Diet Composition and Feeding Intensity of Four-Spotted Megrim, Lepidorhombus boscii (Risso, 1810), in the Eastern Adriatic Sea
Abstract
1. Introduction
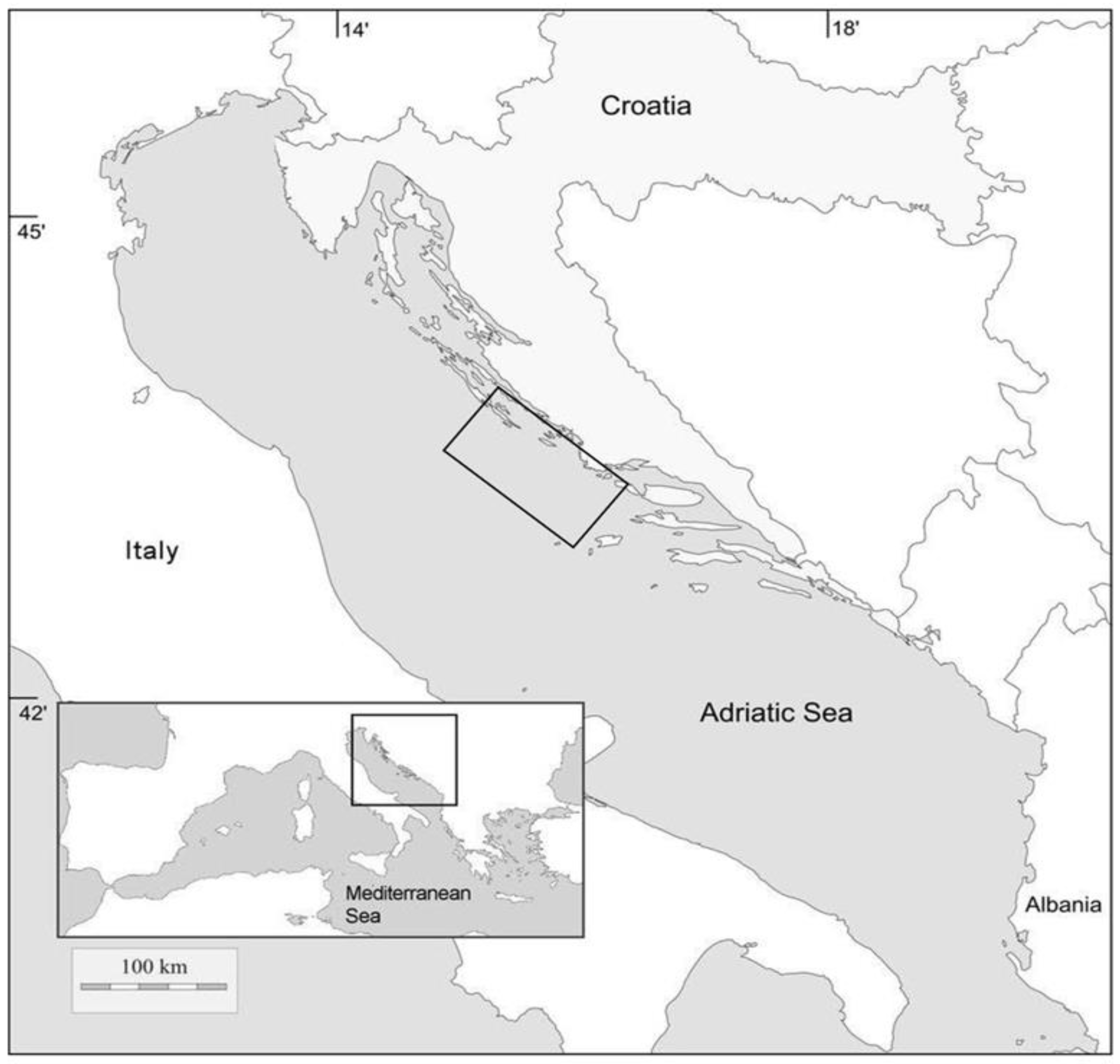
2. Materials and Methods
3. Results
3.1. Feeding Intensity
3.2. Diet Composition
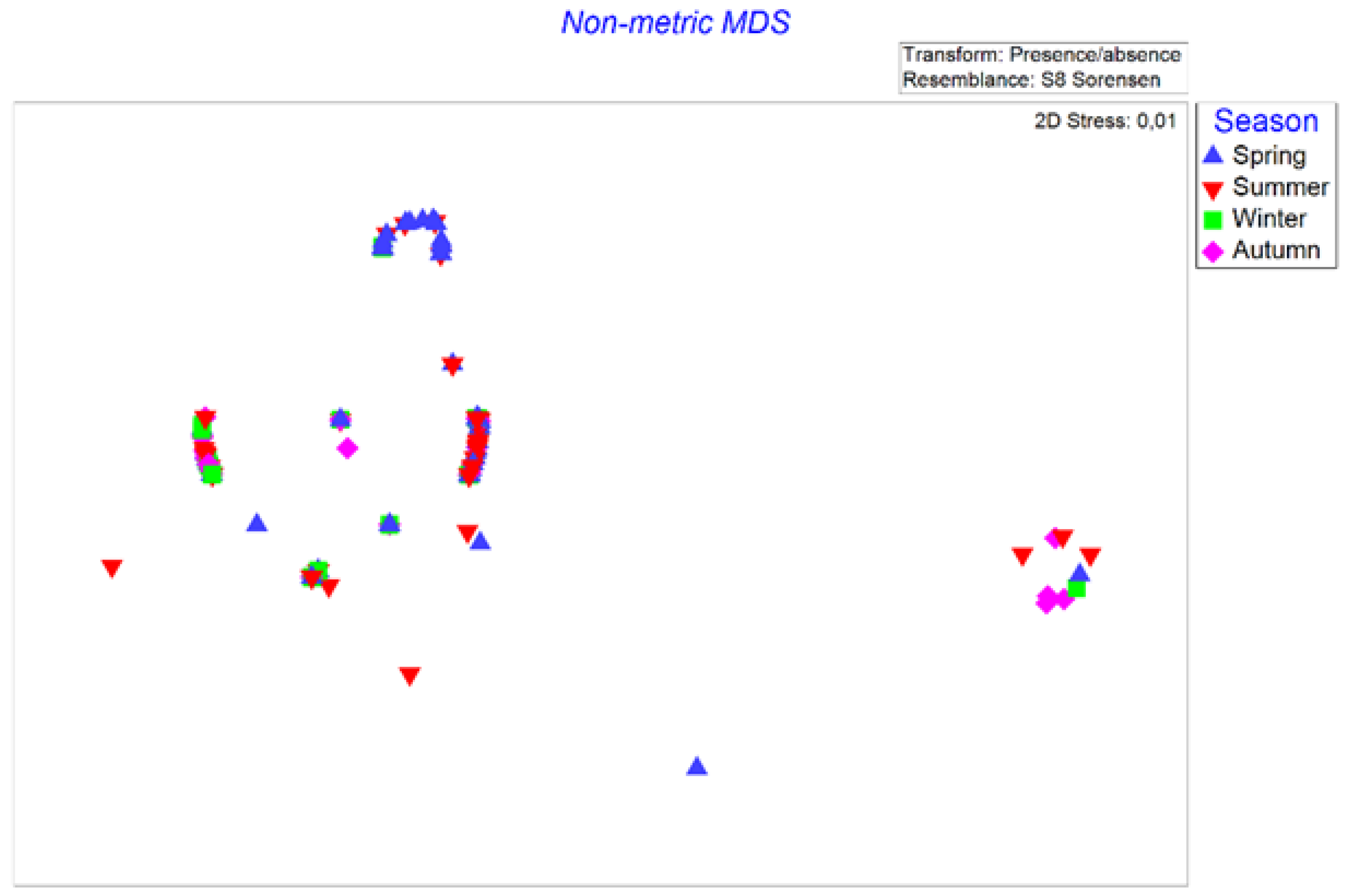
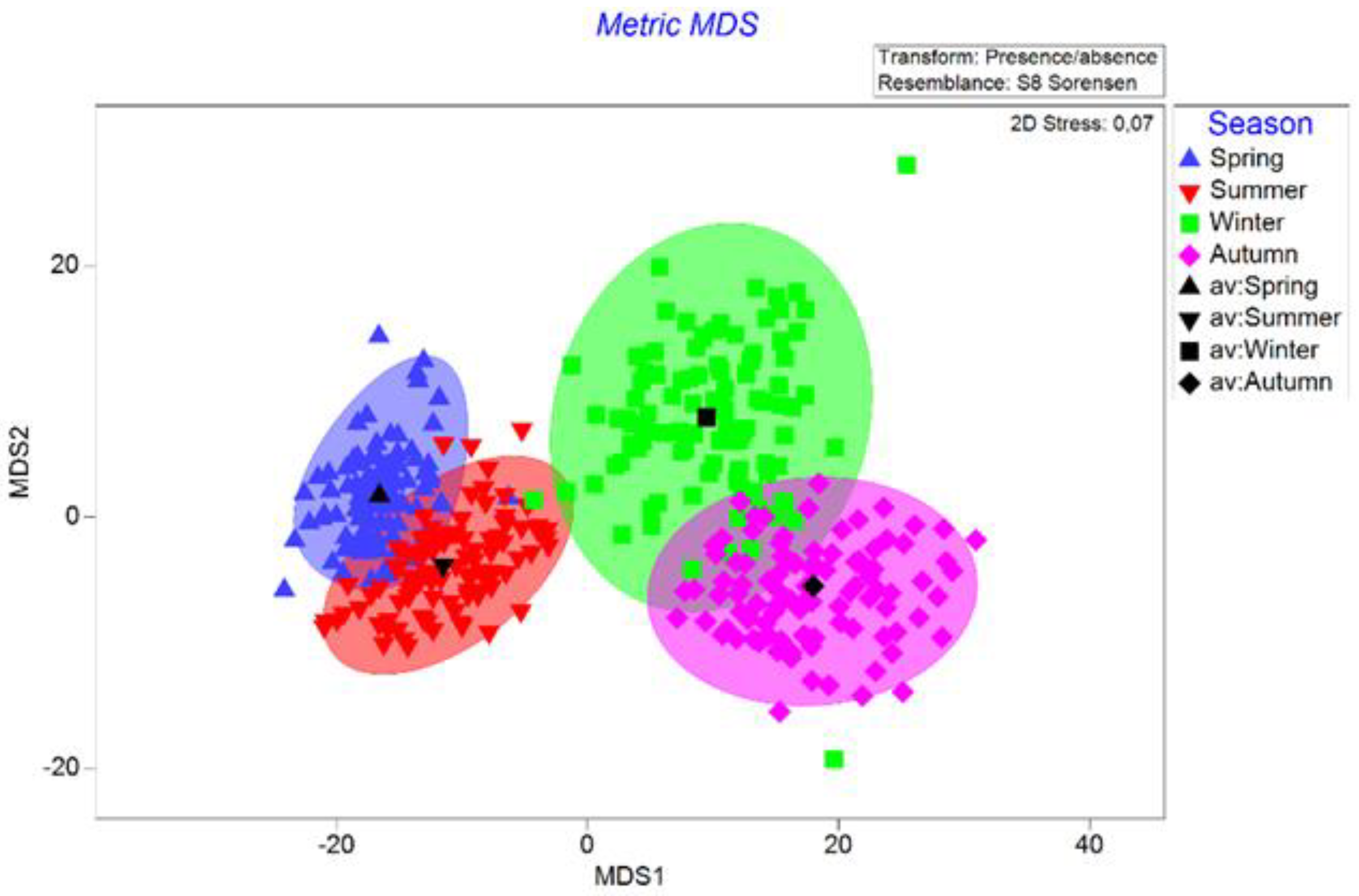
3.3. Seasonal Variation in the Diet Composition
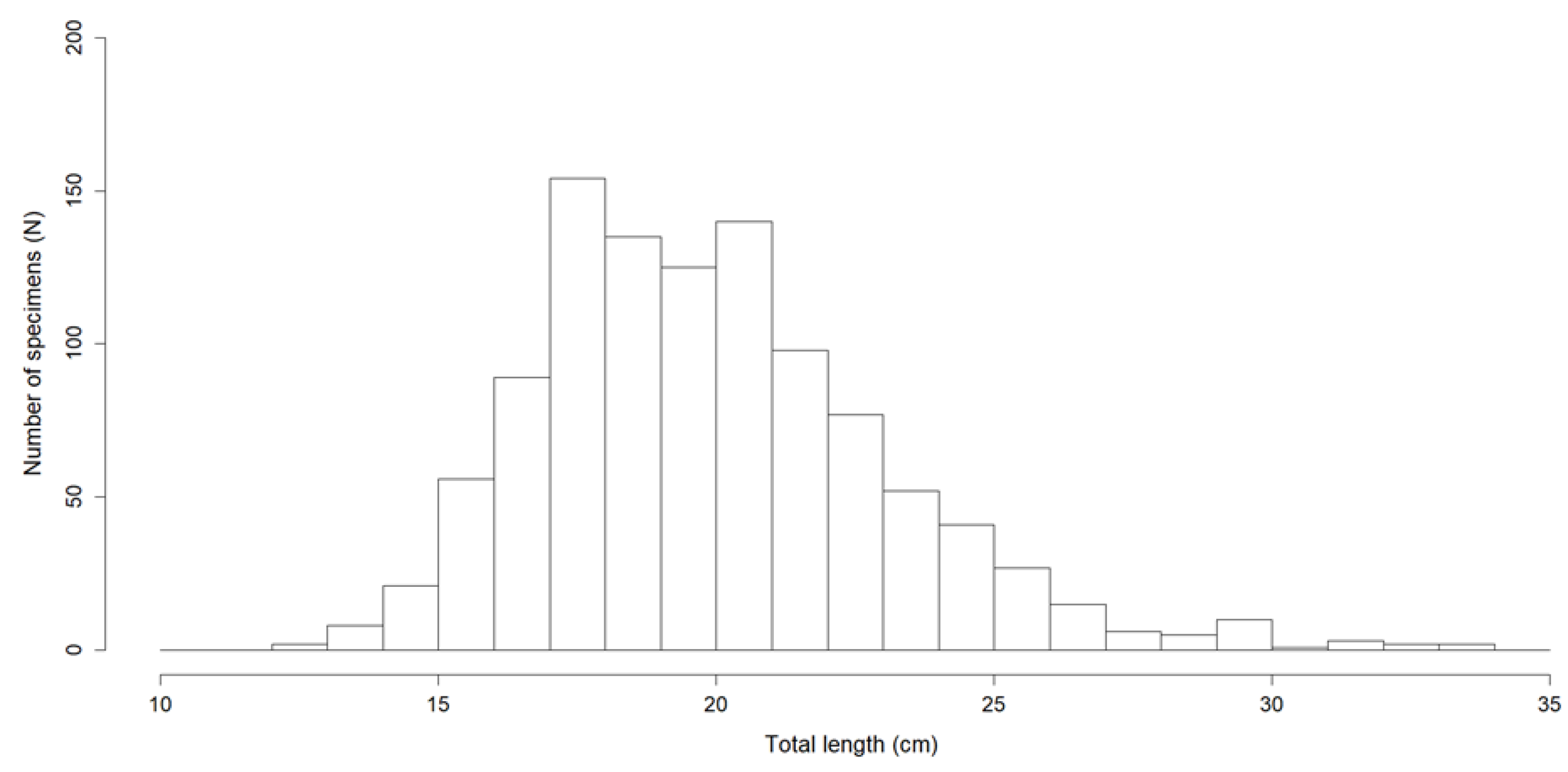
3.4. Food in Relation to Fish Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bauchot, M.L. Poisson Osseux. In Fiches FAO d’identification des Especes pour les Besoins de la Peche (Revision 1), 2nd ed.; Fischer, W., Schneider, M., Bauchot, M.L., Eds.; FAO & Commission des Communautes Europeennes: Rome, Italy, 1987; Volume 2, pp. 761–1530. [Google Scholar]
- Jardas, I. Jadranska Ihtiofauna; Školska knjiga: Zagreb, Croatia, 1996; p. 536. [Google Scholar]
- Ugrin, N.; Paladin, A.; Krstulović Šifner, S. Fecundity, Length at First Sexual Maturity and Gonadal Development of Lepidorhombus boscii in the Eastern Adriatic Sea. Biology 2023, 12, 131. [Google Scholar] [CrossRef]
- Dulčić, J.; Kovačić, M. Ihtiofauna Jadranskog Mora; Golden marketing—Tehnička knjiga, Institut za oceanografiju i ribarstvo: Zagreb, Croatia, 2020; pp. 468–470. [Google Scholar]
- Bostanci, D.; Polat, N. Otolith Structure, Otolith Dimensions-Fish Length Relationships and Age Determination of Fourspotted Megrim Lepidorhombus boscii (Risso, 1810). J. Fish. Sci. 2007, 19, 375–381. [Google Scholar]
- Castilho, R.; Dinis, M.T.; Erzini, K. Age and Growth of Megrim Lepidorhombus boscii, Risso of the Portuguese Continental Coast. Fish. Res. 1993, 16, 339–346. [Google Scholar] [CrossRef]
- Cengiz, Ö.; Ozekinci, U.; Ismen, A.; Oztekin, A. Age and Growth of the Four-Spotted Megrim (Lepidorhombus boscii Risso, 1810) from Saros Bay (Northern Aegean Sea, Turkey). Mediterr. Mar. Sci. 2013, 14, 36–44. [Google Scholar] [CrossRef]
