Abstract
The reef fish communities of the Guanahacabibes National Park have been studied for 20 years using various methodologies that have allowed us to understand aspects of their diversity and structure. However, due to gaps in information about the abundance and distribution of mesopredators (big fish and sharks), a new study was conducted in 2017 to determine their structure, explore the influence of different factors on their spatial variability, and evaluate their behavior. To achieve this, the Baited Remote Underwater Video Surveys (BRUVs) methodology was successfully applied, locating a single set of BRUVs at 90 sites distributed across 9 sectors of the park’s functional zoning. Variability in mesopredator metrics and their potential prey was assessed through a PERMANOVA analysis; a distance-based linear model (DISTLM) was used to explore the relationship between mesopredator abundance and biological, abiotic, and condition variables; and animal behavior was classified as incidental, cautious, or aggressive. A total of 64 fish species were identified, 7 of which were mesopredators, and 3 were sharks. An uneven distribution and abundance were observed among sectors, with the most abundant mesopredators being Carcharhinus perezi, Sphyraena barracuda, and Mycteroperca bonaci. Mesopredator abundance was more closely related to the condition of zone use and its regulations than to biological and abiotic variables. Sharks were more abundant in strictly protected areas, which coincided with relatively murky waters and stronger currents. More than 50% of the observed sharks displayed exploratory and aggressive behavior towards the bait basket. The analyzed metrics validate the effectiveness of the management of the protected area and suggest the presence of healthy and resilient mesopredator fish communities.
Key Contribution:
The presence of abundant sharks and big fish on the Guanahacabibes National Park coral reefs highlights the success and effectiveness of management actions within the protected area. It also indicates the existence of healthy fish communities, which in turn supports the ecological integrity of reefs in Cuba and the Wider Caribbean Region.
1. Introduction
The Guanahacabibes National Park (GNP) encompasses a remote coral reef with low human impact [1], which implies that its coral and fish communities show great diversity, a complex three-dimensional structure, a relatively “good condition”, and great functionality [2,3,4]. In 2012, this marine protected area (MPA) was included in the List of MPAs of the Specially Protected Areas and Wildlife Protocol (SPAW); in 2018, it became part of the Network of Marine Protected Areas of the Gulf of Mexico (RedGolfo), and that same year, it was declared a Site of Hope (the only one of its kind in Cuba), promoted by the Mission Blue-Sylvia Earle Alliance in recognition of the conservation of its coral reef ecosystem, its sustainable use for tourism, and the participation of local communities in marine–coastal management [5].
Several studies have been conducted in the GNP targeting fish communities on coral reefs [3,6,7,8,9,10,11], which have been instrumental in establishing a baseline for the park’s fish associations. They encompass various aspects such as the diversity, composition, and structure of the communities, as well as spatial (habitats) and temporal variations (seasonal and yearly). Additionally, they have helped to assess long-term changes in the fish population and have contributed to a better understanding of coral reefs’ overall functioning.
Different methodologies have been used in these studies, including linear transects [12,13], stationary counts [14], and video transects [15,16]. In general, these techniques allow for the evaluation of the condition of reef fish communities using ecological indicators, and their effectiveness varies depending on the biological associations studied [17,18,19]. These methodologies provide information about different functional groups of reef fish; however, they are not entirely useful for gathering information about mesopredators and are insufficient for gathering information about sharks.
For the GNP, it is highly relevant to know the status of top predators and mesopredators, since they play a significant ecological role in the food chain, controlling prey populations (eliminating weak and diseased ones), preventing imbalances, and contributing to the preservation of the natural balance and species diversity [20,21].
For this study we considered top predators species (bull, tiger, and hammerhead sharks) as those occupying the upper trophic levels in a community, large in size (>300 cm) with a large spatial distribution covering diverse marine ecosystems, and do not have predators and mesopredators species (groupers, snappers, barracudas, and sharks) asthose occupying the highest trophic position in a community and have been observed to have strong effects on trophic dynamics and the diversity of systems [22,23]. In the specific case of sharks, mid-range sharks with intermediate sizes, ranging from 150 to 300 cm in maximum length, are included considering the criteria of the same authors [22,23]. These occupy high trophic levels, tend to be associated with reefs (within a scale of 50 km), and are vulnerable since they can be preyed upon by top predators.
Sharks play various roles in marine ecosystems, including predators, competitors, facilitators, and transporters of nutrients and food. Currently, overfishing and threats arising from climate change have greatly reduced their populations, altering their functions and effects on ecosystems throughout the Caribbean Region [24]. The loss of these predators, which were historically abundant, has been one of the primary arguments for the creation of MPAs, based on the conservation of these threatened mobile species [25,26]. One of the significant challenges faced by MPA managers is monitoring these mobile species using appropriate methodologies that enable the validation of management effectiveness in these areas. Mesopredators require the application of specific methods to attract their presence, as is the case with the BRUVs method [27,28]. This method has become the standard approach for extensive reefs and can detect large, mobile animals, even the most cautious fish, such as sharks [29]. BRUVs are not invasive or destructive and cause minimal damage to the benthic environment [30,31].
In 2015, the “Global FinPrint” https://globalfinprint.org (accessed on 15 November 2024) initiative was launched, bringing together a team of researchers and collaborators dedicated to the study of sharks and rays (elasmobranchs) to assess the conservation status of these species and determine the impacts to which they are subjected, using the BRUVs methodology [32]. Cuba joined this initiative in 2017. Three national parks in southern Cuba were included as study sites (GNP, Punta Francés, and Jardines de la Reina).
This work presents the results obtained in the GNP, implementing this methodology for the first time, with the main objectives being as follows: (1) to determine the structure of the mesopredator communities, including the composition and abundance of species; (2) to explore drivers that may determine possible spatial variability in the structure of mesopredators, including biological, abiotic, and condition variables as predictive variables; and (3) to evaluate the behavior, taking into account the reaction of the individuals to the bait.
2. Material and Methods
2.1. Study Area
The study was conducted at the fringing reefs located south of the Guanahacabibes peninsula, from Cabo Corrientes (21°46′ N; 84°25′ W) to the vicinity of the western limit (21°49′ N; 84°55′ W) of Cabo de San Antonio (Figure 1) [33]. The study area has two characteristic profiles: the eastern profile (La Bajada-Cabo Corrientes), with coral reefs of great structural complexity, composed of two terraces that culminate in a reef slope which coincides with the end of a narrow island platform; and that further west (Veral-Cabo de San Antonio), which shows more homogeneous spur and groove formations, with fewer irregularities and accidents, and with a less steep slope at the end of the platform [8,17,34,35].
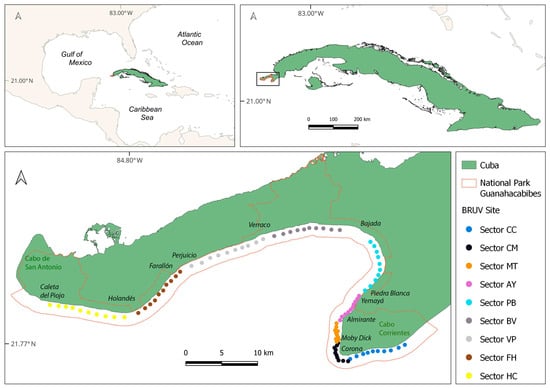
Figure 1.
Geographical location of the sectors and sites where the BRUVs were in the coral reefs of the Guanahacabibes National Park. CC: Cabo Corrientes; CM: Corona-Mobby Dick; MT: Mobby Dick-Tetas de María; AY: Almirante-Yemaya; PB: Piedra Blanca-Bajada; BV: Bajada-Verraco; VP: Verraco-Perjuicio; FH: Farallón-Holandés; HC: Holandés-Caleta del Piojo.
The marine area is affected by a local Cuban countercurrent, which has its main manifestation in intense marine currents in the vicinity of Cabo Corrientes and Cabo San Antonio [36], which favors the aggregation of species such as snappers and groupers in specific sites [5]. The tides usually reach averages of 28 cm at high tide, although the average level reaches 15 cm. With prevailing winds from the southern region, the regular level of the sea waters can rise about 30 cm in the marine area of the national park.
Established in 2001, the GNP encompasses a marine area of 15,950 ha [5]. It includes coastal and marine ecosystems and other ecological and socio-economic values in the Guanahacabibes Peninsula. The functional zonation of the MPA includes non-extractive zones (strict conservation zones, recreational diving zones, administrative zones, and catch-and-release recreational fishing zones) and extractive zones (subsistence fishing zones). Each zone is well defined and has specific regulations. Nine sectors, corresponding to four zones (Zone 1. Strict conservation: [Cabo Corrientes Sector (CC), Verraco-Perjuicio Sector (VP) and Farallón-Holandés Sector (FH)]; Zone 2. Conservation: [Bajada-Verraco Sector (BV) and Holandés-Caleta del Piojo Sector (HC)]; Zone 3. Diving: [Corona-Mobby Dick Sector (CM), Mobby Dick-Tetas de María Sector (MT) and Almirante-Yemaya Sector (AY)]; and Zone 4. Subsistence fishing and public use [Piedra Blanca-Bajada Sector (PB)]) were selected, and within them, a total of 10 sites (per sector) were established (Figure 1, Supplementary Material Table S1).
In the strict conservation zone, only scientific research and monitoring activities included in the GNP Management Plan are allowed. In the diving area, various nautical activities related to SCUBA diving, snorkeling, and boardwalks are allowed. The conservation zone permits a wider range of activities within its boundaries, including research, monitoring, ecotourism, archeology, water sports, and other related pursuits. In the subsistence and bathing fishing zone, sport-recreational fishing is permitted from the coast and from small rowing boats in the maritime waters, extending from the coast to one mile out to sea, along with recreational activities such as bathing or ecotourism, provided that these activities do not exceed the established carrying capacities for the area [5]. The most relevant is the prohibition of spearfishing operations and commercial fishing with trawls, scale dams, and gillnets.
