Selenium, Mercury, and Health Benefit Values of Pelagic Ocean Fish of the Central North Pacific
Abstract
1. Introduction
1.1. Paradigm Change and Advances in Understanding Seafood Safety and Benefits
1.2. Health Benefit Values
2. Materials and Methods
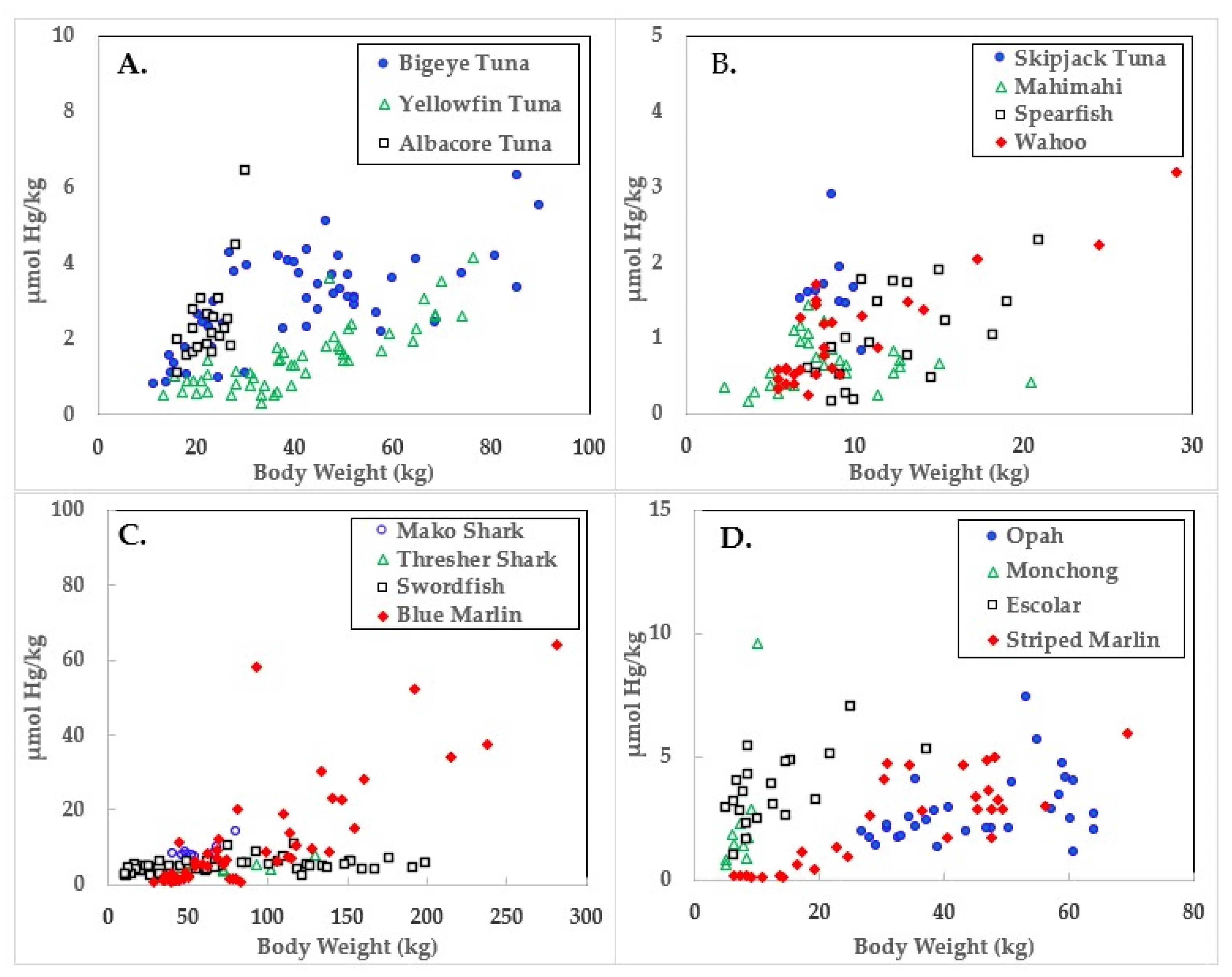
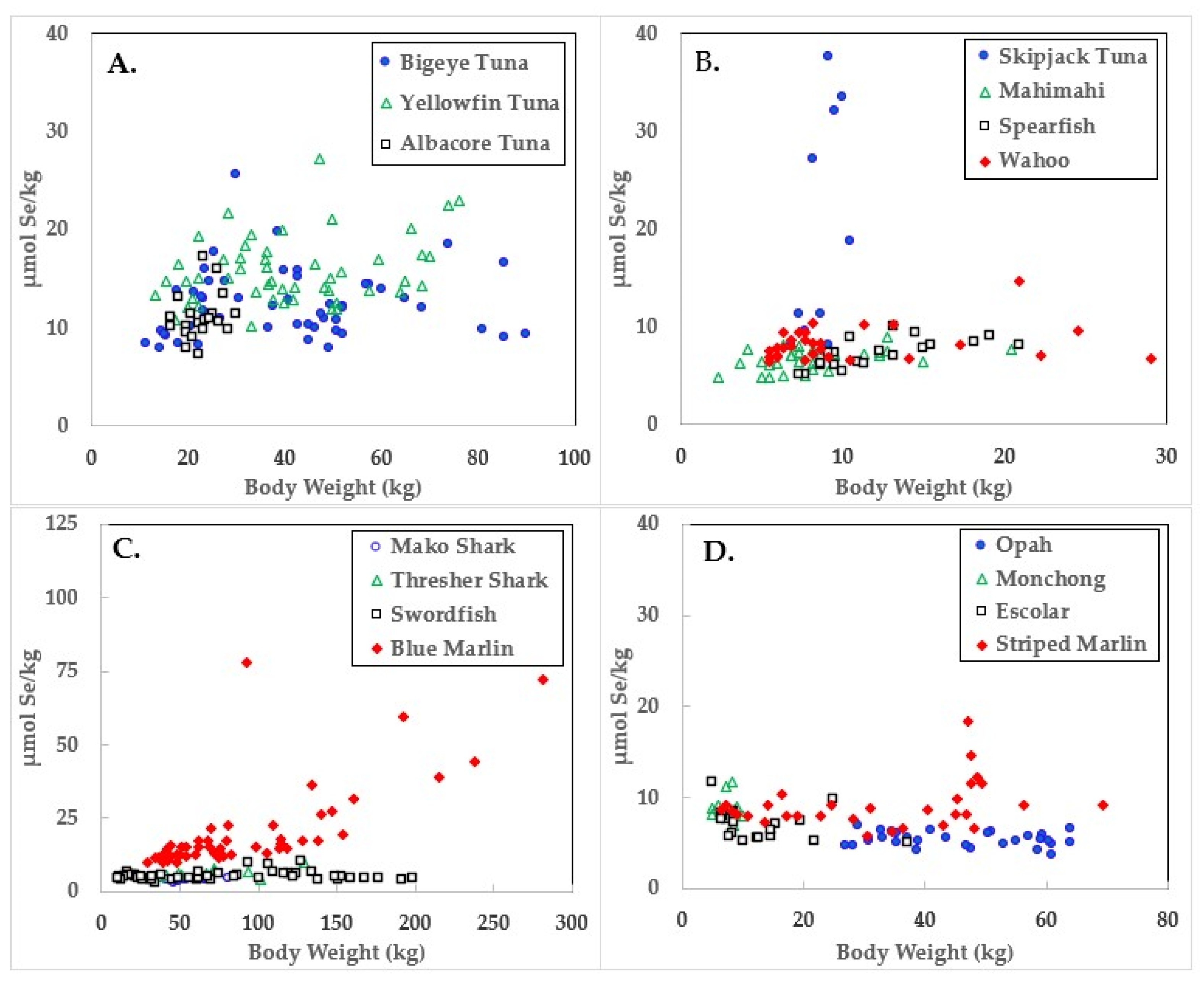
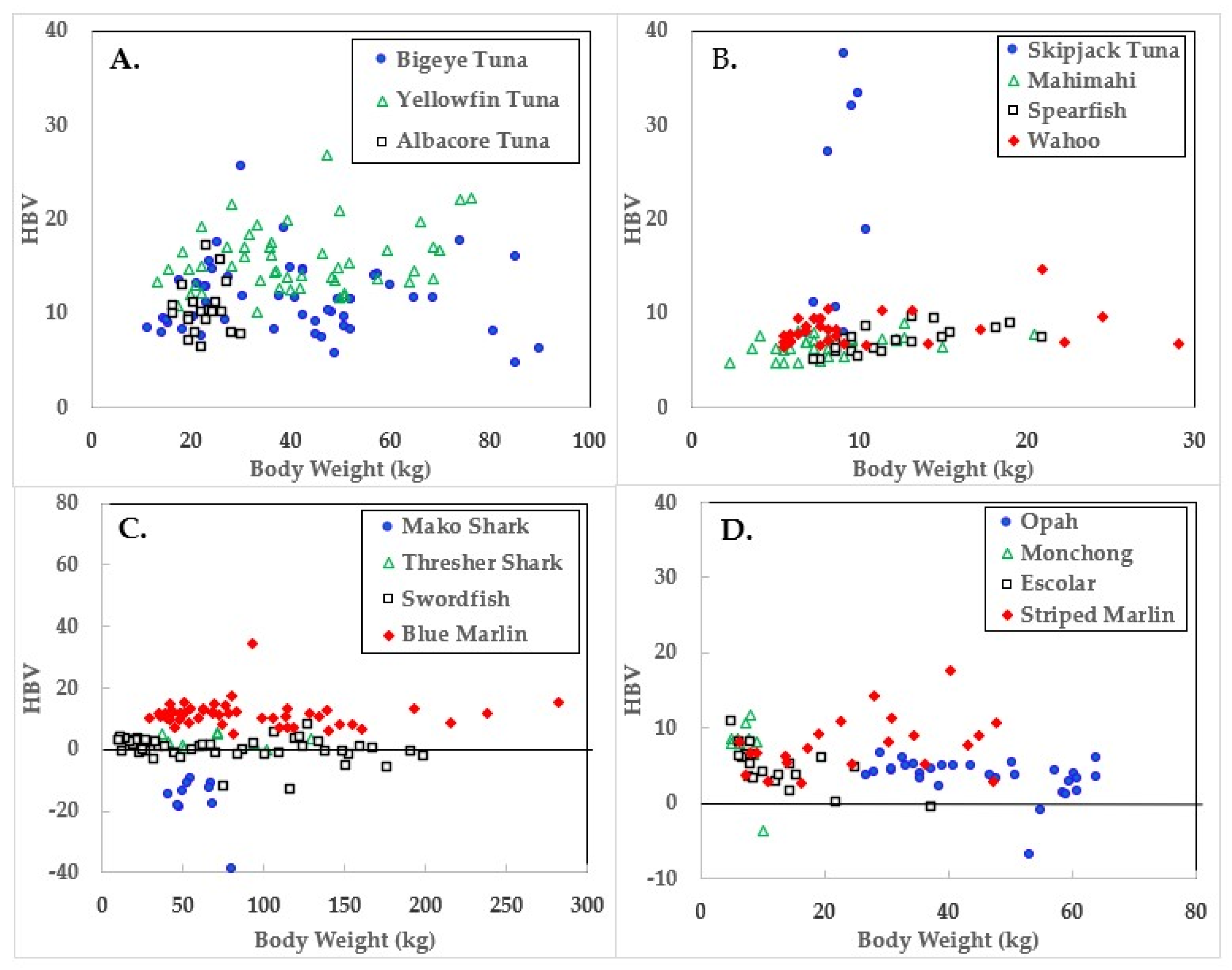
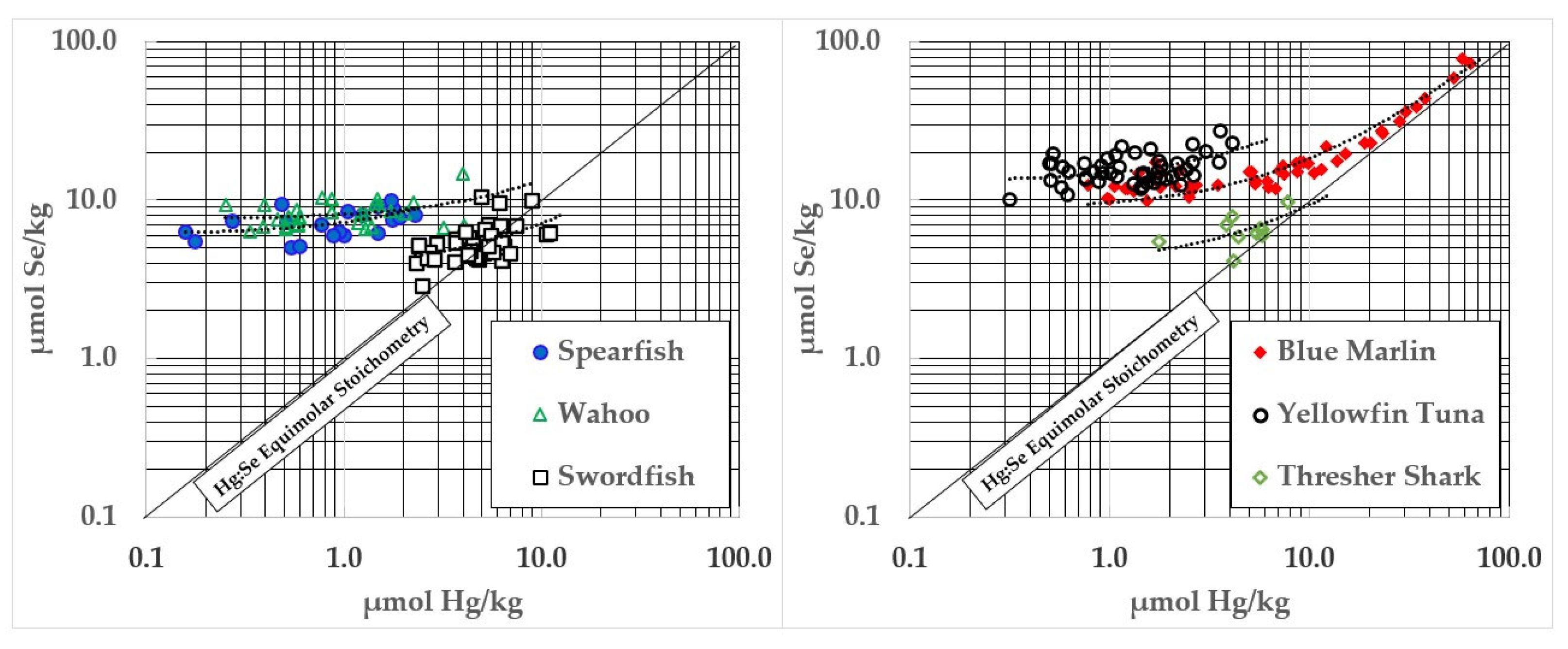
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cd | Cadmium |
| CH3Hg+ | Methylmercury |
| Cys | Cysteine |
| FAO | Food and Agriculture Organization |
| HBV | Health benefit value |
| Hg | Mercury |
| HgSe | Mercury selenide |
| IQ | Intelligence quotient |
| PCB | Polychlorinated biphenyl |
| RfD | Reference dose |
| Se | Selenium |
| Sec | Selenocysteine |
| U.S. EPA | United States Environmental Protection Agency |
| U.S. FDA | United States Food and Drug Administration |
| WHO | World Health Organization |
References
- Calder, P.C.; Yaqoob, P. Omega-3 Polyunsaturated Fatty Acids and Human Health Outcomes. BioFactors 2009, 35, 266–272. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal Seafood Consumption in Pregnancy and Neurodevelopmental Outcomes in Childhood (ALSPAC Study): An Observational Cohort Study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Byrd, K.A.; Thilsted, S.H.; Fiorella, K.J. Fish Nutrient Composition: A Review of Global Data from Poorly Assessed Inland and Marine Species. Public Health Nutr. 2021, 24, 476–486. [Google Scholar] [CrossRef]
- FDA Advice About Eating Fish. Available online: https://www.fda.gov/food/consumers/advice-about-eating-fish (accessed on 4 January 2025).
- Clarkson, T.W.; Magos, L. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C. Mercury’s Neurotoxic Effects on Brain Selenoenzymes. In Handbook of Neurotoxicity; Kostrzewa, R.M., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–27. ISBN 978-3-030-71519-9. [Google Scholar]
- Ralston, N.V.C.; Raymond, L.J. Mercury’s Neurotoxicity Is Characterized by Its Disruption of Selenium Biochemistry. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2405–2416. [Google Scholar] [CrossRef]
- Nishigaki, S.; Harada, M. Methylmercury and Selenium in Umbilical Cords of Inhabitants of the Minamata Area. Nature 1975, 258, 324–325. [Google Scholar] [CrossRef]
