L.; Liu, S.-H.; Liu, T.-Y. Fabrication of in situ magnetic capturing and Raman enhancing nanoplatelets for detection of bacteria and biomolecules. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129189. [Google Scholar] [CrossRef]
- Jing, Z.; Zhang, L.; Xu, X.; Zhu, S.; Zeng, H. Carbon-assistant nanoporous gold for surface-enhanced Raman scattering. Nanomaterials 2022, 12, 1455. [Google Scholar] [CrossRef]
- Hinds, D.T.; Belhout, S.A.; Colavita, P.E.; Ward, A.D.; Quinn, S.J. Microsphere-supported gold nanoparticles for SERS detection of malachite green. Mater. Adv. 2023, 4, 1481–1489. [Google Scholar] [CrossRef]
- Han, L.; Wang, X.; Yu, B.; Qin, X.; Liu, B.; Han, X.; Yuan, H.; Zhao, Z. Development of Fe3O4/DEX/PDA@ Au (Raman reporters)@ Au-MPBA nanocomposites based multi-hotspot SERS probe for ultrasensitive, reliable, and quantitative detection of glucose in sweat. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 326, 125192. [Google Scholar] [CrossRef]
- Xiao, Y.; Luo, S.; Qiu, J.; Zhang, Y.; Liu, W.; Zhao, Y.; Zhu, Y.; Deng, Y.; Lu, M.; Liu, S. Highly sensitive SERS platform for pathogen analysis by cyclic DNA nanostructure@ AuNP tags and cascade primer exchange reaction. J. Nanobiotechnol. 2024, 22, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Bai, J.H.; Zhang, X.; Lv, J.M.; Fan, C.S.; Zhao, Y.M.; Wu, Z.L.; Xu, H.J. Facile synthesis of Au nanoparticle-coated Fe3O4 magnetic composite nanospheres and their application in SERS detection of malachite green. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118532. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, D.-W.; Pu, H.; Chen, L.; Lin, L. Applications of Raman spectroscopic techniques for quality and safety evaluation of milk: A review of recent developments. Crit. Rev. Food Sci. 2019, 59, 770–793. [Google Scholar] [CrossRef] [PubMed]
- Muntean, C.M.; Cuibus, D.; Boca, S.; Falamas, A.; Tosa, N.; Brezeştean, I.A.; Bende, A.; Barbu-Tudoran, L.; Moldovan, R.; Bodoki, E. Gold vs. Silver colloidal nanoparticle films for optimized SERS detection of propranolol and electrochemical-SERS analyses. Biosensors 2023, 13, 530. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. Rapid nondestructive detection of mixed pesticides residues on fruit surface using SERS combined with self-modeling mixture analysis method. Talanta 2020, 217, 120998. [Google Scholar] [CrossRef]
- Machado Da Silva Acioly, T.; Francisco Da Silva, M.; Iannacone, J.; Viana, D.C. Levels of potentially toxic and essential elements in Tocantins River sediment: Health risks at Brazil’s Savanna-Amazon interface. Sci. Rep. 2024, 14, 18037. [Google Scholar] [CrossRef]
- Alves, M.B.; Da Silva Acioly, T.M.; Oliver, J.I.; Jawad, L.J.; De Sousa, A.L.; Dos Santos, D.A.; Viana, D.C. Microplastics and behavioral changes in fish: An integrative review. Cad. Pedagóg. 2024, 21, e8613. [Google Scholar] [CrossRef]
- Alvariño, L.; Guabloche, A.; Da Silva Acioly, T.M.; Viana, D.C.; Iannacone, J. Assessment of potentially toxic metals, metalloids, and non-metals in muscle and liver tissue of two fish species (Mugil cephalus Linnaeus, 1758 and Odontesthes regia (Humboldt, 1821) from the Coastal Area of Callao, Peru. Reg. Stud. Mar. Sci. 2024, 71, 103423. [Google Scholar] [CrossRef]
- Morey, G.M.; Viana, D.C.; Chota, H.R.; Chero, J.D. Three new species of Jainus (Monogenoidea: Dactylogyridae) from the gills of Triportheus angulatus (Characiformes: Triportheidae) collected in the Peruvian Amazonia. Syst. Parasitol. 2025, 102, 1. [Google Scholar] [CrossRef]
- Araújo, K.S.D.S.; Acioly, T.M.D.S.; Nascimento, I.O.; Costa, F.N.; Corrêa, F.; Gagneten, A.M.; Viana, D.C. Biomonitoring of waters and tambacu (Colossoma macropomum × Piaractus mesopotamicus) from the Amazônia Legal, Brazil. Water 2024, 16, 2588. [Google Scholar] [CrossRef]
