Effects of Stocking Density on Phytoplankton Community and Water Quality in Polyculture Ponds of Tegillarca granosa and Litopenaeus vannamei
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Methods of Sample Collection and Measurement of Environmental Parameters
2.2.1. Measurement of Environmental Parameters
2.2.2. Collection of Phytoplankton Samples
2.3. Data Analysis
3. Results
3.1. Water Quality Factors in Ponds
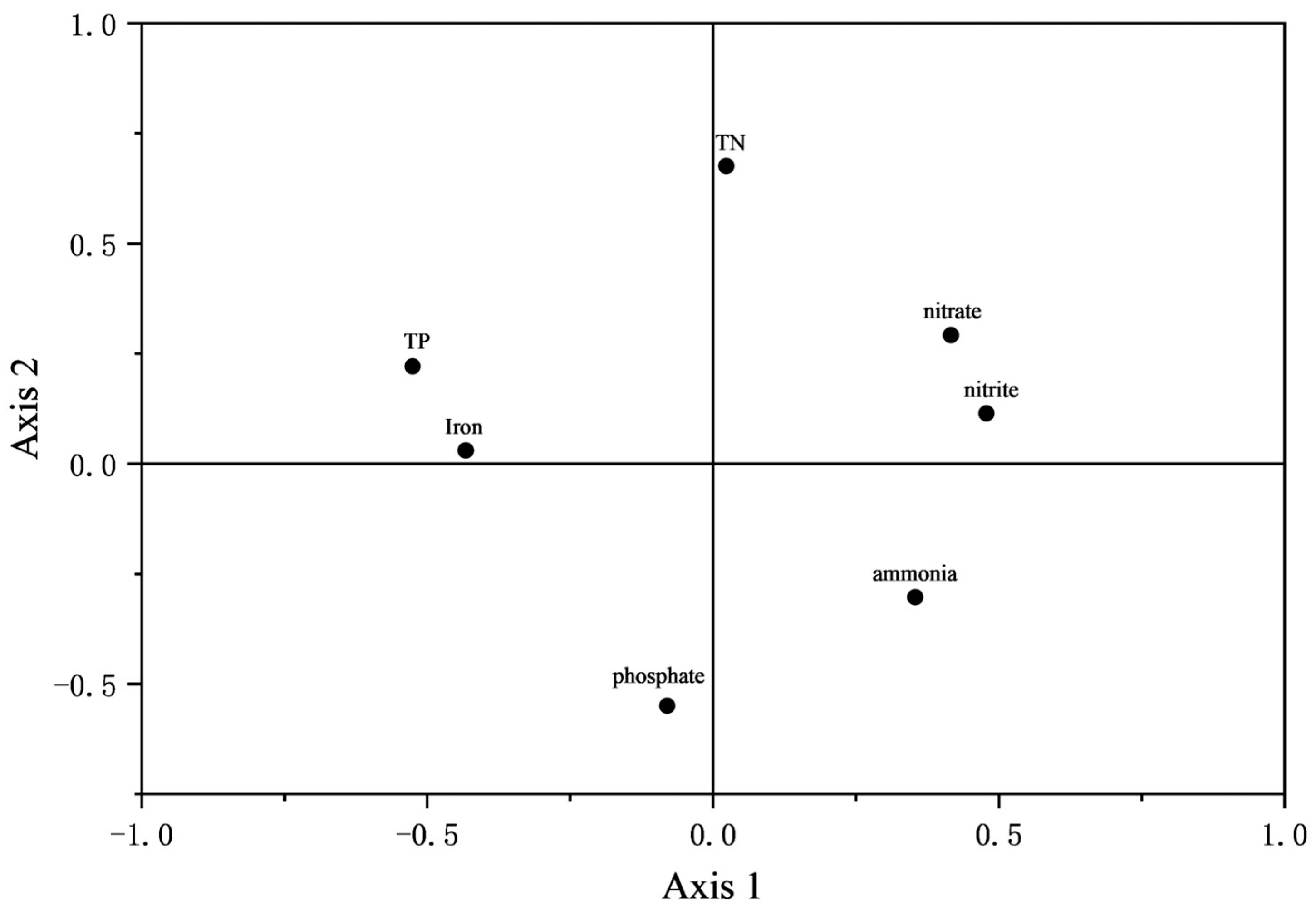
3.2. Water Quality Principal Component Analysis
3.3. Phytoplankton Species Composition and Distribution of Dominant Species
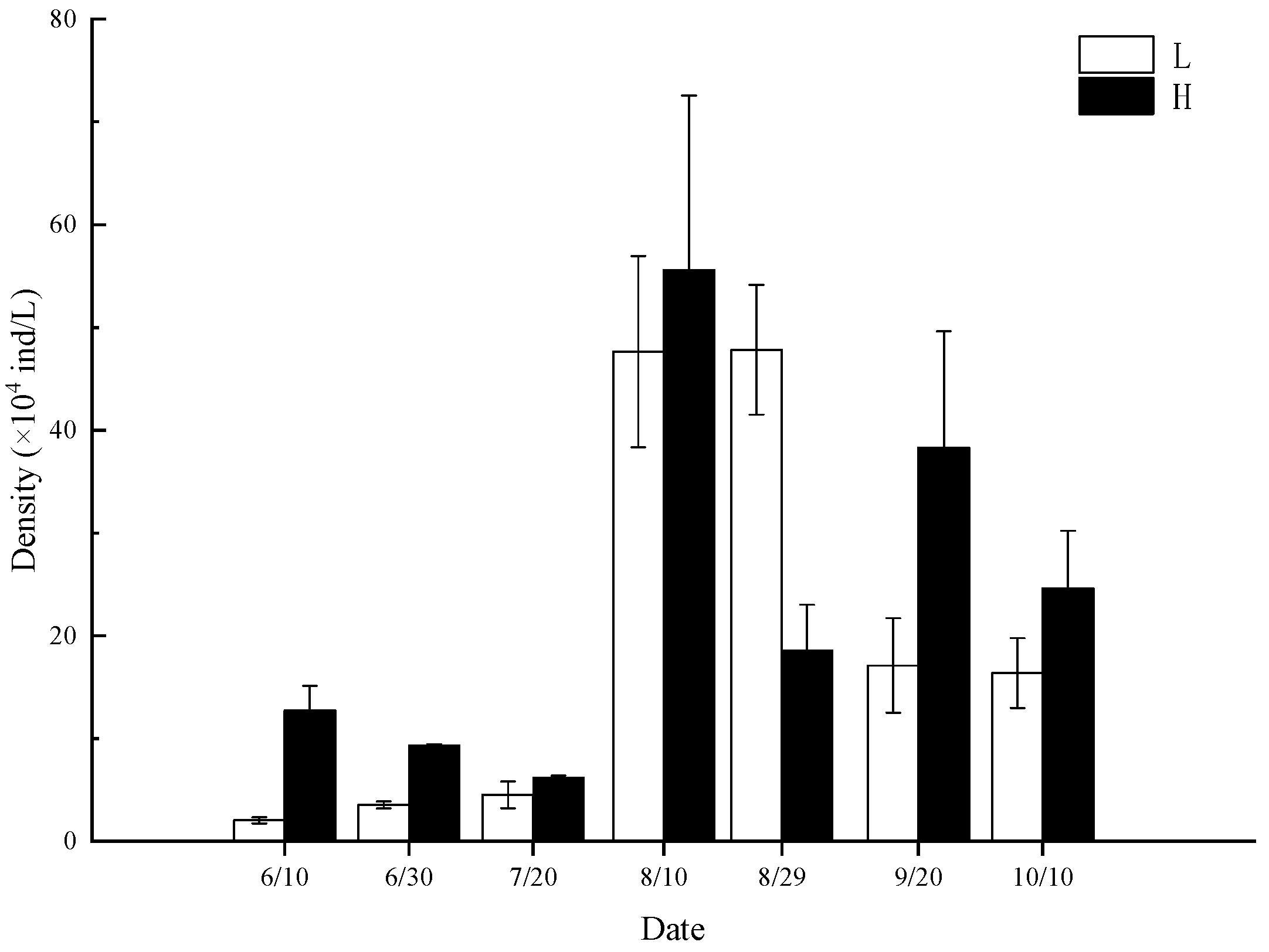
3.4. Changes in Abundance
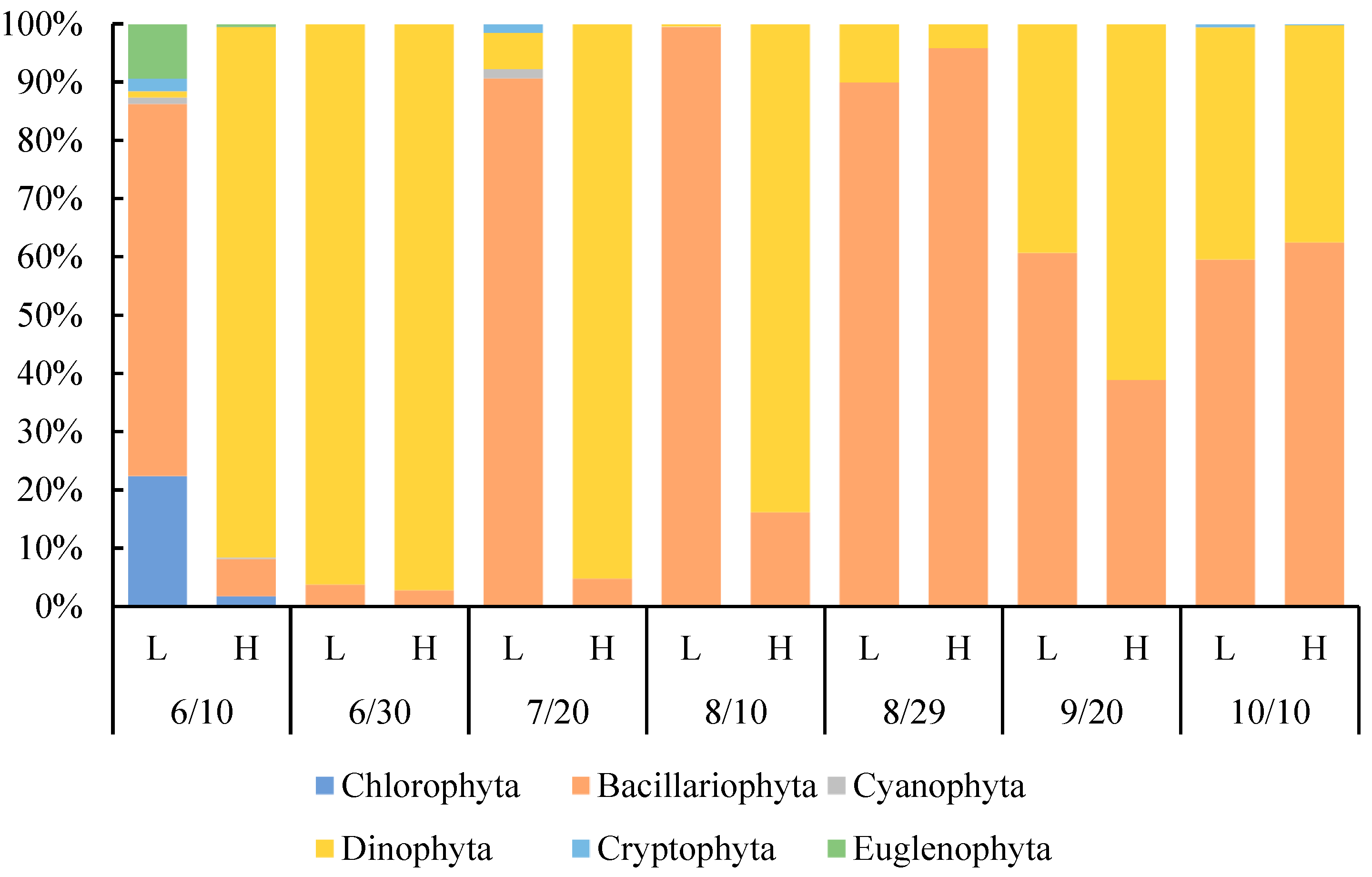
3.5. Changes in Species Composition
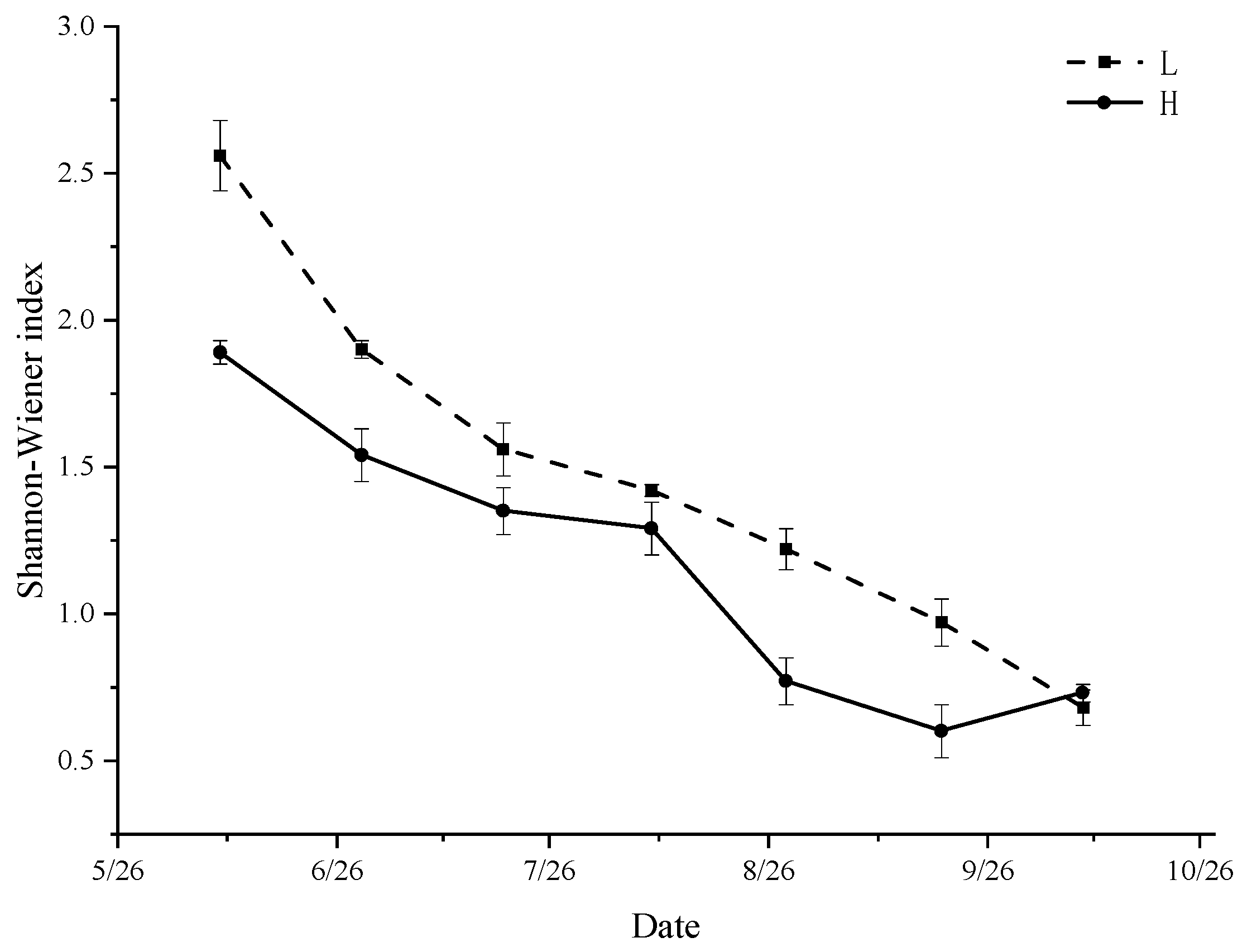
