Effect of Feeding Frequency and Restriction on the Growth Performance, Physiology, and Intestinal Histomorphometry of Colossoma macropomum in a Recirculating Aquaculture System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
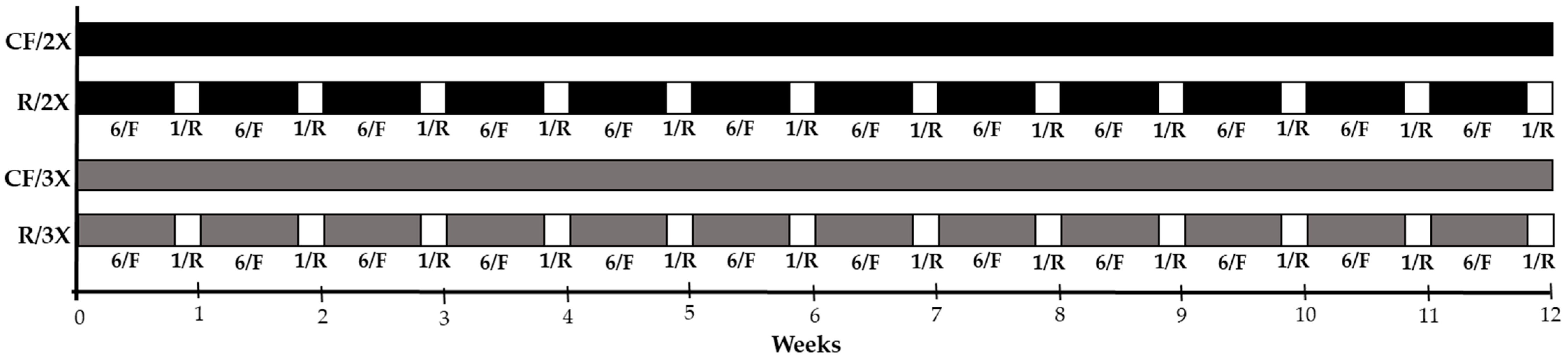
2.2. Experimental Design
- -
- CF/2x: Fish continuously fed, twice a day (08:00 and 16:00), for 12 weeks (84 days);
- -
- R/2x: Fish subjected to feed restriction [weekly cycles of one day of restriction (Sunday) and six days of feeding], twice a day (at 08:00 and 16:00), for 12 weeks (84 days);
- -
- CF/3x: Fish continuously fed, three times a day (08:00, 12:00 and 16:00), for 12 weeks (84 days);
- -
- R/3x: Fish subjected to feed restriction [weekly cycles of one day of restriction (Sunday) and six days of feeding], three times a day (08:00, 12:00 and 16:00), for 12 weeks (84 days).
2.3. Growth Performance Parameters
- -
- Final weight (g);
- -
- Weight gain (g) = final weight (g) − initial weight (g);
- -
- Specific growth rate (%/day) = 100 [(ln final weight − ln initial weight)]/number of days;
- -
- Feed conversion ratio = feed intake (g)/weight gain (g).
2.4. Blood Biochemical Parameters
2.5. Euthanasia, Tissue Collection and Analysis
- -
- Hepatosomatic index (%) = (liver weight/body weight) × 100;
- -
- Mesenteric fat index (%) = (adipose tissue weight/body weight) × 100.
