Bacterial Community Composition and Prevalence of Aeromonas dhakensis in Four Tilapia Freshwater Aquaculture Systems in Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Determination of Physicochemical Water Parameters
2.3. Bacterial Isolation
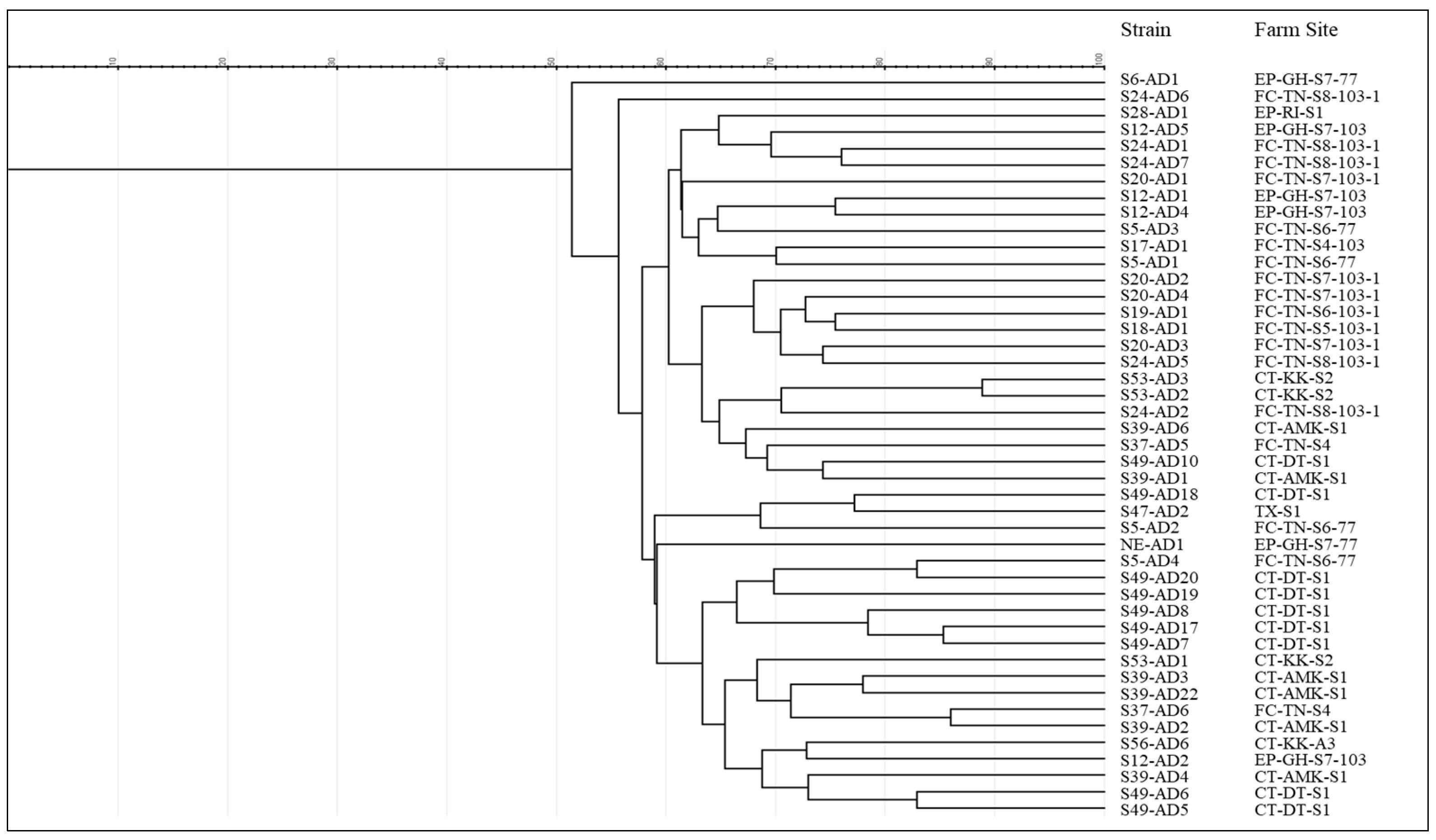
2.4. Identification (Biochemical and Molecular Tests) and Clonality Assessment
2.5. Determination of Microbial Community Composition
2.6. Statistical Analysis
3. Results
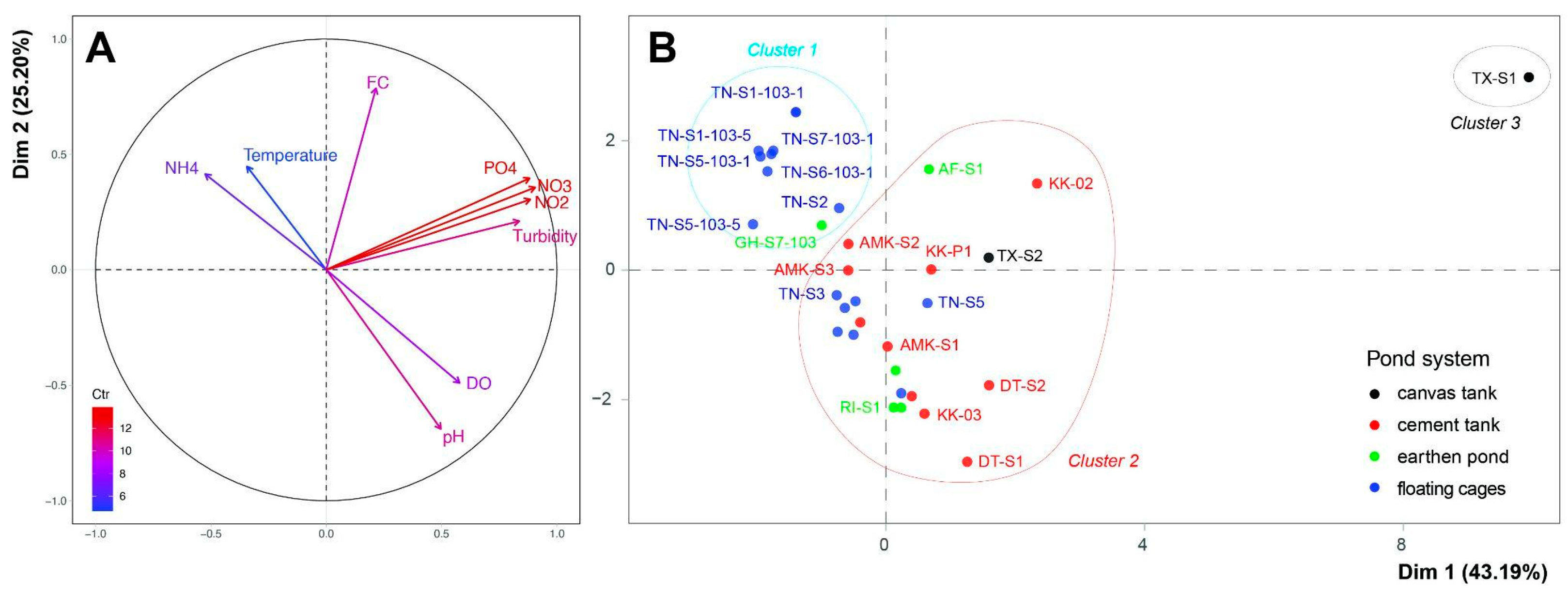
3.1. Physicochemical Variability in Various Tilapia Farming Systems
3.2. Isolation of Aeromonas dhakensis
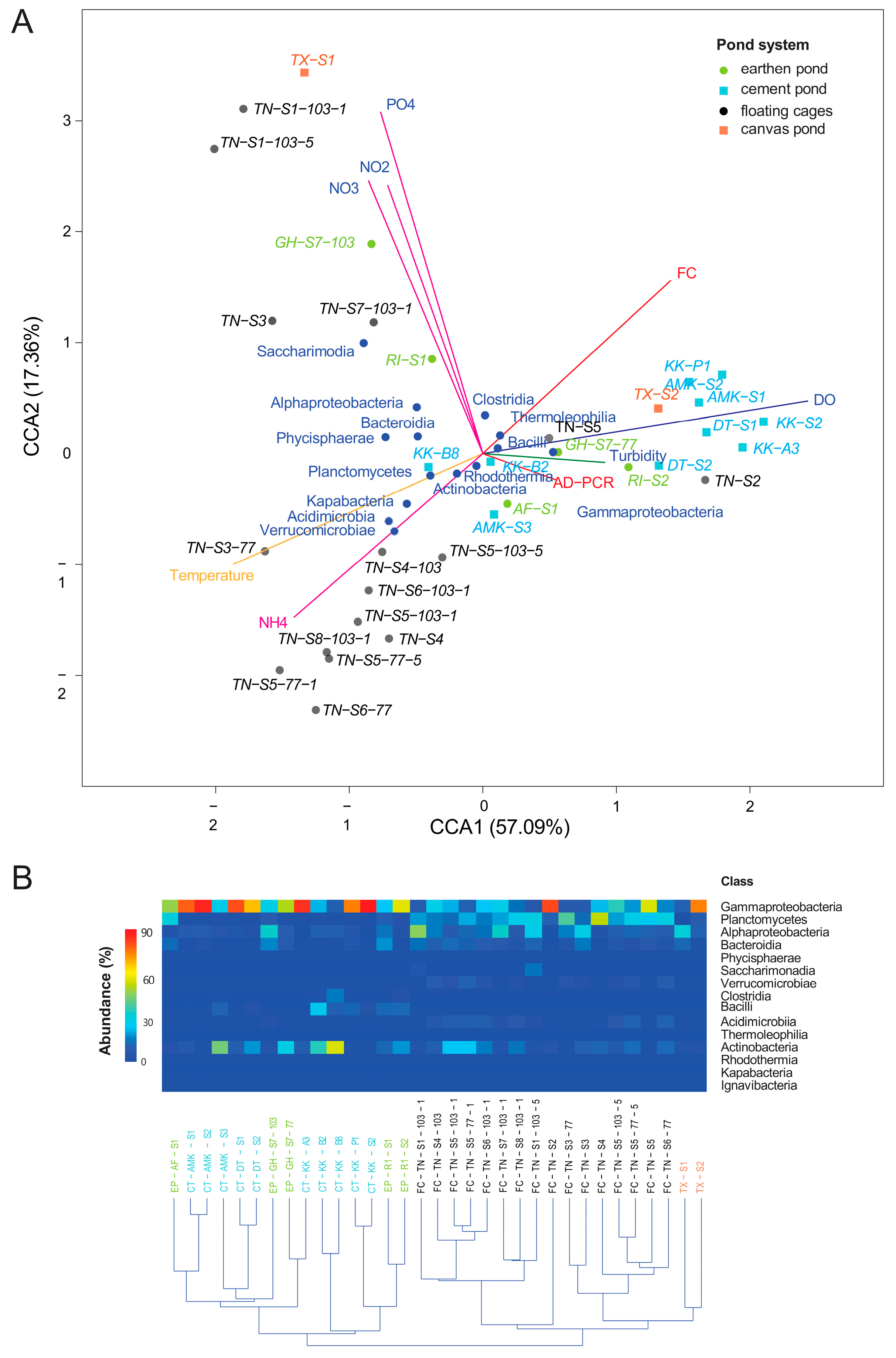
3.3. Microbial Diversity and Community Composition in Tilapia Farming Systems