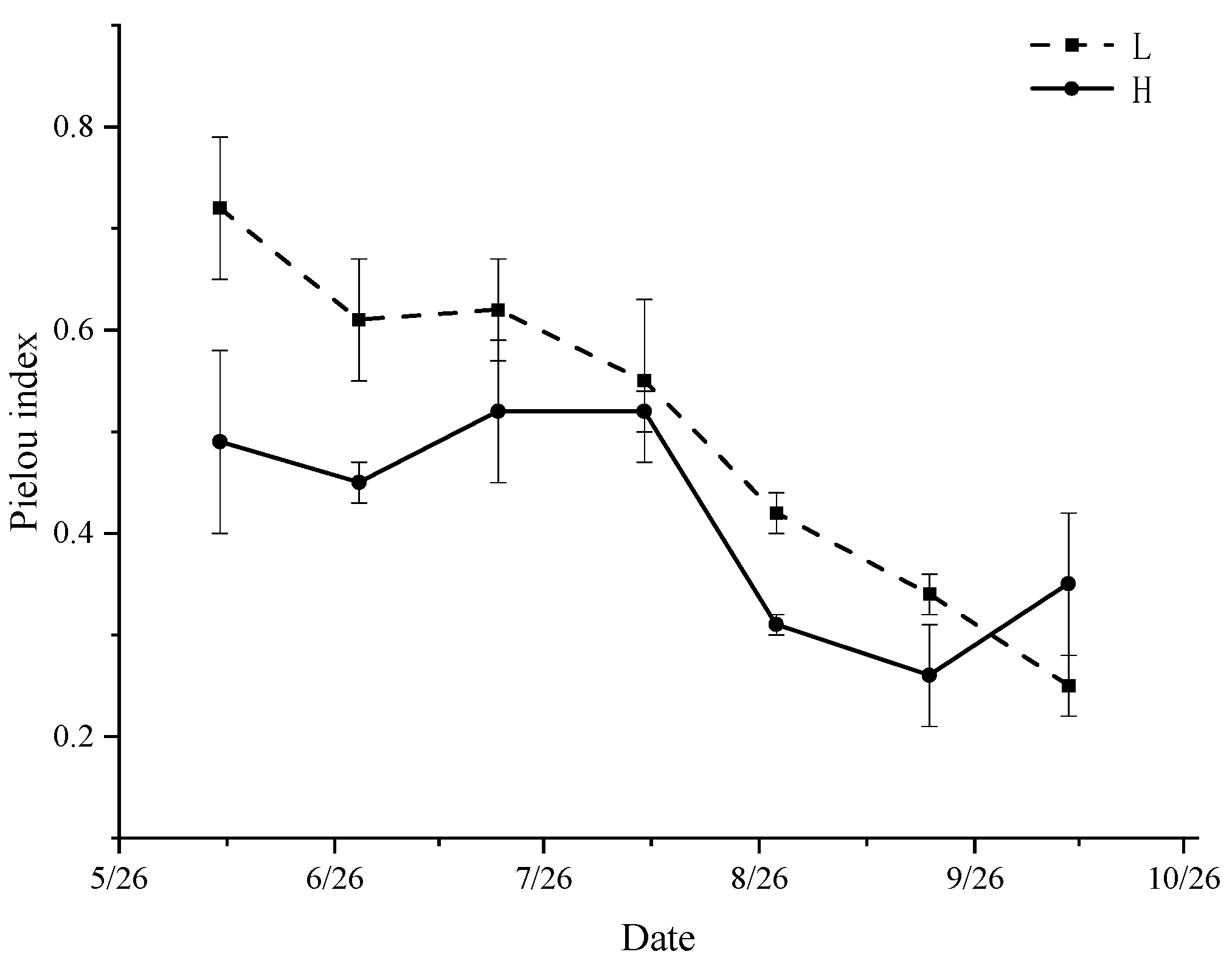
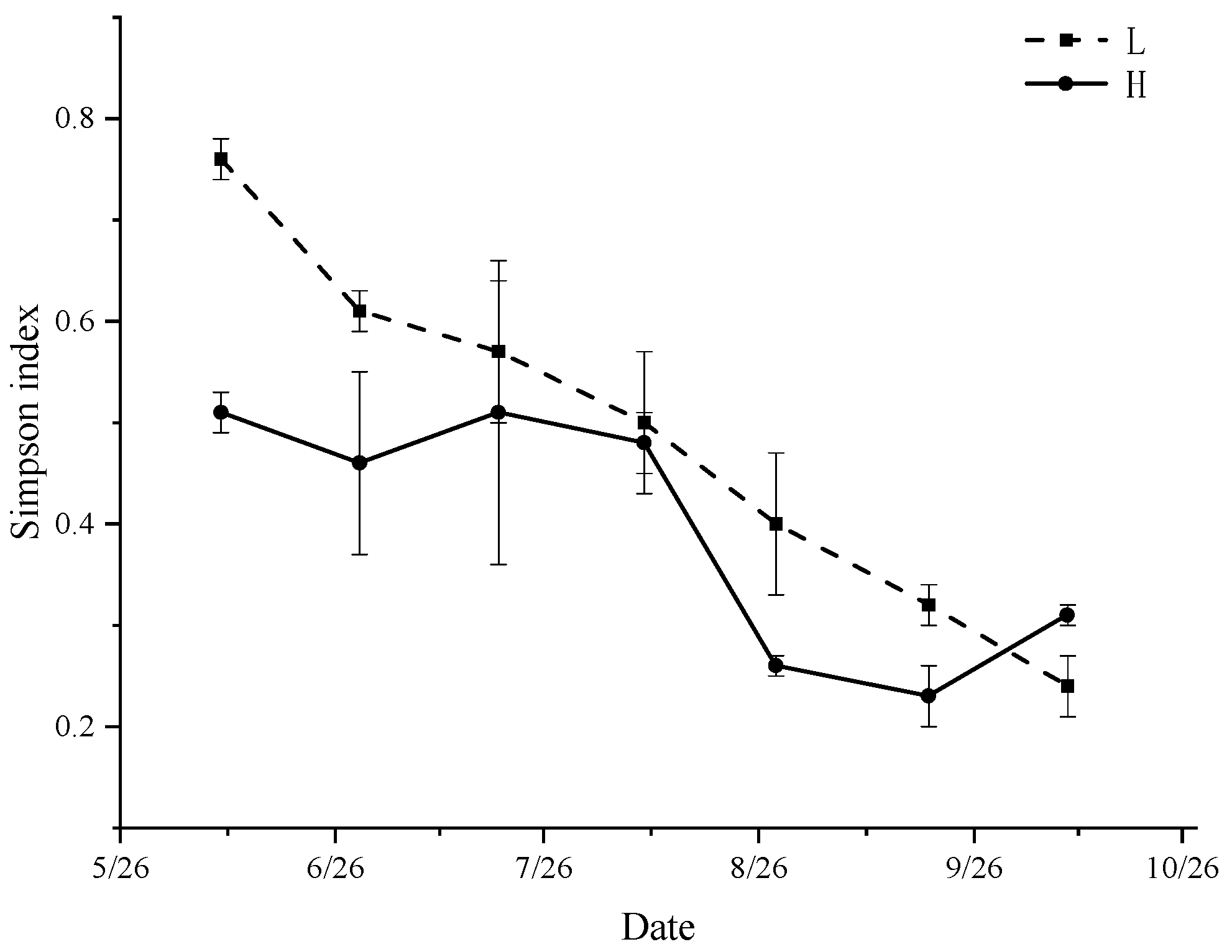
3.6. Diversity Index
3.7. Analysis of the Relationship Between Phytoplankton and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, D.L. Quantitative distribution characteristics of zooplankton from three important mariculture areas of Xiamen. J. Oceanogr. Taiwan Strait 2004, 23, 458–468. [Google Scholar]
- Borghese, J.; Giangrande, A.; Arduini, D.; Trani, R.; Doria, L.; Anglano, M.; Aguilo-Arce, J.; Toso, A.; Putignano, M.; Rizzo, L.; et al. Exploring the Potential Effects of IMTA on Water Column Seston through Intensive Short-Time Cycles Approach. Mar. Pollut. Bull. 2025, 212, 117580. [Google Scholar] [CrossRef] [PubMed]