- Landa, J.; Perez, N.; Pineiro, C. Growth Patterns of the Four-Spot Megrim (Lepidorhombus boscii) in the Northeast Atlantic. Fish. Res. 2002, 55, 141–152. [Google Scholar] [CrossRef]
- Santos, P.T. Growth and Reproduction of the Population of the Four-Spot Megrim (Lepidorhombus boscii Risso) off the Portuguese Coast. Neth. J. Sea Res. 1994, 32, 379–383. [Google Scholar] [CrossRef]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith Atlas for the Western Mediterranean, North and Central Eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Ugrin, N.; Škeljo, F.; Ferri, J.; Krstulović Šifner, S. Use of Otolith Morphology and Morphometry for Species Discrimination of Megrims Lepidorhombus spp. in the Central Eastern Adriatic Sea. J. Mar. Biol. Assoc. U. K. 2021, 101, 735–741. [Google Scholar] [CrossRef]
- Dwivedi, S.N. Comparative Ecology, Morphology and Biology of the Two Species of the Genus Lepidorhombus: L. megastoma (Donovan) and L. boscii (Risso): Study of Their Races and Populations. Rev. Trav. Inst. Pêches Maritimes 1964, 28, 397–399. [Google Scholar]
- Robson, S.M.; King, P.A.; McGrath, D. The Morphometric and Meristic Characteristics of Common Megrim Lepidorhombus whiffiagonis and Four-Spot Megrim Lepidorhombus boscii from off the West Coast of Ireland. Ir. Nat. J. 2005, 28, 116–119. [Google Scholar]
- Ugrin, N.; Krstulović Šifner, S. A Rare Occurrence of Reversal in the Four-Spotted Megrim Lepidorhombus boscii (Risso, 1810) (Pleuronectiformes: Scophthalmidae) in the Central Adriatic Sea. J. Fish Biol. 2022, 101, 1358–1360. [Google Scholar] [CrossRef]
- Ugrin, N.; Krstulović Šifner, S. Biometric Characteristics and Relative Growth of Four-Spot Megrim Lepidorhombus boscii (Risso, 1810) (Pleuronectiformes: Scophthalmidae) in the Eastern Adriatic Sea. Egypt. J. Aquat. Res. 2025, 51, 1–6. [Google Scholar] [CrossRef]