3.4. Relationship Between Microbial Communities and Environmental Variables
4. Discussion
4.1. Characteristics of Microbial Communities and Water Quality in Different Pond Systems
4.2. Prevalence of Aeromonas dhakensis in Tilapia Pond Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2020. Available online: https://www.fao.org/interactive/state-of-fisheries-aquaculture/2020/en/#:~:text=Fish%20is%20crucial%20to%20a (accessed on 31 August 2024).
- Food and Agriculture Organization of the United Nations. Fisheries and Aquaculture Projections, 2022–2032. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/66538eba-9c85-4504-8438-c1cf0a0a3903/content/sofia/2024/fisheries-aquaculture-projections.html (accessed on 11 November 2024).
- Tacon, A.G.J. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 2019, 28, 43–56. [Google Scholar] [CrossRef]
- Naylor, R.L.; Kishore, A.; Sumaila, U.R.; Issifu, I.; Hunter, B.P.; Belton, B.; Bush, S.R.; Cao, L.; Gelcich, S.; Gephart, J.A.; et al. Blue food demand across geographic and temporal scales. Nat. Commun. 2021, 12, 5413. [Google Scholar] [CrossRef]
- Garlock, T.; Asche, F.; Anderson, J.; Bjørndal, T.; Kumar, G.; Lorenzen, K.; Ropicki, A.; Smith, M.D.; Tveterås, R. A global blue revolution: Aquaculture growth across regions, species, and countries. Rev. Fish. Sci. Aquac. 2019, 28, 107–116. [Google Scholar] [CrossRef]
- Department of Fisheries Malaysia. Annual Fisheries Statistics. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 31 August 2024).