- Sakamoto, M.; Marumoto, M.; Haraguchi, K.; Toyama, T.; Saito, Y.; Balogh, S.J.; Tohyama, C.; Nakamura, M. Assessing the Role of Selenium in Minamata Disease through Reanalysis of Historical Samples. Environ. Int. 2025, 195, 109242. [Google Scholar] [CrossRef]
- Bakir, F.; Damluji, S.F.; Amin-Zaki, L.; Murtadha, M.; Khalidi, A.; Al-Rawi, N.Y.; Tikriti, S.; Dhahir, H.I.; Clarkson, T.W.; Smith, J.C.; et al. Methylmercury Poisoning in Iraq: An Interuniversity Report. Science 1973, 181, 230–241. [Google Scholar] [CrossRef]
- Harris, H.H.; Pickering, I.J.; George, G.N. The Chemical Form of Mercury in Fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef]
- Davidson, P.W.; Cory-Slechta, D.A.; Thurston, S.W.; Huang, L.-S.; Shamlaye, C.F.; Gunzler, D.; Watson, G.; van Wijngaarden, E.; Zareba, G.; Klein, J.D.; et al. Fish Consumption and Prenatal Methylmercury Exposure: Cognitive and Behavioral Outcomes in the Main Cohort at 17 Years from the Seychelles Child Development Study. Neurotoxicology 2011, 32, 711–717. [Google Scholar] [CrossRef]
- Klus, J.K.; Thurston, S.W.; Myers, G.J.; Watson, G.E.; Rand, M.D.; Love, T.M.; Yeates, A.J.; Mulhern, M.S.; McSorley, E.M.; Strain, J.J.; et al. Postnatal Methylmercury Exposure and Neurodevelopmental Outcomes at 7 Years of Age in the Seychelles Child Development Study Nutrition Cohort 2. NeuroToxicology 2023, 99, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, M.; Yeates, A.J.; McSorley, E.M.; Watson, G.E.; Van Wijngaarden, E.; Bodin, N.; Govinden, R.; Jean-Baptiste, J.; Desnousse, S.; Shamlaye, C.F.; et al. Dietary Selenium and Mercury Intakes from Fish Consumption during Pregnancy: Seychelles Child Development Study Nutrition Cohort 2. NeuroToxicology 2024, 101, 1–5. [Google Scholar] [CrossRef]
- Spiller, P.; Van Wijngaarden, E.; Adams, H.R.; Strain, J.J.; McSorley, E.M.; Mulhern, M.S.; Conway, M.C.; Yeates, A.J.; Carrington, C.; Bolger, P.M.; et al. Net Effects Explains the Benefits to Children from Maternal Fish Consumption despite Methylmercury in Fish. NeuroToxicology 2023, 99, 195–205. [Google Scholar] [CrossRef]
- Raymond, L.; Ralston, N. Seafood, Selenium, and Pregnancy. In Handbook of Public Health Nutrition: International, National, and Regional Perspectives; Springer International Publishing: Berlin/Heidelberg, Germany, 2025. [Google Scholar]