- He, Q.; Wang, D.; Shao, J.; Li, Y.; Cheng, M.; Dong, L.; Li, Y.; Zhu, J.; Li, H. Multicomponent SERS imprinted bio-membrane based on eggshell membrane for selective detection of spiramycin in water. J. Mol. Struct. 2023, 1289, 135883. [Google Scholar] [CrossRef]
- Xu, Z.; Bi, X.; Huang, Y.; Che, Z.; Chen, X.; Fu, M.; Tian, H.; Yang, S. Sensitive colorimetric detection of Salmonella enteric serovar typhimurium based on a gold nanoparticle conjugated bifunctional oligonucleotide probe and aptamer. J. Food Saf. 2018, 38, e12482. [Google Scholar] [CrossRef]
- Puttige, K.; Nooralabettu, K.P. Effect of homogenization speed and time on the recovery of alkaline phosphatase from the hepatopancreatic tissues of shrimps. Food Sci. Biotechnol. 2012, 21, 461–466. [Google Scholar] [CrossRef]
- Debut, A.; Vizuete, K.; Pazmiño, K.; Calderón, J.; Gallegos, C.; Gaona, V. Effect of visual cognition on the measurement of particle size using ImageJ software. Curr. Mater. Sci. 2021, 14, 141–154. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Cao, Y.; Sang, S.; Guan, L.; Wang, Y.; Wang, J. Preparation of Fe3O4@ PDA@ Au@ GO composite as SERS substrate and its application in the enrichment and detection for phenanthrene. Micromachines 2022, 13, 128. [Google Scholar] [CrossRef]
- Yaroslavtsev, R.N.; Tyumentseva, A.V.; Velikanov, D.A.; Vazhenina, I.G.; Volochaev, M.N.; Stolyar, S.V. Fe3O4/Au nanocomposites: Characterization and cytotoxicity effects in vitro. Mater. Chem. Phys. 2024, 322, 129524. [Google Scholar] [CrossRef]
- Raj, D.; Tayyaba, N.; De Vita, G.; Scaglione, F.; Rizzi, P. Ultrasensitive detection of malachite green isothiocyanate using nanoporous gold as SERS substrate. Materials 2023, 16, 4620. [Google Scholar] [CrossRef]
- Wicaksono, W.P.; Dang, H.; Lee, S.; Choo, J. Electrochemical surface-enhanced Raman spectroscopy analysis of malachite green on gold substrates. Appl. Surf. Sci. 2024, 649, 159163. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, W.; Zhang, J.; Li, Q.; Guo, Z. Water-soluble amino functionalized chitosan: Preparation, characterization, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2022, 217, 969–978. [Google Scholar] [CrossRef]
- He, R.X.; Liang, R.; Peng, P.; Norman Zhou, Y. Effect of the size of silver nanoparticles on SERS signal enhancement. J. Nanopart. Res. 2017, 19, 267. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, X.; Duan, N.; Khan, I.M.; Wang, Z.; Wu, S. A sensitive fluorescent assay based on gold-nanoclusters coated on molecularly imprinted covalent organic frameworks and its application in malachite green detection. Food Chem. 2023, 410, 135425. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Y.; Cui, Y.; Liang, G. Dual-potential electrochemiluminescent film constructed from single AIE luminogens for the sensitive detection of malachite green. Nanoscale 2022, 14, 7711–7719. [Google Scholar] [CrossRef] [PubMed]
- Panapong, K.; Wechakorn, K.; Binhayeeniyi, N.; Teepoo, S. Dual-color immunochromatographic test strip for simultaneous sensitive detection of malachite green and leucomalachite green residues in fish and shrimp meat samples. Food Chem. 2025, 463, 141427. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; He, L.; Zhang, M.; Manyande, A.; Chen, H. Carbon quantum dots derived from fish scales as fluorescence sensors for detection of malachite green. J. Food Meas. Charact. 2023, 17, 3368–3376. [Google Scholar] [CrossRef]
- Singh, R.; Singh, R.K. Detection of malachite green in water using edge excited label free fluorescent probe NCQDs. J. Fluoresc. 2020, 30, 1281–1285. [Google Scholar] [CrossRef]