- Mohamad, S.N.; Noordin, W.N.M.; Ismail, N.F.; Hamzah, A. Red hybrid tilapia (Oreochromis spp.) broodstock development programme in Malaysia: Status, challenges and prospects for future development. Asian Fish. Sci. 2021, 34, 73–81. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Ahmad, A.; Mohd Rahim, N.F.; Mohd Said, N.S.; Alnawajha, M.M.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Ismail, N.I.; Abu Hasan, H. Aquaculture in Malaysia: Water-related environmental challenges and opportunities for cleaner production. Environ. Technol. Innov. 2021, 24, 101913. [Google Scholar] [CrossRef]
- Kelly, A.M.; Renukdas, N.N. Disease management of aquatic animals. In Aquaculture Health Management; Academic Press: Cambridge, MA, USA, 2020; pp. 137–161. [Google Scholar] [CrossRef]
- Azzam-Sayuti, M.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Yusof, M.T.; Annas, S.; Najihah, M.Y.; Liles, M.R.; Monir, M.S.; Zaidi, Z.; Amal, M.N.A. The prevalence, putative virulence genes and antibiotic resistance profiles of Aeromonas spp. isolated from cultured freshwater fishes in peninsular Malaysia. Aquaculture 2021, 540, 736719. [Google Scholar] [CrossRef]
- Bartie, K.L.; Ngo, T.P.H.; Bekaert, M.; Hoàng Oanh, D.T.; Hoare, R.; Adams, A.; Desbois, A.P. Aeromonas hydrophila ST251 and Aeromonas dhakensis are major emerging pathogens of striped catfish in Vietnam. Front. Microbiol. 2023, 13, 1067235. [Google Scholar] [CrossRef]
- Basri, L.; Nor, R.M.; Salleh, A.; Md. Yasin, I.S.; Saad, M.Z.; Abd. Rahaman, N.Y.; Barkham, T.; Amal, M.N.A. Co-Infections of Tilapia Lake Virus, Aeromonas hydrophila and Streptococcus agalactiae in Farmed Red Hybrid Tilapia. Animals 2020, 10, 2141. [Google Scholar] [CrossRef]
- Erickson, V.I.; Khoi, L.M.; Hounmanou, Y.M.G.; Dung, T.T.; Phú, T.M.; Dalsgaard, A. Comparative genomic analysis of Aeromonas dhakensis and Aeromonas hydrophila from diseased striped catfish fingerlings cultured in Vietnam. Front. Microbiol. 2023, 14, 1254781. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Cabanillas-Ramos, J.; Alcaraz, U.; Gomez-Gil, B.; Romalde, J.L. Identification and virulence of Aeromonas dhakensis, Pseudomonas mosselii and Microbacterium paraoxydans isolated from Nile tilapia, Oreochromis niloticus, cultivated in Mexico. J. Appl. Microbiol. 2013, 115, 654–662. [Google Scholar] [CrossRef]
- Yi, S.W.; Chung, T.H.; Joh, S.J.; Park, C.; Park, B.Y.; Shin, G.W. High prevalence of BlaCTX-M group genes in Aeromonas dhakensis isolated from aquaculture fish species in South Korea. J. Vet. Med. Sci. 2014, 76, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Carriero, M.M.; Mendes Maia, A.A.; Moro Sousa, R.L.; Henrique-Silva, F. Characterization of a new strain of Aeromonas dhakensis isolated from diseased pacu fish (Piaractus mesopotamicus) in Brazil. J. Fish Dis. 2016, 39, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Preena, P.G.; Dharmaratnam, A.; Kumar, V.J.R.; Swaminathan, T.R. Plasmid-mediated antimicrobial resistance in motile aeromonads from diseased Nile tilapia (Oreochromis niloticus). Aquac. Res. 2020, 52, 237–248. [Google Scholar] [CrossRef]
- Azzam-Sayuti, M.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Annas, S.; Liles, M.R.; Xu, T.; Amal, M.N.A.; Yusof, M.T. Draft genome sequence of myo-inositol utilizing Aeromonas dhakensis 1P11S3 isolated from striped catfish (Pangasianodon hypopthalmus) in a local fish farm in Malaysia. Data Brief 2022, 41, 107974. [Google Scholar] [CrossRef]
- Hanson, L.A.; Hemstreet, W.G.; Hawke, J.P. Motile Aeromonas Septicemia (MAS) in Fish. Available online: https://srac.msstate.edu/pdfs/Fact%20Sheets/478%20Motile%20Aeromonas%20Septicemia%20(MAS)%20in%20Fish%202019.pdf (accessed on 31 August 2024).