2.2. Survey Methodology
2.2.1. Sampling Methodology for Biological Variables
Ninety sites were sampled continuously in the park’s MPA during April 2017 (Figure 1) using the Baited Remote Underwater Video Surveys (BRUVs) methodology [37]. A BRUV platform was placed at each site, built with steel reinforcement bars arranged in a pyramidal shape for greater stability and with a height of 87 cm (Figure 2). A GoPro Hero 3 (IGoPro, Inc., San Mateo, CA, USA) camera was attached in an underwater housing to the structure, placed 40 cm above the level of the seafloor and with a sunscreen to reduce glare. A box with openings (dimensions: 20 × 14 × 16 cm) was placed on an arm that was 100 cm from the camera mount, containing 1000 g of standardized tuna, used as bait to attract sharks and other predators.

Figure 2.
Overview of the Baited Remote Underwater Video Surveys (BRUVs): (A) BRUV unit with a mounted GoPro Hero3, bait arm, and bait bag prior to deployment; (B) BRUVs in the water and positioned at a reef sampling site.
One BRUV was deployed at each site between 9:00 a.m. and 5:00 p.m. The sites’ depth varied between 15 and 30 m. Each BRUV was deployed for 70 min, of which the first 10 min were considered establishment time and excluded from the analysis. It was assumed that 60 min was sufficient to count as many species and individuals as possible to appear within the camera’s angle of view [38]. The distance between the BRUVs deployed was one mile, considering that 400 m is the minimum distance recommended [39] to reduce the chances of recording individuals (demersal fish) simultaneously during one hour of sampling.
Two biological categories were defined: mesopredators (big fishes and sharks) and prey [23]. For the latter, five subcategories were defined, corresponding to the feeding habit of each prey species: herbivores, invertivores, piscivores, piscivores/invertivores, and planktivores [40]. The list of species per subcategory is presented in the Supplementary Materials (Table S2). Most of the species corresponded to medium and large sizes, which facilitated their identification and quantification. Body markings facilitated observations, mainly in sharks, where each animal was taken as a record [41].
All of the high-definition videos were thoroughly examined for processing. Species were identified and quantified (in terms of the number of individuals per species) by site. When an individual was observed, the video was paused and zoomed in to examine morphological characteristics and confirm the species, following defined identification criteria [42,43,44]. For taxonomic classification by genus and species, the criteria of [45] were followed. The total number of individuals of a species was recorded when they were all observed simultaneously at the same angle of view, in order to avoid overestimating the abundance.
2.2.2. Acquisition of Abiotic Variables
The values of two satellite variables per site were obtained from remote sensing data sets: sea surface temperature (SST) and diffuse attenuation coefficient (KD490). Both parameters belong to the SIMAR platform “https://simar.conabio.gob.mx/explorer/#. (accessed on 18 October 2024)”, and presented a daily frequency data at 1 km, over a period of six years between 2010 and 2015.
The NSST is an operational product that provides an estimate of nocturnal sea surface temperature (°C), derived from multiple satellite observations and supported by in situ data, combining two product sources for two consecutive time periods, and has been re-projected and re-sampled from different spatial resolutions of OSTIA and GHRSST-MUR [46].
KD490 is reprocessed in the SIMAR platform from images provided by the Copernicus Programme maritime service “https://resources.marine.copernicus.eu/?option=com_csw&task=results&pk_vid=53cc7e2f149e9ef9161284869762d2ae (accessed on 22 October 2024)” [47]. KD490 is based on the downwelling irradiance attenuation at 490 nm, obtained from the MODIS sensor on the AQUA satellite using the Morel algorithm [48], and is considered a proxy of seawater turbidity.
In addition to the satellite variables, two abiotic variables were included: depth and distance from the shore. The depth values of each site were taken in situ. The values of distance to the shore of each site were calculated using Qgis 3.30 software tools, based on the geographic coordinates.
2.2.3. Selection of Condition Variables
Eight condition variables were selected: subsistence fishing and swimming sites, dive sites, strict conservation sites, conservation sites, wall sites, spur and groove sites, sand sites, and rocky esplanade sites. Their distribution by site and sector appears in the Supplementary Material Table S3.
2.2.4. Animal Behavior
Animal behavior was analyzed, taking into account the classification used by Bruns and Henderson [49]: incidental (not attracted to the bait, was passing through the site), cautious (clearly attracted to the bait, but kept a safe distance from the structure), exploratory (approached the box with bait and showed interest, but it did not make contact) and aggressive (the animal bit the box in several occasions to obtain the bait). All data were saved and processed in a Microsoft Excel file (2016; 16.0).
2.3. Data Analysis
The abundance of mesopredators (total, big fish and sharks) and prey (total and per category) was represented using box plot graphs with the median (per sector) as a measure of central tendency and percentiles (25–75%) and minimums and maximums as a measure of variability. Abiotic variables were presented using box plot graphs, with the mean as a measure of central tendency and the standard deviation as a measure of variability.
Following the same procedure of Caballero-Aragón et al. [50], for the row values of NSST and KD490, their means and standard deviations were calculated, per year and for each site. Two variables were obtained with the average condition (coded as AVE) and two variables with the condition of variability (coded as SD), for a total of four satellite variables. As a result, six abiotic variables were found to be related to the biological variables.
Mesopredators’ species predominance (species composition) was visualized using a bar graph. A matrix of coral relative abundance (number of colonies per coral species divided by the total number of colonies) was constructed, including only those species that contributed to 95% of the total accumulated abundance, considering the remaining species as rare.
Differences in the metrics of the mesopredators and prey were tested by a PERMANOVA analysis [51], using a similarity matrix based on Euclidean distances for the univariate data and Bray–Curtis for multivariate data, with 9999 permutations and 0.05 significance. A one-way design was applied, using “sector” as a fixed factor. The magnitude of effects was assessed using the estimates of components of variation. Posterior pairwise test analysis was performed.
We used a distance-based linear model (DISTLM, [52]) to explore the relationship between variables per site, using per separated groups as predictable variables: biological, abiotic, and condition variables (categorical variables, expanded to binary form [52]). The abundance and species composition of mesopredators per site were used as response variables (Table 1). Euclidean distance was used as the similarity index for univariate data, while the Bray–Curtis index was selected for multivariate data. DISTLM included the forward procedure method, using the best-fitted model by the percentage of the explained variance (R2), performing 9999 permutations.

Table 1.
Predictor and response variables used in regression analyzes (DISTLM).
Correlations between significant predictors variables (obtained from DISTLM analysis) and univariate response variables (abundance) were visualized using scatter plots. Distance-based redundancy analysis (dbRDA) was used to fit and visualize the results of DISTLM analysis for multivariate data (species composition). Statistical procedures were performed using the PRIMER 6 + PERMANOVA program [52].
3. Results
A total of 10,752 individuals were observed, all of which were identified to the species level (64 species belonging to 17 families). Of these, 7 corresponded to mesopredators (3 sharks and 4 big fish) and 57 to prey (11 herbivores, 16 invertivores, 23 piscivores, 5 piscivores/invertivores, and 2 planktivores). Eleven species identified as threatened by the IUCN were recorded in the nine sectors. The invasive exotic species, lionfish, was also observed in only four sites across two sectors (Table 2).

Table 2.
Total abundance of fish identified in different habitats (reef wall, ridges and buckets, rocky esplanade, and sand) in Guanahacabibes National Park, Cuba. Pterois volitans/miles was considered a species complex because it was difficult to identify using video.
3.1. Mesopredators Abundance
Significant differences were found between sectors regarding the variables of abundance and composition by species of the mesopredators (Table 3, Supplementary Material Table S4). The median for mesopredator abundance was 3 ind. h−1 (range 0–16), with more specimens being observed in the FH, CM, and HC sectors (Figure 3A). The median for shark abundance was 1 ind. h−1 (range 0–7), with the highest number being observed in the FH and HC sectors (Figure 3B). The median for big fish abundance was 2 ind. h−1 (range 0–15), with the CM sector presenting the highest abundance (Figure 3C).

Table 3.
Results of the PERMANOVA analysis among sectors for mesopredators metrics.
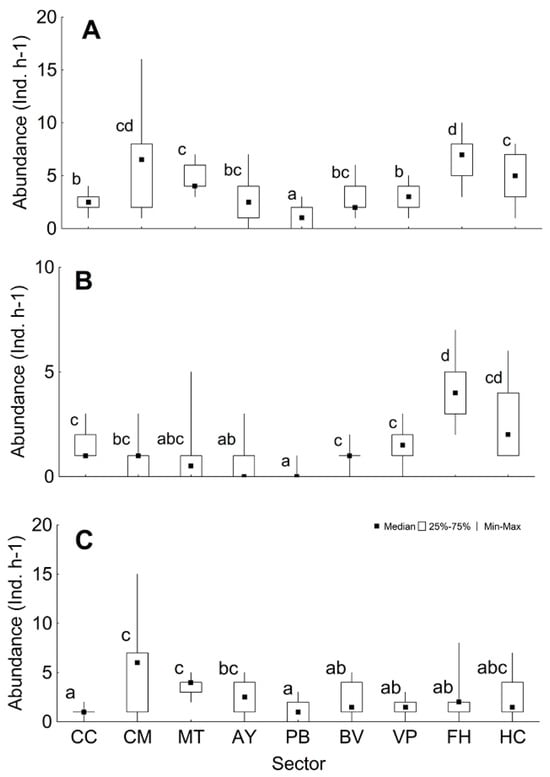
Figure 3.
Mesopredator abundance per sector (median, percentiles, range). (A) Mesopredator total, (B) sharks, and (C) big fish. The lower case letters respond to pairwise test analysis. CC: Cabo Corrientes; CM: Corona-Mobby Dick; MT: Mobby Dick-Tetas de María; AY: Almirante-Yemaya; PB: Piedra Blanca-Bajada; BV: Bajada-Verraco; VP: Verraco-Perjuicio; FH: Farallón-Holandés; HC: Holandés-Caleta del Piojo.
There was a greater predominance of big fish (64%) than sharks (Figure 4A). Most prevalent species were Carcharhinus perezi (33%), followed by Sphyraena barracuda (26%) and Mycteroperca bonaci (18%), with an unequal distribution between sectors (Figure 4B).