- Riisgård, H. Efficiency of Particle Retention and Filtration Rate in 6 Species of Northeast American Bivalves. Mar. Ecol. Prog. Ser. 1988, 45, 217–223. [Google Scholar] [CrossRef]
- Cloern, J. Does the Benthos Control Phytoplankton Biomass in South San Francisco Bay? Mar. Ecol. Prog. Ser. 1982, 9, 191–202. [Google Scholar] [CrossRef]
- Yuan, S.-Y.; Zhu, W.-J.; Neori, A.; Zhang, Y.; Li, M.; Li, J.; Chang, Z.-Q. Benthic Suspension-Feeding Clams Affect Sedimentary Microbial Communities and Nitrogen Cycling in Seawater Pond IMTA. Aquaculture 2023, 563, 738907. [Google Scholar] [CrossRef]
- Batır, E.; Metin, Ö.; Yıldız, M.; Özel, O.T.; Fidan, D. Sustainable Land-Based IMTA: Holistic Management of Finfish, Mussel, and Macroalgae Interactions, Emphasizing Water Quality and Nutrient Dynamics. J. Environ. Manag. 2024, 372, 123411. [Google Scholar] [CrossRef]
- Ni, M.; Yuan, J.; Liu, M.; Gu, Z. Assessment of Water Quality and Phytoplankton Community of Limpenaeus Vannamei Pond in Intertidal Zone of Hangzhou Bay, China. Aquac. Rep. 2018, 11, 53–58. [Google Scholar] [CrossRef]
- Cunha, M.E.; Quental-Ferreira, H.; Parejo, A.; Gamito, S.; Ribeiro, L.; Moreira, M.; Monteiro, I.; Soares, F.; Pousão-Ferreira, P. Understanding the Individual Role of Fish, Oyster, Phytoplankton and Macroalgae in the Ecology of Integrated Production in Earthen Ponds. Aquaculture 2019, 512, 734297. [Google Scholar] [CrossRef]
- Lin, Z.H.; You, Z.H. Intensive mudflat bivalves culture in Zhejiang. Mar. Sci. 2005, 29, 95–99. [Google Scholar]
- Smith, D.W. Biological Control of Excessive Phytoplankton Growth and the Enhancement of Aquacultural Production. Can. J. Fish. Aquat. Sci. 1985, 42, 1940–1945. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.D.; Dong, S.L. The impact of the polycultured filter-feeding animals with peneid shrimp on plankon community. Period. Ocean Univ. China 1999, 29, 75–80. [Google Scholar]
- Li, Q. Influence of silver carp (Hypophthalmichys molitrix C et V) on plankton community in reservoir enclosures. Acta Ecol. Sin. 1993, 13, 30–37. [Google Scholar]
- Tan, K.; Xu, P.; Huang, L.; Luo, C.; Huang, J.; Fazhan, H.; Kwan, K.Y. Effects of Bivalve Aquaculture on Plankton and Benthic Community. Sci. Total Environ. 2024, 914, 169892. [Google Scholar] [CrossRef] [PubMed]
- GB 17378.4-2007; Marine Monitoring Specification—Part 4: Seawater Analysis. Standards Press of China: Beijing, China, 2007.