- Oken, E. Decline in Fish Consumption among Pregnant Women after a National Mercury Advisory. Obstet. Gynecol. 2003, 102, 346–351. [Google Scholar] [CrossRef]
- FAO; WHO. Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2024; ISBN 978-92-5-139107-5. [Google Scholar]
- Oken, E.; Musci, R.J.; Westlake, M.; Gachigi, K.; Aschner, J.L.; Barnes, K.L.; Bastain, T.M.; Buss, C.; Camargo, C.A.; Cordero, J.F.; et al. Demographic and Health Characteristics Associated with Fish and n-3 Fatty Acid Supplement Intake during Pregnancy: Results from Pregnancy Cohorts in the ECHO Programme. Public Health Nutr. 2024, 27, e94. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Raymond, L.J.; Gilman, C.L.; Soon, R.; Seale, L.A.; Berry, M.J. Maternal Seafood Consumption Is Associated with Improved Selenium Status: Implications for Child Health. NeuroToxicology 2024, 101, 26–35. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Raymond, L.J. Dietary Selenium’s Protective Effects against Methylmercury Toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef] [PubMed]
- George, G.N.; MacDonald, T.C.; Korbas, M.; Singh, S.P.; Myers, G.J.; Watson, G.E.; O’Donoghue, J.L.; Pickering, I.J. The Chemical Forms of Mercury and Selenium in Whale Skeletal Muscle. Metallomics 2011, 3, 1232. [Google Scholar] [CrossRef]
- Dyrssen, D.; Wedborg, M. The Sulphur-Mercury(II) System in Natural Waters. Water Air Soil Pollut. 1991, 56, 507–519. [Google Scholar] [CrossRef]
- Nuttall, K.L. A Model for Metal Selenide Formation under Biological Conditions. Med. Hypotheses 1987, 24, 217–221. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Blackwell, J.L.; Raymond, L.J. Importance of Molar Ratios in Selenium-Dependent Protection against Methylmercury Toxicity. Biol. Trace Elem. Res. 2007, 119, 255–268. [Google Scholar] [CrossRef]
- US EPA; Ralston, N.; Raymond, L. Final Report|Fish Selenium Health Benefit Values in Mercury Risk Management|Research Project Database|Grantee Research Project|ORD|US EPA. Available online: https://cfpub.epa.gov/ncer_abstracts//index.cfm (accessed on 18 January 2025).
- Ralston, N.V.C. Concomitant Selenoenzyme Inhibitor Exposures as Etiologic Contributors to Disease: Implications for Preventative Medicine. Arch. Biochem. Biophys. 2023, 733, 109469. [Google Scholar] [CrossRef]
