Comparing Ecosystem Structure and Function of the Geheyan Reservoir Based on the Ecopath Model After a Fishing Ban
Abstract
1. Introduction
2. Materials and Methods
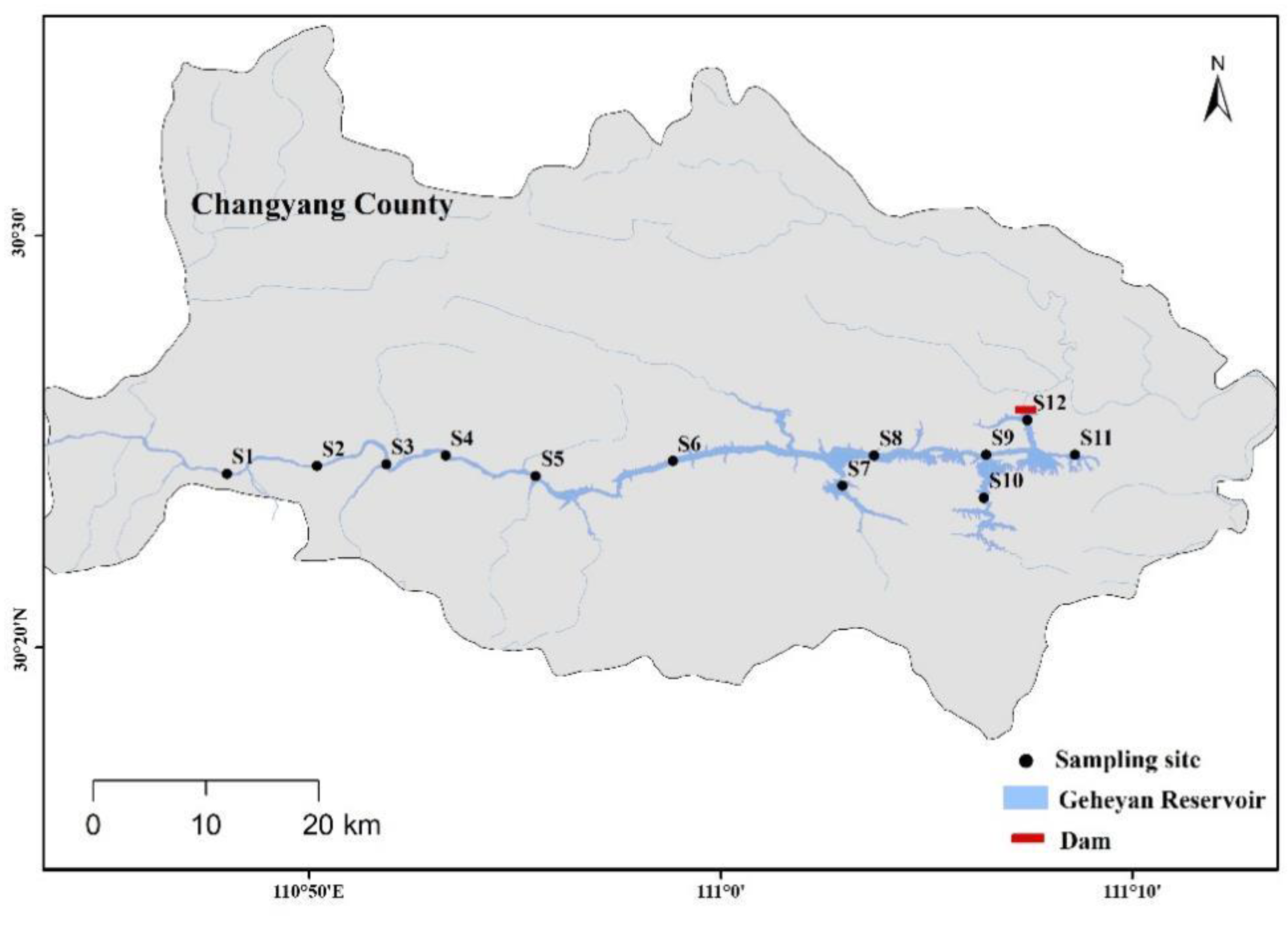
2.1. Study Area and Sampling Procedure
2.2. Modeling Principles
2.3. Functional Grouping
2.4. Data Sources and Parameter Estimation
2.4.1. Biomass (B)
2.4.2. Production (P) and the P/B Coefficient
2.4.3. Q/B Coefficient
2.4.4. EE (Ecological Efficiency)
2.4.5. Detritus
2.4.6. Diet Composition Matrix
2.5. Debugging and Optimisation of the Model
2.6. Comparing Ecosystem Characteristics Pre-And Post-Ban
3. Results
3.1. Model Parameter Output
3.2. Food Web Structure and Trophic Level Analysis
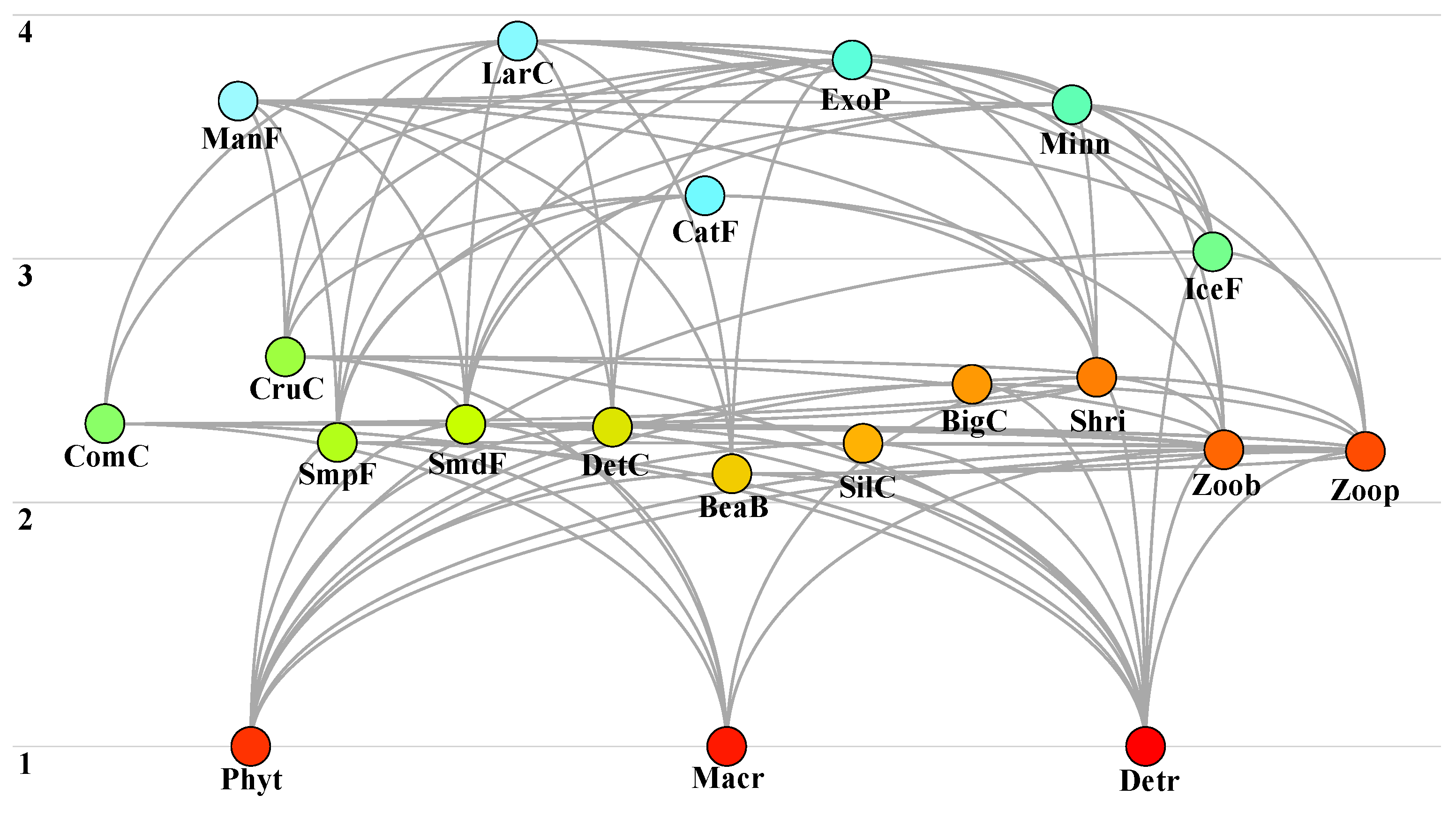
3.2.1. Food Web Structure
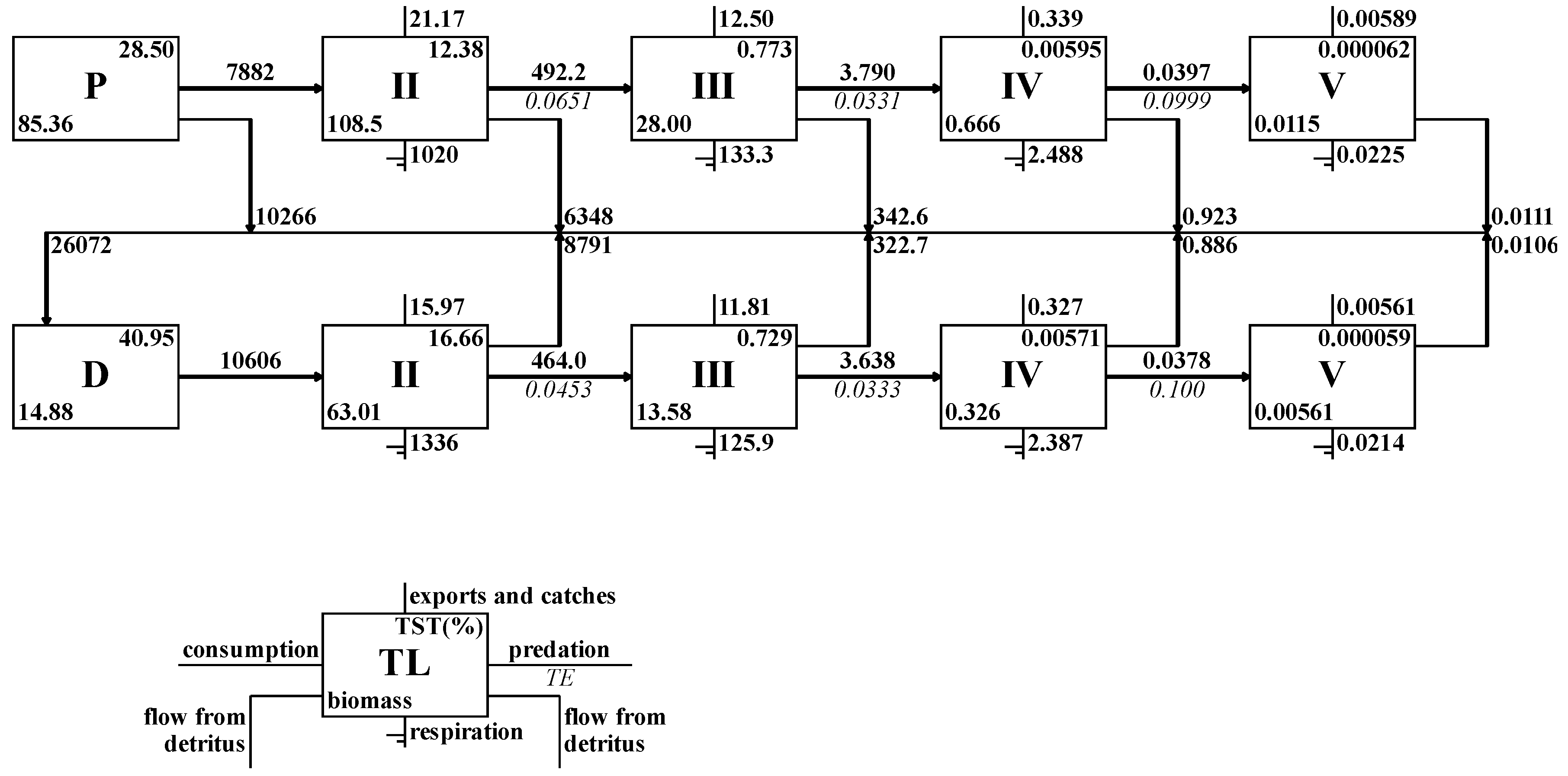
3.2.2. Transfer Efficiency
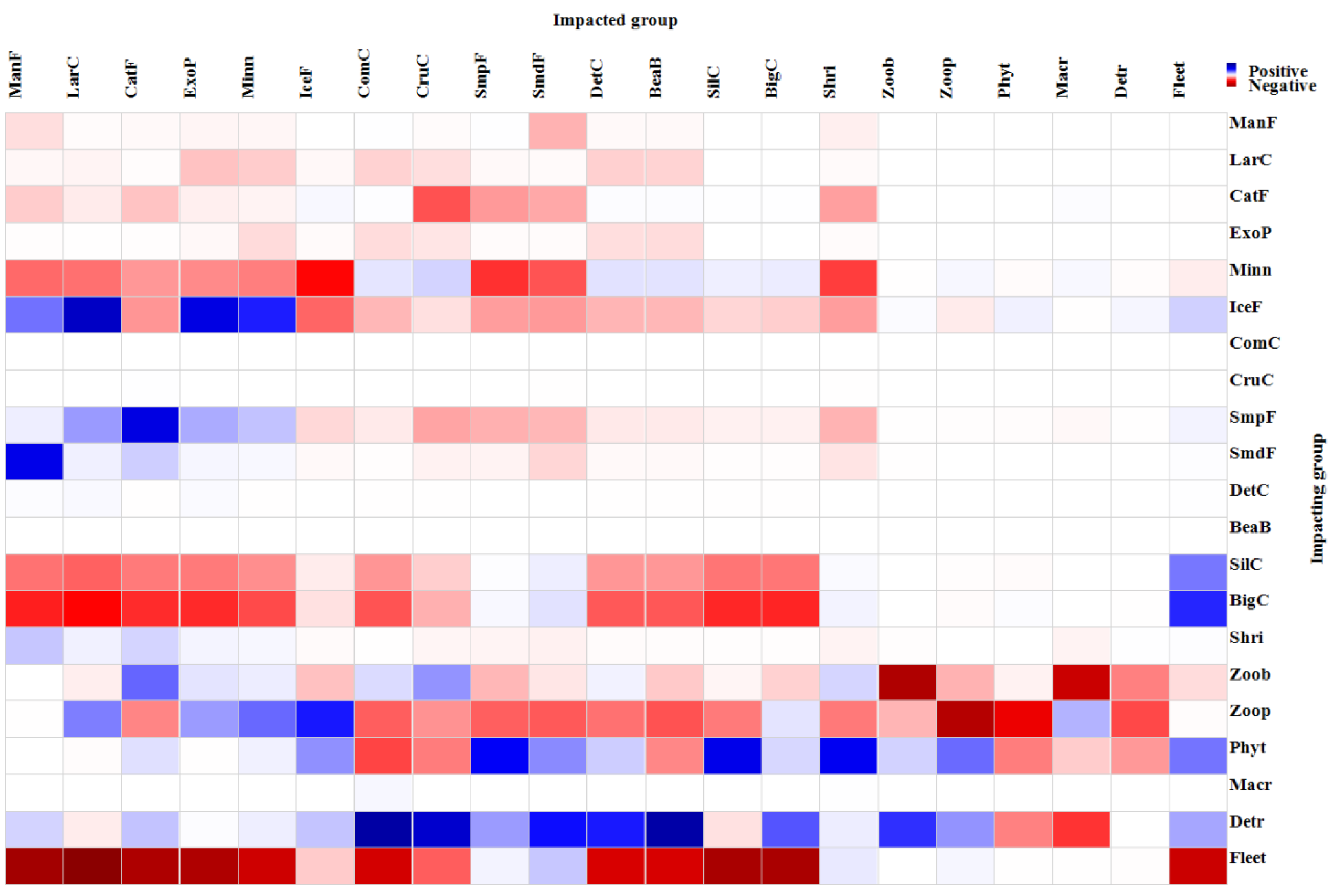
3.2.3. Mixed Trophic Effect
3.3. Ecosystem Parameters
4. Discussion
4.1. Ecopath Model Accuracy Assessment
4.2. Food Web Dynamics and Trophic Interactions
4.3. Comparative Analysis with Similar Water Bodies
4.4. Management Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Functional Groups | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ManF | |||||||||||||||||
| 2 | LarC | |||||||||||||||||
| 3 | CatF | |||||||||||||||||
| 4 | ExoP | 0.0044 | 0.0081 | |||||||||||||||
| 5 | Minn | 0.0431 | 0.079 | 0.0712 | ||||||||||||||
| 6 | IceF | 0.3592 | 0.6576 | 0.5936 | 0.4973 | |||||||||||||
| 7 | ComC | 0.0007 | 0.0007 | |||||||||||||||
| 8 | CruC | 0.0004 | 0.0007 | 0.002 | 0.0007 | |||||||||||||
| 9 | SmpF | 0.1021 | 0.1869 | 0.4647 | 0.1687 | 0.1413 | ||||||||||||
| 10 | SmdF | 0.4019 | 0.0304 | 0.0755 | 0.0274 | 0.0230 | 0.0024 | 0.0043 | ||||||||||
| 11 | DetC | 0.0049 | 0.009 | 0.0080 | ||||||||||||||
| 12 | BeaB | 0.0004 | 0.0007 | 0.0007 | ||||||||||||||
| 13 | SilC | |||||||||||||||||
| 14 | BigC | |||||||||||||||||
| 15 | Shri | 0.0836 | 0.0259 | 0.0644 | 0.0234 | 0.0196 | 0.002 | 0.0037 | 0.001 | |||||||||
| 16 | Zoob | 0.3934 | 0.1056 | 0.1118 | 0.261 | 0.482 | 0.1426 | 0.1383 | 0.0529 | 0.2295 | 0.0969 | |||||||
| 17 | Zoop | 0.001 | 0.2070 | 0.85 | 0.2040 | 0.1206 | 0.1169 | 0.0448 | 0.201 | 0.401 | 0.1941 | 0.0819 | 0.1737 | |||||
| 18 | Phyt | 0.5009 | 0.296 | 0.2871 | 0.1098 | 0.625 | 0.229 | 0.4754 | 0.2011 | 0.4263 | ||||||||
