1. Introduction
The silver scabbardfish (
Lepidopus caudatus, Euphrasen, 1788) is a mesopelagic species of the family Trichiuridae, found in temperate seas worldwide, including the Mediterranean [
1,
2]. This species is typically encountered on both the continental shelf and slope [
3,
4], on sandy and muddy substrates [
5]. Its bathymetric distribution exhibits seasonal shifts: it is more abundant on the continental shelf during winter and migrates to deeper waters in other seasons, particularly in summer [
2,
4,
6]. This species is also known for its vertical migrations from the benthic zone to the water column during the night [
6].
The silver scabbardfish has moderate commercial value, and it is primarily caught as bycatch in different regions, such as New Zealand, the Mediterranean, the Canary Islands and Portugal, by bottom and pelagic trawlers and longliners [
1,
4,
7,
8]. In the Mediterranean, larger individuals are considered economically valuable, particularly in Italy, Malta, Tunisia and Spain, while smaller ones are typically discarded [
6,
9,
10]. Italy accounts for the largest share of the Mediterranean catch of
L. caudatus, consistently exceeding 80% of the total regional catch; however, both Italian and Mediterranean catches have declined since 2018 [
11]. Actually, the Mediterranean experienced an overfishing scenario of
L. caudatus in the Strait of Sicily [
2,
12]. This area in the central Mediterranean has a targeted fishery for the silver scabbardfish based on a new fishing pelagic trawl developed by local fishers [
2]. However, given that this new gear is not regulated by any management measures and the stock status of the exploited species has not been assessed [
2], the collapse of fishing activities and an overfished state of
L. caudatus stock are inevitable.
In the Adriatic, the silver scabbardfish has no commercial value, but several studies have documented its ecological role. Morello and Arneri [
13] highlighted its significance as one of the primary predators of post-larval anchovies in the Manfredonia area, southern Adriatic. Additionally, the population dynamics of
L. caudatus appear to correlate with a decline in chondrichthyan species. During the international bottom trawl survey in the Mediterranean (MEDITS), in the period from 1994 to 2008, a decrease in the densities of chondrichthyans was followed by the expansion of
L. caudatus from deeper waters in the southern Adriatic to the northern basin [
14,
15]. The biological characteristics of the silver scabbardfish, e.g., its predation on fish, crustaceans and cephalopods [
16,
17], rapid growth rate [
6,
9] and strong reproductive capacity [
6,
18], likely contributed to this distribution shift. The ecological implications of such population dynamics are apparent. As a mesopelagic predator,
L. caudatus may influence both the structure and function of food webs through the top-down control of ecologically and commercially valuable species, such as anchovies [
4,
13]. Its expansion following chondrichthyan decline suggests that it may occupy ecological niches that are left empty, with possible cascading effects on the ecosystem balance. These interactions, especially with commercially important species, underline the need to understand the life-history of
L. caudatus better in order to provide crucial information for ecosystem-based fisheries management and predict eventual future shifts under changing environmental or anthropogenic (e.g., exploitation) pressures.
However, despite its worldwide distribution, and its moderate commercial and high ecological importance, knowledge on the biology of
L. caudatus remains limited, especially for the Adriatic, where there are almost no data. In general, most studies have focused on growth [
1,
6,
9,
12,
18], reproduction [
6,
8,
9,
19,
20] and diet [
4]. If we concentrate only on growth studies, which are essential for assessing life-history and population dynamics, some differences in growth parameters appear between locations. So, for example, values of the growth coefficient are higher for the Mediterranean populations compared to the Atlantic ones, indicating that
L. caudatus grows faster in the Mediterranean basin [
1,
6]. The only exception to this observation is the population of
L. caudatus from the Canaries [
8], which is much more similar to the Mediterranean populations in terms of growth characteristics. Also, if we compare the longevity of this species, populations in the Atlantic would reach 95% of their asymptotic length in 20 to 30 years, while those in the Mediterranean take 9 to 11 years [
1,
6]. Although these studies differ in terms of sample size, sampling methodology and fish age structure, their results may indicate spatial heterogeneity in the growth of the silver scabbardfish, which may be important input information for future ecological and fisheries management.