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China; Science Publishing Company: Beijing, China, 2006; pp. 56–95. [Google Scholar]
- Mai, X.W. Study on the Ecological Characteristics of Water Bodies in Greenhouse Desalinated Cultivation of Litopenaeus vannamei; Zhongshan University: Guangzhou, China, 2003. [Google Scholar]
- Ferreira, N.C.; Bonetti, C.; Seiffert, W.Q. Hydrological and Water quality indices as management tools in marine shrimp culture. Aquaculture 2011, 318, 425–433. [Google Scholar] [CrossRef]
- Ma, Z.; Song, X.; Wan, R.; Gao, L. A modified water quality index for intensive shrimp ponds of Litopenaeusvannamei. Ecol. Indic. 2013, 24, 287–293. [Google Scholar] [CrossRef]
- Babalola, O.G. Pollution Studies of Ogun River at Isheri Along Lagos-Ibadan Express Road, Nigeria. Master’s Thesis, University of Ibadan, Ibadan, Nigeria, 2006. [Google Scholar]
- Oketola, A.A.; Osibanjo, O.; Ejelonu, B.C.; Oladimeji, Y.B.; Damazio, O.A. Water quality assessment of River Ogun around the cattle market of Isheri, Nigeria. J. Appl. Sci. 2006, 6, 511–517. [Google Scholar] [CrossRef]
- Ismail, N.I.A.; Amal, M.N.A.; Shohaimi, S.; Saad, M.Z.; Abdullah, S.Z. Associations of water quality and bacteria presence in cage cultured red hybrid tilapia, Oreochromis niloticus × O. mossambicus. Aquaculture 2016, 4, 57–65. [Google Scholar] [CrossRef]
- Lynch, M.; Shapiro, J. Predation, enrichment, and phytoplankton community structure. Limnol. Oceanogr. 1981, 26, 86–102. [Google Scholar] [CrossRef]
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef]
- Chien, Y.H. Water Quality Requirements and Management for Marine Shrimp Culture; World Aquaculture Society: Baton Rouge, LA, USA, 1992; pp. 30–42. [Google Scholar]
- Li, K.Y.; Liu, Z.Y. Plankton of fish and shrimp culture ponds in saline-alkaline wetland. J. Lake Sci. 2002, 14, 369–373. [Google Scholar]
- Li, Y.; Li, Z.; Geng, Y. Effect of N, P concentration on growth rate and biomass of phytoplankton in eutrophical water. Acta Ecol. Sin. 2006, 26, 317–325. [Google Scholar] [CrossRef]
- Sun, W.M.; Dong, S.L.; Zhao, X.D.; Jie, Z.L.; Zhang, L.C.; Zhang, H.W. Plankton community responses to various fertilization combinations in saline-alkaline pond of shrimp (Penaeus vannamei). J. Fish. Sci. China 2007, 14, 30–34. [Google Scholar]
- Martin, J.H.; Gordon, R.M.; Fitzwater, S.E. The case for iron. Limnol. Oceanogr. 1991, 36, 1793–1802. [Google Scholar] [CrossRef]
- Guo, F.; Lin, J.M.; Huang, L.F.; Zhou, S.Q.; Shen, G.Y. Ecological characteristics of phytoplankton in shrimp cultivation area from Pantu, Xiamen. J. Oceanogr. Taiwan Strait 2002, 21, 469–482. [Google Scholar]