| 19 | Macr | 0.10 | 0.01 | 0.0001 | 0.0001 | 0.001 | 0.0001 | |||||||||||
| 20 | Detr | 0.05 | 0.7246 | 0.51 | 0.295 | 0.4397 | 0.4577 | 0.7925 | 0.174 | 0.37 | 0.10 | 0.62 | 0.40 |
References
- Zhang, W.W.; Gao, S.S.; Li, M.L.; Chen, Y.; Fohrer, N.; Costanza, R.; Li, Y.Y.; Chen, Z.J. Vertical Distribution Characteristics and Driving Factors of Bacterioplankton and Nitrogen Phosphorus Cycle Genes in Danjiangkou Reservoir. Huanjing Kexue 2024, 45, 3995–4005. [Google Scholar] [PubMed]
- Kennedy, R.H.; Thornton, K.W.; Ford, D.E. Characterization of the reservoir ecosystem. In Microbial Processes in Reservoirs; Springer: Dordrecht, The Netherlands, 1985; pp. 29–38. [Google Scholar]
- Tundisi, J.G. Reservoirs: New challenges for ecosystem studies and environmental management. Water Secur. 2018, 4, 1–7. [Google Scholar]
- Guo, Z.F.; Boeing, W.J.; Borgomeo, E.; Xu, Y.Y.; Weng, Y. Linking reservoir ecosystems research to the sustainable development goals. Sci. Total Environ. 2021, 781, 146769. [Google Scholar] [CrossRef]
- Polovina, J.J. Model of a coral reef ecosystem: I. The ECOPATH model and its application to French Frigate Shoals. Coral Reefs 1984, 3, 1–11. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J.; Pauly, D. Ecopath with Ecosim: A user’s guide. Fish. Cent. Univ. Br. Columbia Vanc. 2005, 154, 31. [Google Scholar]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Pauly, D. Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature and salinity. Mar. Freshw. Res. 1998, 49, 447–453. [Google Scholar] [CrossRef]
- Sinnickson, D.; Chagaris, D.; Allen, M. Exploring impacts of river discharge on forage fish and predators using ecopath with ecosim. Front. Mar. Sci. 2021, 8, 689950. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol. Modell. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Qi, S.W.; Yan, F.Z.; Wang, S.J.; Xu, R.C. Characteristics, mechanism and development tendency of deformation of Maoping landslide after commission of Geheyan reservoir on the Qingjiang River, Hubei Province, China. Eng. Geol. 2006, 86, 37–51. [Google Scholar] [CrossRef]
- He, Y.; Chen, T. Does the 10-year fishing ban compensation policy in the Yangtze River basin improve the livelihoods of fishing households? Evidence from Ma’anshan City, China. Agriculture 2022, 12, 2088. [Google Scholar] [CrossRef]