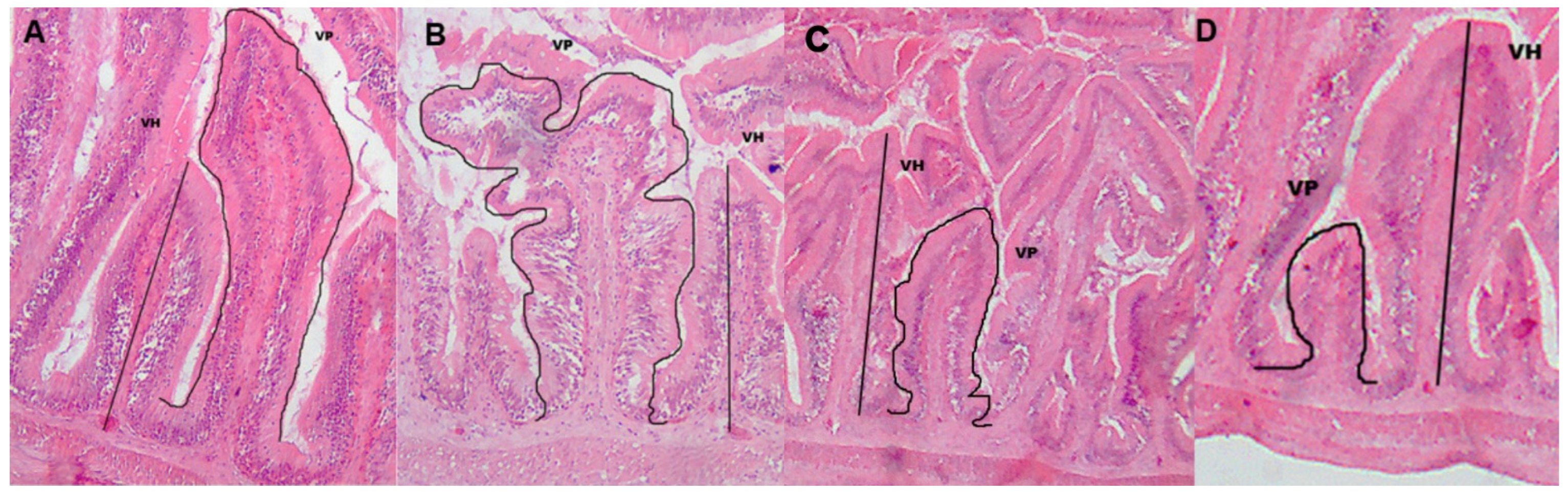
2.6. Intestinal Histomorphometry
2.7. Statistical Analysis
3. Results
3.1. Growth Performance Parameters
3.2. Blood Biochemical Parameters, Somatic Indices and Liver Lipids Levels
3.3. Intestinal Histomorphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations [FAO]. Field guide to the culture of tambaqui (Colossoma macropomum, Cuvier, 1816). In FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2019; 136p. [Google Scholar]
- Guilherme, H.O.; Santos, F.A.; Costa, L.S.; Prado, V.G.; Palheta, G.D.; Melo, N.F.A.C.; Luz, R.K.; Ribeiro, P.A. Feeding, growth, and blood chemistry of the tambaqui (Colossoma macropomum) held under self-feeding and time-restricted automatic feeding conditions. Aquac. Int. 2022, 30, 323–339. [Google Scholar]
- Santos, F.A.; Boaventura, T.P.; Julio, G.S.C.; Cortezzi, P.P.; Figueiredo, L.G.; Favero, G.C.; Palheta, G.D.A.; Melo, N.F.A.C.; Luz, R.K. Growth performance and physiological parameters of Colossoma macropomum in a recirculating aquaculture system: Importance of stocking density and classification. Aquaculture 2021, 534, 736274. [Google Scholar]
- Ferreira, A.L.; Ferreira, N.S.; Almeida, P.G.D.; Takata, R.; Luz, R.K. Effects of stocking density on survival, growth and stress resistance of juvenile tambaqui (Colossoma macropomum) reared in a recirculating aquaculture system (RAS). Aquac. Int. 2021, 29, 609–621. [Google Scholar]
- Tavares-Dias, M.; Araujo, C.S.O.; Porto, S.M.A.; Viana, G.M.; Monteiro, P.C. Sanidade do Tambaqui Colossoma macropomum nas Fases de Larvicultura e Alevinagem; Embrapa Amapá: Macapá, Brazil; Universidade Nilton Lins, Instituto de Pesquisas da Amazônia: Manaus, Brazil, 2013; 46p. [Google Scholar]
- Aroucha, R.J.N.; Ribeiro, F.B.; Bomfim, M.A.D.; Siqueira, J.C.; Marchão, R.S.; Nascimento, D.C.N. Digestible methionine plus cystine requirement in tambaqui (Colossoma macropomum) diets: Growth performance and plasma biochemistry. Aquac. Rep. 2023, 32, 101725. [Google Scholar]