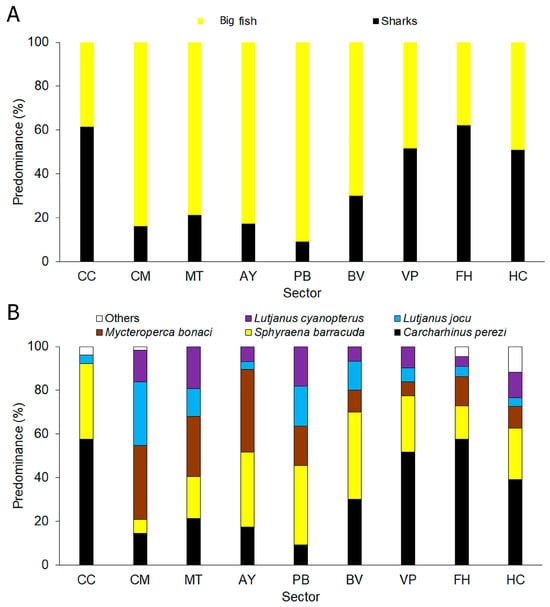
Figure 4.
Mesopredator species composition per sector. (A) Sharks and big fish predominance. (B) Predominance of the most abundant species. CC: Cabo Corrientes; CM: Corona-Mobby Dick; MT: Mobby Dick-Tetas de María; AY: Almirante-Yemaya; PB: Piedra Blanca-Bajada; BV: Bajada-Verraco; VP: Verraco-Perjuicio; FH: Farallón-Holandés; HC: Holandés-Caleta del Piojo.
3.2. Prey Abundance
The median for prey abundance was 74 ind. h−1 (range 10–801), with more specimens being observed in the CM and MT sectors (Figure 5A). The median abundance of herbivores was 11 ind. h−1 (range 0–148) and the median number of fish that eat invertebrates was 1.5 ind. h−1 (range 0–278), without significant differences among sectors in both cases (Figure 5B,C). Piscivores had a median abundance of 13 ind. h−1 (range 1–617), with the largest abundance in the CM and MT sectors (Figure 5D). The piscivores/invertivores group had more individuals in the CM sector (Figure 5E), with a general median of 29 ind. h−1 (Range 3–301). Planktivores were the least abundant and did not display significant differences among sectors (Figure 5F), with median of 0 ind. h−1 (range 0–29).
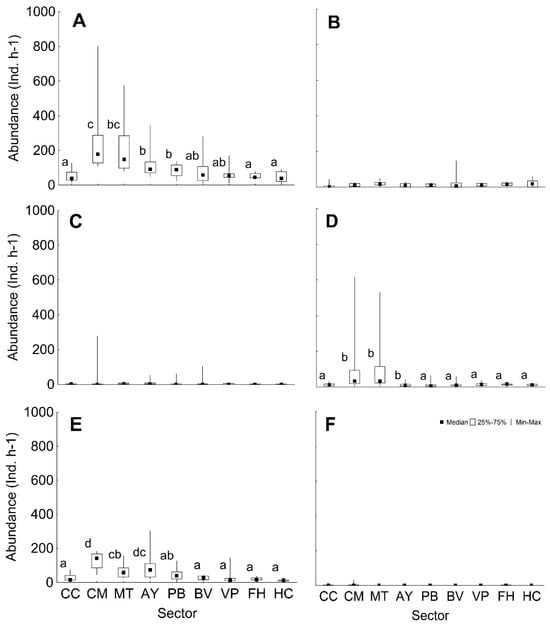
Figure 5.
Abundance of different groups of prey (median, percentiles, range) according to their type of feeding. (A) Prey total, (B) herbivores, (C) invertivores, (D) piscivores, (E) piscivores/invertivores, and (F) planktivores. The lower case letters respond to pairwise test analysis. CC: Cabo Corrientes; CM: Corona-Mobby Dick; MT: Mobby Dick-Tetas de María; AY: Almirante-Yemaya; PB: Piedra Blanca-Bajada; BV: Bajada-Verraco; VP: Verraco-Perjuicio; FH: Farallón-Holandés; HC: Holandés-Caleta del Piojo.
3.3. Abiotic Variables
The mean of AVE SST was 28.5 °C (SD 0.16), and the mean of SD SST was 1.6 °C (SD 0.09), with little variability among sectors in both cases (Figure 6A,B). The means of AVE KD490 and SD KD490 were variable among sectors (Figure 6C,D), with values of 0.03 m−1 (SD 0.001) and 0.007 m−1 (SD 0.001). The working depth varied between 12 and 30 m (Figure 6E), and the sites distanced from the shore between 165 and 1058 m (Figure 6F).
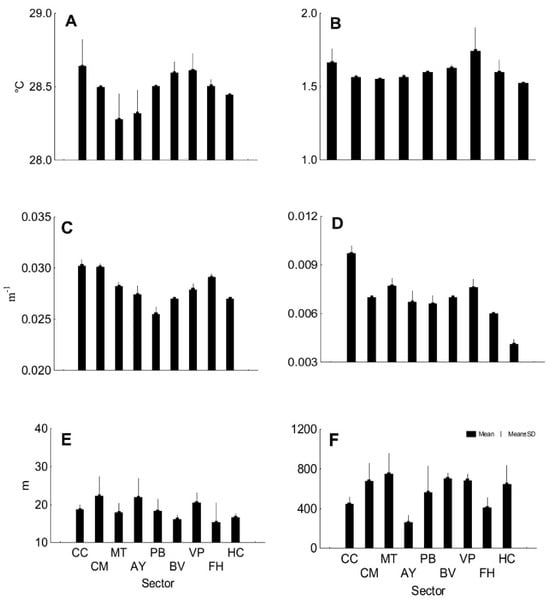
Figure 6.
Mean values per sector of satellite variables. (A) AVE SST, (B) SD SST, (C) AVE KD490, (D) SD KD490, (E) depth, and (F) distance to shore. CC: Cabo Corrientes; CM: Corona-Mobby Dick; MT: Mobby Dick-Tetas de María; AY: Almirante-Yemaya; PB: Piedra Blanca-Bajada; BV: Bajada-Verraco; VP: Verraco-Perjuicio; FH: Farallón-Holandés; HC: Holandés-Caleta del Piojo.
3.4. Regression Analysis
Biological variables explained no more than 13% of the variability in abundance. The abundance of sharks showed no significant relationship with the total abundance of prey (Supplementary Material Table S5). Overall, 4% of the variability in the abundance of big fish was explained by prey abundance; 9% of the variability in the abundance of mesopredators was explained by herbivore abundance; and the abundance of piscivores/invertivores explained 13% of shark abundance variability and 12% of big fish abundance (Figure 7). The abundance of piscivores/invertivores and piscivores explained 13% of the variability in mesopredator species composition (Supplementary Material Table S5). Regarding the relationship between univariate variables, a wide dispersion of data points was observed, reflecting the low determination coefficient values in each case.
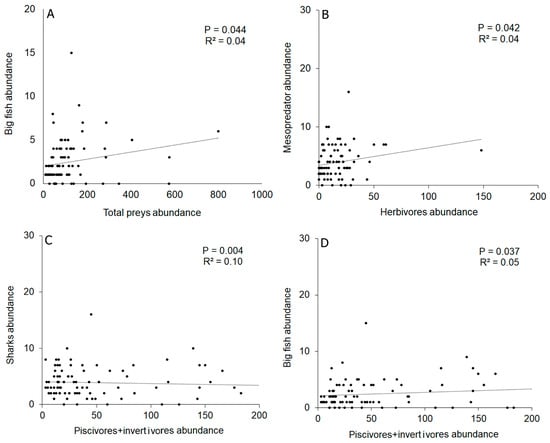
Figure 7.
Relationship between biological univariate variables, predictive (X-axis) and response (Y-axes), that showed significant relationships between them according to DISTLM analyses. P: significance value; R2: determination coefficient. (A) Relationship between the abundance of large fish and total prey; (B) Relationship between the abundance of mesodepredador and herbivores; (C) Relationship between the abundance of sharks and piscivores + invertivores; (D) Relationship between the abundance of big fish and piscivores + invertivores.
Abiotic variables explained 38% of the general mesopredator abundance variability, although only SD KD490, AVE KD490, and shore distance were significant. SD KD490 (19%) and AVE KD490 (8%) had a significant relationship with the shark abundance variability. Big fish abundance variability was explained at a rate of 5% by SD KD490 and 4% by shore distance (Supplementary Material Table S5, Figure 8). In all cases, a great dispersion was observed in the points, which corresponds to the values of R2. General mesopredator species composition was only explained at a rate of 18% by abiotic variables.
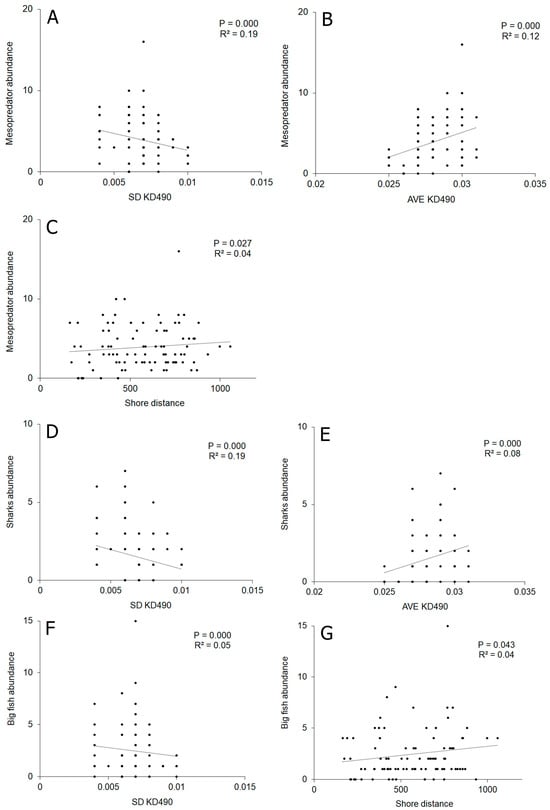
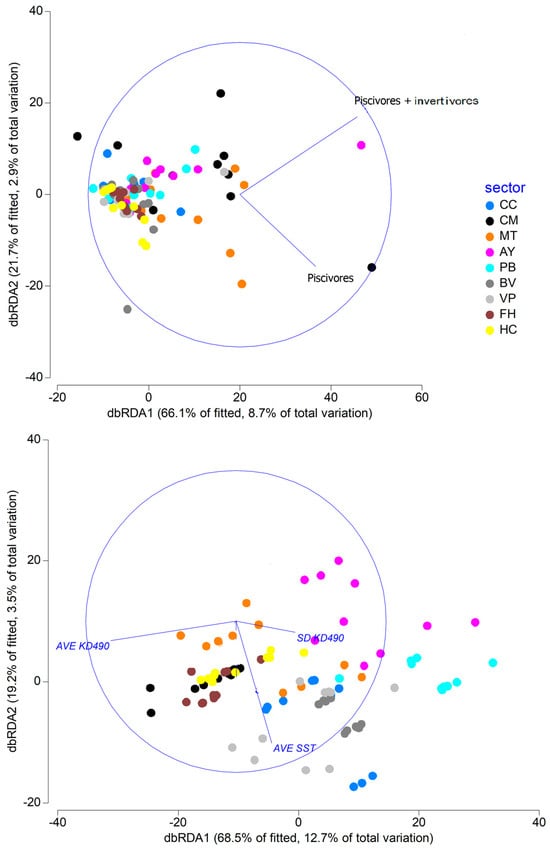

Figure 8.
