Abstract
Understanding the ecology of large pelagic fishes is important for species conservation and maintaining ecosystem dynamics. The Atlantic blue marlin (Makaira nigricans) is usually targeted in recreational fisheries and occasionally captured as bycatch in pelagic longline fisheries, yet it is considered an overexploited stock in the Atlantic. In this study, pop-up archival satellite tags were deployed on twenty-one blue marlins in the Southwest Atlantic Ocean between February 2006 and August 2021. Results show that blue marlin spent an average of 57% of their time in shallow waters (<10 m) and mostly at temperatures between 26 °C and 28 °C during both day and night. Minimum linear distances observed ranged from 124 to 1995 km, with tracking periods lasting 5 to 215 days. Overall, tagged blue marlins remained around the tagging region during the tracking period, except for six individuals that exhibited more directional movements offshore. The results provided in this study are relevant for better understanding the habitat use of blue marlins in the Southwest Atlantic Ocean and to support analysis for stock assessment purposes, which are often affected by uncertainties and large misreporting rates in the region.
Key Contribution:
This study presents the first satellite tagging study on blue marlins in the Southwestern Atlantic, revealing intermediate residency levels within core areas and a mix of localized and long-distance movements. The findings suggest partial migration patterns, where some individuals remain near tagging sites while others undertake extensive displacements, likely influenced by ocean productivity and seasonal temperature shifts.
1. Introduction
Billfishes are large pelagic apex predators that play an ecologically significant role in the marine ecosystem. Therefore, the depletion of their populations might lead to negative cascading effects on the food web structures [1,2,3]. The Atlantic blue marlin (Makaira nigricans Lacepède, 1802) is targeted by recreational fishing in the Atlantic Ocean, and it is one of the several species caught as bycatch by pelagic longline fishery targeting tunas and swordfish (Xiphias gladius Linnaeus, 1758) [4,5]. While this species has low commercial value for the fishing industry, it holds much higher direct and indirect value through sport fishing-related activities around the globe [6,7]. Considered as a single Atlantic stock, blue marlins have been considered overexploited since 2003 [5,8] and more recent population assessments indicated that the stock remains overfished and experiencing overfishing [9]. Other research on tuna and billfish stock status reveals that Atlantic blue marlin stock is heavily depleted and has one of the highest fishing mortality rates, together with the Pacific bluefin tuna and the Atlantic sailfish stocks [10]. Regionally, the Brazilian government has issued a statement prohibiting the catch and sale of blue marlins by the national fleet [11]. Blue marlins are targeted only by recreational fisheries, although they are bycaught by artisanal and commercial fishing [12]. Nevertheless, information on the interactions among these fisheries and the ecology of the species in the South Atlantic are not well understood.
As one of the largest billfish species, blue marlin is a circumtropical species widely distributed throughout the tropical and temperate waters of the Atlantic Ocean, ranging from 45° N to 45° S, usually occupying higher latitudes in the warmer months of the year [13]. It is considered a highly migratory species, performing vast movements across the oceans and occasionally inter-oceanic migrations, being one of the fishes with the greatest recorded distances in tag-recapture studies [14,15]. Since the early 2000s, there has been growing international concern regarding Atlantic blue marlin overfishing, leading to a recommendation of a rebuilding plan for Atlantic stocks [16]. In Brazil, a national sales ban was implemented as a protective measure in 2005, which may have influenced the evident reducing trend in landings up to 2016 [17]. Additionally, there is increasing concern about the uncertainties regarding blue marlin fishing mortality levels in the Atlantic Ocean [18].
Several studies have been conducted in the North Atlantic to investigate the blue marlin’s diving behavior and the effect of oceanographic conditions on their horizontal movements [19,20,21,22,23]. However, despite the recent increase in the use of satellite telemetry for highly migratory species, few studies have been conducted in the South Atlantic [24,25,26,27,28], and even fewer focused specifically on habitat utilization and movements of blue marlins [29,30]. Understanding the habitat use and identifying the essential fish habitats of large pelagic marine species is important to know the processes driving their movements as well as the factors that affect their biology at diverse scales. This knowledge is critical for comprehending the effects of a changing ocean scenario on this species’ distribution.
Broad migratory movements of the billfishes expose them to a wide variation of physicochemical and biological oceanographic features that affect their vertical habitat preferences [31,32]. Light levels, for example, are essential for visual predators such as marlins to forage efficiently and may thus limit their swimming depths. Studies on blue marlin diet indicate a preference for epipelagic surface fishes and squids, despite their ability to consume a wide variety of organisms [33,34,35], while reports of deep-water prey remain rare [13,36]. The vertical distribution of marlins is closely related to temperature-at-depth; temperature can restrict their depth preferences and even influence their cardiac function, resulting in a reduced heart rate in lower water temperatures [37,38]. Similarly, waters with low dissolved oxygen concentrations can also restrict billfish vertical movements [39,40], which might still be influenced by prey abundance.
Blue marlin spawning occurs during the austral summer months (December to April), particularly off the coasts of Bahia and Rio de Janeiro [41,42]. Additionally, studies have shown spatial and seasonal variability in the size of blue marlins in the South Atlantic, suggesting that a cyclic pattern of reproductive migration may occur in this region among different size classes [43,44]. Fishery-dependent data indicate a seasonal, clockwise migratory pattern of large blue marlin in the southwestern Atlantic [43,44,45]. During the first quarter of the year (i.e., austral summertime), individuals are predominantly recorded along the southeastern coast of Brazil, gradually shifting northwards and eastwards throughout the subsequent quarters, thereby establishing a potential migratory loop in the south-central Atlantic. The largest specimens are typically observed in southern regions during the austral summer, followed by a northward movement towards warmer waters during winter. This pattern is likely to be associated with the seasonal displacement of the 25 °C isotherm and supports the hypothesis that spawning occurs off southern Brazil in the first quarter, primarily involving larger individuals [44].
In light of the aforementioned displacement hypothesis, this study aims to investigate the movement patterns and habitat utilization of blue marlins in the southwestern Atlantic Ocean through satellite telemetry and evaluate their residency in the tagging areas.
2. Materials and Methods
This research is based on a compilation of data collected from multiple blue marlin tagging projects conducted in Brazil over a decade. These projects covered two regions along the Brazilian coast using different types of satellite tags. As a result, the data are stratified both spatially and temporally, and certain transmitter features vary depending on the source projects.
2.1. Study Area
The Brazilian coast covers a large portion of the Southwest Atlantic continental shelf. For this study, two primary regions were considered, delimited by the Tropic of Capricorn (~23° S), which represent the limits of the Northeast and Southeast regions of the Brazilian coast (Area I; Area II, respectively). These areas are recognized both for their relevance as sport fishing grounds and proximity to commercial longline fishing hotspots [46,47]. The Royal Charlotte Bank seamount, off Canavieiras in Bahia state coast, is the primary tagging site in Area I and a well-known sport fishing location. In Area II, the upwelling system off the coast of Cabo Frio, in Rio de Janeiro state, is also an important sport fishing ground.
2.2. Data Collection—Tagging
The tags were deployed off the Northeast (Area I; n = 10) and on the Southeast (Area II, n = 11) coasts of Brazil (Figure 1). A total of 21 blue marlins were captured in collaboration with recreational fishermen and tagged between October and March (austral spring and summer) from 2006 to 2021 (Table 1) using three types of Pop-up Satellite Archival Transmitting tags (PSAT; Wildlife Computers, Redmond, WA, USA). The specimens caught before 2016 were tagged with MK-10 (n = 14), while the deployments after 2017 were with miniPAT (n = 6) and one Mark-Report tag (mrPAT). PSATs were programmed to archive light intensity, pressure (with 0.5 m of resolution), and temperature (0.05 °C) for a period ranging from 75 to 215 days (Table 1). The tags were programmed to summarize archived data into 3 to 24 h intervals for satellite data transmission. Datasets were trimmed to reflect the period when a marlin was certainly carrying the tag. Of the 21 tagged animals, 18 (85.7%) tags successfully transmitted some data, while the remaining three failed to report any signal, likely due to technical failure of the tags.
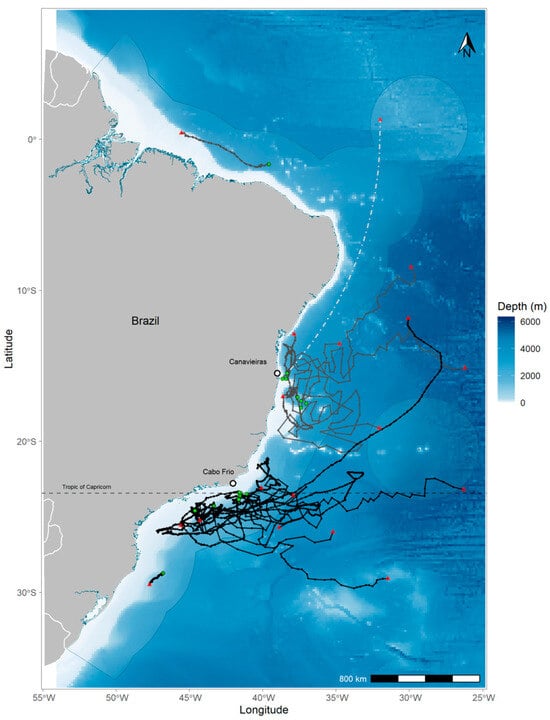
Figure 1.