- Assis, Y.P.A.S.; Porto, L.A.; Melo, N.F.A.C.; Palheta, G.D.A.; Luz, R.K.; Favero, G.C. Feed restriction as a feeding management strategy in Colossoma macropomum juveniles under recirculating aquaculture system (RAS). Aquaculture 2020, 529, 735689. [Google Scholar]
- Garcez, J.R.; Ferreira, R.C.; Santos, G.F.D.; Oliveira, J.; Freire, G.M. Efeito da restrição alimentar no crescimento do tambaqui (Colossoma macropomum, Cuvier 1818) em sistema semi-intensivo. Rev. Cient. Mult. 2023, 4, e483759. [Google Scholar]
- Souza, R.C.; Campeche, D.F.B.; Campos, R.M.L.; Figueiredo, R.A.C.R.; Melo, J.F.B. Frequência de alimentação para juvenis de tambaqui. Arq. Bras. Med. Vet. Zoot. 2014, 66, 927–932. [Google Scholar]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar]
- Santos, E.L.; Soares, A.C.L.; Tenório, O.L.D.; Soares, E.C.; Silva, T.J.; Gusmão Júnior, L.F.; Santos, E.L. Desempenho de tambaquis (Colossoma macropomum) submetidos a restrição alimentar e a realimentação em tanques-rede. Arq. Bras. Med. Vet. Zoot. 2018, 70, 931–938. [Google Scholar]
- Dawood, M.A.O.; Amer, A.A.; Gouda, A.H.; Gewaily, M.S. Interactive effects of cyclical fasting, refeeding, and dietary protein regimes on the growth performance, blood health, and intestinal histology of Nile tilapia (Oreochromis niloticus). Aquaculture 2023, 573, 739620. [Google Scholar]
- Roa, F.G.B.; Silva, S.S.; Hoshiba, M.A.; Silva, L.K.S.; Barros, A.F.; Abreu, J.S. Production performance of tambaqui juveniles subjected to short feed-deprivation and refeeding cycles. Bol. Inst. Pesca 2019, 45, 466. [Google Scholar] [CrossRef]
- Riche, M.; Garling, D. Feeding tilapia in intensive recirculating systems. Extension Fact. Sheets, paper 6 North Central Regional Aquaculture Center, p. 4. 2003. Available online: https://extension.rwfm.tamu.edu/wp-content/uploads/sites/8/2013/09/NCRAC-Fact-Sheet-Series-No.-114-Feeding-Tilapia-in-Intensive-Recirculating-Systems.pdf (accessed on 24 March 2025).