- Choi, A.L.; Budtz-Jørgensen, E.; Jørgensen, P.J.; Steuerwald, U.; Debes, F.; Weihe, P.; Grandjean, P. Selenium as a Potential Protective Factor against Mercury Developmental Neurotoxicity. Environ. Res. 2008, 107, 45–52. [Google Scholar] [CrossRef]
- Budtz-Jørgensen, E.; Grandjean, P.; Weihe, P. Separation of Risks and Benefits of Seafood Intake. Env. Health Perspect. 2007, 115, 323–327. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Ralston, N.V.C. Selenium and Mercury in Pelagic Fish in the Central North Pacific near Hawaii. Biol. Trace Elem. Res. 2007, 119, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Ralston, C.R.; Raymond, L.J. Selenium Health Benefit Values: Updated Criteria for Mercury Risk Assessments. Biol. Trace Elem. Res. 2016, 171, 262–269. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Kaneko, J.J.; Raymond, L.J. Selenium Health Benefit Values Provide a Reliable Index of Seafood Benefits vs. Risks. J. Trace Elem. Med. Biol. 2019, 55, 50–57. [Google Scholar] [CrossRef]
- Dietz, R.; Riget, F.; Born, E. An Assessment of Selenium to Mercury in Greenland Marine Animals. Sci. Total Environ. 2000, 245, 15–24. [Google Scholar] [CrossRef]
- IOM. Toxicological Effects of Methylmercury; National Academies Press: Washington, DC, USA, 2000; ISBN 0-309-56970-2. [Google Scholar]
- Grandjean, P.; Weihe, P.; Jørgensen, P.J.; Clarkson, T.; Cernichiari, E.; Viderø, T. Impact of Maternal Seafood Diet on Fetal Exposure to Mercury, Selenium, and Lead. Arch. Environ. Health Int. J. 1992, 47, 185–195. [Google Scholar] [CrossRef]
- Yang, G.; Wang, S.; Zhou, R.; Sun, S. Endemic Selenium Intoxication of Humans in China. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar] [CrossRef]
- Huggins, F.E.; Raverty, S.A.; Nielsen, O.S.; Sharp, N.E.; Robertson, J.D.; Ralston, N.V.C. An XAFS Investigation of Mercury and Selenium in Beluga Whale Tissues. Environ. Bioindic. 2009, 4, 291–302. [Google Scholar] [CrossRef]
- Korbas, M.; O’Donoghue, J.L.; Watson, G.E.; Pickering, I.J.; Singh, S.P.; Myers, G.J.; Clarkson, T.W.; George, G.N. The Chemical Nature of Mercury in Human Brain Following Poisoning or Environmental Exposure. ACS Chem. Neurosci. 2010, 1, 810–818. [Google Scholar] [CrossRef]