- Feng, J.; Wen, Y.L.; Zhang, H.Y.; Hou, Y.L.; Zhang, Z. Trap or Opportunity: Impact of the Fishing Ban Compensation Policy on the Income of Returning Fishermen in China. Sustainability 2024, 16, 4401. [Google Scholar] [CrossRef]
- Zeller, D.; Reinert, J. Modelling spatial closures and fishing effort restrictions in the Faroe Islands marine ecosystem. Ecol. Modell. 2004, 172, 403–420. [Google Scholar] [CrossRef]
- Gribble, N.A. GBR-prawn: Modelling ecosystem impacts of changes in fisheries management of the commercial prawn (shrimp) trawl fishery in the far northern Great Barrier Reef. Fish. Res. 2003, 65, 493–506. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, X.; Huang, L.; Zheng, X.; Du, J.; Yu, W.; Chen, G.; Hu, W.; Gao, S. The ecological functions and risks of expansive bivalve-macroalgae polyculture: A case study in Sansha Bay, China. Aquaculture 2022, 560, 738549. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Yan, Y.; Liang, Y. Energy flow of macrozoobenthic community in a macrophytic lake, Biandantang Lake. Acta Ecol. Sin. 2023, 23, 527–538. [Google Scholar]
- He, Z.H. Freshwater Ecology; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Huang, G.; Wang, Q.D.; Du, X.; Feng, K.; Ye, S.W.; Yuan, J.; Liu, J.S.; Li, Z.J.; De Silva, S.S. Modeling trophic interaction and impacts of introduced icefish (Neosalanx taihuenis Chen) in three large reservoirs in the Yangtze River basin, China. Hydrobiologia 2020, 847, 3637–3657. [Google Scholar] [CrossRef]
- Park, R.A. A generalized model for simulating lake ecosystems. Simulation 1974, 23, 33–50. [Google Scholar] [CrossRef]
- Scavia, D.; Bloomfield, J.A.; Fisher, J.S.; Nagy, J.; Park, R.A. Documentation of CLEANX: A generalized model for simulating the open-water ecosystems of lakes. Simulation 1974, 23, 51–56. [Google Scholar] [CrossRef]
- Heymans, J.J.; Shannon, L.J.; Jarre, A. Changes in the northern Benguela ecosystem over three decades: 1970s, 1980s, and 1990s. Ecol. Model. 2004, 172, 175–195. [Google Scholar] [CrossRef]
- Guo, C.B.; Chen, Y.S.; Li, W.; Xie, S.G.; Lek, S.; Li, Z.J. Food web structure and ecosystem properties of the largest impounded lake along the eastern route of China′s South-to-North Water Diversion Project. Ecol. Inform. 2018, 43, 174–184. [Google Scholar] [CrossRef]