Relationship between univariate variables (mesopredator variables and abiotic variables), predictive (X-axis) and response (Y-axes), that showed significant relationships between them according to DISTLM analyses. P: significance value; R2: determination coefficient. (A) Relationship between mesopredators abundance and SD KD490; (B) Relationship between mesopredators abundance and AVE KD490; (C) Relationship between mesopredators abundance and shore distance; (D) Relationship between sharks abundance and SD KD490; (E) Relationship between sharks abundance and AVE KD490; (F) Relationship between big fish; abundance and SD KD490; (G) Relationship between big fish abundance and shore distance. In general, the relationship between the variability of biological/abiotic variables and mesopredator species composition was not clear according to dbRDA analysis (Figure 9). The analysis separated some sites from the AY sector and one site from the CM sector, where piscivores/invertivores predominated, as well as a site from the CM sector, where piscivores were more abundant.

Figure 9.
Models of the dbRDA analysis. Biological (above) and abiotic variables (below) and their relationship with the mesopredator species composition. CC: Cabo Corrientes; CM: Corona-Mobby Dick; MT: Mobby Dick-Tetas de María; AY: Almirante-Yemaya; PB: Piedra Blanca-Bajada; BV: Bajada-Verraco; VP: Verraco-Perjuicio; FH: Farallón-Holandés; HC: Holandés-Caleta del Piojo.
Condition variables explained 17% of the mesopredator abundance variability, 30% of shark’s abundance variability, 18% of big fish abundance variability, and 23% of mesopredators species composition variability. In sites of subsistence fishing and swimming condition, lower mesopredator abundance was observed; sharks were more abundant in sites of strict conservation, and big fish were more abundant in diver sites (Table 4).

Table 4.
Relationship between significant predictor variables (condition) and responses variables, according to the results of the DISTLM analysis. P: significant value; R2: coordination coefficient; Cumul.: accumulated value of the R2 of all the predictive variables.
3.5. Behavior
The behavior toward BRUVs varied within and between species. Just under half of all C. perezi (47.4%) showed a cautious attitude, 39.5% showed an exploratory attitude, and 13.2% had an aggressive attitude. G. cirratum tended to be bolder, with only 26.7% showing a cautious attitude and the rest almost equally exploratory (37.8%) or aggressive (35.6%) (Figure 10).

Figure 10.
Examples of shark and ray behavior observed during the study ((A) incidental, (B) cautious, (C) exploratory, and (D) aggressive). Images extracted by Dorka Cobián Rojas during the analysis of the videos (BRUVs).
4. Discussion
Previous studies carried out in the GNP have provided a valuable comparison of the condition of the different communities of reef fish that inhabit its shoreline. However, the efficiency of each methodology used has been variable. Visual censuses using linear routes have proven to be very effective in assessing fish diversity, density, and biomass in general. This method covers a large reef area and is very useful for smaller mobile organisms with patchy distribution [8]. Stationary counting has efficiently assessed the frequency and relative abundance, with many replicates that give it high statistical reliability. However, it covers a smaller area and has limitations in evaluating density and biomass [3]. Likewise, video transects have made it possible to cover a larger area in less time and have guaranteed a permanent record of high-quality images that facilitate their reproduction [11]. In a coral reef, each biological association studied will have its most optimal sampling methodology [17,18,19].
Despite the advantages of the previously applied techniques, no shark species had been reported during the sampling, even though there were reports of sightings by divers and local fishermen in different areas of the park. Our study successfully applied the BRUVs methodology, which made it possible to determine the distribution of sharks and rays in different habitats of the GNP. Various studies have applied BRUVs in coral reefs to validate the presence of mesopredators and sharks [53,54,55,56,57] and demonstrate the effectiveness of the management of these species [58,59,60]. In this single study, the BRUVs attracted a total of 64 fish species out of the 201 recorded for the park [8], including individuals from the families Carcharhinidae, Urolophidae, and Sphyrnidae, which had not yet been reported, in addition to recording other functional groups such as fish herbivores, investors, piscivores, piscivores/invertivores, and planktivores. Along with the demonstrated efficiency, another benefit of this methodology for the study of sharks is its non-destructive and non-extractive nature, which makes it a suitable alternative with more benefits than more traditional methods of capture [30,31].
The presence of large mesopredators in the GNP indicates a healthy condition of the fish communities. Snappers, groupers, jacks, serranids, and sharks are frequently observed to play an important ecological role, as they belong to critical functional groups responsible for the top-down control of the different trophic levels. Several authors suggest that the presence of healthy populations of these species indicates high water quality, a well-structured habitat, and a balanced ecosystem [61,62,63]. The abundance values of mesopredators in the GNP are similar to those recorded in some areas of the Jardines de la Reina National Park [13,64,65] and higher than those reported in the Cayos de San Felipe [66] and Punta Francés national parks [67], as well as on the northwest coast of Cuba [68].The abundance values are also higher than those reported in studies conducted in Mexico [69] and the Dominican Republic [70].
The abundance values of mesopredator fish and sharks were spatially heterogeneous. The highest abundance values were observed in the strict conservation (sharks) and diving (fish) zones, wherein in the first zone, only research and monitoring activities are carried out, and in the second, nautical activities with specific regulations are carried out. Both locations are difficult for poachers to access and are far from the local communities in the Guanahacabibes Peninsula. In this case, the distribution of mesopredators responds adequately to the management actions established in the park. One of the most important and effective actions taken by the park administration is the collaboration agreements established with institutions that operate in the coastal–marine zone, such as the International Diving Center, the Forest Ranger Corps, and the Border Guards. Protection of this area primarily involves marine and coastal patrols conducted by accredited inspectors, who enforce regulations outlined in the Management Plan by imposing fines or sanctions on individuals engaging in illegal fishing activities [5].
Sharks were dominant in the Cabo Corrientes sector, located to the east of the peninsula, and in the Verraco-Perjuicio, Farallón-Holandés, and Holandés-Caleta del Piojo sectors, situated to the west and all located within the strict conservation zones. These areas are located at the extremes, and in addition to their management condition, they are exposed to strong marine currents, which could be one of the causes that favor the greater abundance of this group in these localities. A previous study [71] has demonstrated that sharks use regions of predicted upwelling and change their central area of space use based on tidal status (incoming versus outgoing). They also estimated that sharks’ routine metabolic rates can be reduced by 10% to 15% in updraft zones. In addition, a telemetry study [72] with species of the family Carcharhinidae in Florida, showed that the tidal current significantly influenced their swimming direction to reduce the energy cost and facilitate the capture of prey.
Studies of elasmobranchs in different regions of the world have recorded varying numbers of species, although most have been dominated by one or two species, regardless of the richness of the area [73,74,75,76]. In the GNP, C. perezi, declared endangered by the International Union for Conservation of Nature [77], was the most abundant species, with a dominance of 33%, even higher than that of G. cirratum, which has a greater distribution and abundance indices at the Caribbean level [53]. Up to seven individuals of C. perezi were observed at one site during sampling. Global studies using BRUVs have found that most deployments do not exceed a maximum number of eight sharks [78,79]. The species C. perezi and G. cirratum have generally been the most abundant recorded by BRUV studies in the region [49], as is the case of the Bahamas [29], Grand Cayman [80], and Turks and Caicos Islands [49]. Based on this evidence and supported by other authors’ criteria [23,39], C. perezi is the mesopredator shark most associated with reefs, where it spends most of its life cycle [5], and it has an ecological role like the predatory fish of these ecosystems in the Caribbean.
Deployments in the GNP showed adult females, males, and juveniles in all assessed habitats. The family Carcharhinidae uses coastal areas as nursery grounds, and that this group is the most diverse and abundant in tropical coastal waters [73]. Sharks’ use of coastal areas exposes their most critical stages to potential threats from human interaction [81]. In the case of the GNP, we can infer that it is relatively free of these threats since it has specific regulations prohibiting all types of fishing [5], in addition to being one of the most effectively protected areas in Cuba [82]. Based on the evidence in this study (high abundance of C. perezi and conservation status of habitats), we can recommend our MPA as an essential area for shark conservation in Cuba and the Caribbean region.
In diving areas of the GNP, individuals of H. americanus and A. narinari are frequently observed during SCUBA and snorkeling activities. They are the elasmobranchs most reported in reef habitats and sandy areas [49]. However, in this study, their abundance was low, possibly due to the lack of attraction to the bait used. In this case, fish belonging to the genus Sarda was used, and the rays are likely more attracted to invertivores than to fish as bait. Various authors [83,84,85] demonstrated that different types of bait affect species richness, relative abundance, and the size of individuals attracted to BRUVs.