- Taylan, B.; Uluturk, E. Determination of Fecundity in the Four-Spot Megrim Lepidorhombus boscii (Risso, 1810) (Pisces: Scophthalmidae) from the Aegean Sea. CBM-Cah. Biol. Mar. 2017, 58, 213–217. [Google Scholar]
- Teixeira, C.M.; Batisat, M.I.; Cabral, H.N. Diet, Growth and Reproduction of Four Flatfishes on the Portuguese Coast. Sci. Mar. 2010, 74, 223–233. [Google Scholar] [CrossRef]
- Vassilopoulou, V.; Haralabous, J. Effects of Sexual Maturity and Feeding on Condition of a Deep-Sea Flatfish, Lepidorhombus boscii, in North-Eastern Mediterranean Waters. J. Nat. Hist. 2008, 42, 695–720. [Google Scholar] [CrossRef]
- Sartor, P.; De Ranieri, S. Food and Feeding Habits of Lepidorhombus boscii (Pisces, Scophthalmidae) in the Southern Tuscan Archipelago, Tyrrhenian Sea. Vie Milieu 1996, 46, 57–64. [Google Scholar]
- Morte, S.; Redon, M.J.; Sanz-Brau, A. Feeding Ecology of Two Megrims Lepidorhombus boscii and Lepidorhombus whiffiagonis in the Western Mediterranean (Gulf of Valencia, Spain). J. Mar. Biol. Assoc. U. K. 1999, 79, 161–169. [Google Scholar] [CrossRef]
- Vasilopoulou, V. Dietary Habits of the Deep-Sea Flatfish Lepidorhombus boscii in North-Eastern Mediterranean Waters. J. Fish Biol. 2006, 69, 1202–1220. [Google Scholar] [CrossRef]
- Braga, R.R.; Bornatowski, H.; Vitule, J.R.S. Feeding Ecology of Fishes: An Overview of Worldwide Publications. Rev. Fish Biol. Fish. 2012, 22, 915–929. [Google Scholar] [CrossRef]
- Hureau, J.C. Biologie Comparée de Quelques Poissons Antarctiques (Nototheniidae). Bulletin de l’Institut Océanographique de Monaco 1970, 68, 1–244. [Google Scholar]
- Berg, J. Discussion of the Methods of Investigating the Food of Fishes, with Reference to a Preliminary Study of the Food of Gobiusculus flavescens (Gobiidae). Mar. Biol. 1979, 50, 263–273. [Google Scholar] [CrossRef]