- Azzam-Sayuti, M.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Annas, S.; Yusof, M.T.; Monir, M.S.; Mohamad, A.; Muhamad-Sofie, M.H.N.; Lee, J.Y.; Chin, Y.K.; et al. Comparative pathogenicity of Aeromonas spp. in cultured red hybrid tilapia (Oreochromis niloticus × O. mossambicus). Biology 2021, 10, 1192. [Google Scholar] [CrossRef]
- Chiquito-Contreras, R.G.; Hernandez-Adame, L.; Alvarado-Castillo, G.; Martínez-Hernández, M.d.J.; Sánchez-Viveros, G.; Chiquito-Contreras, C.J.; Hernandez-Montiel, L.G. Aquaculture—Production system and waste management for agriculture fertilization—A review. Sustainability 2022, 14, 7257. [Google Scholar] [CrossRef]
- Verdegem, M.; Buschmann, A.H.; Win Latt, U.; Dalsgaard, A.J.T.; Lovatelli, A. The contribution of aquaculture systems to global aquaculture production. J. World Aquac. Soc. 2023, 54, 206–250. [Google Scholar] [CrossRef]
- Masser, M.P. Cage culture in freshwater and protected marine areas. In Aquaculture Production Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 119–134. [Google Scholar] [CrossRef]
- David, G.S.; Carvalho, E.D.; Lemos, D.; Silveira, A.N.; Dall’Aglio-Sobrinho, M. Ecological carrying capacity for intensive tilapia (Oreochromis niloticus) cage aquaculture in a large hydroelectrical reservoir in Southeastern Brazil. Aquacult. Eng. 2015, 66, 30–40. [Google Scholar] [CrossRef]
- Menon, S.; Kumar, A.; Middha, S.K.; Paital, B.; Mathur, S.; Johnson, R.; Kademan, A.; Usha, T.; Hemavathi, K.N.; Dayal, S.; et al. Water physicochemical factors and oxidative stress physiology in fish, a review. Front. Environ. Sci. 2023, 11, 1240813. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Effects of dissolved oxygen and fish size on Nile tilapia, Oreochromis niloticus (L.): Growth performance, whole-body composition, and innate immunity. Aquac. Int. 2015, 23, 1261–1274. [Google Scholar] [CrossRef]
- Sundh, H.; Finne-Fridell, F.; Ellis, T.; Taranger, G.L.; Niklasson, L.; Pettersen, E.F.; Wergeland, H.I.; Sundell, K. Reduced water quality associated with higher stocking density disturbs the intestinal barrier functions of Atlantic salmon (Salmo salar L.). Aquaculture 2019, 512, 734356. [Google Scholar] [CrossRef]
- McMurtrie, J.; Alathari, S.; Chaput, D.L.; Bass, D.; Ghambi, C.; Nagoli, J.; Delamare-Deboutteville, J.; Mohan, C.V.; Cable, J.; Temperton, B.; et al. Relationships between pond water and tilapia skin microbiomes in aquaculture ponds in Malawi. Aquaculture 2022, 558, 738367. [Google Scholar] [CrossRef]
- Sun, F.; Wang, C.; Yang, H. Physicochemical factors drive bacterial communities in an aquaculture environment. Front. Environ. Sci. 2021, 9, 709541. [Google Scholar] [CrossRef]
- Liu, Z.; Iqbal, M.; Zeng, Z.; Lian, Y.; Zheng, A.; Zhao, M.; Li, Z.; Wang, G.; Li, Z.; Xie, J. Comparative analysis of microbial community structure in the ponds with different aquaculture model and fish by high-throughput sequencing. Microb. Pathog. 2020, 142, 104101. [Google Scholar] [CrossRef] [PubMed]
- Rieder, J.; Kapopoulou, A.; Bank, C.; Adrian-Kalchhauser, I. Metagenomics and metabarcoding experimental choices and their impact on microbial community characterization in freshwater recirculating aquaculture systems. Environ. Microbiome 2023, 18, 8. [Google Scholar] [CrossRef]
- Soh, M.; Er, S.; Low, A.; Jaafar, Z.; de Boucher, R.; Seedorf, H. Spatial and temporal changes in gut microbiota composition of farmed Asian seabass (Lates calcarifer) in different aquaculture settings. Microbiol. Spectr. 2025, e01989-24. [Google Scholar] [CrossRef]