Big fish (groupers, snappers, and barracudas) dominated the park’s diving area. It seems that these fish are better adapted to the presence of divers than sharks, which tend to be more cautious. Anecdotal reports from divers and researchers who frequent the area indicate that sharks tend to move away quickly as soon as they sense the presence of divers; C. perezi likely remains at a greater depth or out of the field of vision of divers in sectors that correspond to intense diving activity. Various studies have shown that the presence of divers causes immediate impacts on the behavior of sharks (e.g., [86,87,88,89]). However, other study [90], stated that such changes do not persist or create long-term effects. These authors argue that responsible, well-regulated shark diving tourism can be achieved without undermining conservation goals. Nonetheless, to give divers the best chance of seeing sharks up close, many dive operators use different methods to attract them, with a feeding incentive being the most common [91]. In the case of the diving area managed by the María la Gorda International Diving Center, located within the limits of the GNP, the feeding of fish by tour operators or visitors is not allowed, so sharks are not concentrated around the dive sites.
The applied regression studies did not find a strong relationship between mesopredators and their potential prey. That is, the distribution and abundance of mesopredators apparently are not very dependent on the distribution and abundance of their food. However, it should be considered that a single sampling was carried out, and both groups can generally present a great deal of seasonal variability [92,93,94]. The regression studies applied in this work have been somewhat exploratory. Without the hope of finding definitive results, the ideal would be to use them with a higher frequency of sampling to obtain more representative results of the behavior or dependence of both groups. Consequently, the primary results achieved do not have to be conclusive. Theoretically, the relationship between mesopredators and their prey is fundamental to the health of marine ecosystems, influencing the evolution and behavior of both groups. The different species of prey, based on being part of the diet of mesopredators, can affect their distribution and abundance [9]. In turn, many sharks, for example, feed on different prey at different stages, from small fish to larger prey [95], and thus exert other pressure on their abundance. However, this study could not show this.
We can infer that both the abundance of mesopredators and their potential prey is more closely related to the protection conditions established within the park and that sharks and rays have enough food within the limits of the GNP. The potential prey of mesopredators presented greater abundances in the diving area, sites more protected from fishermen due to their difficult access and the greater structural complexity of the reef [1,8,35]. The twenty sites evaluated in the diving area were located on the reef wall of the GNP, where the highest abundance of fish is reported due to greater availability of shelters and food [8,9]. The rest of the areas of the park had lower values of prey abundance; these corresponded to habitats of ridges and channels, rocky terraces, and sand, with less structural complexity [1,8,35].
Nor was a strong relationship observed between mesopredators and abiotic variables, and this may be because the GNP is relatively homogeneous in terms of its environmental variability, whose reefs have transparent waters with relatively small spatial differences in the value of the remaining predominant abiotic variables [1]. The most significant relationship was observed between the abundance of sharks and the conditions of greater water turbidity (it is important to clarify that the sites with the greatest turbidity had values of Kd490 = 0.030, low values when compared to other areas of Cuba [96]). Turbidity can help sharks during hunting, making it difficult for their prey to see them and allowing them to approach undetected. Turbidity can also influence shark behavior, favoring social interactions that can result in groupings or cooperative hunting [97]. Some studies [98,99] found that water turbidity, along with temperature, dissolved oxygen, and PH, plays an essential role in the distribution and abundance of some shark species, mainly in their juvenile stages in estuarine areas and reefs of Florida. The distribution and abundance of sharks and rays can be influenced by salinity, depending on their life stage (juvenile or adult) and the habitats they occupy [72]. In the GNP, salinity does not exhibit significant variability across different reef areas [1], which may help to explain the observed patterns in the presence of these species.
The bait used (bonito, genus Sarda) had a positive effect on C. perezi. The observed behaviors of the sharks reaffirm that the bait used was effective, where more than 50% of the observed individuals displayed exploratory and aggressive behavior (rapid movements towards the basket with bait and bites to the basket) towards the device. The first specimens to arrive at the BRUVs were species belonging to the families Serranidae, Lutjanidae, Scaridae, Haemulidae, Muraenidae, and Acanthuridae, which correspond to territorial species that move on a smaller scale. Sharks took longer to arrive compared to other species, as they are distributed over larger spatial scales. However, at many of our sites, sharks arrived within the first 20 min, which could be related to the time it takes for the smell of the bait to disperse in the water column [49]. It has been confirmed that this group can detect odors over a long distance. For example, one study [88] estimated that with the BRUVs method (90 min), the bait has a shark attraction radius of hundreds of meters. However, these authors consider that small behavioral changes in spatial terms could be undetectable with this methodology since sharks differ in the size and morphology of their olfactory bulbs [100,101], and therefore likely differ in their relative attraction and ability to detect odor particles that are dispersed during BRUV deployments. The sharks’ ability to detect chemical cues over distance also depends on prevailing environmental conditions, including turbidity, current strength, sea surface temperature (SST), and substrate composition, all of which influence the diffusion and directionality of scents in the water column [102].
We only had one sighting of Sphyrna lewini, who displayed exploratory behavior when reaching the bait. This large species, considered critically endangered by the IUCN [77], is included among the top transit predators due to its body size and trophic position [23]. It is a highly mobile species with low site fidelity, whose territorial behavior spans large areas, as it can migrate between pelagic and neritic zones. This species exhibits seasonal migratory patterns, likely associated with currents and water temperature [103]. Therefore, a study of these abiotic variables at different depths in the reefs of GNP is recommended. The presence of top transit predators at the GNP adds significant value to this MPA, as it encompasses part of their life cycle and may offer partial protection to these highly impacted and threatened species. Further research is needed to determine whether these species are residents or only transient in the area.
5. Conclusions
The spatial variability of the abundance and distribution of mesopredators in the GNP was heterogeneous. It was better explained by the zones’ usage conditions and their regulations than by their relationship with biological and abiotic variables. The sharks and rays of the GNP appear to have enough food within the park’s boundaries, and in the relatively murkier waters of the park, they displayed increased abundance and distribution. The GNP is a suitable habitat for C. perezi shark populations, as indicated by the number of specimens observed per site. The sites in the subsistence fishing and bathing area had the lowest abundances of mesopredators; these sectors are the most accessible to fishermen and cover a small area concerning the total marine area of the park. The abundance of sharks and large fish on the reef validates the management actions and their effectiveness in the protected area and suggests the presence of healthy fish communities, which contributes to the ecological integrity of the reefs of Cuba and the Greater Caribbean region.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10040169/s1, Table S1: The geographic coordinates of each site, zone name, sector name, distance from the shore, and depth of work; Table S2: The list of species per subcategory; Table S3: Eight condition variables were selected; Table S4: Significant differences were found between sectors, regarding the variables of abundance and composition by species of the mesopredators; Table S5: Biological variables explained the variability in abundance at a rate of no more than 13%.
Author Contributions
Conceptualization, D.C.-R., J.A.-V. and H.C.-A.; methodology, D.C.-R., J.A.-V., H.C.-A., L.V.G.-L., P.P.C.-M. and J.I.H.-A.; validation, D.C.-R., J.A.-V., H.C.-A., P.P.C.-M., L.V.G.-L., S.P.-V. and J.I.H.-A.; formal analysis, D.C.-R., H.C.-A., S.P.-V., J.A.-V., P.P.C.-M. and J.I.H.-A.; data curation, D.C.-R., H.C.-A., P.P.C.-M. and J.A.-V.; writing—original draft preparation, D.C.-R., S.P.-V., H.C.-A., P.P.C.-M. and J.A.-V.; writing—review and editing, D.C.-R., S.P.-V., H.C.-A. and J.A.-V.; visualization, D.C.-R., S.P.-V., H.C.-A., J.I.H.-A., P.P.C.-M. and J.A.-V.; supervision, D.C.-R.; project administration, D.C.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data utilized in this study are available upon request from the corresponding author.
Acknowledgments
We thank the organizations that supported and made this research possible, especially “Global FinPrint”, specialists from the Guanahacabibes National Park, and workers from the María la Gorda International Diving Center and the Quick Travel Agency for ensuring the logistics of the scientific expedition.