First of all, this study aims to determine the age and growth of L. caudatus through the analysis of sagittal otoliths of the eastern Adriatic population. In this way, significant gaps that exist in our understanding of the growth patterns of this species in the Adriatic will be filled. Based on all previous studies, we hypothesised that the sampled fish in the Adriatic are fast growing and have a short lifespan. Our results will allow us to compare our sample population with previously studied Mediterranean and Atlantic populations and to identify similarities and/or differences. We further hypothesise that our results provide more evidence of the spatial stratification in the age structure and growth pattern of L. caudatus populations. Also, given the known ecological role of the investigated species in the Adriatic, the life-history data we provide can contribute to predicting possible future scenarios in population dynamics and responding in a timely manner in the context of protection and/or sustainable fisheries management.
3. Results
A total of 295 specimens of the silver scabbardfish were examined; they included 64 (21.7%) males and 20 (6.8%) females, and most individuals were juveniles and specimens with poorly developed gonads (211 (71.5%)). The sex ratio was significantly biased in favour of males (
p < 0.00001). Total length range of all specimens was from 20.7 to 123.0 cm. The male and female total lengths ranged from 41.5 to 123.0 cm and from 66.5 to 103.9 cm, respectively (
Table 1).
The length frequency distributions between males and females were significantly different (Kolmogorov–Smirnov test; p = 0.02), with a higher proportion of males in the smallest and largest length classes compared to females. Females only prevailed in classes from 75.0 to 85.0 cm. On the contrary, the mean length of males (74.3 ± 17.19 cm) was not significantly different than that of females (73.5 ± 11.31 cm) (t-test, df = 82, p = 0.91).