- Ismail, N.I.A.; Amal, M.N.A.; Shohaimi, S.; Saad, M.Z.; Abdullah, S.Z. Associations of water quality and bacteria presence in cage cultured red hybrid tilapia, Oreochromis niloticus × O. mossambicus. Aquac. Rep. 2016, 4, 57–65. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, K.; Li, Z.; Xie, J.; Wang, G.; Li, H.; Yu, E.; Xia, Y.; Tian, J.; Gong, W. Analysis of the structure and function of microbial community in late-stage of grass carp (Ctenopharyngodon idella) farming ponds. Aquac. Rep. 2023, 30, 101556. [Google Scholar] [CrossRef]
- Khor, W.C.; Puah, S.M.; Tan, J.; Puthucheary, S.D.; Chua, K.H. Phenotypic and genetic diversity of Aeromonas species isolated from freshwater lakes in Malaysia. PLoS ONE 2015, 10, e0145933. [Google Scholar] [CrossRef]
- Puah, S.M.; Khor, W.C.; Kee, B.P.; Tan, J.A.M.A.; Puthucheary, S.D.; Chua, K.H. Development of a species-specific PCR-RFLP targeting rpoD gene fragment for discrimination of Aeromonas species. J. Med. Microbiol. 2018, 67, 1271–1278. [Google Scholar] [CrossRef]
- Szczuka, E.; Kaznowski, A. Typing of clinical and environmental Aeromonas sp. strains by random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus sequence PCR. J. Clin. Microbiol. 2004, 42, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Heras, J.; Domínguez, C.; Mata, E.; Larrea, C.; Pascual, V.; Lozano, C.; Torres, C.; Zarazaga, M. GelJ—A tool for analyzing DNA fingerprint gel images. BMC Bioinform. 2015, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Komiya, S.; Yasumizu, Y.; Yasuoka, Y.; Mizushima, K.; Takagi, T.; Kryukov, K.; Fukuda, A.; Morimoto, Y.; Naito, Y.; et al. Full-length 16S rRNA gene amplicon analysis of human gut microbiota using MinIONTM nanopore sequencing confers species-level resolution. BMC Microbiol. 2021, 21, 35. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 14 October 2024).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- R Core Development Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 14 October 2024).
- Wickham, H. Programming with ggplot2. In ggplot2; Springer: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Huang, Z.; Wang, H. Tree Visualization by one table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. Spec. Publ. 2023, 51, W587–W592. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Available online: https://github.com/vegandevs/vegan (accessed on 14 October 2024).
- Blancheton, J.P.; Attramadal, K.J.K.; Michaud, L.; d’Orbcastel, E.R.; Vadstein, O. Insight into bacterial population in aquaculture systems and its implication. Aquacult. Eng. 2013, 53, 30–39. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Vargas-Albores, F. Microbial metagenomics in aquaculture: A potential tool for a deeper insight into the activity. Rev. Aquac. 2017, 9, 42–56. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; El-Ghobashy, H.; Shaban, Y.; Elsheikh, F.; El-Reheem, S.A.; Aboegela, M. Mathematical model for predicting oxygen concentration in tilapia fish farms. Sci. Rep. 2021, 11, 24130. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Monier, M.N.; Hoseinifar, S.H.; Faggio, C. Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019, 45, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Schafer, N.; Matousek, J.; Rebl, A.; Stejskal, V.; Brunner, R.M.; Goldammer, T.; Verleih, M.; Korytar, T. Effects of chronic hypoxia on the immune status of pikeperch (Sander lucioperca Linnaeus, 1758). Biology 2021, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cao, J.; Qin, X.; Qiu, W.; Mei, J.; Xie, J. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and tissue structure in fish exposed to ammonium nitrogen: A review. Animals 2021, 11, 3304. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, S.; Edziyie, R.E.; Obirikorang, K.A.; Adjei-Boateng, D.; Skov, P.V. Nile tilapia (Oreochromis niloticus) show high tolerance to acute ammonium exposure but lose metabolic scope during prolonged exposure at low concentration. Aquat. Toxicol. 2024, 271, 106932. [Google Scholar] [CrossRef]