Conflicts of Interest
Lázaro Valentín García-López was employed by the company Conceptos Arkipelago, SA. de CV. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Caballero-Aragón, H.; Perera-Valderrama, S.; Cobián-Rojas, D.; González, Z.H.; Méndez, J.G.; De la Guardia, E. A decade of study on the condition of western Cuban coral reefs, with low human impact. PeerJ 2023, 11, e15953. [Google Scholar] [CrossRef] [PubMed]
- Perera- Valderrama, S.; Hernández-Arana, H.A.; Ruiz-Zárate, M.Á.; Alcolado, P.M.; Caballero-Aragón, H.; González-Cano, J.; Vega-Zepeda, A.; Cobián Rojas, D. Condition assessment of coral reefs of two marine protected areas under different regimes of use in the north-western Caribbean. Ocean. Coast. Manag. 2016, 127, 16–25. [Google Scholar] [CrossRef]
- Cobián-Rojas, D.; Schmitter-Soto, J.J.; Aguilar -Betancourt, C.M.; Aguilar-Perera, A.; Ruiz-Zárate, M.Á.; González-Sansón, G.; Chevalier-Monteagudo, P.P.; Herrera-Pavón, R.; García-Rodríguez, A.; Corrada-Wong, R.I.C.; et al. The community diversity of two Caribbean MPAs invaded by lionfish does not support the biotic resistance hypothesis. J. Sea Res. 2018, 134, 26–33. [Google Scholar] [CrossRef]
- Cobián-Rojas, D.; Perera-Valderrama, S.; Chevalier-Monteagudo, P.P.; Schmitter-Soto, J.J.; Corrada Wong, R.I.; de la Guardia Llansó, E.; Mendez, J.G.; García-Rodríguez, A.; Hernández-Albernas, J.; Márquez-Llauger, L.; et al. Guanahacabibes National Park: Research, Monitoring, and Management for the Conservation of Coral Reefs. In Coral Reefs of Cuba; Springer International Publishing: Cham, Switzerland, 2023; pp. 339–358. [Google Scholar]
- Márquez, L.; Cobián-Rojas, D.; Camejo, J.A.; Linares, J.L.; Borrego, O.; Sosa, A.; Varela, R. Plan de Manejo del Parque Nacional Guanahacabibes, Periodo 2024–2028; Centro de Investigaciones y Servicios Ambientales, ECOVIDA, CITMA: Pinar del Río, Cuba, 2023; 312p. [Google Scholar]
- Claro, R.; Cantelar, K. Rapid assessment of the coral communities of María la Gorda, Southeast Ensenada de Corrientes, Cuba (Part 2: Reef fishes). Atoll. Res. Bull. 2003, 496, 278–293. [Google Scholar] [CrossRef][Green Version]
- Cobián-Rojas, D.; Chevalier Monteagudo, P.P. Evaluación de las asociaciones de peces de los arrecifes coralinos del Centro Internacional de Buceo María la Gorda, Parque Nacional Guanahacabibes, Cuba. Rev. Mar. Coast. Sci. 2009, 1, 111–125. [Google Scholar] [CrossRef]
- Cobián-Rojas, D.; Claro, R.; Chevalier, P.C.; Perera, S.; Caballero, H. Estructura de las asociaciones de peces en los arrecifes coralinos del Parque Nacional Guanahacabibes, Cuba. Rev. Cienc. Mar. Costeras 2011, 3, 153–169. [Google Scholar] [CrossRef]
- Cobián- Rojas, D.; Chevalier Monteagudo, P.P.; Schmitter-Soto, J.J.; Corrada Wong, R.I.; Salvat Torres, H.; Cabrera Sansón, E.; García Rodríguez, A.; Fernández Osorio, A.; Espinosa Pantoja, L.; Cabrera Guerra, D.; et al. Density, size, biomass, and diet of lionfish in Guanahacabibes National Park, western Cuba. Aquat. Biol. 2016, 24, 219–226. [Google Scholar] [CrossRef]
- Cobián-Rojas, D.; Schmitter-Soto, J.J.; Aguilar-Perera, A.; Betancourt, C.M.A.; Ruiz-Zárate, M.Á.; González-Sansón, G.G.; Perera-Valderrama, S.; Aragón, H.C.; de la Guardia, E. Diversity of native reef fish communities in two protected areas in the Caribbean Sea and its relationship to the invasive lionfish. Rev. Biol. Trop. 2018, 66, 189–203. [Google Scholar]
- de la Guardia, E.; Perera-Valderrama, S.; Rojas, D.C.; Espinosa-Pantoja, L.; García-López, L.; Hernández-González, Z.; Angulo-Valdés, J. Comparación de ensambles de corales y peces de arrecife cubanos registrados por censo visual y técnica de video estéreo submarino. Indicadores Ecológicos 2021, 121, 107220. [Google Scholar]
- Brock, V.E. A preliminary report on a method of estimating reef fish populations. J. Wildl. Manag. 1954, 18, 297–308. [Google Scholar] [CrossRef]
- Pina-Amargós, F.; Salvat-Torres, H.; López-Fernández, N. Ictiofauna del archipiélago Jardines de la Reina, Cuba. Rev. Investig. Mar. 2012, 32, 54–65. [Google Scholar]
- Bohnsack, J.A.; Bannerot, S.P. A stationary visual census technique for quantitatively assessing community structure of coral reef fishes. NOAA Tech. Rep. NMFS 1986, 41, 1–15. [Google Scholar]
- Watson, D.L.; Harvey, E.S.; Anderson, M.J.; Kendrick, G.A. A comparison of temperate reef fish assemblages recorded by three underwater stereo-video techniques. Mar. Biol. 2005, 148, 415–425. [Google Scholar] [CrossRef]
- Delacy, C.R. Latitudinal Patterns in Reef Fish Assemblage Structure: The Influence of Long-Term and Short-Term Processes; University of Western Australia: Nedlands, Australia, 2008. [Google Scholar]
- Shin, Y.J.; Bundy, A.; Shannon, L.J.; Simier, M.; Coll, M.; Fulton, E.A.; Link, J.S.; Jouffre, D.; Ojaveer, H.; Mackinson, S.; et al. Can simple be useful and reliable? Using ecological indicators to represent and compare the states of marine ecosystems. J. Mar. Sci. 2010, 67, 717–731. [Google Scholar] [CrossRef]
- Andradi-Brown, D.A.; Gress, E.; Wright, G.; Exton, D.A.; Rogers, A.D. Reef fish community biomass and trophic structure changes across shallow to upper-mesophotic reefs in the Mesoamerican Barrier Reef, Caribbean. PLoS ONE 2016, 11, e0156641. [Google Scholar] [CrossRef]
- Lam, V.Y.; Doropoulos, C.; Mumby, P.J. The influence of resilience-based management on coral reef monitoring: A systematic review. PLoS ONE 2017, 12, e0172064. [Google Scholar] [CrossRef]
- Bascompte, J.; Melián, C.J.; Sala, E. Interaction strength combinations and the overfishing of a marine food web. Proc. Natl. Acad. Sci. USA 2005, 102, 5443–5447. [Google Scholar] [CrossRef]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- Heupel, M.R.; Knip, D.M.; Simpfendorfer, C.A.; Dulvy, N.K. Sizing up the ecological role of sharks as predators. Mar. Ecol. Prog. Ser. 2014, 495, 291–298. [Google Scholar] [CrossRef]
- Roff, G.; Doropoulos, C.; Rogers, A.; Bozec, Y.M.; Krueck, N.C.; Aurellado, E.; Priest, M.; Birrell, C.; Mumby, P.J. The ecological role of sharks on coral reefs. Trends Ecol. Evol. 2016, 31, 395–407. [Google Scholar] [CrossRef]
- Dedman, S.; Moxley, J.H.; Papastamatiou, Y.P.; Braccini, M.; Caselle, J.E.; Chapman, D.D.; Cinner, J.E.; Dillon, E.M.; Dulvy, N.K.; Dunn, R.E.; et al. Ecological roles and importance of sharks in the Anthropocene Ocean. Science 2024, 385, adl2362. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.J.; Brownscombe, J.W.; Alsudairy, N.A.; Casagrande, A.B.; Fu, C.; Harding, L.; Harris, S.D.; Hammerschlag, N.; Howe, W.; Huertas, A.D.; et al. Tiger sharks support the characterization of the world’s largest seagrass ecosystem. Nat. Commun. 2022, 13, 6328. [Google Scholar] [CrossRef] [PubMed]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Meekan, M.; Cappo, M.; Carleton, J.; Marriott, R. Surveys of Sharks and Fin-Fish Abundance on Reefs Within the MOU74 Box and Rowley Shoals Using Baited Remote Underwater Video Systems; Department of the Environment and Heritage; Australian Institute of Marine Science: Townsville, Australia, 2006. [Google Scholar]
- Malcolm, H.A.; Gladstone, W.; Lindfield, S.; Wraith, J.; Lynch, T.P. Variación espacial y temporal en los ensambles de peces de arrecife de los parques marinos de Nueva Gales del Sur, Australia: Observaciones de vídeo con cebo. Ser. Prog. Ecol. Mar. 2007, 350, 277–290. [Google Scholar] [CrossRef]
- Brooks, E.J.; Sloman, K.A.; Sims, D.W.; Danylchuk, A.J. Validating the use of baited remote underwater video surveys for assessing the diversity, distribution, and abundance of sharks in the Bahamas. Endanger. Species Res. 2011, 13, 231–243. [Google Scholar] [CrossRef]
- Cappo, M.; Speare, P.; De’ath, G. Comparison of baited remote underwater video stations (BRUVs) and prawn (shrimp) trawls for assessments of fish biodiversity in interreefal areas of the Great Barrier Reef Marine Park. J. Exp. Mar. Biol. Ecol. 2004, 302, 123–152. [Google Scholar] [CrossRef]
- Cappo, M.; Harvey, E.; Shortis, M. Counting and measuring fish with baited video an overview. In Cutting-Edge Technologies in Fish and Fisheries Science, Proceedings of the Australian Society for Fish Biology Workshop, Hobart, Tasmania, 28–29 August 2006; Lyle, J.M., Furlani, D.M., Buxton, C.D., Eds.; Australian Society of Fish Biology: Hobart, Tasmania, 2006; pp. 101–115. [Google Scholar]
- Cappo, M.; Speare, P.; Wassenberg, T.; Harvey, E.; Rees, M.; Heyward, A.; Pitcher, R. The use of baited remote underwater video stations (BRUVs) to survey demersal fish stocks-how deep and meaningful. In Video Sensing of the Size and Abundance of Target and Non-Target Fauna in Australian Fisheries: A National Workshop; Fisheries Research Development Corporation: Canberra, Australia, 2001; pp. 63–71. [Google Scholar]
- González-Ferrer, S.; Caballero, H.; Alcolado, P.M.; Jiménez, A.; Martín, F.; Cobián, D. Diversidad de corales pétreos en once sitios de buceo recreativo de “María la Gorda”, Cuba. Rev. Inv. Mar. 2007, 28, 121–130. [Google Scholar]
- Caballero-Aragón, H.; González, S.; Cobián-Rojas, D.; Álvarez, S.; Alcolado, P.M. Evaluación AGRRA del bento en diez sitios de buceo de “María 920 la Gorda”, Bahía de Corrientes, Cuba. Rev. Investig. Mar. 2007, 28, 131–138. [Google Scholar]
- Perera-Valderrama, S.; Alcolado, P.M.; Caballero Aragón, H.; de la Guardia Llansó, E.; Cobián Rojas, D. Condición de los arrecifes coralinos del Parque Nacional Guanahacabibes, Cuba. Rev. Cienc. Mar. Costeras 2013, 5, 69–86. [Google Scholar] [CrossRef]
- Pérez-Santos, I.; Schneider, W.; Fernández-Vila, L. Variabilidad y características de la contracorriente cubana en la cuenca de Yucatán, mar Caribe. Cienc. Mar. 2015, 41, 65–83. [Google Scholar] [CrossRef][Green Version]
- Harvey, E.; Cappo, M.; Butler, J.J.; Hall, N.; Kendrick, G.A. Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Mar. Ecol. Prog. Ser. 2007, 350, 245–254. [Google Scholar] [CrossRef]
- Watson, D.L.; Harvey, E.S.; Fitzpatrick, B.M.; Langlois, T.J.; Shedrawi, G. Assessing reef fish assemblage structure: How do different stereo-video techniques compare? Mar. Biol. 2010, 157, 1237–1250. [Google Scholar] [CrossRef]
- Bond, T.; Partridge, J.C.; Taylor, M.D.; Langlois, T.J.; Malseed, B.E.; Smith, L.D.; McLean, D.L. Fish associated with a subsea pipeline and adjacent seafloor of the Northwest Shelf of Western Australia. Mar. Environ. Res. 2018, 141, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Micheli, F.; Mumby, P.J.; Brumbaugh, D.R.; Broad, K.; Dahlgren, C.P.; Harborne, A.R.; Holmes, K.E.; Kappel, C.V.; Litvin, S.Y.; Sanchirico, J.N. High vulnerability of ecosystem function and services to diversity loss in Caribbean coral reefs. Biol. Conserv. 2014, 171, 186–194. [Google Scholar] [CrossRef]
- Sherman, C.S.; Chin, A.; Heupel, M.R.; Simpfendorfer, C.A. Are we underestimating elasmobranch abundances on baited remote underwater video systems (BRUVs) using traditional metrics? J. Exp. Mar. Biol. Ecol. 2018, 503, 80–85. [Google Scholar] [CrossRef]
- Böhlke, J.E.; Chaplin, C.C. Fishes of the Bahamas and Adjacent Tropical 912 Waters; University of Texas Press: Austin, TX, USA, 1993. [Google Scholar]
- Humann, P.; Deloach, N. Reef Fish Identification (Florida-Caribbean-Ba1087 Hamas), 3rd ed.; New World Publications; Star Standard Industries: Singapore, 2002. [Google Scholar]
- Carpenter, K.E. (Ed.) The living marine resources of the Western Central Atlantic. In Volume 1: Introduction, Molluscs, Crustaceans, Hagfishes, Sharks, Batoid Fishes, and Chimaeras; FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5; FAO: Rome, Italy, 2002; pp. 1–600. [Google Scholar]
- Eschmeyer, W.N.; Fricke, R.; Van der Laan, R. Catalog of Fishes: Genera, 1036 Species, References. 2020. Available online: https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 7 July 2024).