- Zhang, Y. The Study of Fishery Resource and Ecopath Model in Jinshahe Reservoir Ecosystem, Hubei Province. Master’s Dissertation, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Mitra, A.; Flynn, K.J.; Tillmann, U.; Raven, J.A.; Caron, D.; Stoecker, D.K.; Not, F.; Hansen, P.J.; Hallegraeff, G.; Sanders, R.; et al. Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: Incorporation of diverse mixotrophic strategies. Protist 2016, 167, 106–120. [Google Scholar]
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015, 347, 1257594. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, K.; Wang, X.; Zhang, J.A.; Duan, J.; Hu, Z.; Liu, Q. Food Web Structure and Ecosystem Functions of the Water Source in the Middle Route of China’s South-to-North Water Diversion Project. Fishes 2024, 9, 202. [Google Scholar] [CrossRef]
- Fortuna, C.M.; Fortibuoni, T.; Bueno-Pardo, J.; Coll, M.; Franco, A.; Giménez, J.; Stranga, Y.; Peck, M.A.; Claver, C.; Brasseur, S.; et al. Top predator status and trends: Ecological implications, monitoring and mitigation strategies to promote ecosystem-based management. Front. Mar. Sci. 2024, 11, 1282091. [Google Scholar] [CrossRef]
- Barnes, A.D.; Jochum, M.; Lefcheck, J.S.; Eisenhauer, N.; Scherber, C.; O’Connor, M.I.; de Ruiter, P.; Brose, U. Energy flux: The link between multitrophic biodiversity and ecosystem functioning. Trends Ecol. Evol. 2018, 33, 186–197. [Google Scholar] [CrossRef]
- Östman, Ö.; Eklöf, J.; Eriksson, B.K.; Olsson, J.; Moksnes, P.O.; Bergström, U. Top-down control as important as nutrient enrichment for eutrophication effects in North Atlantic coastal ecosystems. J. Appl. Ecol. 2016, 53, 1138–1147. [Google Scholar] [CrossRef]
- Pringle, R.M.; Hutchinson, M.C. Resolving food-web structure. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 55–80. [Google Scholar] [CrossRef]
- Thompson, R.M.; Brose, U.; Dunne, J.A.; Hall, R.O., Jr.; Hladyz, S.; Kitching, R.L.; Martinez, Z.D.; Rantala, H.; Romanuk, T.N.; Stouffer, D.B.; et al. Food webs: Reconciling the structure and function of biodiversity. Trends Ecol. Evol. 2012, 27, 689–697. [Google Scholar] [CrossRef]
- Qiu, L.H.; Qiu, Y.H.; Peng, L.G.; Shen, J.Z.; Li, G.Y.; Li, J.G. Enhancing Fishery Management in Tanghe Reservoir, China: Insights from Food Web Structure and Ecosystem Analysis. Water 2024, 16, 200. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Lawrence, M.J.; Deslauriers, D.; Filgueira, R.; Boyd, R.J.; Cooke, S.J. Applied fish bioenergetics. Fish Physiol. 2022, 39, 141–188. [Google Scholar]