- Riche, M.; Haley, D.I.; Oetker, M.; Garbrecht, S.A.R.A.H.; Garling, D.L. Effect of feeding frequency on gastric evacuation and the return of appetite in tilapia Oreochromis niloticus (L.). Aquaculture 2004, 234, 657–673. [Google Scholar] [CrossRef]
- Jobling, M. Mathematical models of gastric emptying and the estimation of daily rates of food consumption for fish. J. Fish Biol. 1981, 19, 245–257. [Google Scholar] [CrossRef]
- Islam, M.A.; Mely, S.S.; Alam, M.S.; Rahman, M.H. Effect of different feeding rates on growth performance and survival rate of tilapia (Oreochromis niloticus L. 1758) fingerlings reared in rectangular hapas. Asian J. Fish. Aquac. Res. 2023, 25, 18–30. [Google Scholar] [CrossRef]
- López-Olmeda, J.F.; Noble, C.; Sánchez-Vázquez, F.J. Does feeding time affect fish welfare? Fish Physiol. Biochem. 2012, 38, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Aydin, I.; Ak, O.; Küçük, E.; Polat, H.; Ceylan, B. Optimum temperature and growth performance of hatchery reared Black Sea flounder (Platichthys flesus luscus Pallas, 1814). Turk. J. Vet. An. Sci. 2012, 36, 101–106. [Google Scholar] [CrossRef]
- Aydin, I. The effect of ploidy on growth and feeding pattern of diploid and triploid turbot Scophthalmus maximus under communal rearing condition. Turk. J. Fish. Aquat. Sci. 2021, 21, 275–281. [Google Scholar] [CrossRef]
- Boaventura, T.P.; Oliveira, C.G.; Santos, F.A.C.; Correia, R.O.; Silva, S.S.; Souza, A.S.; Ananias, I.M.C.; Silva, N.C.S.; Oliveira, J.E.; Luz, R.K. Use of the essential oil of Thymus vulgaris (thyme) and its nanoemulsion as an anesthetic during the cultivation of tambaqui (Colossoma macropomum): Anesthesia induction and recovery curve, physiology, performance, and feed consumption. Aquac. Int. 2024, 32, 9375–9391. [Google Scholar] [CrossRef]
- Mattioli, C.C.; Takata, R.; Leme, F.O.P.; Costa, D.C.; Luz, R.K. Physiological and metabolic responses of juvenile Lophiosilurus alexandri catfish to air exposure. Fish Physiol. Biochem. 2019, 45, 455–467. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J.A. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Liu, W.; Lu, X.; Jiang, M.; Wu, F.; Tian, J.; Yang, C.; Yu, L.; Wen, H. Effects of dietary manipulation on compensatory growth of juvenile genetically improved farmed tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2019, 45, 21–32. [Google Scholar]
- Py, C.; Elizondo-González, R.; Peña-Rodríguez, A. Compensatory growth: Fitness cost in farmed fish and crustaceans. Rev. Aquac. 2022, 14, 1389–1417. [Google Scholar]
- Won, E.T.; Borski, R.J. Endocrine regulation of compensatory growth in fish. Front. Endocrinol. 2013, 4, 74. [Google Scholar]
- Ji, W.; Ping, H.-C.; Wei, K.-J.; Zhang, G.-R.; Shi, Z.-C.; Yang, R.-B.; Zou, G.-W.; Wang, W.-M. Ghrelin, neuropeptide Y (NPY) and cholecystokinin (CCK) in blunt snout bream (Megalobrama amblycephala): cDNA cloning, tissue distribution and mRNA expression changes responding to fasting and refeeding. Gen. Comp. Endocrinol. 2015, 223, 108–119. [Google Scholar] [PubMed]