- Cerdeira-Estrada, S.; Martell-Dubois, R.; Valdéz, J.; Ressl, R. Daily 1-km Satellite Nighttime Sea Surface Temperature (NSST) (L4-Blended, Daily, 1-km, Since 1-Oct-1981). Satellite-Based Ocean Monitoring System (SATMO). Marine-Coastal Information and Analysis System (SIMAR). CONABIO. México. 2020. Available online: https://simar.conabio.gob.mx/explorer/?satmo=nsst (accessed on 7 July 2024).
- Cerdeira-Estrada, S.; Martell-Dubois, R.; Valdéz, J.; Ressl, R. Daily 1-km Satellite Diffuse Attenuation Coefficient of the Downwelling Irradiance at 490 nm (KD490). Satellite-Based Ocean Monitoring System (SATMO). Marine-Coastal Information and Analysis System (SIMAR). CONABIO. México. 2018. Available online: https://simar.conabio.gob.mx/explorer/?satmo=nsst (accessed on 7 July 2024).
- Morel, A.; Huot, Y.; Gentili, B.; Werdell, P.J.; Hooker, S.B.; Franz, B.A. Examining the consistency of products derived from various ocean color sensors in open ocean (Case 1) waters in the perspective of a multi-sensor approach. Remote Sens. Environ. 2007, 111, 69–88. [Google Scholar] [CrossRef]
- Bruns, S.; Henderson, A.C. A baited remote underwater video system (BRUVS) assessment of elasmobranch diversity and abundance on the eastern Caicos Bank (Turks and Caicos Islands); an environment in transition. Environ. Biol. Fishes 2020, 103, 1001–1012. [Google Scholar] [CrossRef]
- Caballero-Aragón, H.; Armenteros, M.; Perera-Valderrama, S.; Martell-Dubois, R.; Rey-Villiers, N.; Rosique-de la Cruz, L.; Cerdeira-Estrada, S. Wave exposure and temperature drive coral community structure at regional scale in the Cuban archipelago. Coral Reefs 2022, 42, 43–61. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014; Available online: http://plymsea.ac.uk/id/eprint/7656 (accessed on 7 July 2024).
- Tavares, R.A.T. Fishery biology of the Caribbean reef sharks, Carcharhinus perezi (Poey, 1876), in a Caribbean insular platform: Los Roques Archipelago National Park, Venezuela. Pan.-Am. J. Aquat. Sci. 2009, 4, 500–512. [Google Scholar]
- Bond, M.E.; Babcock, E.A.; Pikitch, E.K.; Abercrombie, D.L.; Lamb, N.F.; Chapman, D.D. Reef sharks exhibit site-fidelity and higher relative abundance in marine reserves on the Mesoamerican Barrier Reef. PLoS ONE 2012, 7, e32983. [Google Scholar] [CrossRef]
- Goetze, J.S.; Fullwood LA, F. Fiji’s largest marine reserve benefits reef sharks. Coral Reefs 2013, 32, 121–125. [Google Scholar] [CrossRef]
- Pejdo, D.; Kruschel, C.; Schultz, S.; Zubak, I.; Kanski, D.; Markov, M.; Peleš, P. Fish Monitoring in Kornati National Park: Baited, Remote, Underwater Video (BRUV) Versus Trammel Net Sampling. Pomor. Zb. 2016, 253–260. [Google Scholar] [CrossRef]
- Osgood, G.J.; McCord, M.E.; Baum, J.K. Using baited remote underwater videos (BRUVs) to characterize chondrichthyan communities in a global biodiversity hotspot. PLoS ONE 2019, 14, e0225859. [Google Scholar] [CrossRef] [PubMed]
- Bornt, K.; McLean, D.; Langlois, T.; Harvey, E.; Bellchambers, L.; Evans, S.; Newman, S. Targeted demersal fish species exhibit variable responses to long-term protection from fishing at the Houtman Abrolhos Islands. Coral Reefs 2015, 34, 1297–1312. [Google Scholar] [CrossRef]
- Coleman, M.A.; Bates, A.E.; Stuart-Smith, R.D.; Malcolm, H.A.; Harasti, D.; Jordan, A.; Knott, N.A.; Edgar, G.J.; Kelaher, B.P. Functional traits reveal early responses in marine reserves following protection from fishing. Divers. Distrib. 2015, 21, 876–887. [Google Scholar] [CrossRef]
- Whitmarsh, S.K.; Fairweather, P.G.; Huveneers, C. What is Big BRUVver up to? Methods and uses of baited underwater video. Rev. Fish. Biol. Fish. 2017, 27, 53–73. [Google Scholar] [CrossRef]
- Grorud-Colvert, K.; Sullivan-Stack, J.; Roberts, C.; Constant, V.; Horta e Costa, B.; Pike, E.P.; Kingston, N.; Laffoley, D.; Sala, E.; Claudet, J.; et al. The MPA guide: A framework to achieve global goals for the ocean. Science 2021, 373, eabf0861. [Google Scholar] [CrossRef]
- Pinna, M.; Zangaro, F.; Saccomanno, B.; Scalone, C.; Bozzeda, F.; Fanini, L.; Specchia, V. An overview of ecological indicators of fish to evaluate the anthropogenic pressures in aquatic ecosystems: From traditional to innovative DNA-based approaches. Water 2023, 15, 949. [Google Scholar] [CrossRef]
- Perisic, N.; Hickerson, L.; Helwitt, D.; Norwood, D.; Shipley, O.N.; Bervoets, T.; Gallagher, A.J. Reef fish biodiversity and occurrence of endangered sharks within a small marine protected area off Sint Maarten, Dutch Caribbean. Environ. Biol. Fishes 2024, 1–12. [Google Scholar] [CrossRef]
- Navarro-Martínez, Z.M.; Armenteros, M.; Espinosa, L.; Lake, J.J.; Apprill, A. Taxonomic and functional assemblage structure of coral reef fishes from Jardines de la Reina (Caribbean Sea, Cuba). Mar. Ecol. Prog. Ser. 2022, 690, 113–132. [Google Scholar] [CrossRef]
- Pina-Amargós, F.; González-Díaz, P.; González-Sansón, G.; Aguilar-Betancourt, C.; Rodríguez-Cueto, Y.; Olivera-Espinosa, Y.; Figueredo-Martín, T.; Rey-Villiers, N.; Barreto, R.A.; Cobián-Rojas, D.; et al. Status of Cuban Coral Reefs. In Coral Reefs of Cuba; Springer International Publishing: Cham, Switzerland, 2023; pp. 283–307. [Google Scholar]
- de la Guardia, E.; Cobián-Rojas, D.; Martínez-Daranas, B.; González-Díaz, P. Componentes más comunes de la flora y la fauna marina del Parque Nacional Cayos de San Felipe, Cuba. Rev. Investig. Mar. 2018, 38, 21–43. [Google Scholar]
- Navarro Martínez, Z. Ictiofauna Arrecifal de Punta Francés, Cuba: Estructura y Estado de Conservación en el Período 2011–2014. Master Dissertation, Centro de Investigaciones Marinas, Universidad de la Habana, La Habana, Cuba, 2015. [Google Scholar]
- González-Sansón, G.; Aguilar, C.; Hernández, I.; Cabrera, Y.; Curry, R.A. The influence of habitat and fishing on reef fish assemblages in Cuba. Gulf Caribb. Res. 2009, 21, 13–21. [Google Scholar] [CrossRef][Green Version]
- Schmitter-Soto, J.J.; Aguilar-Perera, A.; Cruz-Martínez, A.; Herrera-Pavón, R.L.; Morales-Aranda, A.A.; Cobián-Rojas, D. Interdecadal trends in composition, density, size, and mean trophic level of fish species and guilds before and after coastal development in the Mexican Caribbean. Biol. Conserv. 2017, 27, 459–474. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Innes-Gold, A.A.; Brandl, S.J.; Steneck, R.S.; Torres, R.E.; Rasher, D.B. Tropical fish diversity enhances coral reef functioning across multiple scales. Sci. Adv. 2019, 5, eaav6420. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Iosilevskii, G.; Di Santo, V.; Huveneers, C.; Hattab, T.; Planes, S.; Ballesta, L.; Mourier, J. Sharks surf the slope: Current updrafts reduce energy expenditure for aggregating marine predators. J. Anim. Ecol. 2021, 90, 2302–2314. [Google Scholar] [CrossRef]
- Steiner, P.A.; Michel MA RC, E.L.; O’Donnell, P.M. Effects of tidal current on the movement patterns of juvenile bull sharks and blacktip sharks. In American Fisheries Society Symposium; American Fisheries Society: Washington, DC, USA, 2007; Volume 50, p. 251. [Google Scholar]
- Beer, A.J.E. Diversity and Abundance of Sharks in No-Take and Fished Sites in the Marine Protected Area Network of Raja Ampat, West Papua, Indonesia, Using Baited Remote Underwater Video (BRUVs). Master Dissertation, University of Royal Roads, Victoria, BC, Canada, 2015. [Google Scholar]
- Spaet, J.L.Y.; Nanninga, G.B.; Berumen, M.L. Decline of shark populations in the Eastern Red Sea. Biol. Conserv. 2016, 201, 20–28. [Google Scholar] [CrossRef]