- Rossechi, E.; Nouaze, Y. Comparaison de Cinq Indices Alimentaires Utilisés dans l’Analyse des Contenus Stomacaux. Rev. Trav. Inst. Pêches Marit. 1987, 49, 111–123. [Google Scholar]
- Pinkas, L.; Oliphant, M.S.; Iversen, I.L.K. Food Habits of Albacore, Bluefin Tuna and Bonito in California Waters. Fish. Bull. 1971, 152, 1–105. [Google Scholar]
- Clarke, K.R.; Green, R.H. Statistical Design and Analysis for a ‘Biological Effects’ Study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Somerfield, P.J.; Clarke, K.R.; Gorley, R.N. A Generalised Analysis of Similarities (ANOSIM) Statistic for Designs with Ordered Factors. Austral Ecol. 2021, 46, 901–910. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- De Groot, S.J. On the Interrelationships Between Morphology of the Alimentary Tract, Food and Feeding Behaviour in Flatfishes (Pisces: Pleuronectiformes). Neth. J. Sea Res. 1971, 5, 121–196. [Google Scholar] [CrossRef]
- Ferrari, I.; Chieregato, A.R. Feeding Habits of Juvenile Stages of Sparus aurata L., Dicentrarchus labrax L. and Mugilidae in a Brackish Embayment of the Po River Delta. Aquaculture 1981, 25, 243–257. [Google Scholar] [CrossRef]
- Paradinović, A. Population Dynamics of Anuropodione amphiandra on the Host Munida speciosa in the Eastern Adriatic Sea. Master’s Thesis, University of Split, Split, Croatia, 2021. [Google Scholar]
- Boyko, C.B.; Williams, J.D.; Öktener, A. Redescription of the Mediterranean Endemic Parasitic Isopod Anuropodione amphiandra (Codreanu, Codreanu & Pike, 1966) n. comb. (Crustacea: Isopoda: Bopyridae) from Munida rutllanti Zariquiey Alvarez, 1952, with New Records from Turkey and a Review of the Genus Anuropodione Bourdon, 1967. Mediterr. Mar. Sci. 2018, 19, 611–619. [Google Scholar]
- Arias-Gonzalez, J.E.; Morand, S. Trophic Functioning with Parasites: A New Insight for Ecosystem Analysis. Mar. Ecol. Prog. Ser. 2006, 320, 45–53. [Google Scholar] [CrossRef]