- Jönsson, E. The role of ghrelin in energy balance regulation in fish. Gen. Comp. Endocrinol. 2013, 187, 79–85. [Google Scholar]
- Amole, N.; Unniappan, S. Fasting induces preproghrelin mRNA expression in the brain and gut of zebrafish, Danio rerio. Gen. Comp. Endocrinol. 2009, 161, 133–137. [Google Scholar] [PubMed]
- Murashita, K.; Kurokawa, T.; Nilsen, T.O.; Ronnestad, I. Ghrelin, cholecystokinin, and peptide YY in Atlantic salmon (Salmo salar): Molecular cloning and tissue expression. Gen. Comp. Endocrinol. 2009, 160, 223–235. [Google Scholar] [PubMed]
- Unniappan, S.; Canosa, L.F.; Peter, R.E. Orexigenic actions of ghrelin in goldfish: Feeding-induced changes in brain and gut mRNA expression and serum levels, and responses to central and peripheral injections. Neuroendocrinology 2004, 79, 100–108. [Google Scholar]
- Hosomi, N.; Furutami, T.; Takahashi, N.; Masumoto, T.; Fukada, H. Yellowtail neuropeptide Y: Molecular cloning, tissue distribution, and response to fasting. Fish. Sci. 2014, 80, 483–492. [Google Scholar]
- Carmo, T.L.L.; Lima, M.C.M.; Lima, J.L.V.; Souza, S.S.; Val, A.L. Tissue distribution of appetite regulation genes and their expression in the Amazon fish Colossoma macropomum exposed to climate change scenario. Total Sci. Environ. 2023, 854, 158729. [Google Scholar]
- Lui, T.A.; Silva, W.P.; Nervis, J.A.L.; Brum, J.M.D.; Bittencourt, F.; Neu, D.H.; Boscolo, W.R. Food restriction in Nile tilapia juveniles (Oreochromis niloticus). Span. J. Agric. Res. 2020, 18, e0607. [Google Scholar]
- Takahashi, L.S.; Biller, J.D.; Criscuolo-Urbinati, E.; Urbinati, E.C. Feeding strategy with alternate fasting and refeeding: Effects on farmed pacu production. J. Anim. Physiol. Anim. Nutr. 2011, 95, 259–266. [Google Scholar]
- Yengkokpam, S.; Sahu, N.P.; Pal, A.K.; Debnath, D.; Kumar, S.; Jain, K.K. Compensatory growth, feed intake and body composition of Labeo rohita fingerlings following feed deprivation. Aquac. Nutr. 2013, 20, 101–108. [Google Scholar]
- El-Araby, D.A.; Amer, S.A.; Khalil, A.A. Effect of different feeding regimes on the growth performance, antioxidant activity, and health of Nile tilapia, Oreochromis niloticus. Aquaculture 2020, 528, 735572. [Google Scholar]
- Baloi, M.; Carvalho, C.V.A.; Sterzelecki, F.C.; Passini, G.; Cerqueira, V.R. Effects of feeding frequency on growth, feed efficiency and body composition of juveniles Brazilian sardine, Sardinella brasiliensis (Steindacher 1879). Aquac. Res. 2016, 47, 554–560. [Google Scholar]
- Başçınar, N.; Çakmak, E.; Çavdar, Y.; Aksungur, N. The effect of feeding frequency on growth performance and feed conversion rate of black sea trout (Salmo trutta labrax Pallas, 1811). Turk. J. Fish. Aquat. Sci. 2007, 7, 13–17. [Google Scholar]
- Jamabo, N.A.; Fubana, R.I.; Dienye, H.E. Feeding frequency on growth and feed conversion of Clarias gariepinus (Burchell, 1822) fingerlings. Int. J. Fish. Aquat. Stud. 2015, 3, 353–356. [Google Scholar]
- Karadal, O.; Gürog, D.; Türkmen, G. Effects of feeding frequency and Spirulina on growth performance, skin coloration and seed production on kenyi cichlids (Maylandia lombardoi). Aquac. Int. 2017, 25, 121–134. [Google Scholar]