Map of the study region with estimated tracks between tag (green circle) and pop-off (red triangle) locations from the tagged blue marlins across the Atlantic Ocean. Individuals tagged in Area I are represented with grey lines and in Area II with black lines. Individual BUM14 (white dashed line) was tagged with a mark report tag, showing only an indicative line between tagging and pop-off location. Light-blue shaded area represents the Brazilian Exclusive Economic Zone. The dashed line indicates the Tropic of Capricorn limit.

Table 1.
Summary data for blue marlins (Makaira nigricans) tagged with pop-up satellite tags in the Southwest Atlantic Ocean, off the Brazilian coast. Size is a measure of lower jaw fork length (LJFL cm). MLD refers to the minimum linear distance, calculated as the straight-line between the geographic coordinates of the tagging location and the pop-up location in km. * Denotes the tracks processed with the TrackIt state space model.
Hooked blue marlins were brought to the side of the boat, the size was visually estimated by the experienced fishermen by comparing the fish with the boat (lower jaw fork length, LJFL, in centimeters) and the fish were tagged with PSATs by inserting a nylon anchor into the dorsal musculature of the fish in order to lock it with the pterygiophore on the dorsal fin [48,49,50]. Once tagged, the fishing line was cut off as close as possible to the fish mouth, allowing it to swim away from the vessel. Only individuals considered to be in good conditions to survive were released after tagging. Criteria included the absence of major bleeding, active and coordinated swimming behavior, and good tail propulsion. After capture and tagging, fish were gently towed behind the boat at a slow speed to promote gill ventilation and facilitate recovery from handling stress before release [51]. Deployment locations were recorded using the vessels’ onboard GPS equipment, and the first transmissions of good Argos quality (i.e., Location class 3 to 0) were used as pop-up locations.
2.3. Data Processing
The horizontal movements of tagged marlins were estimated by processing the data received from the Argos satellite system using the manufacturer’s light-based geolocation software (Global Position Estimator, versions: GPE2 and GPE3, available at: www.wildlifecomputers.com, accessed 25 April 2025). Light-level geolocation utilizes ambient light intensity to estimate sunrise and sunset times, which helps determine longitude by comparing local noon timing to UTC and latitude based on the length of the day [52].
Due to the partial loss of the original files (BUM03 to BUM10) required to fully decode the raw satellite telemetry data, two different geolocation models were employed. Nine tags were initially processed using the TrackIt model [53] (Table 1); however, the original files were later lost, which precluded reprocessing (i.e., decode) their transmitted data. For the geolocation data processed with GPE2, the Kalman filter space state model TrackIt [54] was used to improve and reconstruct the daily positions. The model integrates a correlated random walk movement process with observation error structures and incorporates sea surface temperature (SST) matching to refine latitude estimates and reduce positional uncertainty. Since convergence was rarely achieved with the SST inclusion [53], the model was applied without incorporation of the sea surface temperature (SST) due to the temperature homogeneity of the study area [54].
For the remaining tags (n = 12), the light geolocations were processed using the GPE3, a discretized hidden Markov model (HMM), which combines observations of light level, sea surface temperature, maximum depths, and any known locations from different sources, and incorporates a movement model based on a speed parameter chosen by the user [55]. The daily positions were averaged by calculating the centroid of the two 12 h light locations and then filtered by removing interpolated locations within gaps larger than three days. Although different models were employed, the resultant datasets were considered comparable due to the consistent spatial patterns observed in visual track inspection and kernel density estimates (see below). The data eligible for GPE3 were not reprocessed utilizing TrackIt, as the latter does not facilitate the integration of sea surface temperature, depth, and speed (movement model). Conversely, GPE3 employs an HMM that incorporates these variables to enhance geolocation accuracy [55]. The utilization of TrackIt instead of GPE3 would have diminished the precision of position estimates and compromised the integrity of the higher-resolution GPE3 tracks.
2.4. Data Analysis
Minimum linear distance (MLD) over the entire tracking period was calculated as the straight-line Euclidean distance between the geographic coordinates of the tagging location and the pop-up location at the first good-quality ARGOS transmission. Displacement rates were also estimated using the MLD divided by the deployment duration of each tag.
The fixed Kernel home range analysis estimated the marlins’ utilization distributions across the Southwestern Atlantic Ocean. Kernel density estimates animal distributions by modeling movements among locations (i.e., tracking estimated locations), transforming points distribution into density estimates [56,57]. The Kernel utilization distributions (KUDs) were estimated using all marlin available tracks (i.e., TrackIt and filtered GPE3) from individuals with more than five relocations from the two tagging periods with the R package adehabitatHR [58]. The “href” ad hoc approach was used to estimate the smoothing parameter (h). This method extrapolates the animals’ time spent at each location point to a broader distribution area [22,59]. The KUDs were computed from daily positional data, with the 50% and 95% isopleths denoting the core utilization area and the surrounding home range area, respectively. These percentiles correspond to spatial domains where the animals are estimated to spend 50% and 95% of their time based on the probability density of their movements. The coordinates were projected using the EPSG:5641 (SIRGAS 2000/Brazil Polyconic) reference system to ensure distance measurements were performed in meters. The spatial grid used for the kernel estimation was the default value (grid = 100) of the kernelUD function across the spatial range of the data, resulting in an approximate cell size of 50 km. This resolution is consistent with the spatial uncertainty expected for filtered light-level geolocation data (e.g., GPE3 and TrackIt) and provides an appropriate balance between smoothing animal movement uncertainty and retaining ecological relevance at regional scales. Despite the generation of geolocation data via two distinct models (TrackIt and GPE3), the resultant tracks demonstrated broadly comparable spatial characteristics with respect to movement direction, extent, and core utilization areas. Although formal statistical validation was not conducted, a visual inspection of the tracks, in conjunction with separate kernel density estimations, indicated consistent spatial patterns between the two datasets. This permitted the cautious integration of both outputs into a comprehensive kernel density analysis designed to encapsulate a more complete understanding of habitat utilization. This methodology maximized the utility of the data while ensuring transparency regarding the potential implications of geolocation methodology on spatial interpretation. However, due to inherent differences in model assumptions and the quality of input data, a certain degree of spatial uncertainty must be acknowledged and considered when interpreting the combined results.
In order to examine spatial and temporal patterns in diving behavior and thermal habitats, the proportion of time-at-depth (TAD) and temperature (TAT) and the profiles of depth and temperature (PDT) data were analyzed for each marlin tagged. A summary of the luminosity data identifying periods of dawn and dusk was used to separate TAD and TAT data into day, night, and crepuscular periods and diel differences in time spent were examined. The Kruskal–Wallis rank sum test was used to assess whether median diel depth (MDD) and median diel temperature (MDT) differed significantly between day, night, and crepuscular periods. When significant differences were detected, post hoc pairwise comparisons were performed using Dunn’s test with Bonferroni correction to control for multiple comparisons. Due to data loss or poor quality (i.e., amount of successfully transmitted data), depth and temperature histogram data were processed from eight of the tagged fish. All calculations were performed using R language for statistical computing version 4.1.2 [60].
3. Results
From the 21 tags deployed, 18 (85.7% of the sample) successfully reported, from which a part of data was impacted by tag premature releases (n = 15; 70%) due to unknown reasons. However, PSAT tags had a mean deployment time of 93.8 ± 59 days, with a range of time at liberty from 5 to 215 days (Table 1). Tagged blue marlins ranged in size from 175 to 390 cm in lower jaw fork length (LJFL), with a mean size of 250 ± 51.2 cm.
3.1. Horizontal Movement
Tracking results revealed a wide range movement patterns of blue marlins in the Southwest Atlantic Ocean, with six individuals undertaking long-distance displacements of over 1000 km. However, most of the individuals (n = 12) remained near the tagging location (Figure 1). Latitudinal and longitudinal movements were limited, with the majority of individuals (n = 14) estimated to move within a 10-degree range. However, four fish (BUM03, BUM09, BUM10 and BUM17) performed larger longitudinal movements beyond 10°. The MLDs were variable among individuals within and between tagging areas, with no clear patterns of residency to the tagging region. Minimum linear distances observed between release and pop-off locations ranged from 124 to 1995.7 km, with an average for all blue marlins of 754 ± 603.4 km. The MLD was similar between areas, with an average of 846 and 682 km for Area I and Area II, respectively. Displacement rates varied from 0.8 to 41.4 km per day, with a mean of 12 ± 11.6 km per day.