- Jiao, X.Y. Species diversity of aquerrantia in the prawn pool and coast of Dongxiaomo. Chin. Biodivers. 1996, 4, 7–13. [Google Scholar]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984; pp. 143–165. [Google Scholar]
- Zhang, H.H.; Li, Z.J.; Guo, Z.X.; Jia, X.P. Study on the influences of probiotics on ecological characteristics of plankton in the maricultural ponds. South China Fish. Sci. 2005, 1, 7–14. [Google Scholar]
- Proulx, M.; Pick, F.R.; Mazumder, A.; Hamilton, P.B.; Lean, D.S. Experimental evidence for interactive impacts of human activities on lake algalspecies richness. Oikos 1996, 76, 191–195. [Google Scholar] [CrossRef]
- Li, Q.H.; Chen, L.L.; Chen, F.F.; Gao, T.J.; Li, X.F.; Liu, S.P.; Li, C.X. MaixiRiverestuary to the Baihua Reservoir in the Maotiao River catchment: Phytoplankton community and environmental factors. Chin. J. Oceanol. Limnol. 2013, 31, 290–299. [Google Scholar] [CrossRef]
- Jiang, Y.J.; He, W.; Liu, W.X.; Qin, N.; Ouyang, H.L.; Wang, Q.M.; Kong, X.Z.; He, Q.S.; Yang, C.; Yang, B.; et al. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a largeeutrophic Chinese lake (Lake Chaohu). Ecol. Indic. 2014, 40, 58–67. [Google Scholar] [CrossRef]
- Peng, C.C.; Li, Z.J.; Cao, Y.C.; Wen, G.D.; Liu, X.Z.; Hu, X.J. Characteristics of the evolutionary influence of the planktonic microalgae community survival on the Penaeus monodon’s tidal shrimp culture. J. Saf. Environ. 2012, 12, 95–101. [Google Scholar]
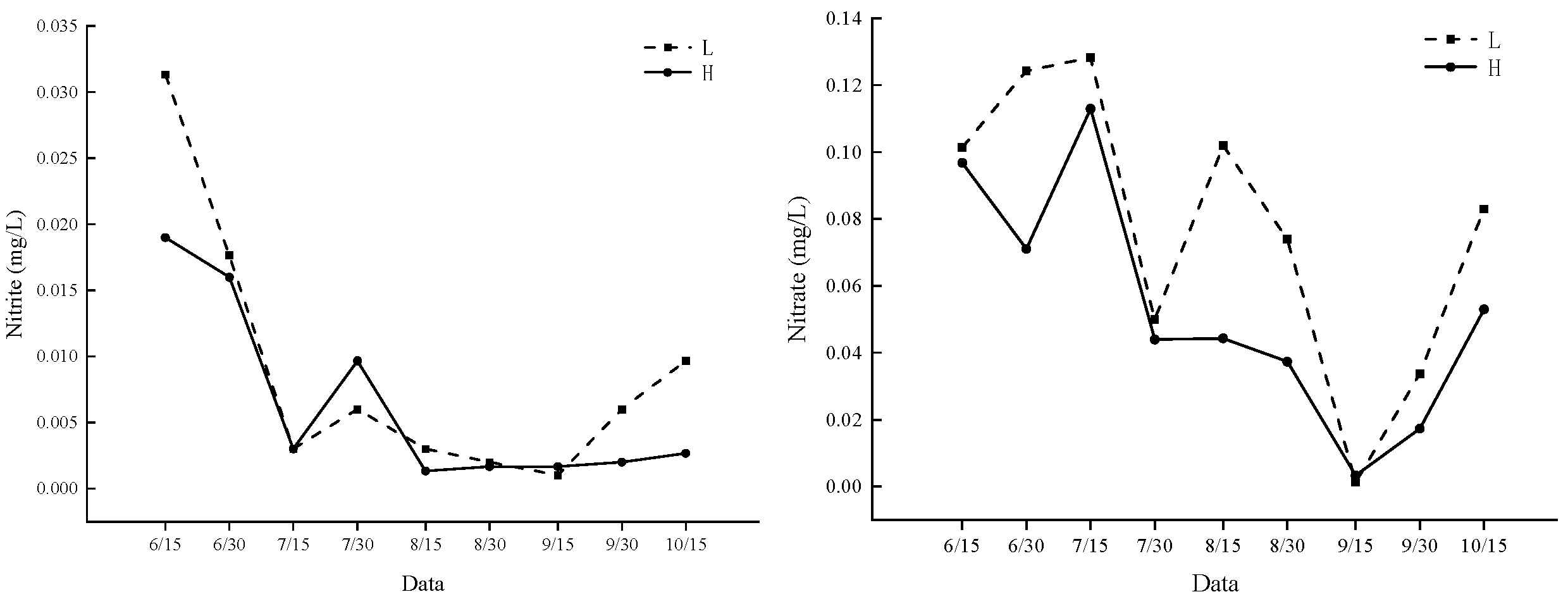
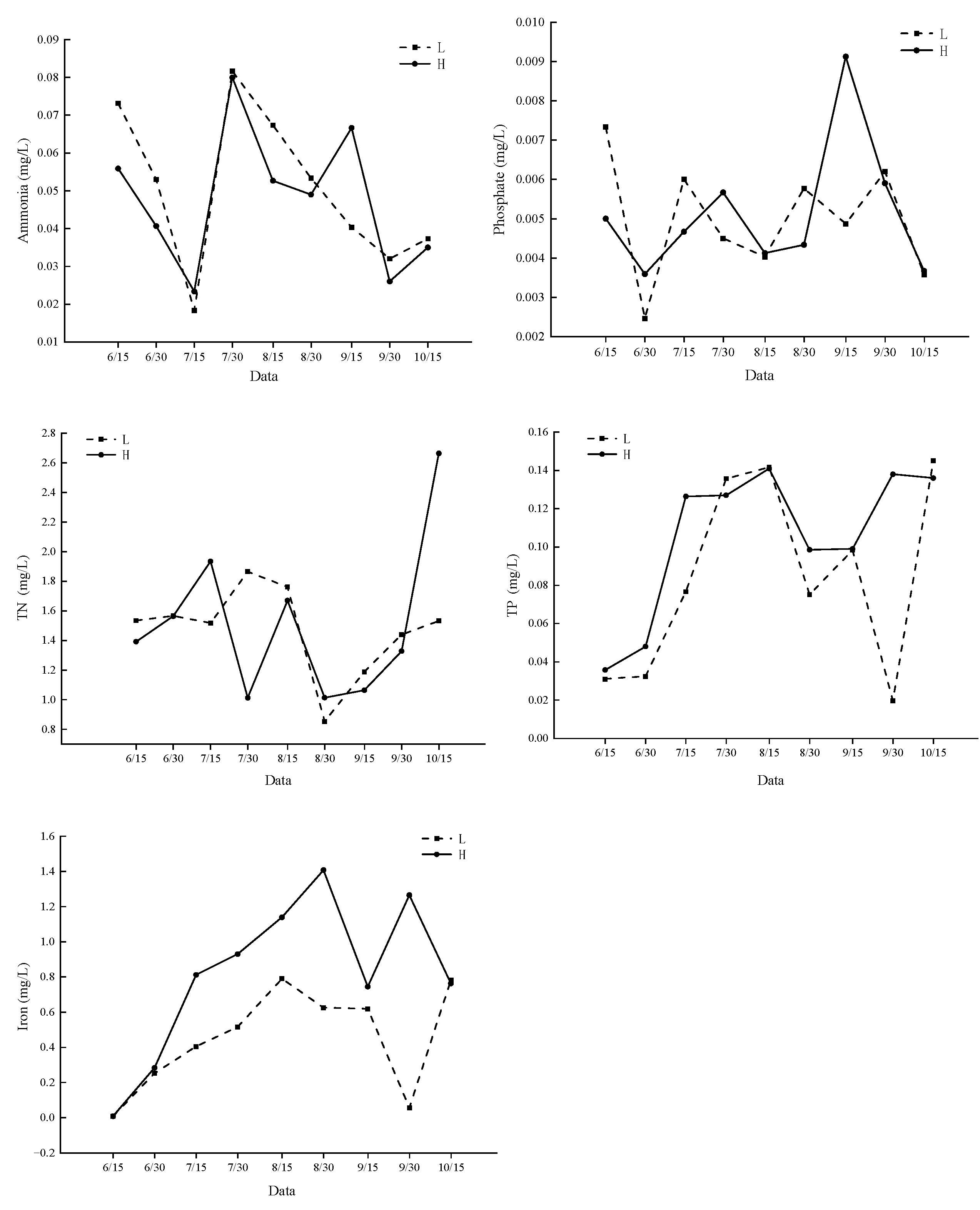

| Team | Mean (mg/L) | SD | Minimun (mg/L) | Maximum (mg/L) | |
|---|---|---|---|---|---|
| Nitrite | L | 0.012 | 0.015 | 0.000 | 0.061 |
| H | 0.006 | 0.006 | 0.001 | 0.022 | |
| Nitrate | L | 0.093 | 0.099 | 0.001 | 0.343 |
| H | 0.084 | 0.080 | 0.001 | 0.291 | |
| Ammonia | L | 0.066 | 0.049 | 0.009 | 0.203 |
| H | 0.055 | 0.031 | 0.017 | 0.142 | |
| Phosphate | L | 0.005 | 0.003 | 0.002 | 0.015 |
| H | 0.005 | 0.002 | 0.001 | 0.008 | |
| Total nitrite | L | 1.473 | 0.412 | 0.735 | 2.353 |
| H | 1.459 | 0.631 | 0.679 | 3.969 | |
| Total phosphate | L | 0.084 | 0.050 | 0.011 | 0.187 |
| H | 0.106 | 0.042 | 0.029 | 0.180 | |
| Iron | L | 0.482 * | 0.335 | 0.007 | 1.382 |
| H | 0.818 * | 0.565 | 0.002 | 2.330 |
| Team | Mean | SD | Minimun | Maximum | |
|---|---|---|---|---|---|
| Transparency | L | 41 | 22 | 26 | 106 |
| (cm) | H | 42 | 21 | 27 | 103 |
| pH | L | 7.88 | 0.36 | 7.19 | 8.32 |
| H | 7.93 | 0.40 | 7.28 | 8.51 | |
| Salinity | L | 22.64 | 2.24 | 18.98 | 26.57 |
| H | 22.77 | 2.11 | 18.66 | 26.00 | |
| DO (mg/L) | L | 5.61 | 1.56 | 3.98 | 9.96 |