- Xie, C.; Dai, B.G.; Wu, J.J.; Liu, Y.Z.; Jiang, Z.G. Initial recovery of fish faunas following the implementation of pen-culture and fishing bans in floodplain lakes along the Yangtze River. JEM 2022, 319, 115743. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, Z.R.; Zhang, Y.M.; Qiao, Q. How can governments and fishermen collaborate to participate in a fishing ban for ecological restoration? JEM 2024, 360, 120958. [Google Scholar] [CrossRef] [PubMed]
- Ngupula, G.W.; Kayanda, R. Benthic macrofauna community composition, abundance and distribution in the Tanzanian and Ugandan inshore and offshore waters of Lake Victoria. Afr. J. Aquat. Sci. 2010, 35, 185–192. [Google Scholar] [CrossRef]
- Aura, C.M.; Musa, S.; Yongo, E.; Okechi, J.K.; Njiru, J.M.; Ogari, Z.; Wanyama, R.; Charo-Karisa, H.; Mbugua, H.; Kidera, S.; et al. Integration of mapping and socio-economic status of cage culture: Towards balancing lake-use and culture fisheries in Lake Victoria, Kenya. Aquac. Res. 2017, 49, 532–545. [Google Scholar]
- Xia, Z.J.; Wang, Q.; Brosse, S.; Heino, J.; Wang, Z.X.; Liao, Z.H.; Li, X.H.; He, Y.F.; Liu, F.; Wang, J.W. Trait-based analyses reveal the recovery of riverine fish communities after a fishing ban. Rev. Fish Biol. Fish. 2024, 35, 431–445. [Google Scholar]
- Zhou, L.B.; Luo, M.Y.; Hong, P.B.; Leroux, S.; Chen, F.Z.; Wang, S.P. Energy transfer efficiency rather than productivity determines the strength of aquatic trophic cascades. Ecology 2025, 106, e4482. [Google Scholar]
- Cebrian, J. Energy flows in ecosystems. Science 2015, 349, 1053–1054. [Google Scholar] [CrossRef]
- Li, H.X.; Jin, L.; Si, Y.J.; Mu, J.D.; Liu, Z.N.; Liu, C.Q.; Zhang, Y.J. Lake Restoration Improved Ecosystem Maturity Through Regime Shifts—A Case Study of Lake Baiyangdian, China. Sustainability 2024, 16, 9372. [Google Scholar] [CrossRef]
- MacArthur, R. Fluctuations of animal populations and a measure of community stability. Ecology 1955, 36, 533–536. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Rooney, N.; McCann, K.S. Integrating food web diversity, structure and stability. Trends Ecol. Evol. 2012, 27, 40–46. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, R.; Dowling, N.; Cohen, J.E. Longer food chains in pelagic ecosystems: Trophic energetics of animal body size and metabolic efficiency. Am. Nat. 2016, 188, 76–86. [Google Scholar] [CrossRef]