The shape of the sagittal otoliths of the silver scabbardfish was triangular and elongated, with a long and pointed rostrum and an absent antirostrum in the anterior region. This region was peaked, while the posterior region of the otoliths was oblique. No significant differences were observed in the morphometric measurements between left and right otoliths (paired
t-test,
p > 0.05 for all measurements) and between females and males (ANCOVA,
p > 0.05 for all measurements). As a result, the data were combined, and we used the mean values for each otolith pair in all subsequent analyses (
Table 2). The relationships between fish total length and otolith length, width, thickness and mass were linear. All these relationships were described with high coefficients of determination (≥0.828) (
Table 3).
Age was determined independently by two readers, with both readers agreeing on the age of 159 fish (59.3%). The variability of IAPE and CV indices was 11.2% and 16.9%, respectively. The estimated age ranged from 0 to 4 years, i.e., five age classes. The whole population was dominated by the youngest 0
+-year-old fish, which represented approximately 70% of the successfully aged fish (N = 268 readable otoliths). The maximum age of the sampled silver scabbardfish was 4 years for males (
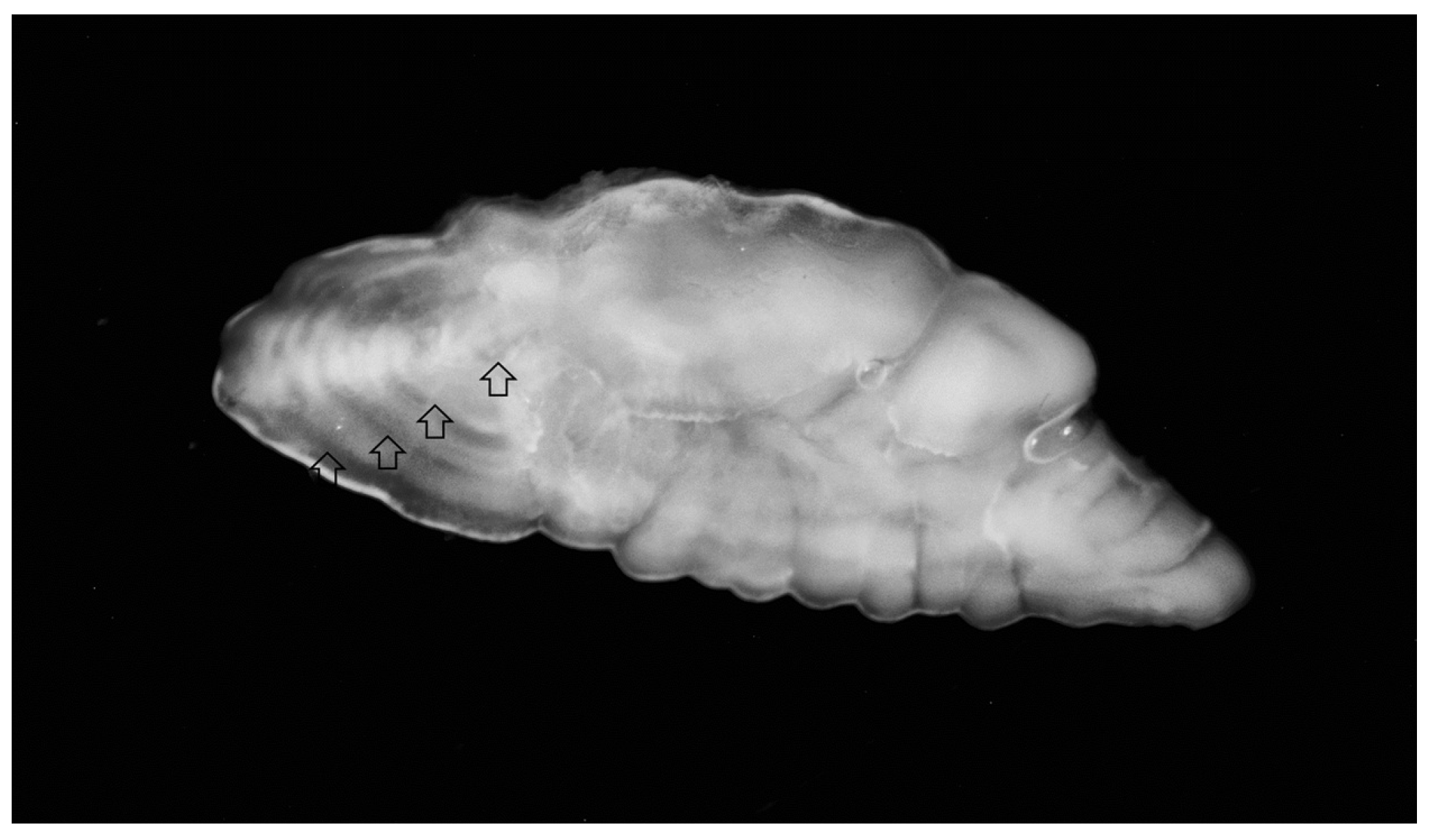
Figure 1) and 2 years for females. After age 2, males dominated the older age classes, but we found only six (2.2%) 3- and 4-year-old individuals. A distance measurements analysis revealed consistency in forming annuli in that first growth annulus forms at 2.00 mm (±0.11 SD) from the otolith centre. In addition, the second, third and fourth growth annuli form at 2.5 mm (±0.42 SD), 3.3 mm (±0.21 SD) and 3.5 mm (±0.16 SD) from the centre, respectively.
Figure 1.
Sagittal otolith of 4-year-old silver scabbardfish.
Figure 1.
Sagittal otolith of 4-year-old silver scabbardfish.