- Gerking, S.D. Feeding Ecology of Fish; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Platell, M.E.; Potter, I.C. Distributions, Size Compositions and Diets of Two Abundant Benthic Ambush-Feeding Teleosts in Coastal Waters of South-Western Australia. J. Mar. Biol. Assoc. U. K. 1998, 78, 587–608. [Google Scholar] [CrossRef]
- Draper, D.L. Food Habit Variability of Arrowtooth Flounder (Atheresthes stomias) along the U.S. West Coast. Fish. Res. 2022, 248, 106205. [Google Scholar] [CrossRef]
- Bișinicu, E.; Harcotă, G.E.; Lazăr, L.; Niță, V.; Țoțoiu, A.; Țiganov, G. Fish Abundance and Mesozooplankton Resource: A Study on Sprattus sprattus (Linnaeus, 1758) (Actinopterygii: Clupeidae) in the Romanian Black Sea Waters. Acta Zool. Bulg. 2024, 76, 215–224. [Google Scholar] [CrossRef]
- Vučetić, T. Zooplankton and Circulation Pattern of the Water Masses in the Adriatic. Neth. J. Sea Res. 1973, 7, 112–121. [Google Scholar] [CrossRef]
- Grubišić, F. Fishes, Crustaceans and Mussels of the Adriatic Sea; ITRO Naprijed: Zagreb, Croatia; GRO Liburnija: Rijeka, Croatia, 1988. (In Croatian) [Google Scholar]
- Bisinicu, E.; Harcota, G.E. Baseline Assessment of Black Sea Food Web Integrity Using a Zooplankton-Based Approach Under the Marine Strategy Framework Directive. J. Mar. Sci. Eng. 2025, 13, 713. [Google Scholar] [CrossRef]
- Bowman, R.E.; Bowman, E.W. Diurnal Variation in the Feeding Intensity and Catchability of Silver Hake (Merluccius bilinearis). Canadian J. Fish. Aquat. Sci. 1980, 37, 1565–1572. [Google Scholar] [CrossRef]
- Zore-Armanda, M.; Bone, M.; Dadić, V.; Morović, M.; Ratković, D.; Stojanoski, L.; Vukadin, I. Hydrographic Properties of the Adriatic Sea in the Period from 1971 through 1983. Acta Adriat. 1991, 32, 6–554. [Google Scholar]