- Fu, G.; Weng, H.; Lai, Z.; Lin, Z.; Huang, Z. Detection of malachite green residue in aquaculture water by using a rare earth fluorescence probe. J. Chem. Sci. 2021, 133, 54. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Li, J.-J.; Liu, Q.-A.; Weng, G.-J.; Zhu, J.; Zhao, J.-W. Sensitive detection of malachite green in aquaculture water by surface enhanced Raman scattering using silver coated urchin-like gold nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131485. [Google Scholar] [CrossRef]
- Zeng, W.-Y.; Wang, Z.-F.; Lin, D.-Z.; Cheng, I.-C. Pore evolution and size effect of nanoporous silver on SERS for malachite green detection. Appl. Surf. Sci. 2023, 640, 158306. [Google Scholar] [CrossRef]
- Tian, X.; Fan, Q.; Guo, J.; Yu, Q.; Xu, L.; Kong, X. Surface-enhanced Raman scattering of flexible cotton fiber-Ag for rapid adsorption and detection of malachite green in fish. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120174. [Google Scholar] [CrossRef]



| Spiked Concentration (M) | Raman Strength (a. u.) | Test Concentration (M) | Recovery (%) | Variable Coefficient (%) |
|---|---|---|---|---|
| Aquaculture water | ||||
| 5 × 10−4 | 1499.82 | 5.7412 × 10−4 | 114.824 | 5.63 |
| 5 × 10−5 | 745.151 | 4.7098 × 10−5 | 94.196 | 1.52 |
| 5 × 10−6 | 341.765 | 4.6679 × 10−6 | 93.358 | 2.92 |
| fish | ||||
| 5 × 10−4 | 465.957 | 5.455 × 10−4 | 109.1 | 5.23 |
| 5 × 10−5 | 216.953 | 5.664 × 10−5 | 113.28 | 3.66 |
| 5 × 10−6 | 101.864 | 5.745 × 10−6 | 114.9 | 9.86 |
| prawn | ||||
| 5 × 10−4 | 244.324 | 4.0129 × 10−4 | 80.258 | 3.27 |
| 5 × 10−5 | 123.608 | 5.0844 × 10−5 | 101.688 | 6.74 |
| 5 × 10−6 | 65.9874 | 5.27165 × 10−6 | 105.433 | 5.08 |
| Test Method | Linearity Range (M) | LOD (M) | Reference |
|---|---|---|---|
| Molecular imprinting | 1.5 × 10−7–10−8 M | 2.78 × 10−9 M | [41] |
| Colorimetric method | 2.38 × 10−6−6 × 10−8 M | 4.57 × 10−8 M | [1] |
| Electrochemistry | 10−5−10−10 M | 7.6 × 10−11 M | [42] |
| Test strip | 4.93 × 10−9−1.91 × 10−9 M | 6.05 × 10−10 M | [43] |
| Fluorescence | 0−1.5 × 10−4 M | 4.4 × 10−6 M | [44] |
| Fluorescence | 8 × 10−5−10−5 M | 5.16 × 10−6 M | [45] |
| Fluorescence | 5 × 10−7−2 × 10−8 M | 1.1729 × 10−7 M | [46] |
| SERS | 10−3−10−8 M | 2.5 × 10−9 M | [47] |
| SERS | 10−3−10−5 M | 1 × 10−5 M | [48] |
| SERS | 2.74 × 10−4−2.74 × 10−6 M | 1.37 × 10−7 M | [49] |
| SERS | 10−3–10−10 M | 1 × 10−10 M | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Huang, T.; Hu, S.; Ye, H.; Xing, J.; Zhan, S. Core–Shell Magnetic Gold Nanoparticles with Chitosan Coating as a SERS Substrate: A Rapid Detection Strategy for Malachite Green Contamination in Aquatic Foods. Fishes 2025, 10, 221. https://doi.org/10.3390/fishes10050221
Yang Y, Huang T, Hu S, Ye H, Xing J, Zhan S. Core–Shell Magnetic Gold Nanoparticles with Chitosan Coating as a SERS Substrate: A Rapid Detection Strategy for Malachite Green Contamination in Aquatic Foods. Fishes. 2025; 10(5):221. https://doi.org/10.3390/fishes10050221
Chicago/Turabian StyleYang, Yihui, Tao Huang, Sijia Hu, Hang Ye, Jiali Xing, and Shengnan Zhan. 2025. "Core–Shell Magnetic Gold Nanoparticles with Chitosan Coating as a SERS Substrate: A Rapid Detection Strategy for Malachite Green Contamination in Aquatic Foods" Fishes 10, no. 5: 221. https://doi.org/10.3390/fishes10050221
APA StyleYang, Y., Huang, T., Hu, S., Ye, H., Xing, J., & Zhan, S. (2025). Core–Shell Magnetic Gold Nanoparticles with Chitosan Coating as a SERS Substrate: A Rapid Detection Strategy for Malachite Green Contamination in Aquatic Foods. Fishes, 10(5), 221. https://doi.org/10.3390/fishes10050221