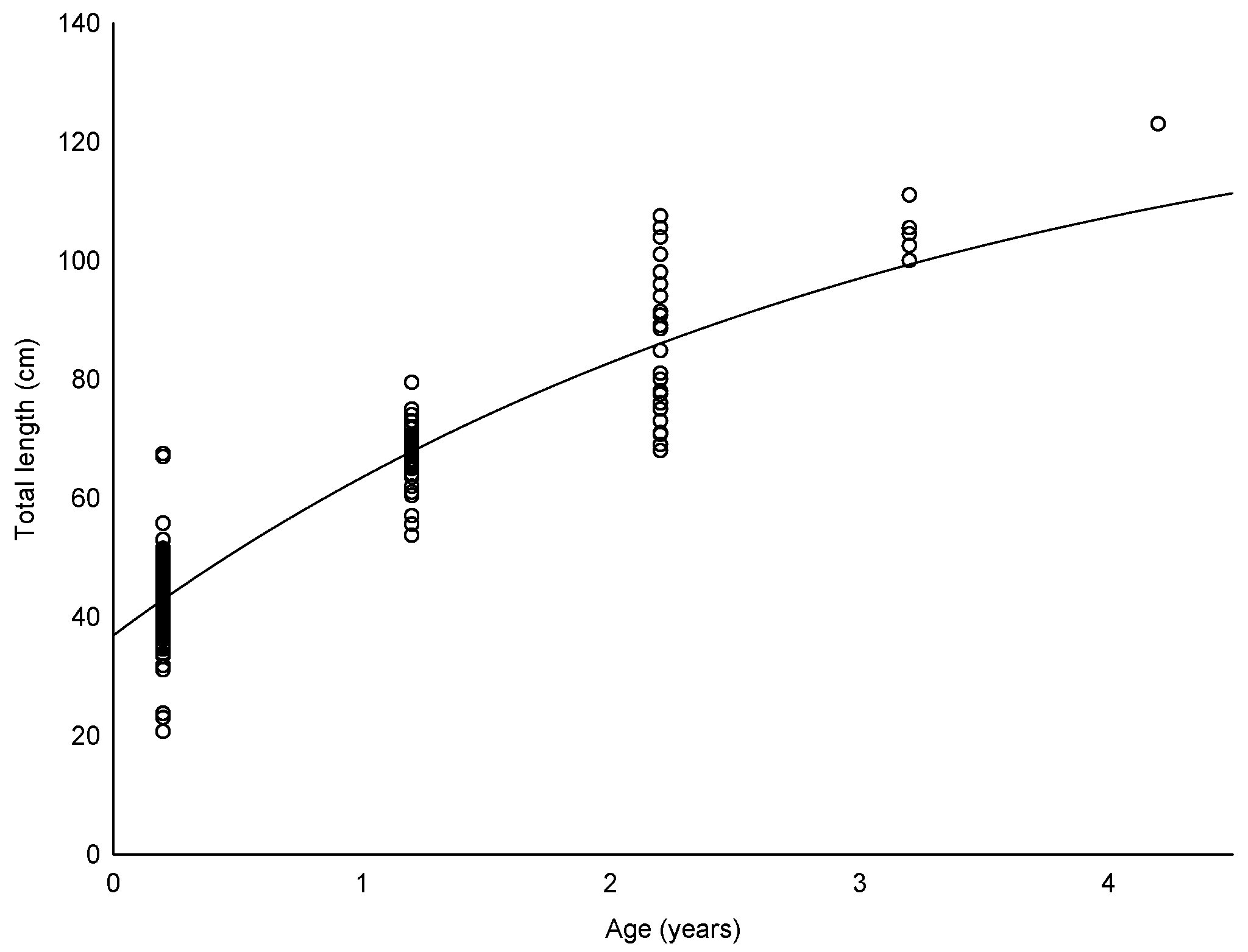
The estimated parameters of the von Bertalanffy growth model were L
∞ = 134.98 cm, K = 0.32 year
−1, and t
0 = −1.01 year (R
2 = 0.930) (
Figure 2). We found that the silver scabbardfish has an estimated lifespan of 8.35 years. Furthermore, the growth performance index of the sampled fish was 3.77. The relationships between estimated fish age and otolith morphometrics are shown in
Table 4. The linear model accounted for 75.2% to 85.4% of the variation in age. The best age prediction can be obtained by simply measuring the otolith length.