In Area I, three blue marlins exhibited large displacement (>1000 km) movements toward north (BUM14), east (BUM03), and northeast (BUM06) during the fourth quarter of the year (austral spring/summer). The other six marlins tagged in this region showed relatively lower displacement during a similar recorded time at liberty. However, two marlins (BUM00 and BUM11) performed strongly directional movements considering the short monitoring time of 17 and 10 days, respectively. BUM00 performed a westward directional movement, and BUM11 went in the northward direction almost in a straight line, showing the highest displacement rates (41.4 and 34 km/day, respectively) of all tagged marlins in this study (Figure S1).
In Area II, BUM09 and BUM10 displayed a directed eastward movement away from the tagging site (>1000 km) during the first quarter of the year (Figure S1). Additionally, BUM17 underwent a large northward displacement until pop-off during the second quarter and was the only tagged animal to cross between regions (Area II to Area I) (Figure S1). All other blue marlins tagged in Area II exhibited spiral movements around a large portion related to the tagging region (Figure 1).
The kernel density analysis revealed two primary core areas used by blue marlins: one located near the seamount Royal Charlotte Bank (in Area I) and the other off the coast of Cabo Frio in Rio de Janeiro state (in Area II) (Figure 2 and Figure S2). Despite the overlapping home range utilization distributions of blue marlins tagged in both Area I and II, they exhibited distinct core locations centered around the deployment sites, predominantly situated on the offshore banks and continental slopes. In Area II, the core area was considerably larger and covered the platform, continental slope, and some offshore regions (Figure 2 and Figure S2). Movements in Area II appeared to be less directional and more constrained to the continental shelf and slope. Conversely, individuals tagged in Area I exhibited more linear and extensive offshore displacements, which indicates potential regional differences in spatial behavior and habitat utilization.
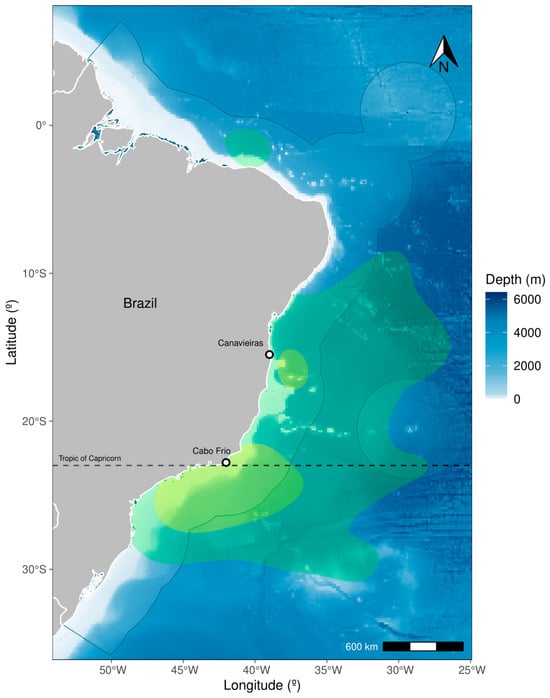
Figure 2.
Combined utilization distributions for blue marlins tagged with PSATs in the Southwest Atlantic Ocean. The green shades represent the home range (95% KUD), and yellow shades the core (50% KUD) areas. Light-blue shaded area represents the Brazilian Exclusive Economic Zone. The dashed line indicates the Tropic of Capricorn limit.
3.2. Vertical Habitat
Tagged individuals spent 46.6% of their time within the top 5 m of the water column, as indicated by the time-at-depth histograms of vertical habitat utilization (Figure 3). The diel pattern analysis showed that blue marlins spent a large portion of their tracked time in shallow waters (depth range 0–5 m) during both day (30.3%) and nighttime (63.7%) (Figure 3). Other peaks of time at depth were observed at 25–50 m (12.5%), 50–75 m (14.0%), and 100–150 m (15.8%) depths during the daytime. Occasionally, tagged blue marlins were recorded at depths greater than 200 m, with most deep descent occurring during the day. The deepest recorded dive for a blue marlin in this study was 374 m during the day by BUM15 tagged in Area II.
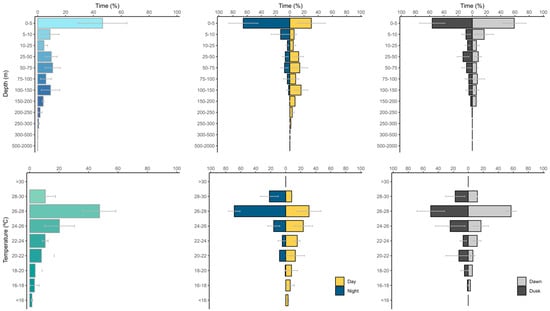
Figure 3.
Time-at-depth (upper panel) and time-at-temperature (lower panel) histograms for eight blue marlins tagged across the Southwest Atlantic Ocean. Middle panels show histograms divided by night and daytime, and right panels by dawn and dusk periods.
Throughout the daytime, blue marlins had a mean depth of 72.0 ± 68.7 m, while at nighttime the mean depth was 13.5 ± 24.9 m, and at crepuscular periods 26.4 ± 38.6. Depth profiles were identified as significantly different based on Kruskall–Wallis test results MDD (p < 0.001). Post hoc Dunn’s tests revealed significant differences between all diel periods, suggesting that blue marlins dive deeper during the day compared to both the crepuscular and night periods (adjusted p < 0.001). Depths during the crepuscular period were also significantly greater than those at night (adjusted p < 0.001). These results indicate a clear diel pattern in vertical distribution, with blue marlins primarily occupying shallow waters during the night and twilight periods while making deeper and more frequent dives during the daytime.
Temperature data showed that blue marlins spent 47.5% of their time within a relatively narrow temperature range of 26.0–28.0 °C. Their diel temperature patterns showed that they remained within this range for 31.0% of the day and 65.2% of the night (Figure 3). Significant differences were found between the mean temperatures experienced during the day (22.9 ± 3.4 °C), at crepuscular periods (25.0 ± 2.6 °C), and at night (26.2 ± 1.7 °C) (MDT, p < 0.001). Post hoc Dunn’s tests showed that blue marlin experienced significantly higher temperatures during the night compared to both the crepuscular and daytime periods (adjusted p < 0.001). Crepuscular temperatures were also significantly warmer than those observed during the day (adjusted p < 0.001). These results suggest a clear diel pattern in thermal habitat use, with individuals occupying cooler waters during the day and progressively warmer layers during twilight and night hours.
4. Discussion
While previous studies have been conducted in other ocean basins, this research represents the first satellite tagging study on blue marlins in the Southwestern Atlantic region. The findings of this study reveal that blue marlins exhibit intermediate levels of residency within core areas, with some individuals displaying a looping swimming pattern around the tagging site region. These results support a growing number of research indicating that pelagic species show site fidelity within core areas, although some individuals also undertake long-range movements [61,62,63]. Nevertheless, the observed highly directed, long-distance movements of certain individuals suggest that blue marlin may exhibit partial migration whereby only a subset of the population undertakes extensive displacements. This behavioural pattern is analogous to that observed in other species in which individuals undergo prolonged growth periods prior to reaching reproductive maturity [64,65] However, it is essential to recognize that premature tag detachment and a maximum monitoring time of 215 days may restrict our ability to capture the full extent of individual migratory trajectories. Consequently, some longer or more complex movements may remain undetected, and interpretations regarding the presence of partial migration should therefore be approached with caution.
Such long-distance movements, mainly representing movements between foraging and reproduction zones, have been documented in many marine animals, including sea turtles [66], teleosts [67], sharks [63], seabirds [68], and mammals [69]. The timing of these migrations is often associated with seasonal peaks in ocean productivity and seasonal temperature changes that are suitable for reproduction [67,68]. Long-distance movements are presumably a trade-off between the energy expended during migration, the energetic returns from foraging, and the benefits to reproductive success [70,71]. The decision to undertake long-distance migrations depends on many factors, and if regions close to suitable reproductive areas are productive and can sustain local populations, there may be less need for extensive movements away from those areas. Therefore, conducting a tagging study to unravel the contributing factors to animal movement requires careful selection of appropriate spatial and temporal scales for such analysis.