- Tyler, A.Y. Monthly Changes in Stomach Contents of Demersal Fishes in Passamaquoddy Bay (N.B.). Fish. Res. Board Can. 1971, 288, 114. [Google Scholar]
- Garrido, S.; Murta, A.G.; Moreira, A.; Ferreira, M.J.M. Angelico Horse Mackerel (Trachurus trachurus) Stomach Fullness off Portugal: Index Calibration and Spatio-Temporal Variations in Feeding Intensity. ICES J. Mar. Sci. 2008, 65, 1662–1669. [Google Scholar] [CrossRef]
- Kock, K.H.; Wilhelms, S.; Everson, I.; Goger, J. Variations in the Diet Composition and Feeding Intensity of Mackerel Icefish Champsocephalus gunnari at South Georgia (Antarctic). Mar. Ecol. Prog. Ser. 1994, 108, 43–57. [Google Scholar] [CrossRef]
- Šantić, M.; Podvinski-Markov, M.; Palaoro, A.; Jardas, I.; Kirinčić, M. Feeding Habits of Megrim, Lepidorhombus whiffiagonis (Walbaum, 1792), from the Central Adriatic Sea. J. Appl. Ichthyol. 2009, 25, 417–422. [Google Scholar] [CrossRef]
| Food Items | %F | %N | %W | IRI | %IRI |
|---|---|---|---|---|---|
| PISCES TELEOSTEI | |||||
| Argentina sphyraena | 0.25 | 0.24 | 0.11 | 0.08 | <0.1 |
| Callionymus fasciatus | 0.25 | 0.24 | 0.09 | 0.08 | <0.1 |
| Cepola rubescens | 0.50 | 0.48 | 1.06 | 0.77 | <0.1 |
| Lesueurigobius friessi | 0.25 | 0.24 | 0.13 | 0.09 | <0.1 |
| Merluccius merluccius | 9.92 | 9.48 | 10.08 | 194.03 | 2.16 |
| Non-identified Teleostei | 3.30 | 3.16 | 2.18 | 17.62 | 0.19 |
| Total Teleostei | 14.47 | 13.84 | 13.65 | 397.78 | 4.44 |
| CRUSTACEA DECAPODA | |||||
| Alpheus glaber | 0.25 | 0.24 | 0.27 | 0.12 | <0.1 |
| Eriphia spinifrons | 0.25 | 0.24 | 0.03 | 0.06 | <0.1 |
| Goneplax rhomboides | 18.06 | 18.00 | 11.38 | 530.60 | 5.92 |
| Munida intermedia | 5.34 | 5.10 | 2.78 | 42.07 | 0.46 |
| Munida nexa | 1.52 | 1.49 | 0.64 | 3.23 | <0.1 |
| Iridonida speciosa | 2.03 | 1.94 | 1.35 | 6.67 | <0.1 |
| Munida spp. | 1.01 | 1.21 | 0.55 | 1.77 | <0.1 |
| Parapenaeus longirostris | 23.40 | 22.62 | 21.34 | 1028.66 | 11.48 |