- Salama, A.J. Effects of different feeding frequency on the growth, survival and feed conversion ratio of the Asian sea bass Lates calcarifer juveniles reared under hypersaline seawater of the Red Sea. Aquac. Res. 2008, 39, 561–567. [Google Scholar]
- Tian, H.-Y.; Zhang, D.-D.; Li, X.-F.; Zhang, C.-N.; Qian, Y.; Lui, W.-B. Optimum feeding frequency of juvenile blunt snout bream Megalobrama amblycephala. Aquaculture 2015, 437, 60–66. [Google Scholar]
- Lee, S.-M.; Cho, S.H.; Kim, D.-J. Effects of feeding frequency and dietary energy level on growth and body composition of juvenile flounder, Paralichthys olivaceus (Temminck & Schlegel). Aquac. Res. 2000, 31, 917–921. [Google Scholar]
- Barcellos, L.J.G.; Marqueze, A.; Trapp, M.; Quevedo, R.M.; Ferreira, D. The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture 2010, 300, 231–236. [Google Scholar]
- Laiz-Carrión, R.; Viana, I.R.; Cejas, J.R.; Ruiz-Jarabo, I.; Jerez, S.; Martos, J.A.; Eduardo, A.B.; Mancera, J.M. Influence of food deprivation and high stocking density on energetic metabolism and stress response in red porgy, Pagrus pagrus L. Aquac. Int. 2012, 20, 585–599. [Google Scholar]
- Li, H.; Xu, W.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Liu, H.; Xie, S. Effects of starvation on glucose and lipid metabolism in gibel carp (Carassius auratus gibelio var. CAS III). Aquaculture 2018, 496, 166–175. [Google Scholar]
- Nebo, C.; Gimbo, R.Y.; Kojima, J.T.; Overturf, K.; Dal-Pai-Silva, M.; Portella, M.C. Depletion of stored nutrients during fasting in Nile tilapia (Oreochromis niloticus) juveniles. J. Appl. Aquac. 2013, 30, 157–173. [Google Scholar] [CrossRef]
- Honorato, C.A.; Cruz, C.; Carneiro, D.J.; Machado, M.R.F. Histologia e histoquímica do intestino anterior de tilápia do Nilo (Oreochromis niloticus) alimentadas com dietas contendo silagem de peixe. Braz. J. Vet. Res. Anim. Sci. 2011, 48, 281–288. [Google Scholar]
- Zaldúa, N.; Naya, D.E. Digestive flexibility during fasting in fish: A review. Comp. Biochem. Physiol. A 2014, 169, 7–14. [Google Scholar]
- Cavali, J.; Nóbrega, B.A.; Saturnino, K.C.; Ferreira, E.; Dantas Filho, J.V.; Schons, S.V.; Porto, M.O. Intestinal histomorphometry and biometric indexes of tambaqui (Colossoma macropomum) grown under different feeding rates. Braz. J. Dev. 2020, 6, 80546–80561. [Google Scholar]
- Shaibani, M.E.; Amiri, B.M.; Khodabandeh, S. Starvation and refeeding effects on pyloric caeca structure of Caspian salmon (Salmo trutta caspius, Kessler 1877) juvenile. Tissue Cell 2013, 45, 204–210. [Google Scholar]
- Eroldogan, O.T.; Kumlu, M.; Aktas, M. Optimum feeding rates for European sea bass Dicentrarchus labrax L. reared in seawater and freshwater. Aquaculture 2004, 231, 501–515. [Google Scholar]
- Ferreira, C.M.; Antoniassi, N.A.B.; Silva, F.G.; Povh, J.A.; Potença, A.; Moraes, T.C.H.; Silva, T.K.S.T.; Abreu, J.S. Características histomorfométricas do intestino de juvenis de tambaqui após uso de probiótico na dieta e durante transporte. Pesq. Vet. Bras. 2014, 34, 1258–1260. [Google Scholar]