This study was conducted at a broad spatial scale, encompassing the Southwest Atlantic Ocean, and spanned a substantial temporal range, with data collected sparsely over nine years and individual tracking durations of up to 215 days. Our results recorded two types of movement behavior in the studied region. While some individuals exhibited unidirectional movements following tag deployment, others displayed residency behavior (e.g., BUM15 and BUM16), remaining in the same region throughout the entire tracking period (Figure S1). It is worth noting that several tracks overlapped temporally with the species’ presumed spawning period during the first quarter of the year, when blue marlins are thought to migrate southward along the Brazilian coast following the 25 °C isotherm. This temporal overlap suggests that at least some of the observed displacements may reflect reproductive-related movements. Nevertheless, we acknowledge that longer monitoring periods (e.g., one to two years) would be necessary to capture complete migratory cycles and better assess the drivers of movement patterns, particularly in relation to spawning dynamics. Furthermore, tagging was conducted over a decade-long timeframe, during which interannual environmental variability, including potential climate-driven changes, may have influenced horizontal movement behavior. Such variability can alter the distribution of thermal fronts, prey availability, and migratory cues, potentially affecting the spatial patterns observed in different years.
The results of spatial displacement showed that the mean distance (754 km) between tag and pop-off locations falls within the range reported in similar previous studies using either satellite telemetry or conventional tagging [15,21,22]. The average displacement rate at the study region (12 km·day⁻1) was similar to that recorded in the Gulf of Mexico (11.7 km·day⁻1) [21] but much lower than rates reported in the North Atlantic Ocean [22,48,72,73]. Although the track durations vary greatly, the reduced average MLD (754 ± 603.4 km) might reflect a seasonal site fidelity shown by most of the tagged blue marlins, which can also be observed through the utilization distribution results of Kernel analysis. For example, the BUM15, the fish with the longest time at liberty (215 days), displayed a large area of tracking signature, but the tag popped off during winter and very close to the tagging site, which supports the description of a residency behavior (Figure 2 and Figure S1).
From the density analysis, KUD core areas are related to continental shelf, slope, and upwelling areas, highly productive zones that are likely preferred by billfishes for feeding and possibly spawning [38]. Eleven out of the eighteen individuals tracked remained near the tagging region, presumably for foraging, and initiated unidirectional migratory movements only toward the end of the tracking durations, which may have constrained observations of their full migratory trajectories. Although this pattern might indicate a methodological bias, as greater density values close to the tagging site may arise from prolonged residency right after deployment [50,74], it aligns with the species’ ecology and earlier observations of billfish movement patterns. The results indicate a larger core area in Area II, likely due to the high productivity driven by the upwelling system in this region [75]. These findings suggest that the identified core areas, which also overlap with important sport fishing grounds, are indeed zones of high blue marlin density in the Southwest Atlantic region [43,44]. Although blue marlins are highly migratory, their tendency to remain within productive regions for extended periods rather than undertaking immediate long-distance movements has also often been reported for billfishes [14,39].
Even with some tags attached for a relatively short period, interesting movement patterns of blue marlins in the South Atlantic Ocean could be detected, such as the more pronounced long-distance and directed movements observed. The first hypothesis regarding the migratory behavior of blue marlins in the Southwest Atlantic Ocean was proposed based on spatial and seasonal patterns in the CPUE data from the Brazilian longline fishery operating in the region [45]. This study identified high catch rates of blue marlin off the northeast coast of Brazil (Area I in the present study) during the third quarter of the year (winter), as well as off the southeast coast (Area II) during the fourth and first quarters (spring/summer). This observation suggests seasonal shifts in distribution. The identified spatial and seasonal patterns constitute the foundation of the first hypothesis concerning the migration of blue marlin in the Southwest Atlantic. Although certain tags were deployed for relatively short durations, our findings revealed consistent movement patterns, including long-distance and directional displacements, which correspond to these hypothesized seasonal shifts, particularly the transition from Area II to Area I between the summer and winter months.
It has been suggested that the blue marlins undertake a southward migration along the Brazilian coast, following the displacement of the 25 °C isotherm to spawn in this region during the first quarter of the year [44]. Supporting this seasonal pattern, other fishery-based studies have suggested similar latitudinal movements. More recently, analyses of catch data have described the size composition of blue marlin in the equatorial and southwestern Atlantic, proposing that adult individuals migrate from the southeastern to the northeastern coast of Brazil during the second quarter and subsequently move eastward towards the equatorial region in the third quarter [43]. These findings align with part of our telemetry data. Blue marlin BUM17, tagged during the first quarter, exhibited a northward displacement during the second quarter until tag detachment (Figure S1). Conversely, BUM03 and BUM06, both tagged in Area I at the onset of the fourth quarter, began to move away from the region within the three-month deployment period, with trajectories oriented eastward and northward, respectively. These movement patterns diverge from those previously described using fishery-based data, which reported a predominantly southward migration during the first quarter [44]. Together, these results suggest that blue marlin movements off the Brazilian coast may exhibit greater spatial and temporal variability than previously documented by fishery-dependent data, potentially influenced by interannual oceanographic conditions, individual behavioral differences, or spawning site plasticity.
Other unexpected directional movements worth noting are the ones observed from BUM09 and BUM10 which travelled eastward from the tagging site in Area II during the first quarter of the year rather than moving northward toward the equatorial region along the continental shelf (Figure S1). However, since the tags from nine individuals (BUM03, BUM06, BUM08, BUM09, BUM10, and BUM14 to BUM17) detached after approximately three months, their subsequent movements remained unknown. Given the substantial variation in migratory movements and residency behavior observed in this study, we propose that blue marlins exhibit highly seasonal shifts in habitat use. These findings also raise the possibility of more individual-specific migration strategies across the Atlantic Ocean.
The blue marlin demonstrated a marked preference for the upper 25 m of the water column and temperatures ranging between 26 °C and 28 °C throughout the tracking period, underscoring their dependence on the warm, stable surface layer. Nonetheless, diurnal variations in behavior were apparent. During daylight hours, blue marlins were observed with greater frequency at depths between 25 and 75 m and in somewhat cooler waters (24–26 °C), as observed in the Atlantic Ocean [22,29,48], suggesting potential foraging dives or thermoregulatory behavior. In contrast, at night, individuals consistently inhabited the upper 5 m and remained within the warmest temperature range (≥26 °C), likely reflecting reduced vertical activity and closer association with the surface layer [20,30]. These patterns suggest that marlin may optimize their vertical movements to balance energetic efficiency and prey availability while also minimizing thermal stress. Considering the species’ noticeable association with the surface mixed layer, anticipated ocean warming and modifications in mixed layer depth under climate change scenarios have the potential to substantially alter the distribution, behavior, and prey accessibility of blue marlin [76,77]. Additionally, the mixed-layer shallowing may restrict the availability of suitable habitats, whereas thermal stratification could potentially influence vertical foraging strategies [20,38]. From an ecosystem management perspective, this sensitivity emphasizes the necessity of integrating vertical habitat preferences and oceanographic variability into predictive models and conservation frameworks, particularly concerning spatial planning and fisheries regulation.
The amount of dissolved oxygen has also been considered to affect the vertical distribution of istiophorids, acting as a physical barrier to limit vertical movement and increasing predator–prey interactions due to habitat compression [39,76,77]. However, the southwest tropical Atlantic is a high dissolved oxygen (DO) concentration area [75,78], which might indicate that temperature is more relevant than DO as a limiting factor for blue marlin vertical habitat utilization in this region. Moreover, temperature directly limits cardiac output and consequently swimming performance in tunas and billfishes [37], which does not imply that incursions to deeper water are not possible.
Although most of the time spent by blue marlin was restricted to warmer waters near the surface, our results showed diel patterns of vertical distribution for some individuals diving deeper during the day and swimming mainly near the surface at night, a similar pattern as observed in previous studies in the Atlantic Ocean [20,21,22]. This diel pattern is associated with the variation in the range of light levels; as they are visual predators, deep dives can be considered a foraging behavior aimed at locating deeper-living organisms during the daytime [79]. The strong presence in the surface layer is also reflected in the blue marlin diet, which consists mainly of epipelagic fishes and squids [33,34,35], with few studies reporting deep water prey items [13,36].
Assessing how much fish core habitats, estimated from survey data (e.g., animal tracks), overlap with areas used by the fisheries is an important prerequisite for estimating fishing impacts [80]. We believe that a larger amount of tags than we deployed is needed to better understand the migratory patterns of blue marlin and its interaction with fishing across the Southwest Atlantic Ocean. However, the synoptic nature of the PSAT data and the detailed information about horizontal and vertical movements that these tags can provide are unique.
Blue marlin seem to shift between offshore pelagic zones (where they are generally found year-round) and coastal areas during the breeding season [34,43,44]. This is likely driven by a combination of water temperature and food availability. Our study revealed core areas used by tagged blue marlins, which overlap considerably with major fishing grounds for Brazilian longline fishing fleets in the Southwest Atlantic Ocean [81]. This overlap is reflected in the presence of blue marlin catches in commercial longline fisheries operating in this region [4,46,82]; thus, some effect of horizontal overlap could be smoothed out by taking management measures such as time-area closure [42].