- Ralston, N.; Azenkeng, A.; Ralston, C.; Blackwell, J.L.; Raymond, L. Seafood Science: Advances in Chemistry, Technology and Applications; CRC Press: Boca Raton, FL, USA, 2015; pp. 433–457. [Google Scholar]
- Polak-Juszczak, L. Selenium and Mercury Molar Ratios in Commercial Fish from the Baltic Sea: Additional Risk Assessment Criterion for Mercury Exposure. Food Control 2015, 50, 881–888. [Google Scholar] [CrossRef]
- Bridges, K.N.; Furin, C.G.; Gerlach, R.F. Subsistence Fish Consumption in Rural Alaska: Using Regional Monitoring Data to Evaluate Risk and Bioavailability of Dietary Methylmercury. Sci. Total Environ. 2020, 736, 139676. [Google Scholar] [CrossRef]
- Zhu, Y.; Ho, Q.T.; Dahl, L.; Azad, A.M.; Bank, M.S.; Boitsov, S.; Kjellevold, M.; Kögel, T.; Lien, V.S.; Lundebye, A.-K.; et al. Predicting Essential and Hazardous Element Concentrations in Marine Fish from the Northeast Atlantic Ocean: A Bayesian Approach. Sci. Total Environ. 2025, 968, 178748. [Google Scholar] [CrossRef]
- Ruelas-Inzunza, J.; Šlejkovec, Z.; Mazej, D.; Fajon, V.; Horvat, M.; Ramos-Osuna, M. Bioaccumulation of As, Hg, and Se in Tunas Thunnus Albacares and Katsuwonus Pelamis from the Eastern Pacific: Tissue Distribution and As Speciation. Env. Sci. Pollut. Res. 2018, 25, 19499–19509. [Google Scholar] [CrossRef]
- Ordiano-Flores, A.; Rosíles-Martínez, R.; Galván-Magaña, F. Biomagnification of Mercury and Its Antagonistic Interaction with Selenium in Yellowfin Tuna Thunnus Albacares in the Trophic Web of Baja California Sur, Mexico. Ecotoxicol. Environ. Saf. 2012, 86, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.J.; Butler, E.C.V.; Macleod, C.K. Spatial Variability in Selenium and Mercury Interactions in a Key Recreational Fish Species: Implications for Human Health and Environmental Monitoring. Mar. Pollut. Bull. 2013, 74, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef]
- Lazarini, T.E.D.M.; Milani, R.F.; Morgano, M.A. Selenium, Total Mercury and Methylmercury in Sardine: Study of Molar Ratio and Protective Effect on the Diet. J. Environ. Sci. Health Part B 2019, 54, 387–393. [Google Scholar] [CrossRef]
- Melgar, M.J.; Núñez, R.; García, M.Á. Selenium Intake from Tuna in Galicia (Spain): Health Risk Assessment and Protective Role against Exposure to Mercury and Inorganic Arsenic. Sci. Total Environ. 2019, 694, 133716. [Google Scholar] [CrossRef] [PubMed]