- Wang, Y.; Xie, S.; Nie, Z.; Li, Q.; Sun, Y.; Shao, N.; Gao, J.; Hu, J.; Xu, P.; Xu, G. Optimum feeding frequency of juvenile largemouth bass (Micropterus salmoides) reared in in-pond raceway recirculating culture system. Fish Physiol. Biochem. 2020, 46, 2197–2212. [Google Scholar] [PubMed]
| 0–42 days | Final weight (g) | Weight gain (g) | SGR (%/Day) | Feed intake (g/Fish) | Feed conversion ratio |
| Means for feeding protocol | |||||
| Continuously fed | 140.20 ± 37.54 | 39.38 ± 10.76 | 1.55 ± 0.31 | 30.83 ± 9.87 b | 0.79 ± 0.22 |
| Restricted | 136.70 ± 34.78 | 44.88 ± 12.74 | 1.82 ± 0.40 | 43.27 ± 6.23 a | 1.00 ± 0.16 |
| Means for feeding frequency | |||||
| Twice a day (2x) | 138.72 ± 38.31 | 45.38 ± 13.23 | 1.80 ± 0.43 | 38.58 ± 8.02 | 0.88 ± 0.18 |
| Three times a day (3x) | 138.14 ± 34.02 | 38.88 ± 9.81 | 1.56 ± 0.29 | 35.52 ± 12.46 | 0.91 ± 0.26 |
| p-values | |||||
| Feeding protocol | 0.584 | 0.382 | 0.161 | 0.012 | 0.059 |
| Feeding frequency | 0.928 | 0.304 | 0.240 | 0.479 | 0.781 |
| Interaction | 0.379 | 0.747 | 0.470 | 0.313 | 0.665 |
| 43–84 days | Final weight (g) | Weight gain (g) | SGR (%/day) | Feed intake (g/fish) | Feed conversion ratio |
| Means for feeding protocol Continuously fed (CF) | |||||
| Restricted (R) | 217.60 ± 68.48 | 58.63 ± 25.83 | 0.80 ± 0.23 b | 79.52 ± 26.07 | 1.42 ± 0.24 a |
| Means for feeding frequency | 208.20 ± 66.86 | 81.50 ± 29.37 | 1.06 ± 0.25 a | 76.49 ± 19.01 | 0.98 ± 0.15 b |
| Twice a day (2x) | 205.00 ± 65.39 | 71.63 ± 26.81 | 0.94 ± 0.22 | 81.08 ± 23.71 | 1.18 ± 0.21 |
| Three times a day (3x) | 220.90 ± 69.28 | 68.50 ± 33.23 | 0.93 ± 0.33 | 74.93 ± 21.51 | 1.22 ± 0.38 |
| p-values | |||||
| Feeding protocol | 0.432 | 0.109 | 0.023 | 0.799 | <0.001 |
| Feeding frequency | 0.183 | 0.817 | 0.600 | 0.606 | 0.589 |
| Interaction | 0.019 | 0.096 | 0.023 | 0.302 | 0.024 |
| Feeding Protocol | Feeding Frequency | Final Weight (g) | SGR (%/Day) | Feed Conversion Ratio |
|---|---|---|---|---|
| Continuously fed (CF) | Twice a day (2x) | 222.28 ± 66.55 Aa | 0.94 ± 0.23 Aa | 1.28 ± 0.24 Aa |
| Three times a day (3x) | 187.63 ± 60.34 Bb | 0.67 ± 0.15 Bb | 1.55 ± 0.18 Aa | |
| Restricted (R) | Twice a day (2x) | 213.00 ± 71.10 Aa | 0.94 ± 0.24 Aa | 1.06 ± 0.11 Ab |
| Three times a day (3x) | 228.81 ± 67.60 Aa | 1.19 ± 0.22 Aa | 0.89 ± 0.14 Ba |
| 0–42 Days | Glucose (mg/dL) | Triglycerides (mg/dL) | Cholesterol (mg/dL) | Total Proteins (g/dL) |
| Means for feeding protocol | ||||
| Continuously fed (CF) | 61.43 ± 7.52 | 178.71 ± 49.47 | 187.47 ± 64.78 | 5.85 ± 0.35 |
| Restricted (R) | 65.67 ± 14.80 | 186.30 ± 32.08 | 219.84 ± 35.48 | 5.85 ± 0.14 |
| Means for feeding frequency | ||||
| Twice a day (2x) | 64.95 ± 11.55 | 173.87 ± 27.82 | 207.62 ± 36.01 | 5.78 ± 0.22 |
| Three times a day (3x) | 62.14 ± 12.06 | 191.13 ± 50.74 | 199.70 ± 68.44 | 5.91 ± 0.28 |
| p-values | ||||
| Feeding protocol | 0.346 | 0.614 | 0.084 | 1.000 |
| Feeding frequency | 0.531 | 0.257 | 0.665 | 0.176 |