The systematic issue of overfishing trends in the Atlantic blue marlin population demands new information to support stock assessments and reduce uncertainties [9,18], thereby posing new challenges for population modeling. One of these challenges requires incorporating spatial patterns of species into stock assessment modeling [31,83,84]. There is considerable variability in both the depths at which pelagic longlines are set [85] and the diel vertical behavior of the animals. These factors strongly influence the encounters between marlins and fishing gear, making catch data a relatively weak abundance estimator [20,86,87]. Therefore, standardizing catch rates for factors other than abundance is needed since catch rates are a critical source for most fish stock assessments [19,88,89]. Archival tagging data can improve fisheries analysis with great potential to enhance spatiotemporal models and fish population dynamics estimates [90]. This includes, for example, the improvement of gear selectivity characteristics to apply to assessment models [91], the use in integrated stock assessment models [92], multi-stock age-structured tag integrated assessment (MAST) [93], or habitat-based standardization [89].
Therefore, data collected as part of this study may represent valuable information on movement patterns for the use in stock assessment methods in an Atlantic-wide future analysis. Besides having such information on blue marlin migratory dynamics off the coast of Brazil, it is also very important to extend studies to other regions of the South Atlantic that have not been previously explored for this species. This could provide additional insights into their behavior and contribute to a broader perception of their migration patterns, adding a few more pieces to the puzzle.
5. Conclusions
The present research reveals that blue marlins in the Southwestern Atlantic Ocean spend almost half of their time occupying surface waters (up to 5 m deep) while also occasionally diving beyond 350 m. A clear diel pattern is observed, with individuals occupying deeper waters during the day, likely for foraging, and remaining in shallower layers at night. Temperature preferences are also narrow, with most time spent in waters between 26.0 and 28.0 °C.
The horizontal movement data show a broad distribution across the study area, with blue marlins exhibiting both directional dispersal and residency behavior. These findings suggest a partial migration strategy, where some individuals remain in core activity areas while others undertake long-distance movements, likely driven by seasonal changes in ocean productivity and temperature. Kernel density analysis identifies two primary core areas of habitat use, indicating site fidelity to productive offshore banks and continental slope regions.
In recent years, research on habitat use of large pelagic species in the South Atlantic has become increasingly important for the conservation of target species and the bycatch of commercial fisheries. Despite the key insights into the spatial ecology of the blue marlin in this study, the issue of overfishing can only be thoroughly assessed through additional research, incorporating the movement data into stock assessment and population models.
The findings of this research represent a substantial advance in understanding of the behavior and ecology of blue marlin in the South Atlantic Ocean. Although long-term data collection remains essential, the findings clarify existing uncertainties and facilitate more informed science-based decision-making in international management and conservation initiatives. Further studies are necessary to investigate the environmental drivers and interactions with fisheries influencing blue marlin movement patterns in the southwestern Atlantic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10050201/s1, Figure S1: Track for tagged blue marlins divided into the four quarters of the year across the study area. Circles indicate tagging locations, and red triangles mark the last location data (pop-off) of each individual track. The light-blue shaded area represents the Brazilian Exclusive Economic Zone. The dashed line indicates the Tropic of Capricorn limit.; Figure S2: Utilization distributions for blue marlins tagged with PSATs in the Southwest Atlantic Ocean. The left panel represents KUD areas from tags locations processed with GPE3 model, and the right panel is the KUD results from the tags processed with TrackIt geolocation. The green shades represent the home range (95% KUD), and yellow shades represent the core areas (50% KUD). The light-blue shaded area represents the Brazilian Exclusive Economic Zone. The dashed line indicates the Tropic of Capricorn limit.
Author Contributions
Conceptualization, O.C.-N., B.C.L.M., B.M. and A.F.A.; methodology, O.C.-N., B.C.L.M., and A.F.A.; formal analysis, O.C.-N., B.C.L.M.; investigation, O.C.-N., B.M., B.C.L.M.; resources, A.F.A., E.G.P., E.W.W.; data curation, O.C.-N., J.C.P., E.W.W., E.G.P.; writing—original draft preparation, O.C.-N.; writing—review and editing, O.C.-N., B.C.L.M., B.M., A.F.A., J.C.P., E.W.W., E.G.P.; visualization, O.C.-N., B.C.L.M.; supervision, A.F.A. and B.M.; project administration, A.F.A. and B.M.; funding acquisition, A.F.A., B.M., E.W.W., E.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The data used for this research was made possible by the financial support of multiple projects from many sources, including the Science Without Borders (SWB) program from CNPq- Conselho Nacional de Desenvolvimento Científico e Tecnológico, PROTUNA project (Processo: 445810/2015-7)/ Brazilian Ministry of Fisheries and Aquaculture (Ministério da Pesca e Aquicultura—MPA), Guy Harvey Foundation, FAO (Food and Agriculture Organization), and FAPESP- Fundação de Amparo à Pesquisa do Estado de São Paulo- (Processo: 2016/05259-0, Migração de marlim-azul). OC and BCLM acknowledge the FCT—Foundation for Science and Technology, I.P., under the project UIDB/05634/2025 and UIDP/05634/2025 and to the Regional Government of the Azores through the project M1.1.A/FUNC.UI&D/003/2021-2024.
Institutional Review Board Statement
The tagging methods used in this research were approved by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) of the Brazilian Ministry of the Environment (protocol no. 41163882), and the Ethics Committee on the Use of Animals in Experimentation (CEUA)/UFRPE (protocol no. 23082.009679/2009).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank all captains, sportfishermen, and fishermen for their support in animal capture and tagging processes. We are deeply grateful to all the scientists who supported the fieldwork process and data collection, including Carlos Eduardo Malavasi and Mariana Alberto. We thank J. Graves and D. Kerstetter for their support and partnership to pioneering the tagging efforts in the study region, initiated in 2006. The authors are extremely thankful for the scientific support on the research design and critical reviews by Fábio Hazin (in memoriam) and Felipe Carvalho. We also acknowledge the reviewers for their valuable inputs for the improvement of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pace, M.; Cole, J.; Carpenter, S.; Kitchell, J. Trophic Cascades Revealed in Diverse Ecosystems. Trends Ecol. Evol. 1999, 14, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Kitchell, J.F.; Martell, S.J.D.; Walters, C.J.; Jensen, O.P.; Kaplan, I.C.; Watters, J.; Essington, T.E.; Boggs, C.H. Billfishes in an Ecosystem Context. Bull. Mar. Sci. 2006, 79, 669–682. [Google Scholar]
- Casini, M.; Hjelm, J.; Molinero, J.-C.; Lövgren, J.; Cardinale, M.; Bartolino, V.; Belgrano, A.; Kornilovs, G. Trophic Cascades Promote Threshold-like Shifts in Pelagic Marine Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 197–202. [Google Scholar] [CrossRef]
- Uozumi, Y.; Nakano, H. A historical review of Japanese longline fishery and billfish catches in the Atlantic Ocean. Col. Vol. Sci. Pap. ICCAT 1994, 41, 233–243. [Google Scholar]
- Restrepo, V.; Prince, E.D.; Scott, G.P.; Uozumi, Y. ICCAT Stock Assessments of Atlantic Billfish. Mar. Freshw. Res. 2003, 54, 361–367. [Google Scholar] [CrossRef]
- Ditton, R.B.; Stoll, J.R. Social and Economic Perspective on Recreational Bill Fish Fisheries. Mar. Freshw. Res. 2003, 54, 545–554. [Google Scholar] [CrossRef]
- Gentner, B. The Value of Billfish Resources to Both Commercial and Recreational Sectors in the Caribbean. In FAO Fisheries and Aquaculture Circular; FAO Subregional Office for Latin America and the the Caribbean: Bridgetown, Barbados, 2016; Volume 1125, p. 42. [Google Scholar]
- Hazin, H.G.; Hazin, F.; Carvalho, F.; Mourato, B.; Frédou, T.; Travassos, P.; Pacheco, J.C. Standardized Cpue Series of Blue Marlin and White Marlin Caught By Brazilian Tuna Longline Fisheries in the Southwestern Atlantic Ocean (1980–2010). Collect. Vol. Sci. Pap. ICCAT 2012, 68, 1531–1542. [Google Scholar]
- Mourato, B.L.; Winker, H.; Carvalho, F.; Ortiz, M. Stock Assessment of Atlantic Blue Marlin (Makaira nigricans) Using a Bayesian State-Space Surplus Production Model JABBA. Collect. Vol. Sci. Pap. ICCAT 2018, 75, 1003–1025. [Google Scholar]
- Pons, M.; Branch, T.A.; Melnychuk, M.C.; Jensen, O.P.; Brodziak, J.; Fromentin, J.M.; Harley, S.J.; Haynie, A.C.; Kell, L.T.; Maunder, M.N.; et al. Effects of Biological, Economic and Management Factors on Tuna and Billfish Stock Status. Fish Fish. 2016, 18, 1–21. [Google Scholar] [CrossRef]
- Normas Brasil. Instrução Normativa SEAP/PR nº 12, de 14 de julho de 2005. Estabelece Normas e Procedimentos para Captura e Comercialização dos Agulhões Brancos (Tetrapturus albidus), Agulhões Negros (Makaira nigricans), Agulhões Verdes (Tetrapturus pfluegeri) e Agulhões Vela (Istiophorus albicans), nas Águas Jurisdicionais Brasileiras e Alto-Mar. Diário Oficial da União. 15 July 2005. Available online: https://www.normasbrasil.com.br/norma/instrucao-normativa-12-2005_75831.html (accessed on 2 March 2025).