4. Discussion
This study represents the first comprehensive investigation of the age and growth patterns of the silver scabbardfish in the Adriatic Sea, contributing valuable insights into the species’ life-history and population structure.
The length structure and age estimation of the silver scabbardfish in the Adriatic revealed a population dominated by juvenile and immature individuals, with the majority of the fish being 0
+ years old (approximately 70%). This result is consistent with previous studies, which have reported a larger proportion of juveniles when commercial bottom trawl is used to sample populations [
6,
9,
12]. This gear type is size-selective and features a relatively small vertical opening, which tends to capture mostly smaller, immature specimens living on the continental shelf [
9]. In addition to a large number of juvenile and young individuals, our sampled population was also characterised by a relatively low maximum total length (123 cm) in comparison with the previously reported maximum length of 210 cm [
26] and data from other studies. Most studies of
L. caudatus in the Mediterranean and Atlantic waters have analysed populations with maximum total lengths not below 160 cm [
1,
6,
9]. These studies were conducted at greater depths (up to 800 m), and the lack of larger individuals in our sample can be explained by trawl sampling in shallower waters, only down to 200 m. Therefore,
L. caudatus is definitely a species whose populations show a specific spatial distribution with respect to depth. The length structure of the sampled populations also affected the age structure. And while the maximum estimated age of our individuals was four years, other data indicate that the oldest
L. caudatus live in the north-western Mediterranean [
6] and mid-Atlantic Ocean [
1] and are aged 8 and 7 years, respectively.
As for sex, the Adriatic population showed differences compared to other studies of the silver scabbardfish. The prevalence of females is a common tendency in deep-sea fauna [
12,
27], and in the case of the studied scabbardfish, this observation has been confirmed in almost all previous studies in Mediterranean and Atlantic waters [
1,
9,
12]. Also, females were represented in larger length classes and older age classes, and had a lower growth rate compared to males [
1,
12]. All of these results suggest that this species has a sex-specific life-history strategy. This strategy was also confirmed for the Adriatic population, except that in our case, males were generally more numerous, more represented in larger length classes, and older than females. This is not entirely non-specific for members of the Trichiuridae family, and, as already documented, male dominance is possible in the spring–summer period, i.e., the spawning period of some species [
28]. Previous studies have indicated possible sex segregation in such a way that females dominate populations during autumn and winter, and males dominate in the remaining period of the year [
28,
29]. Given that the samples in our study originated exclusively from the spring period, the obtained result is not surprising. However, it should be considered that less than 30% of individuals in our sample were successfully sexed, so we are talking about a relatively small sample. Additionally, we believe that this result could also be due to sampling bias (the type of fishing gear and sampling depth). Nevertheless, these findings carry important implications for fisheries management. A reduced proportion of females in the population could indicate lower reproductive potential, particularly if females are more vulnerable to fishing pressure due to their size, behaviour or habitat preferences [
30]. Given that females often reach larger sizes and older ages in many
L. caudatus populations [
1,
9,
12], targeted removal of these individuals could disproportionately affect stock productivity. For effective and sustainable management, it is crucial to monitor sex ratios and the timing of sexual maturity. Incorporating sex-specific life-history traits into stock assessment models would improve accuracy, help in differing vulnerabilities between males and females and support more resilient population dynamics.
Due to the small number of males and females, we modelled age on the total sample, regardless of sex. The von Bertalanffy growth model described the growth of
L. caudatus well, with an estimated asymptotic length of 134.98 cm and a growth constant of 0.32 year
−1. These values suggest a high growth rate of the species, with individuals approaching their maximum length in the sample in approximately 4 to 5 years. However, we acknowledge that there are some uncertainties regarding the observed length at time 0 from the growth model. While this model suggests an initial length close to 40.0 cm, which is larger than the size of the smallest collected individual (20.7 cm), this value should not be interpreted literally as the length at hatching. As noted by Robertson [
7],
L. caudatus hatches at a total length of 4.75 to 5.03 mm. So, the back-calculated length at time 0 represents an extrapolation of the von Bertalanffy growth curve based on the size range of the analysed samples. This pattern is consistent with findings from other studies on
L. caudatus [
6,
8,
9], where the observed length at time 0 often exceeds the known hatching size and corresponds more closely with the lower length from the length range of the sampled fish. These observations may indicate that the length at time 0 in such models primarily reflects the available data rather than the true size at hatching. In future work, incorporating more empirical data on early life stages and, more importantly, expanding the size range of samples (including the smallest individuals) could help refine the growth model parameters and reduce this source of uncertainty.