- Vega-Sánchez, B.; Ortega-García, S.; Ruelas-Inzunza, J.; Frías-Espericueta, M.; Escobar-Sánchez, O.; Jara-Marini, M. Selenium and Mercury in Dolphinfish (Coryphaena hippurus) from the Gulf of California: Inter-Annual Variations and Selenium Health Benefit Value. Env. Sci. Pollut. Res. 2020, 27, 2311–2318. [Google Scholar] [CrossRef]
- Medina-Morales, S.A.; Corro-Espinosa, D.; Escobar-Sánchez, O.; Delgado-Alvarez, C.G.; Ruelas-Inzunza, J.; Frías-Espericueta, M.G.; Jara-Marini, M.E.; Páez-Osuna, F. Mercury (Hg) and Selenium (Se) Content in the Shark Mustelus Henlei (Triakidae) in the Northern Mexican Pacific. Env. Sci. Pollut. Res. 2020, 27, 16774–16783. [Google Scholar] [CrossRef]
- Teixeira, G.; Raimundo, J.; Goulart, J.; Costa, V.; Menezes, G.M.; Caetano, M.; Pacheco, M.; Martins, I. Hg and Se Composition in Demersal Deep-Sea Fish from the North-East Atlantic. Env. Sci. Pollut. Res. 2020, 27, 33649–33657. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Meleleo, D.; Dambrosio, A.; Garofalo, R.; Busco, A.; Storelli, M.M. Levels of Mercury, Methylmercury and Selenium in Fish: Insights into Children Food Safety. Toxics 2021, 9, 39. [Google Scholar] [CrossRef]
- Rudershausen, P.J.; Cross, F.A.; Runde, B.J.; Evans, D.W.; Cope, W.G.; Buckel, J.A. Total Mercury, Methylmercury, and Selenium Concentrations in Blue Marlin Makaira Nigricans from a Long-Term Dataset in the Western North Atlantic. Sci. Total Environ. 2023, 858, 159947. [Google Scholar] [CrossRef]
- Cusack, L.K.; Eagles-Smith, C.; Harding, A.K.; Kile, M.; Stone, D. Selenium: Mercury Molar Ratios in Freshwater Fish in the Columbia River Basin: Potential Applications for Specific Fish Consumption Advisories. Biol. Trace Elem. Res. 2017, 178, 136–146. [Google Scholar] [CrossRef]
- Peterson, S.A.; Ralston, N.V.C.; Whanger, P.D.; Oldfield, J.E.; Mosher, W.D. Selenium and Mercury Interactions with Emphasis on Fish Tissue. Environ. Bioindic. 2009, 4, 318–334. [Google Scholar] [CrossRef]
- Johnson, T.K.B.; LePrevost, C.E.; Kwak, T.J.; Cope, W.G. Selenium, Mercury, and Their Molar Ratio in Sportfish from Drinking Water Reservoirs. Int. J. Environ. Res. Public Health 2018, 15, 1864. [Google Scholar] [CrossRef]