- Peel, E.; Nelson, R.; Goodyear, C.P. Managing Atlantic Marlin as Bycatch under ICCAT. The Fork in the Road: Recovery or Collapse. Mar. Freshw. Res. 2003, 54, 575–584. [Google Scholar] [CrossRef]
- Nakamura, I. FAO Species Catalogue. Vol. 5: Billfishes of the World. An Annotated and Illustrated Catalogue of Marlins, Sailfishes, Spearfishes and Swordfishes Known to Date. FAO Fish. Synop. 1985, 125, 65. [Google Scholar]
- Ortiz, M.; Prince, E.D.; Serafy, J.E.; Holts, D.B.; Davy, K.B.; Pepperell, J.G.; Lowry, M.B.; Holdsworth, J.C. Global Overview of the Major Constituent-Based Billfish Tagging Programs and Their Results since 1954. Mar. Freshw. Res. 2003, 54, 489–507. [Google Scholar] [CrossRef]
- Orbesen, E.S.; Hoolihan, J.P.; Serafy, J.E.; Snodgrass, D.; Peel, E.M.; Prince, E.D. Transboundary Movement of Atlantic Istiophorid Billfishes Among International and U.S. Domestic Management Areas Inferred from Mark-Recapture Studies. Mar. Fish. Rev. 2008, 70, 14–23. [Google Scholar]
- Recommendation by ICCAT to Amend the Plan to Rebuild Blue Marlin and White Marlin Populations. ICCAT Recommendation 01-10; International Commission for the Conservation of Atlantic Tunas ICCAT: Madrid, Spain, 2001; Available online: https://www.iccat.int/Documents/Recs/compendiopdf-e/2001-10-e.pdf (accessed on 26 April 2025).
- Report of the 2018 ICCAT Blue Marlin Stock Assessment Meeting. ICCAT: Madrid, Spain, 2018. Available online: https://www.iccat.int/Documents/Meetings/Docs/2018/REPORTS/2018_BUM_SA_ENG.pdf (accessed on 26 April 2025).
- Mourato, B.; Sant’ana, R.; Kikuchi, E.; Cardoso, L.G.; Ngom, F.; Ruiz, M.N.; Arocha, F.; Kimoto, A.; Ortiz, M. Preliminary Assessment of Atlantic Blue Marlin (Makaira nigricans) Using JABBA Model (1956–2022). Collect. Vol. Sci. Pap. ICCAT 2024, 81, 1–21. [Google Scholar]
- Graves, J.E.; Kerstetter, D.W.; Luckhurst, B.E.; Prince, E.D. Habitat Preferences of Istiophorid Billfishes in the Western North Atlantic: Applicability of Archival Tag Data to Habitat- Based Stock Assessment Methodologies. Collect. Vol. Sci. Pap. ICCAT 2003, 55, 594–602. Available online: https://nsuworks.nova.edu/occ_facreports/65/ (accessed on 10 January 2020).
- Goodyear, C.P.; Luo, J.; Prince, E.; Hoolihan, J.; Snodgrass, D.; Orbesen, E.; Serafy, J. Vertical Habitat Use of Atlantic Blue Marlin Makaira nigricans: Interaction with Pelagic Longline Gear. Mar. Ecol. Prog. Ser. 2008, 365, 233–245. [Google Scholar] [CrossRef][Green Version]
- Kraus, R.; Wells, R.J.D.; Rooker, J. Horizontal Movements of Atlantic Blue Marlin (Makaira nigricans) in the Gulf of Mexico. Mar. Biol. 2011, 158, 699–713. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Mikles, C.S.; Dale, J.J.; Castleton, M.; Block, B.A. Seasonal and Diel Habitat Use of Blue Marlin Makaira nigricans in the North Atlantic Ocean. ICES J. Mar. Sci. 2023, 80, 1002–1015. [Google Scholar] [CrossRef]
- Dale, J.J.; Brodie, S.; Carlisle, A.B.; Castleton, M.; Hazen, E.L.; Bograd, S.J.; Block, B.A. Global Habitat Loss of a Highly Migratory Predator, the Blue Marlin (Makaira nigricans). Divers. Distrib. 2022, 28, 2020–2034. [Google Scholar] [CrossRef]
- Mourato, B.L.; Hazin, F.; Bigelow, K.; Musyl, M.; Carvalho, F.; Hazin, H. Spatio-Temporal Trends of Sailfish, Istiophorus Platypterus Catch Rates in Relation to Spawning Ground and Environmental Factors in the Equatorial and Southwestern Atlantic Ocean. Fish. Oceanogr. 2014, 23, 32–44. [Google Scholar] [CrossRef]
- Afonso, A.S.; Hazin, F.H.V. Vertical Movement Patterns and Ontogenetic Niche Expansion in the Tiger Shark, Galeocerdo Cuvier. PLoS ONE 2015, 10, e0116720. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Ahrens, R.; Murie, D.; Bigelow, K.; Aires-da-Silva, A.; Maunder, M.; Hazin, F. Using Pop-up Satellite Archival Tags to Inform Selectivity in Fisheries Stock Assessment Models: A Case Study for the Blue Shark in the South Atlantic Ocean. ICES J. Mar. Sci. 2015, 72, 1715–1730. [Google Scholar] [CrossRef]
- Santos, J.C.P. Estudo Do Comportamento Dos Agulhões Branco (Kajikia Albida—Poey, 1860) e Negro (Makaira nigricans—Lacepede, 1802). Ph.D. Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2015; p. 84. [Google Scholar]
- Tolotti, M.T.; Bach, P.; Hazin, F.; Travassos, P.; Dagorn, L. Vulnerability of the Oceanic Whitetip Shark to Pelagic Longline Fisheries. PLoS ONE 2015, 10, e0141396. [Google Scholar] [CrossRef] [PubMed]
- Yokawa, K.; Saito, H. Use of Pop-up Tags to Esstimate Vertical Distribution of Atlantic Blue Marlin (Makaira nigricans) Released from the Commercial and Research Longline Cruise during 2002 and 2003. Collect. Vol. Sci. Pap. ICCAT 2006, 59, 252–264. [Google Scholar]
- Madigan, D.J.; Richardson, A.J.; Carlisle, A.B.; Weber, S.B.; Brown, J.; Hussey, N.E. Water Column Structure Defines Vertical Habitat of Twelve Pelagic Predators in the South Atlantic. ICES J. Mar. Sci. 2021, 78, 867–883. [Google Scholar] [CrossRef]
- Brill, R.W.; Lutcavage, M.E. Understanding Environmental Influences on Movements and Depth Distributions of Tunas and Billfishes Can Significantly Improve Population Assessments. Am. Fish. Soc. Symp. 2001, 25, 179–198. [Google Scholar]
- Bernal, D.; Sepulveda, C.; Musyl, M.; Brill, R. The Eco-Physiology of Swimming and Movement Patterns of Tunas, Billfishes, and Large Pelagic Sharks. In Fish Locomotion–an Etho-Ecological Perspective; Science Publishers: Enfield, UK, 2009; pp. 436–483. [Google Scholar]
- Brock, R.E. A Contribution To the Trophic Biology of the Blue Marlin Makaira-Nigricans in Hawaii Usa. Pac. Sci. 1984, 38, 141–149. [Google Scholar]
- Vaske, T., Jr.; Travassos, P.E.; Pinheiro, P.B.; Hazin, F.H.V.; Tolotti, M.T.; Barbosa, T.M. Diet of the Blue Marlin (Makaira nigricans, Lacepède 1802) (Perciformes: Istiophoridae) of the Southwestern Equatorial Atlantic Ocean. Braz. J. Aquat. Sci. Technol. 2011, 15, 65–70. [Google Scholar] [CrossRef]
- Shimose, T.; Shono, H.; Yokawa, K. Food and Feeding Habits of Blue Marlin, Makaira nigricans, around Yonaguni Island, Southwestern Japan. Bull. Mar. Sci. 2006, 79, 761–775. [Google Scholar]
- Harvey, G.C. An Historical Review of Recreational and Artisanal Fisheries for Billfish in Jamaica, 1976–1988. Col. Vol. Sci. Pap. ICCAT 1989, 30, 440–450. [Google Scholar]
- Brill, R.W.; Lowe, T.E.; Cousins, K.L. How Water Temperature Really Limits the Vertical Movements of Tunas and Billfishes—It’S the Heart Stupid. In Proceedings of the International Congress on Biology of Fish. American Fisheries Society, Towson University, Baltimore, MD, USA, 27–30 July 1998; p. 4. [Google Scholar]