| H | 5.46 | 1.28 | 4.10 | 8.73 |
| Team | Data | Mean | SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6/15 | 6/30 | 7/15 | 7/30 | 8/15 | 8/30 | 9/15 | 9/30 | 10/15 | |||
| L | 49.47 | 48.34 | 19.82 | 13.75 | 12.44 | 11.37 | 12.08 | 73.17 | 10.56 | 27.89 | 23.07 |
| H | 39.01 | 32.59 | 15.30 | 7.97 | 11.84 | 10.28 | 10.75 | 9.62 | 19.59 | 17.44 | 11.08 |
| Phylum | Species | Richness |
|---|---|---|
| Bacillariophyta | Gyrosigma | + |
| Pleurosigma sp. | +++ | |
| Pleurosigma. formosum | +++ | |
| Synedra. tabulata | ++ | |
| Coscinodiscus. lineatus | + | |
| Nitzschia. pungens | ++ | |
| Navicula. cryptocephala | + | |
| Synedra. ulna | + | |
| Triceratium sp. | + | |
| Melosira. moniliformis | ++ | |
| Cyclotella sp. | +++ | |
| Rhizosoleniales sp. | + | |
| Climacosphenia moniligera | +++ | |
| Nitzschia. cocconeiformis | + | |
| Navicular sp. | +++ | |
| Amphora sp. | + | |
| Cymbella sp. | ++ | |
| Asteromphalus sp. | + | |
| Licmophora sp. | ++ | |
| Cyanophyta | Phormidium sp. | + |
| Aphanocapsa sp. | + | |
| Oscillatoria. princeps | + | |
| Aphanothece sp. | + | |
| Lyngbya sp. | ++ | |
| Dactylococcopsis sp. | + | |
| Chlorophyta | Closteriopsis sp. | + |
| Eudorina. elegans | + | |
| Chlorella. ellipsoidea | ++ | |
| Chlorococcum sp. | + | |
| Platymonas sp. | + | |
| Cladopgoraceae sp. | + | |
| Dinophyta | Gymnodiniaceae sp. | +++ |
| Glenodinium sp. | +++ | |
| Dactyliosolen. mediterraneus | + | |
| Cryptophyta | Cryptomonas sp. | +++ |
| Euglenophyta | Astasia sp. | + |
| Chrysophyta | Cocconeiaceae sp. | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; He, L.; Lin, Z.; Xu, Y. Effects of Stocking Density on Phytoplankton Community and Water Quality in Polyculture Ponds of Tegillarca granosa and Litopenaeus vannamei. Fishes 2025, 10, 222. https://doi.org/10.3390/fishes10050222
He J, He L, Lin Z, Xu Y. Effects of Stocking Density on Phytoplankton Community and Water Quality in Polyculture Ponds of Tegillarca granosa and Litopenaeus vannamei. Fishes. 2025; 10(5):222. https://doi.org/10.3390/fishes10050222
Chicago/Turabian StyleHe, Jing, Lin He, Zhihua Lin, and Yongjian Xu. 2025. "Effects of Stocking Density on Phytoplankton Community and Water Quality in Polyculture Ponds of Tegillarca granosa and Litopenaeus vannamei" Fishes 10, no. 5: 222. https://doi.org/10.3390/fishes10050222
APA StyleHe, J., He, L., Lin, Z., & Xu, Y. (2025). Effects of Stocking Density on Phytoplankton Community and Water Quality in Polyculture Ponds of Tegillarca granosa and Litopenaeus vannamei. Fishes, 10(5), 222. https://doi.org/10.3390/fishes10050222