Although the estimated asymptotic length is much lower than the results obtained in other studies (≥182 cm) [
1], when analysing the growth constant, the Adriatic population has a growth rate similar to other Mediterranean populations (0.20–0.30 year
−1) [
6,
9], suggesting that
L. caudatus is a fast-growing species. Interestingly, the Atlantic populations, from Azores, show somewhat slower growth, and the only exception is the population around the Canaries, which is much closer in its growth pattern to the Mediterranean specimens [
8]. The slower growth that characterises populations in the Atlantic waters is a commonly accepted characteristic of deep-water species [
1,
31]. Such species, as k-strategists, tend to exhibit slow growth and attain high longevity [
31]. As a contribution to this strategy, the calculated longevity of Atlantic populations (except those from the Canary Islands) is high, ranging from 20 to 30 years [
1]. Considering the above, the rapid growth of this fish in the Canary Islands and the Mediterranean (Catalan, Ionian and Adriatic Seas), with a relatively low longevity of 8 to 11 years [
6,
9], may seem surprising at first. However, in addition to examples for
L. caudatus, faster growth in deep-sea species has been recorded for another member of the Trichiuridae family,
Aphanopus carbo [
31], as well as for some other deep-water fish families, e.g., Macrouridae and Bathysauridae [
32].
In addition to previous comparisons, we also used the growth performance index, an invaluable tool for comparing the growth curves of various populations within the same fish species [
33]. So, calculated from published data of the von Bertalanffy growth parameters, the Adriatic population of
L. caudatus is, according to the values, placed between the Atlantic (3.67–3.71) and Mediterranean ones (3.98–3.99) [
1,
6]. This variation pattern in the performance indices of different populations can be added to the previously mentioned differences, and enables us to elucidate the size and age distinction inherent to Atlantic and Mediterranean populations. Differences in growth parameters might be observed due to the different habitat characteristics of each study area, and, therefore, they highlight the influence of spatial heterogeneity on growth processes and the importance of considering this kind of stratification in analysed fish. This could be important information in the future for the effective management and conservation of this species, as separate units are closely linked to their location. The relevance of these findings extends beyond biological characterisation and could have important implications for regional fisheries assessments. The relatively fast growth and short lifespan of
L. caudatus in the Adriatic, combined with the dominance of juveniles/immature fish and absence of large individuals, may indicate a population structure that is especially sensitive to exploitation. Although there is currently no targeted fishery for this species in the Adriatic, the overfishing scenario documented in the central Mediterranean [
2,
12] raises concerns about the vulnerability of similar populations if fishing pressure increases. Furthermore, if stock assessments are based on generalised parameters without accounting for regional specifics and variations in growth and life-history traits, they risk misrepresenting the true status of the species. Our findings support the need for incorporating Adriatic-specific biological data into management models to ensure that potential future exploitation will not lead to the same overexploitation pattern observed elsewhere in the Mediterranean. In this sense, our results not only fill a regional knowledge gap but also provide essential data for responsible, location-specific stock management. Given the frequent need for more efficient, faster and cheaper methods of estimating the age of fish [
34], we also tested relationships that could facilitate age estimation. The linear relationships found between fish total length and age, on one hand, and otolith morphometrics, on the other hand, and their high coefficients of determination suggest that otolith measurements can be used to infer the size and age of the Adriatic population with a high degree of accuracy.