- Braun, C.D.; Kaplan, M.B.; Horodysky, A.Z.; Llopiz, J.K. Satellite Telemetry Reveals Physical Processes Driving Billfish Behavior. Anim. Biotelem. 2015, 3, 2. [Google Scholar] [CrossRef]
- Prince, E.D.; Goodyear, C.P. Hypoxia-Based Habitat Compression of Tropical Pelagic Fishes. Fish. Oceanogr. 2006, 15, 451–464. [Google Scholar] [CrossRef]
- Carlisle, A.B.; Kochevar, R.E.; Arostegui, M.C.; Ganong, J.E.; Castleton, M.; Schratwieser, J.; Block, B.A. Influence of Temperature and Oxygen on the Distribution of Blue Marlin (Makaira nigricans) in the Central Pacific. Fish. Oceanogr. 2017, 26, 34–48. [Google Scholar] [CrossRef]
- Silva, C.M.T. Biologia Reprodutiva Do Agulhão Negro (Makaira nigricans Lacépède, 1803) No Atlântico Oeste Tropical. Master’s Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2007. [Google Scholar]
- de Oliveira, Í.A.; Hazin, H.G.; Hazin, F.H.V.; Travassos, P.E.P.; da Silva, G.B.; Mourato, B.L.; Carvalho, F. Distribuição Do Agulhão Negro No Atlântico Sul e Equatorial e Potencial de Estratégia de Manejo Espacial. Bol. Inst. Pesca 2015, 41, 607–617. [Google Scholar]
- Frédou, T.; Frédou, F.L.; Hazin, F.H.V.; Travassos, P. Length Composition and Spatio-Temporal Distribution of Blue Marlin (Makaira nigricans) in the South Atlantic Ocean. Collect. Vol. Sci. Pap. ICCAT 2012, 68, 1524–1530. [Google Scholar]
- Amorim, A.; Arfelli, C.; Hazin, F.; Antero-Silva, J.; Lessa, R.; Arraes, R. Blue Marlin (Makaira nigricans) Fisheries off Brazilian Coast by National and Leased Longliners (1971–1991). Col. Vol. Sci. Pap. ICCAT 1994, 41, 208–213. [Google Scholar]
- Hazin, F.H.V. Fisheries-Oceanographical Study on Tunas, Billfishes and Sharks in the Southwestern Equatorial Atlantic Ocean. Ph.D. Thesis, University of Fisheries, Tokyo, Japan, 1994. [Google Scholar]
- Hazin, F.H.V.; Hazin, H.G.; Travassos, P.; Oliveira, M. Standardized Catch Per Unit of Effort of White Marlin, Tetrapturus Albidus, and Blue Marlin, Makaira nigricans, Caught By Brazilian Tuna Longline Fleet. Fish 2007, 60, 1652–1662. [Google Scholar]
- Mourato, B.L.; Amorim, A.F.; Arfelli, C.A.; Hazin, H.G.; Hazin, F.H.V.; Wor, C. Standardized CPUE of Atlantic Sailfish (Istiophorus Platypterus) Caught by the Recreational Fishery in Southern Brazil (1996–2007). Collect. Vol. Sci. Pap. 2009, 64, 1941–1950. [Google Scholar]
- Graves, J.E.; Luckhurst, B.E.; Prince, E.D. An Evaluation of Pop-up Satellite Tags Forestimating Postrelease Survival of Blue Marlin (Makaira nigricans) from a Recreational Fishery. Fish. Bull. 2002, 100, 134–142. [Google Scholar]
- Domeier, M.L.; Dewar, H.; Nasby-Lucas, N. Mortality Rate of Striped Marlin (Tetrapturus audax) Caught with Recreational Tackle. Mar. Freshw. Res. 2003, 54, 435–445. [Google Scholar] [CrossRef]
- Horodysky, A.Z.; Graves, J.E. Application of Pop-up Satellite Archival Tag Technology to Estimate Postrelease Survival of White Marlin (Tetrapturus albidus) Caught on Circle and Straight-Shank (“J”) Hooks in the Western North Atlantic Recreational Fishery. Fish. Bull. 2005, 103, 84–96. [Google Scholar]
- Prince, E.D.; Ortiz, M.; Venizelos, A.; Rosenthal, D.S. In-Water Conventional Tagging Techniques Developed by the Cooperative Tagging Center for Large, Highly Migratory Species. Am. Fish. Soc. Symp. 2002, 2002, 155–171. [Google Scholar]
- Ekstrom, P.A. An Advance in Geolocation by Light. Mem. Natl. Inst. Polar Res. 2004, 58, 210–226. [Google Scholar]
- Crespo, O. Utilização de Habitat e Movimentos Migratórios Do Agulhão Negro (Makaira nigricans) No Oceano Atlântico Sul. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2016. [Google Scholar]
- Lam, C.H.; Nielsen, A.; Sibert, J.R. Incorporating Sea-Surface Temperature to the Light-Based Geolocation Model TrackIt. Mar. Ecol. Prog. Ser. 2010, 419, 71–84. [Google Scholar] [CrossRef]
- Pedersen, M.W.; Patterson, T.A.; Thygesen, U.H.; Madsen, H. Estimating Animal Behavior and Residency from Movement Data. Oikos 2011, 120, 1281–1290. [Google Scholar] [CrossRef]
- Worton, B.J. Kernel Methods for Estimating the Utilization Distribution in Home-Range Studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Horne, J.S.; Garton, E.O. Likelihood Cross-Validation versus Least Squares Cross-Validation for Choosing the Smoothing Parameter in Kernel Home-Range Analysis. J. Wildl. Manag. 2006, 70, 641–648. [Google Scholar] [CrossRef]
- Calenge, C. The Package “Adehabitat” for the R Software: A Tool for the Analysis of Space and Habitat Use by Animals. Ecol. Modell. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Péron, G. Modified Home Range Kernel Density Estimators That Take Environmental Interactions into Account. Mov. Ecol. 2019, 7, 16. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R Core Team, R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Campana, S.E.; Dorey, A.; Fowler, M.; Joyce, W.; Wang, Z.; Wright, D.; Yashayaev, I. Migration Pathways, Behavioural Thermoregulation and Overwintering Grounds of Blue Sharks in the Northwest Atlantic. PLoS ONE 2011, 6, e16854. [Google Scholar] [CrossRef]
- Howey-Jordan, L.A.; Brooks, E.J.; Abercrombie, D.L.; Jordan, L.K.B.; Brooks, A.; Williams, S.; Gospodarczyk, E.; Chapman, D.D. Complex Movements, Philopatry and Expanded Depth Range of a Severely Threatened Pelagic Shark, the Oceanic Whitetip (Carcharhinus Longimanus) in the Western North Atlantic. PLoS ONE 2013, 8, e56588. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.C.; Foley, D.G.; Ganong, J.E.; Perle, C.; Shillinger, G.L.; Block, B.A. Migration of an Upper Trophic Level Predator, the Salmon Shark Lamna Ditropis, between Distant Ecoregions. Mar. Ecol. Prog. Ser. 2008, 372, 253–264. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Partial Migration: Niche Shift versus Sexual Maturation in Fishes. Rev. Fish. Biol. Fish. 1993, 3, 348–365. [Google Scholar] [CrossRef]
- Chapman, B.B.; Hulthén, K.; Brodersen, J.; Nilsson, P.A.; Skov, C.; Hansson, L.A.; Bronmark, C. Partial Migration in Fishes: Causes and Consequences. J. Fish. Biol. 2012, 81, 456–478. [Google Scholar] [CrossRef] [PubMed]
- James, M.C.; Ottensmeyer, C.A.; Myers, R.A. Identification of High-Use Habitat and Threats to Leatherback Sea Turtles in Northern Waters: New Directions for Conservation. Ecol. Lett. 2005, 8, 195–201. [Google Scholar] [CrossRef]