- Gogoi, M.; Bhattacharya, P.; Kumar Sen, S.; Mukherjee, I.; Bhushan, S.; Chaudhuri, S.R. Aquaculture effluent treatment with ammonium remover Bacillus albus (ASSF01). J. Environ. Chem. Eng. 2021, 9, 105697. [Google Scholar] [CrossRef]
- Wiegand, S.; Jogler, M.; Jogler, C. On the maverick Planctomycetes. FEMS Microbiol. Rev. 2018, 42, 739–760. [Google Scholar] [CrossRef]
- Ruiz, P.; Vidal, J.M.; Sepúlveda, D.; Torres, C.; Villouta, G.; Carrasco, C.; Aguilera, F.; Ruiz-Tagle, N.; Urrutia, H. Overview and future perspectives of nitrifying bacteria on biofilters for recirculating aquaculture systems. Rev. Aquac. 2019, 12, 1478–1494. [Google Scholar] [CrossRef]
- Li, J.; Jin, Q.; Liang, Y.; Geng, J.; Xia, J.; Chen, H.; Yun, M. Highly efficient removal of nitrate and phosphate to control eutrophication by the dielectrophoresis-assisted adsorption method. Int. J. Environ. Res. Public Health 2022, 19, 1890. [Google Scholar] [CrossRef]
- Olmos, J.; Acosta, M.; Mendoza, G.; Pitones, V. Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch. Microbiol. 2019, 202, 427–435. [Google Scholar] [CrossRef]
- Duan, Y.; Xiong, D.; Li, Y.; Ding, X.; Dong, H.; Wang, W.; Zhang, J. Changes in the microbial communities of the rearing water, sediment and gastrointestinal tract of Lateolabrax maculatus at two growth stages. Aquac. Rep. 2021, 20, 100742. [Google Scholar] [CrossRef]
- Bartie, K.L.; Desbois, A.P. Aeromonas dhakensis: A zoonotic bacterium of increasing importance in aquaculture. Pathogens 2024, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, C.; Peng, L.; Song, C.; Li, X.; Tao, L.; Li, G. Different distribution patterns of microorganisms between aquaculture pond sediment and water. J. Microbiol. 2021, 59, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Zhu, Y.; Zhu, T.; Wu, X.; Yang, D. Microbial community structure and its driving environmental factors in black carp (Mylopharyngodon piceus) aquaculture pond. Water 2021, 13, 3089. [Google Scholar] [CrossRef]
- Cheok, Y.Y.; Puah, S.M.; Chua, K.H.; Tan, J.A.M.A. Isolation and molecular identification of Aeromonas species from the tank water of ornamental fishes. Acta Vet. Hung. 2020, 68, 130–139. [Google Scholar] [CrossRef]
- Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Monitoring and managing microbes in aquaculture—Towards a sustainable industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef]
- Wang, C.; Chuprom, J.; Wang, Y.; Fu, L. Beneficial bacteria for aquaculture: Nutrition, bacteriostasis and immunoregulation. J. Appl. Microbiol. 2019, 128, 28–40. [Google Scholar] [CrossRef]



| Physiochemical Parameters | Pond System | |||||||
|---|---|---|---|---|---|---|---|---|
| Earthen Pond | Floating Cage | Canvas Tank | Cement Tank | |||||
| Range | Mean ± Standard Deviation | Range | Mean ± Standard Deviation | Range | Mean ± Standard Deviation | Range | Mean ± Standard Deviation | |
| Temperature (°C) | 28.70–38.00 | 31.68 ± 3.68 | 31.20–34.00 | 32.17 ± 0.63 | 29.20–30.00 | 29.60 ± 0.57 | 25.50–32.10 | 29.01 ± 2.46 |
| pH | 6.50–7.30 | 7.00 ± 0.44 | 6.10–6.70 | 6.45 ± 0.25 | 6.70–6.90 | 6.80 ± 0.14 | 6.00–7.10 | 6.68 ± 0.31 |
| DO (mg L−1) | 0.21–6.22 | 3.10 ± 2.37 | 0.17–11.20 | 3.22 ± 3.62 | 5.32–9.34 | 7.33 ± 2.84 | 2.20–8.59 | 5.53 ± 2.05 |