- Gido, K.B.; Brown, J.H. Invasion of north American drainages by alien fish species. Freshw. Biol. 2010, 42, 387–399. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Valero-Rodriguez, J.M.; Peñalver-García, J.; Izquierdo-Gomez, D.; Sanchez-Jerez, P. Linking coastal aquaculture of meagre Argyrosomus regius and Western Mediterranean coastal fisheries through escapes incidents. Fish. Manag. Ecol. 2015, 22, 317–325. [Google Scholar] [CrossRef]
- Allan, J.D.; Abell, R.; Hogan, Z.E.B.; Revenga, C.; Taylor, B.W.; Welcomme, R.L.; Winemiller, K. Overfishing of inland waters. BioScience 2005, 55, 1041–1051. [Google Scholar] [CrossRef]
- Gao, X.; Liang, J.; Zhu, Z.; Li, W.; Lu, L.; Qiu, X.; Li, S.; Tang, N.; Li, X. Unraveling the impact of drought on waterbird community assembly and conservation strategies. J. Environ. Manag. 2025, 373, 123685. [Google Scholar] [CrossRef]
| No | Code | Functional Group | Composition |
|---|---|---|---|
| 1 | ManF | Mandarin fish | Siniperca chuatsi (Basilewsky, 1855), Siniperca knerii Garman, 1912 |
| 2 | LarC | Large culter | Culter alburnus Basilewsky, 1855 |
| 3 | CatF | Catfish | Pseudobagrus vachelli (Richardson, 1846), Pelteobagrus fulvidraco (Richardson, 1846), Silurus meridionalis Chen, 1977 |
| 4 | ExoP | Exotic perch | Sander lucioperca (Linnaeus, 1758) |
| 5 | Minn | Minnow | Opsariichthys bidens Günther, 1873 |
| 6 | IceF | Icefish | Neosalanx taihuensis Chen, 1956 |
| 7 | ComC | Common carp | Cyprinus carpio Linnaeus, 1758 |
| 8 | CruC | Crucian carp | Carassius auratus (Linnaeus, 1758) |
| 9 | SmpF | Small pelagic fish | Hemiculter leucisculus (Basilewsky, 1855), Rhodeus ocellatus (Kner, 1866), Rhodeus sinensis Günther, 1868 |
| 10 | SmdF | Small demersal fish | Rhinogobius giurinus (Rutter, 1897), Saurogobio dabryi Bleeker, 1871 |
| 11 | BeaB | Beardless sucking bard | Garra imberaba Garman, 1912 |
| 12 | SilC | Silver carp | Hypophthalmichthys molitrix (Valenciennes, 1844) |
| 13 | BigC | Bighead carp | Hypophthalmichthys nobilis (Richardson, 1845) |
| 14 | DetC | Detritivorous carp | Distoechodon tumirostris Peters, 1881, Hemibarbus maculatus Bleeker, 1871 |
| 15 | Shri | Shrimp | Macrobrachium nipponense (De Haan, 1849), Palaemon modestus (Heller, 1862) |