- Patterson, T.A.; Evans, K.; Carter, T.I.; Gunn, J.S. Movement and Behaviour of Large Southern Bluefin Tuna (Thunnus maccoyii) in the Australian Region Determined Using Pop-up Satellite Archival Tags. Fish. Oceanogr. 2008, 17, 352–367. [Google Scholar] [CrossRef]
- Shaffer, S.A.; Tremblay, Y.; Weimerskirch, H.; Scott, D.; Thompson, D.R.; Sagar, P.M.; Moller, H.; Taylor, G.A.; Foley, D.G.; Block, B.A.; et al. Migratory Shearwaters Integrate Oceanic Resources across the Pacific Ocean in an Endless Summer. Proc. Natl. Acad. Sci. USA 2006, 103, 12799–12802. [Google Scholar] [CrossRef]
- Mate, B.; Mesecar, R.; Lagerquist, B. The Evolution of Satellite-Monitored Radio Tags for Large Whales: One Laboratory’s Experience. Deep. Sea Res. 2 Top. Stud. Oceanogr. 2007, 54, 224–247. [Google Scholar] [CrossRef]
- Alerstam, T.; Hedenstrom, A.; Akesson, S. Long-Distance Migration: Evolution and Determinants. Oikos 2003, 103, 247–260. [Google Scholar] [CrossRef]
- Evans, K.; Kolody, D.; Abascal, F.; Holdsworth, J.; Maru, P.; Sippel, T. Spatial Dynamics of Swordfish in the South Pacific Ocean Inferred from Tagging Data; WCPFC-SC8-2012/SA-IP-05; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2012; p. 36. [Google Scholar]
- Kerstetter, D.W.; Luckhurst, B.E.; Prince, E.D.; Graves, J.E. Use of Pop-up Satellite Archival Tags to Demonstrate Survival of Blue Marlin (Makaira nigricans) Released from Pelagic Longline Gear. Fish. Bull. 2003, 101, 939–948. [Google Scholar]
- Freitas, C.; Freitas, M.; Andrzejaczek, S.; Dale, J.J.; Whippen, W.; Block, B.A. First Insights into the Movements and Vertical Habitat Use of Blue Marlin (Makaira nigricans) in the Eastern North Atlantic. Anim. Biotelem. 2022, 10, 12. [Google Scholar] [CrossRef]
- Hoolihan, J.P.; Luo, J.; Abascal, F.J.; Campana, S.E.; De Metrio, G.; Dewar, H.; Domeier, M.L.; Howey, L.A.; Lutcavage, M.E.; Musyl, M.K.; et al. Evaluating Post-Release Behaviour Modification in Large Pelagic Fish Deployed with Pop-up Satellite Archival Tags. ICES J. Mar. Sci. 2011, 68, 880–889. [Google Scholar] [CrossRef]
- Braga, E.d.S.; Niencheski, L.F.H. Composição Das Massas de Água e Seus Potenciais Produtivos Na Área Entre o Cabo de São Tomé (RJ) e o Chuí (RS). In O Ambiente Oceanografia da Plataforma Continental e do Talude na Região Sudeste-Sul do Brasil; Edusp: Sao Paulo, Brazil, 2006; pp. 161–218. ISBN 10:8531409489. [Google Scholar]
- Prince, E.D.; Luo, J.; Phillip Goodyear, C.; Hoolihan, J.P.; Snodgrass, D.; Orbesen, E.S.; Serafy, J.E.; Ortiz, M.; Schirripa, M.J. Ocean Scale Hypoxia-Based Habitat Compression of Atlantic Istiophorid Billfishes. Fish. Oceanogr. 2010, 19, 448–462. [Google Scholar] [CrossRef]
- Stramma, L.; Prince, E.D.; Schmidtko, S.; Luo, J.; Hoolihan, J.P.; Visbeck, M.; Wallace, D.W.R.; Brandt, P.; Körtzinger, A. Expansion of Oxygen Minimum Zones May Reduce Available Habitat for Tropical Pelagic Fishes. Nat. Clim. Change 2011, 2, 33–37. [Google Scholar] [CrossRef]
- Castro Filho, B.M.; Miranda, L.B. Physical Oceanography of the Western Atlantic Continental Shelf Located between 4 N and 34 S: Coastal Segment (4,W). In The Sea; John Wiley & Sons: Oxford, UK, 1998; Volume 11. [Google Scholar]
- Young, T.; Pincin, J.; Neubauer, P.; Ortega-García, S.; Jensen, O.P. Investigating Diet Patterns of Highly Mobile Marine Predators Using Stomach Contents, Stable Isotope, and Fatty Acid Analyses. ICES J. Mar. Sci. 2018, 75, 1583–1590. [Google Scholar] [CrossRef]
- Vinther, M.; Eero, M. Quantifying Relative Fishing Impact on Fish Populations Based on Spatio-Temporal Overlap of Fishing Effort and Stock Density. ICES J. Mar. Sci. 2013, 70, 618–627. [Google Scholar] [CrossRef]
- Mourato, B.; Sant, R.; Kikuchi, E.; Gustavo Cardoso, L. Catch Rates of Sailfish from Brazilian Longline Fisheries in the western Atlantic (1991–2022). Collect. Vol. Sci. Pap. ICCAT 2023, 80, 226–235. [Google Scholar]
- Amorim, A.F.; Arfelli, C.A.; Antero-Silva, J.N.; Fagundes, L.; Costa, F.E.S.; Assumpção, R. Blue Marlin (Makaira nigricans) and White Marlin (Tetrapturus albidus) Caught off the Brazilian Coast. Col. Vol. Sci. Pap. ICCAT 1998, 47, 163–184. [Google Scholar]
- Reuchlin-Hugenholtz, E.; Shackell, N.L.; Hutchings, J.A. The Potential for Spatial Distribution Indices to Signal Thresholds in Marine Fish Biomass. PLoS ONE 2015, 10, e0120500. [Google Scholar] [CrossRef]
- Carson, S.; Shackell, N.; Mills Flemming, J. Local Overfishing May Be Avoided by Examining Parameters of a Spatio-Temporal Model. PLoS ONE 2017, 12, e0184427. [Google Scholar] [CrossRef]
- Rice, P.H.; Goodyear, C.P.; Prince, E.D.; Snodgrass, D.; Serafy, J.E. Use of Catenary Geometry to Estimate Hook Depth during Near-Surface Pelagic Longline Fishing: Theory versus Practice. N. Am. J. Fish. Manag. 2007, 27, 1148–1161. [Google Scholar] [CrossRef]
- Ward, P.; Hindmarsh, S. An Overview of Historical Changes in the Fishing Gear and Practices of Pelagic Longliners, with Particular Reference to Japan’s Pacific Fleet. Rev. Fish. Biol. Fish. 2007, 17, 501–516. [Google Scholar] [CrossRef]
- Su, N.J.; Sun, C.L.; Punt, A.E.; Yeh, S.Z. Environmental and Spatial Effects on the Distribution of Blue Marlin (Makaira nigricans) as Inferred from Data for Longline Fisheries in the Pacific Ocean. Fish. Oceanogr. 2008, 17, 432–445. [Google Scholar] [CrossRef]
- Bigelow, K.A.; Maunder, M.N. Does Habitat or Depth Influence Catch Rates of Pelagic Species? Can. J. Fish. Aquat. Sci. 2007, 64, 1581–1594. [Google Scholar] [CrossRef]
- Goodyear, C.P.; Bigelow, K.A. Preliminary Analyses of Simulated Longline Atlantic Blue Marlin CPUE with HBS and Generalized Linear Models. Collect. Vol. Sci. Pap. ICCAT 2012, 68, 1510–1523. [Google Scholar]
- Sippel, T.; Paige Eveson, J.; Galuardi, B.; Lam, C.; Hoyle, S.; Maunder, M.; Kleiber, P.; Carvalho, F.; Tsontos, V.; Teo, S.L.H.; et al. Using Movement Data from Electronic Tags in Fisheries Stock Assessment: A Review of Models, Technology and Experimental Design. Fish. Res. 2014, 163, 152–160. [Google Scholar] [CrossRef]
- Carvalho, F.C. Spatial Distribution of Blue Shark Catch Rate and Catch Probability of Juveniles in the Southwest Atlantic. ICES J. Mar. Sci. 2011, 68, 890–900. [Google Scholar] [CrossRef]
- Maunder, M.N.; Punt, A.E. A Review of Integrated Analysis in Fisheries Stock Assessment. Fish. Res. 2013, 142, 61–74. [Google Scholar] [CrossRef]
- Taylor, N.G.; Mcallister, M.K.; Lawson, G.L.; Carruthers, T.; Block, B.A. Atlantic Bluefin Tuna: A Novel Multistock Spatial Model for Assessing Population Biomass. PLoS ONE 2011, 6, 27693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).