| Turbidity (mg L−1) | 65.67–1161.66 | 328.00 ± 467.35 | 22.00–897.67 | 194.86 ± 228.93 | na | na | 81.00–172.66 | 126.83 ± 64.81 |
| NO3 (mg L−1) | 0.60–1.00 | 0.76 ± 0.18 | 0.50–1.15 | 0.74 ± 0.17 | 48.20 | - | 0.30–1.50 | 0.79 ± 0.36 |
| NO2 (mg L−1) | 0.002–0.010 | 0.005 ± 0.003 | 0.002–0.015 | 0.008 ± 0.004 | 0.591 | - | 0.005–0.102 | 0.017 ± 0.032 |
| NH4 (mg L−1) | 0.00–0.44 | 0.26 ± 0.19 | 0.10–1.27 | 0.87 ± 0.32 | 0.13 | - | 0.02–1.18 | 0.33 ± 0.35 |
| PO4 (mg L−1) | 0.07–0.34 | 0.19 ± 0.11 | 0.01–0.80 | 0.17 ± 0.21 | 5.46 | - | 0.10–0.96 | 0.36 ± 0.33 |
| Pond Systems | Sample ID | Presumptive Colonies | Number of Oxidase- Positive Colonies | Number of Representative ERIC-PCR-Positive Isolates | Strain |
|---|---|---|---|---|---|
| Earthen pond | EP-RI-S1 | 15 | 14 | 1 | S28-AD1 |
| EP-RI-S2 | 25 | 25 | 0 | - | |
| EP-AF-S1 | 25 | 25 | 0 | - | |
| EP-GH-S7-103 | 127 | 117 | 4 | S12-AD1, S12-AD2, S12-AD4, S12-AD5 | |
| EP-GH-S7-77 | 50 | 19 | 2 | S6-AD1, NE-AD1 | |
| Floating cages | FC-TN-S1-103-1 | 29 | 20 | 0 | - |
| FC-TN-S2 | 25 | 25 | 0 | - | |
| FC-TN-S3 | 25 | 23 | 0 | - | |
| FC-TN-S3-77 | 14 | 14 | 0 | - | |
| FC-TN-S4 | 25 | 23 | 2 | S37-AD5, S37-AD6 | |
| FC-TN-S4-103 | 68 | 63 | 1 | S17-AD1 | |
| FC-TN-S5 | 25 | 25 | 0 | - | |
| FC-TN-S5-103-1 | 60 | 30 | 1 | S18-AD1 | |
| FC-TN-S5-77-1 | 25 | 25 | 0 | - | |
| FC-TN-S6-77 | 35 | 28 | 4 | S5-AD1, S5-AD2, S5-AD3, S5-AD4 | |
| FC-TN-S6-103-1 | 80 | 77 | 1 | S19-AD1 | |
| FC-TN-S7-103-1 | 61 | 54 | 4 | S20-AD1, S20-AD2, S20-AD3, S20-AD4 | |
| FC-TN-S8-103-1 | 77 | 61 | 5 | S24-AD1, S24-AD2, S24-AD5, S24-AD6, S24-AD7 | |
| Canvas tank | TX-S1 | 25 | 25 | 1 | S47-AD2 |
| TX-S2 | 14 | 14 | 0 | - | |
| Cement tank | CT-AMK-S1 | 25 | 25 | 6 | S39-AD1, S39-AD2, S39-AD3, S39-AD4, S39-AD6, S39-AD22 |
| CT-AMK-S2 | 25 | 23 | 0 | - | |
| CT-AMK-S3 | 25 | 19 | 0 | - | |
| CT-DT-S1 | 25 | 25 | 9 | S49-AD5, S49-AD6, S49-AD7, S49-AD8, S49-AD10, S49-AD17, S49-AD18, S49-AD19, S49-AD20 | |
| CT-DT-S2 | 25 | 25 | 0 | - | |
| CT-KK-S2 | 25 | 19 | 3 | S53-AD1, S53-AD2, S53-AD3 | |
| CT-KK-P1 | 25 | 21 | 0 | - | |
| CT-KK-B2 | 25 | 25 | 0 | - | |
| CT-KK-A3 | 25 | 25 | 1 | S56-AD6 | |
| CT-KK-B8 | 21 | 21 | 0 | - | |
| Total | 1076 | 935 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.L.; Puah, S.M.; Baharudin, S.N.; Mohd Razalan, N.I.; Hii, K.S.; Khor, W.C.; Lim, Y.C.; Aung, K.T.; Chua, K.H.; Lim, P.T.; et al. Bacterial Community Composition and Prevalence of Aeromonas dhakensis in Four Tilapia Freshwater Aquaculture Systems in Malaysia. Fishes 2025, 10, 204. https://doi.org/10.3390/fishes10050204
Lim SL, Puah SM, Baharudin SN, Mohd Razalan NI, Hii KS, Khor WC, Lim YC, Aung KT, Chua KH, Lim PT, et al. Bacterial Community Composition and Prevalence of Aeromonas dhakensis in Four Tilapia Freshwater Aquaculture Systems in Malaysia. Fishes. 2025; 10(5):204. https://doi.org/10.3390/fishes10050204
Chicago/Turabian StyleLim, Sook Ling, Suat Moi Puah, Siti Nursyuhada Baharudin, Nur Insyirah Mohd Razalan, Kieng Soon Hii, Wei Ching Khor, Yen Ching Lim, Kyaw Thu Aung, Kek Heng Chua, Po Teen Lim, and et al. 2025. "Bacterial Community Composition and Prevalence of Aeromonas dhakensis in Four Tilapia Freshwater Aquaculture Systems in Malaysia" Fishes 10, no. 5: 204. https://doi.org/10.3390/fishes10050204
APA StyleLim, S. L., Puah, S. M., Baharudin, S. N., Mohd Razalan, N. I., Hii, K. S., Khor, W. C., Lim, Y. C., Aung, K. T., Chua, K. H., Lim, P. T., & Leaw, C. P. (2025). Bacterial Community Composition and Prevalence of Aeromonas dhakensis in Four Tilapia Freshwater Aquaculture Systems in Malaysia. Fishes, 10(5), 204. https://doi.org/10.3390/fishes10050204