- Muñoz, R.C.; Burton, M.L. Comparison of video and diver observations of sharks from a fishery-independent trap-video survey off east-Central Florida, including utility of an alternative method of video analysis. Fish. Bull. 2019, 117, 87–96. [Google Scholar] [CrossRef]
- Murray, R.; Conales, S.; Araujo, G.; Labaja, J.; Snow, S.J.; Pierce, S.J.; Songco, A.; Ponzo, A. Tubbataha reefs Natural Park: The first comprehensive elasmobranch assessment reveals global hotspot for reef sharks. J. Asia-Pac. Biodivers 2019, 12, 49–56. [Google Scholar] [CrossRef]
- Callejas-Arrioja, A.V.; Jiménez, J.C.P. Categorías de conservación UICN para tiburones que se distribuyen en aguas del Golfo de México y Mar Caribe mexicanos. Bioagrociencias 2021, 14, 51–58. [Google Scholar] [CrossRef]
- Kilfoil, J.P.; Wirsing, A.J.; Campbell, M.D.; Kiszka, J.J.; Gastrich, K.R.; Heithaus, M.R.; Zhang, Y.; Bond, M.E. Baited Remote Underwater Video surveys undercount sharks at high densities: Insights from full-spherical camera technologies. Mar. Ecol. Prog. Ser. 2017, 585, 113–121. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Chapman, D.D.; Heupel, M.; Simpfendorfer, C.A.; Heithaus, M.; Meekan, M.; Harvey, E.; Goetze, J.; Kiszka, J.; Bond, M.E.; et al. Global status and conservation potential of reef sharks. Nature 2020, 583, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Ormond, R.; Gore, M.; Bladon, A.; Dubock, O.; Kohler, J.; Millar, C. Protecting Cayman Island Sharks: Monitoring, Movement and Motive Protegiendo a los Tiburones de las Islas de Caimán: Monitoreo, Movimiento y Motivo Protection des Requins aux Iles Cayman: Surveillance, Mouvement et Motivation. In Proceedings of the 69th Gulf and Caribbean Fisheries Institute, Grand Cayman, Cayman Islands, 7–11 November 2016. [Google Scholar]
- Simpfendorfer, C.A.; Heithaus, M.R.; Heupel, M.R.; MacNeil, M.A.; Meekan, M.; Harvey, E.; Sherman, C.S.; Currey-Randall, L.M.; Goetze, J.S.; Kiszka, J.J.; et al. Widespread diversity deficits of coral reef sharks and rays. Science 2023, 380, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Perera-Valderrama, S.; González-Méndez, J.; Hernández-Ávila, A.; Estrada-Estrada, R.; Cobián-Rojas, D.; Ramón-Puebla, A.; de la Guardia-Llansó, E.; Ferro-Azcona, H.; Hernández-Albernas, J.; Hernández-González, Z.; et al. Coral Reefs in Cuban Marine-Protected Areas. In Coral Reefs of Cuba; Springer International Publishing: Cham, Switzerland, 2023; pp. 375–391. [Google Scholar]
- Bailey, D.M.; King, N.J.; Priede, I.G. Cameras and carcasses: Historical and current methods for using artificial food falls to study deep-water animals. Mar. Ecol. Prog. Ser. 2007, 350, 179–191. [Google Scholar] [CrossRef]
- Wraith, J.; Lynch, T.; Minchinton, T.E.; Broad, A.; Davis, A.R. Bait type affects fish assemblages and feeding guilds observed at baited remote underwater video stations. Mar. Ecol. Prog. Ser. 2013, 477, 189–199. [Google Scholar] [CrossRef]
- Last, P.R.; Naylor, G.J.P.; Séret, B.; White, W.T.; de Carvalho, M.R.; Stehmann, M.F.W. Rays of the World; CSIRO Publishing: Collingwood, Australia, 2016. [Google Scholar]
- Quiros, A.L. Tourist compliance to a code of conduct and the resulting effects on whale shark (Rhincodon typus) behavior in Donsol, Philippines. Fish. Res. 2007, 84, 102–108. [Google Scholar] [CrossRef]
- Smith, K.; Scarr, M.; Scarpaci, C. Grey nurse shark (Carcharias taurus) diving tourism: Tourist compliance and shark behaviour at Fish Rock, Australia. Env. Manag. 2010, 46, 699–710. [Google Scholar] [CrossRef]
- Cubero-Pardo, P.; Herrón, P.; González-Pérez, F. Shark reactions to scuba divers in two marine protected areas of the Eastern Tropical Pacific. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 239–246. [Google Scholar] [CrossRef]
- Baronio, M. The Use of a Micro Remotely Operated Vehicle as a Tool for Studies of Shark Behavior and diver Impact. Ph.D. Thesis, Southern Cross University, Lismore, Australia, 2012. [Google Scholar]
- Bradley, D.; Papastamatiou, Y.P.; Caselle, J.E. No persistent behavioral effects of SCUBA diving on reef sharks. Mar. Ecol. Prog. Ser. 2017, 567, 173–184. [Google Scholar] [CrossRef]
- Vignon, M.; Sasal, P.; Johnson, R.L.; Galzin, R. Impact of sharkfeeding tourism on surrounding fish populations off Moorea Island (French Polynesia). J. Fish. Biol. 2010, 163–169. [Google Scholar]
- González-Rodríguez, E.; Trasviña-Castro, A.; Ramos-Rodríguez, A. El Bajo de Espíritu Santo; punto caliente de abundancia biológica afuera de Bahía de La Paz. CICIMAR Oceánides. 2018, 33, 13–24. [Google Scholar]
- Zambrano, S.; Croquer, A.; Evangelista, D.Y.; King, S.; Delance, J.; Romero-Mujalli, D. Patrones espacio-temporales en la abundancia y biomasa de peces loro (Perciformes: Scaridae) en la costa norte de la República Dominicana. Novit. Caribaea 2024, 24, 19–36. [Google Scholar] [CrossRef]
- Pardo-Gandarillas, M.C.; Duarte, F.; Chong, J.; Ibáñez, C.M. Dieta de tiburones juveniles Prionace glauca (Linnaeus, 1758) (Carcharhiniformes: Carcharhinidae) en la zona litoral centro-sur de Chile. Rev. Biol. Mar. Oceanogr. 2007, 42, 365–369. [Google Scholar] [CrossRef]
- Torres Huerta, A. Distribución, Abundancia y Hábitos Alimentarios de Juveniles del Tiburón Martillo Sphyrna lewini Griffith y Smith (Sphyrnidae) en la Costa de Sinaloa, México Durante el Evento el niño 1997–1998. Master Dissertation, Universidad del Mar, Puerto Escondido, Mexico, 2004. Available online: http://localhost:8383/jspui/handle/123456789/867 (accessed on 20 December 2024).
- Valle-Pombrol, A.; Avila-Alonso, D.; Muñoz-Caravaca, A.; Cárdenas-Ortiz, R.; Castro-Rodríguez, D.J. Variación espacio-temporal del coeficiente de atenuación de la luz en la Bahía de Cienfuegos, Cuba. Rev. Investig. Mar. 2017, 37, 40–51. [Google Scholar]
- Chávez-Calderón, E. Movimientos y uso del Hábitat del Tiburón toro (Carcharhinus leucas) en el Estero Coyote, Guanacaste, Heredia, Costa Rica. Master’s Thesis, Universidad Nacional, Heredia, Costa Rica, 2017. [Google Scholar]
- Ortega Lori, A. Movement and Distribution of Juvenile Bull Sharks, Carcharhinus leucas, in Response to Water Quality and Quantity Modifications in a Florida Nursery. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2008. Available online: https://digitalcommons.usf.edu/etd/436 (accessed on 20 December 2024).
- Reyier, E.; Ahr, B.; Iafrate, J.; Scheidt, D.; Lowers, R.; Watwood, S.; Back, B. Sharks associated with a large sand shoal complex: Community insights from longline and acoustic telemetry surveys. PLoS ONE 2023, 18, e0286664. [Google Scholar] [CrossRef]
- Meredith, T.L.; Kajiura, S.M. Olfactory morphology and physiology of elasmobranchs. J. Exp. Biol. 2010, 213, 3449–3456. [Google Scholar] [CrossRef]
- Yopak, K.E.; Lisney, T.J.; Collin, S.P. Not all sharks are “swimming noses”: Variation in olfactory bulb size in cartilaginous fishes. Brain Struct. Funct. 2015, 220, 1127–1143. [Google Scholar] [CrossRef]
- Gardiner, J.M.; Atema, J.; Hueter, R.E.; Motta, P.J. Multisensory integration and behavioral plasticity in sharks from different ecological niches. PLoS ONE 2014, 9, e93036. [Google Scholar] [CrossRef]
- Nalesso, E. Distribución Espacio-Temporal de los Tiburones Martillo, Sphyrna lewini, Alrededor de la Isla del Coco (2005–2013), Pacífico Tropical Oriental. Doctoral Dissertation, Tesis de Maestría, Centro de Investigación Científica y de Educación Superior, Baja California, México, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).