| Processa canaliculata | 0.50 | 0.48 | 0.33 | 1.02 | <0.1 |
| Solenocera membranacea | 1.27 | 1.21 | 2.19 | 4.31 | <0.1 |
| Upogebia pulsilla | 0.25 | 0.24 | 0.20 | 0.11 | <0.1 |
| Non-identified Crustacea | 0.76 | 0.72 | 0.79 | 1.14 | <0.1 |
| Total Decapoda | 54.64 | 53.49 | 41.85 | 5209.37 | 58.18 |
| MYSIDA | |||||
| Lophogaster typicus | 2.29 | 2.43 | 1.88 | 9.86 | 0.11 |
| Total Mysida | 2.29 | 2.43 | 1.88 | 9.86 | 0.11 |
| ISOPODA | |||||
| Anuropodione amphiandra | 7.12 | 7.05 | 9.77 | 119.75 | 1.33 |
| Total Isopoda | 7.12 | 7.05 | 9.77 | 119.75 | 1.33 |
| MOLLUSCA CEPHALOPODA | |||||
| Alloteuthis media | 11.45 | 10.94 | 18.18 | 333.42 | 3.72 |
| Illex coindetii | 1.78 | 1.70 | 2.78 | 7.87 | <0.1 |
| Loligo vulgaris | 1.01 | 0.97 | 2.78 | 3.78 | <0.1 |
| Sepia officinalis | 4.58 | 4.37 | 2.86 | 33.11 | 0.36 |
| Sepiola rondeletii | 0.25 | 0.24 | 0.21 | 0.11 | <0.1 |
| Non-identified Cephalopoda | 1.78 | 1.70 | 0.76 | 4.37 | <0.1 |
| Total Cephalopoda | 20.85 | 19.98 | 27.57 | 978.57 | 10.93 |
| GASTROPODA | |||||
| Non-identified Gastropoda | 0.76 | 0.72 | 0.36 | 0.82 | <0.1 |
| Total Gastropoda | 0.76 | 0.72 | 0.36 | 0.82 | <0.1 |
| BIVALVIA | |||||
| Nucula spp. | 0.25 | 0.24 | 0.12 | 0.09 | <0.1 |
| Nuculana pella | 0.25 | 0.24 | 0.06 | 0.07 | <0.1 |
| Non-identified Bivalvia | 0.50 | 0.48 | 0.18 | 0.33 | <0.1 |
| Total Bivalvia | 3.81 | 3.64 | 1.95 | 21.29 | 0.23 |
| IRI (%IRI) | ||||
|---|---|---|---|---|
| Group of Prey | Summer | Autumn | Winter | Spring |
| Teleostei | 156.25 (3.34) | 277.39 (4.70) | 642.72 (23.70) | 201.82 (3.27) |
| Bivalvia | 3.69 (0.07) | - | - | - |
| Gastropoda | - | - | - | 0.56 (0.009) |
| Cephalopoda | 133.13 (2.85) | 4283.76 (72.70) | 603.05 (22.23) | 95.53 (1.55) |
| Isopoda | 33.28 (0.71) | - | 17.85 (0.65) | 213.69 (3.46) |
| Mysida | 8.32 (0.17) | 13.71 (0.23) | 1.98 (0.07) | - |