- Plessl, C.; Gilbert, B.M.; Sigmund, M.F.; Theiner, S.; Avenant-Oldewage, A.; Keppler, B.K.; Jirsa, F. Mercury, Silver, Selenium and Other Trace Elements in Three Cyprinid Fish Species from the Vaal Dam, South Africa, Including Implications for Fish Consumers. Sci. Total Environ. 2019, 659, 1158–1167. [Google Scholar] [CrossRef]
- Liu, C.; Ralston, N.V.C. Seafood and Health: What You Need to Know? In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 97, pp. 275–318. ISBN 978-0-12-824580-4. [Google Scholar]
| Name | n | Scientific Name | Name | n | Scientific Name |
|---|---|---|---|---|---|
| Bigeye | 50 | Thunnus obesus | Mako shark | 10 | Isurus oxyrinchus |
| Yellowfin | 50 | Thunnus albacares | Thresher shark | 10 | Alopius vulpinus |
| Albacore | 20 | Thunnus alalunga | Swordfish | 49 | Xiphias gladius |
| Skipjack | 10 | Katsuwonus pelamis | Blue marlin | 50 | Makaira mazara |
| Wahoo | 30 | Acanthocybium solandri | Opah | 30 | Lampris guttatus |
| Spearfish | 20 | Tetrapturus angustirostris | Striped marlin | 30 | Tetrapturus audax |
| Escolar | 20 | Lepidocybium flavobrunneum | Mahimahi | 30 | Coryphaena hippurus |
| Monchong | 10 | Taractichthys steindachneri |
| Species | Body wt. (kg) | Hg µmol/kg | Se µmol/kg | HBV 2 | |
|---|---|---|---|---|---|
| ± SD | min–max | ± SD g | ± SD | ||
| Skipjack Tuna | 8.7 ± 1.2 | 6.4–10.4 | 1.7 ± 0.5 | 19.8 ± 11.7 | 19.6 ± 11.8 |
| Yellowfin Tuna | 41.1 ± 16.7 | 13.2–76.2 | 1.5 ± 0.3 | 15.8 ± 3.4 | 15.6 ± 3.4 |
| Blue Marlin | 97.9 ± 63.8 | 33.1–318.9 | 11.9 ± 14.9 | 20.2 ± 14.8 | 11.5 ± 4.2 |
| Bigeye Tuna | 41.2 ± 20.4 | 11.3–89.8 | 3.0 ± 1.2 | 12.4 ± 3.5 | 11.4 ± 3.8 |
| Albacore Tuna | 22.6 ± 3.8 | 16.3–29.9 | 2.5 ± 1.2 | 11.1 ± 2.4 | 10.4 ± 2.7 |
| Wahoo | 10.4 ± 6.2 | 5.0–29.0 | 1.2 ± 1.0 | 8.3 ± 1.7 | 8.0 ± 1.8 |
| Striped Marlin | 32.2 ± 17.1 | 6.4–69.4 | 2.3 ± 1.8 | 9.1 ± 2.6 | 8.1 ± 3.2 |
| Mahimahi | 8.3 ± 3.8 | 1.8–20.4 | 0.7 ± 0.3 | 6.7 ± 1.1 | 6.6 ± 1.1 |
| Monchong | 7.3 ± 1.7 | 5.0–10.0 | 2.3 ± 2.6 | 9.0 ± 1.4 | 7.5 ± 4.2 |
| Spearfish | 12.2 ± 3.9 | 7.3–18.6 | 1.0 ± 0.6 | 7.3 ± 1.5 | 7.1 ± 1.4 |
| Escolar | 12.8 ± 8.0 | 5.0–37.2 | 3.7 ± 1.4 | 7.1 ± 1.7 | 4.8 ± 2.7 |
| Opah | 45.4 ± 12.4 | 26.8–64.0 | 2.8 ± 1.4 | 5.4 ± 0.8 | 3.5 ± 2.5 |
| Thresher Shark | 71.3 ± 29.4 | 36.7–129.3 | 4.9 ± 1.6 | 6.5 ± 1.5 | 2.6 ± 2.0 |
| Swordfish | 77.2 ± 55.9 | 10.4–198.7 | 5.0 ± 1.8 | 5.4 ± 1.5 | 0.3 ± 3.7 |