| 16 | Zoob | Zoobenthos | Branchiura sowerbyi Beddard, 1892, Limnodrilus hoffmeisteri Claparède, 1862, Bellamya sp., Corbicula fluminea (Müller, 1774), Chironomus sp., Polypedilum sp. |
| 17 | Zoop | Zooplankton | Rotifer, Nauplius, Strobilidium sp., Difflugia globulosa Dujardin, 1837, Tintinnopsis entzii Daday, 1892 |
| 18 | Phyt | Phytoplankton | Cyanophyta, Chlorophyta, Bacillariophyta, Cryptophyta |
| 19 | Macr | Macrophyte | Potamogeton crispus L. |
| 20 | Detr | Detritus | Bacteria, Organic debris |
| Code | Trophic Level | Biomass (t/km2) | P/B (Year−1) | Q/B (Year−1) | EE | P/Q |
|---|---|---|---|---|---|---|
| ManF | 3.65 | 0.04 | 1.16 | 5.99 | 0.86 | 0.19 |
| LarC | 3.89 | 0.13 | 1.56 | 6.32 | 0.64 | 0.25 |
| CatF | 3.26 | 0.20 | 1.70 | 7.51 | 0.59 | 0.23 |
| ExoP | 3.82 | 0.10 | 1.79 | 6.40 | 0.60 | 0.28 |
| Minn | 3.63 | 0.95 | 1.27 | 12.68 | 0.89 | 0.10 |
| IceF | 3.03 | 7.92 | 7.80 | 31.93 | 0.24 | 0.24 |
| ComC | 2.32 | 0.01 | 1.59 | 5.26 | 0.70 | 0.30 |
| CruC | 2.60 | 0.01 | 2.23 | 13.12 | 0.66 | 0.17 |
| SmpF | 2.25 | 2.25 | 2.31 | 43.48 | 0.95 | 0.05 |
| SmdF | 2.32 | 0.37 | 2.50 | 20.93 | 0.97 | 0.12 |
| DetC | 2.31 | 0.11 | 1.37 | 8.28 | 0.82 | 0.17 |
| BeaB | 2.12 | 0.01 | 1.96 | 26.40 | 0.57 | 0.07 |
| SilC | 2.24 | 19.13 | 1.31 | 6.00 | 0.76 | 0.22 |
| BigC | 2.49 | 30.61 | 1.51 | 5.10 | 0.66 | 0.30 |
| Shri | 2.51 | 0.31 | 3.09 | 41.23 | 0.74 | 0.07 |
| Zoob | 2.22 | 40.67 | 20.21 | 186.16 | 0.90 | 0.11 |
| Zoop | 2.21 | 34.39 | 105.91 | 420.78 | 0.95 | 0.25 |
| Phyt | 1.00 | 84.40 | 215.00 | 0.43 | ||
| Macr | 1.00 | 0.96 | 1.25 | 0.65 | ||
| Detr | 1.00 | 14.88 | 0.69 |
| Parameters | Value | Unit |
|---|---|---|
| Ecosystem properties | ||
| Sum of all consumption | 22,698.69 | t/(km2·year) |
| Sum of all exports | 15,527.75 | t/(km2·year) |
| Sum of all respiratory flows | 2619.88 | t/(km2·year) |
| Sum of all flows into detritus | 26,071.92 | t/(km2·year) |
| Total system throughput | 66,918.24 | t/(km2·year) |
| Sum of all production | 22,753.85 | t/(km2·year) |
| Mean trophic level of the catch | 2.50 | - |
| Gross efficiency | 0.003 | - |
| Calculated total net primary production | 18,147.63 | t/(km2·year) |
| Total biomass (excluding detritus) | 222.55 | t/km2 |
| Total catch | 62.13 | t/(km2·year) |
| Net system production | 15,527.75 | t/(km2·year) |
| Ecosystem maturity | ||
| Total primary production/total respiration | 6.93 | |