| Decapoda | 4201.18 (89.98) | 1316.87 (22.35) | 1446.14 (53.32) | 5650.97 (91.69) |
| Pairwise Tests | ||
|---|---|---|
| Season | R | p |
| Spring–Summer | 0.004 | 0.231 |
| Spring–Winter | 0.122 | 0.001 |
| Spring–Autumn | 0.149 | 0.0003 |
| Summer–Winter | 0.089 | 0.0015 |
| Summer–Autumn | 0.11 | 0.0003 |
| Winter–Autumn | 0.019 | 0.0852 |
| IRI (%IRI) | |||
|---|---|---|---|
| Group of Prey | 12.5–19 cm | 19–24 cm | 24–34 cm |
| Teleostei | 439.67 (14.87) | 948.34 (5.09) | 316.75 (3.31) |
| Bivalvia | 1.97 (0.06) | - | 1.99 (0.02) |
| Gastropoda | - | - | 2.22 (0.02) |
| Cephalopoda | 984.8 (33.31) | 2524.82 (13.55) | 63.05 (0.65) |
| Isopoda | - | 224.51 (1.20) | 930.03 (9.73) |
| Mysida | 14.57 (0.49) | 2.73 (0.01) | 1.99 (0.20) |
| Decapoda | 1515.16 (51.25) | 14,920.41 (80.12) | 8241.83 (86.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugrin, N.; Šantić, M.; Trumbić, Ž.; Krstulović Šifner, S. Diet Composition and Feeding Intensity of Four-Spotted Megrim, Lepidorhombus boscii (Risso, 1810), in the Eastern Adriatic Sea. Fishes 2025, 10, 211. https://doi.org/10.3390/fishes10050211
Ugrin N, Šantić M, Trumbić Ž, Krstulović Šifner S. Diet Composition and Feeding Intensity of Four-Spotted Megrim, Lepidorhombus boscii (Risso, 1810), in the Eastern Adriatic Sea. Fishes. 2025; 10(5):211. https://doi.org/10.3390/fishes10050211
Chicago/Turabian StyleUgrin, Nika, Mate Šantić, Željka Trumbić, and Svjetlana Krstulović Šifner. 2025. "Diet Composition and Feeding Intensity of Four-Spotted Megrim, Lepidorhombus boscii (Risso, 1810), in the Eastern Adriatic Sea" Fishes 10, no. 5: 211. https://doi.org/10.3390/fishes10050211
APA StyleUgrin, N., Šantić, M., Trumbić, Ž., & Krstulović Šifner, S. (2025). Diet Composition and Feeding Intensity of Four-Spotted Megrim, Lepidorhombus boscii (Risso, 1810), in the Eastern Adriatic Sea. Fishes, 10(5), 211. https://doi.org/10.3390/fishes10050211