| Mako Shark | 57.4 ± 12.5 | 40.8–80.3 | 9.0 ± 2.0 | 4.1 ± 0.5 | −16.4 ± 8.6 |
| Species | Hg vs. Body wt. | p Value | Se vs. Body wt. | p Value | Hg × Se | p Value |
|---|---|---|---|---|---|---|
| Skipjack Tuna | NS | NS | NS | |||
| Yellowfin Tuna | y = 0.043x − 0.253 | p < 0.0001 | y = 0.058x + 13.4 | p = 0.04 | y = 1.794x + 13.09 | p < 0.001 |
| Blue Marlin | y = 0.213x − 7.357 | p < 0.0001 | y = 0.190x + 3.02 | p < 0.0001 | y = 0.964x + 8.72 | p < 0.001 |
| Bigeye Tuna | y = 0.040x + 1.314 | p < 0.0001 | NS | NS | ||
| Albacore Tuna | y = 0.199x + 2.021 | p = 0.002 | NS | NS | ||
| Wahoo | y = 0.139x − 0.219 | p < 0.0001 | NS | y = 0.589x + 7.55 | p = 0.08 | |
| Striped Marlin | y = 0.086x − 0.441 | p < 0.0001 | NS | NS | ||
| Mahimahi | NS | y = 0.134x + 5.538 | p = 0.01 | NS | ||
| Monchong | y = 1.019x − 5.088 | p = 0.04 | NS | NS | ||
| Spearfish | y = 0.098x − 0.157 | p < 0.0001 | y = 0.258x + 4.149 | p < 0.001 | y = 1.180x + 6.06 | p = 0.02 |
| Escolar | y = 0.194x + 2.280 | p < 0.01 | NS | NS | ||
| Opah | y = 0.040x + 0.738 | p = 0.02 | NS | NS | ||
| Thresher Shark | y = 0.032x + 2.576 | p = 0.07 | NS | y = 0.547x + 3.89 | p = 0.08 | |
| Swordfish | y = 0.012x + 4.069 | p = 0.01 | NS | y = 0.346x + 3.68 | p = 0.002 | |
| Mako Shark | y = 0.111x + 2.658 | p = 0.03 | y = 0.030x + 2.336 | p = 0.008 | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralston, N.V.C.; Kaneko, J.J.; Raymond, L.J. Selenium, Mercury, and Health Benefit Values of Pelagic Ocean Fish of the Central North Pacific. Fishes 2025, 10, 158. https://doi.org/10.3390/fishes10040158
Ralston NVC, Kaneko JJ, Raymond LJ. Selenium, Mercury, and Health Benefit Values of Pelagic Ocean Fish of the Central North Pacific. Fishes. 2025; 10(4):158. https://doi.org/10.3390/fishes10040158
Chicago/Turabian StyleRalston, Nicholas V. C., J. John Kaneko, and Laura J. Raymond. 2025. "Selenium, Mercury, and Health Benefit Values of Pelagic Ocean Fish of the Central North Pacific" Fishes 10, no. 4: 158. https://doi.org/10.3390/fishes10040158
APA StyleRalston, N. V. C., Kaneko, J. J., & Raymond, L. J. (2025). Selenium, Mercury, and Health Benefit Values of Pelagic Ocean Fish of the Central North Pacific. Fishes, 10(4), 158. https://doi.org/10.3390/fishes10040158