| Total primary production/total biomass | 81.54 | |
| Total biomass/total throughput | 0.003 | /year |
| Food web structure | ||
| Connectivity index | 0.25 | |
| System omnivory index | 0.23 | |
| Finn’s cycling index | 24.61 | % |
| Finn’s mean path length | 3.69 | |
| Model reliability | ||
| Pedigree index | 0.47 | |
| Measure of fit | 2.20 |
| Parameters | Qiandao Lake | Fanshuijiang Reservoir | Manwan Reservoir | Study Area |
|---|---|---|---|---|
| Sum of all consumption (t/(km2·year)) | 5337.5 | 24,102 | 1563.3 | 22,698.69 |
| Sum of all exports (t/(km2·year)) | 3083.1 | 23,809.5 | 1274.3 | 15,527.75 |
| Sum of all respiratory flows (t/(km2·year)) | 1131.5 | 5671.0 | 868.1 | 2619.88 |
| Sum of all flows into detritus (t/(km2·year)) | 5990.3 | 2357.0 | 1844.0 | 26,071.92 |
| Total system throughput (t/(km2·year)) | 15,543.0 | 78,938.0 | 5550.0 | 66,918.24 |
| Sum of all production (t/(km2·year)) | 4436.0 | 29,240.9 | 2524.8 | 22,753.85 |
| Mean trophic level of the catch | 2.65 | 2.75 | 2.47 | 2.50 |
| Total catch (t/km2) | 10.187 | 114.06 | - | 62.13 |
| Total primary production/total respiration | 1.944 | 4.820 | 2.470 | 6.93 |
| Total primary production/total biomass | 73.96 | 161.30 | 18.90 | 81.54 |
| Connectivity index | 0.220 | 0.206 | 0.34 | 0.25 |
| System omnivory index | 0.087 | 0.062 | 0.130 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, M.; Liu, H.; Wei, N.; Meng, Z.; Hu, F.; Li, X. Comparing Ecosystem Structure and Function of the Geheyan Reservoir Based on the Ecopath Model After a Fishing Ban. Fishes 2025, 10, 168. https://doi.org/10.3390/fishes10040168
Xiang M, Liu H, Wei N, Meng Z, Hu F, Li X. Comparing Ecosystem Structure and Function of the Geheyan Reservoir Based on the Ecopath Model After a Fishing Ban. Fishes. 2025; 10(4):168. https://doi.org/10.3390/fishes10040168
Chicago/Turabian StyleXiang, Miao, Haoran Liu, Nian Wei, Zihao Meng, Feifei Hu, and Xuemei Li. 2025. "Comparing Ecosystem Structure and Function of the Geheyan Reservoir Based on the Ecopath Model After a Fishing Ban" Fishes 10, no. 4: 168. https://doi.org/10.3390/fishes10040168
APA StyleXiang, M., Liu, H., Wei, N., Meng, Z., Hu, F., & Li, X. (2025). Comparing Ecosystem Structure and Function of the Geheyan Reservoir Based on the Ecopath Model After a Fishing Ban. Fishes, 10(4), 168. https://doi.org/10.3390/fishes10040168