| Interaction | 0.522 | 0.662 | 0.088 | 0.488 |
| 43–84 days | Glucose (mg/dL) | Triglycerides (mg/dL) | Cholesterol (mg/dL) | Total proteins (g/dL) |
| Means for feeding protocol | ||||
| Continuously fed (CF) | 54.22 ± 6.04 b | 278.51 ± 26.19 | 182.92 ± 83.69 | 6.02 ± 0.31 |
| Restricted (R) | 67.88 ± 10.78 a | 284.86 ± 25.13 | 223.15 ± 49.02 | 5.94 ± 0.45 |
| Means for feeding frequency | ||||
| Twice a day (2x) | 60.17 ± 9.99 | 277.36 ± 25.20 | 209.83 ± 71.43 | 5.98 ± 0.42 |
| Three times a day (3x) | 61.92 ± 12.28 | 286.02 ± 25.76 | 196.24 ± 71.20 | 5.98 ± 0.36 |
| p-values | ||||
| Feeding protocol | <0.001 | 0.498 | 0.115 | 0.587 |
| Feeding frequency | 0.582 | 0.357 | 0.587 | 0.928 |
| Interaction | 0.414 | 0.392 | 0.460 | 0.153 |
| Hepatosomatic Index (%) | Mesenteric Fat Index (%) | Liver Lipid Levels (mg/g) | |
|---|---|---|---|
| Means for feeding protocol | |||
| Continuously fed (CF) | 1.45 ± 0.50 | 2.17 ± 1.08 | 49.92 ± 12.49 |
| Restricted (R) | 1.66 ± 0.59 | 1.95 ± 0.85 | 54.50 ± 12.55 |
| Means for feeding frequency | |||
| Twice a day (2x) | 1.52 ± 0.71 | 1.80 ± 0.93 | 51.39 ± 16.00 |
| Three times a day (3x) | 1.59 ± 0.33 | 2.32 ± 0.96 | 53.02 ± 8.24 |
| p-values | |||
| Feeding protocol | 0.308 | 0.514 | 0.455 |
| Feeding frequency | 0.725 | 0.131 | 0.788 |
| Interaction | 0.902 | 0.159 | 0.083 |
| Villi Perimeter (µm) | Villi Height (µm) | |
|---|---|---|
| Means for feeding protocol | ||
| Continuously fed (CF) | 306.03 ± 98.55 b | 102.52 ± 29.58 b |
| Restricted (R) | 712.78 ± 558.13 a | 235.13 ± 164.80 a |
| Means for feeding frequency | ||
| Twice a day (2x) | 649.13 ± 554.28 a | 211.69 ± 164.66 a |
| Three times a day (3x) | 329.77 ± 95.42 b | 111.31 ± 33.13 b |
| p-values | ||
| Feeding protocol | <0.001 | <0.001 |
| Feeding frequency | <0.001 | <0.001 |
| Interaction | <0.001 | <0.001 |
| Feeding Protocol | Feeding Frequency | Villi Perimeter (µm) | Villi Height (µm) |
|---|---|---|---|
| Continuously fed (CF) | Twice a day (2x) | 289.13 ± 83.70 Bb | 98.39 ± 24.50 Bb |
| Three times a day (3x) | 330.74 ± 116.05 Aa | 108.39 ± 35.92 Aa | |
| Restricted (R) | Twice a day (2x) | 1051.49 ± 581.61 Aa | 340.10 ± 161.54 Aa |
| Three times a day (3x) | 328.92 ± 77.55 Ba | 114.00 ± 31.56 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assis, Y.P.A.S.; Amorim, M.P.S.; de Assis Porto, L.; de Oliveira, P.E.C.M.; Santos, C.A.; Reis, G.P.A.; Luz, R.K.; Favero, G.C. Effect of Feeding Frequency and Restriction on the Growth Performance, Physiology, and Intestinal Histomorphometry of Colossoma macropomum in a Recirculating Aquaculture System. Fishes 2025, 10, 148. https://doi.org/10.3390/fishes10040148
Assis YPAS, Amorim MPS, de Assis Porto L, de Oliveira PECM, Santos CA, Reis GPA, Luz RK, Favero GC. Effect of Feeding Frequency and Restriction on the Growth Performance, Physiology, and Intestinal Histomorphometry of Colossoma macropomum in a Recirculating Aquaculture System. Fishes. 2025; 10(4):148. https://doi.org/10.3390/fishes10040148
Chicago/Turabian StyleAssis, Yhago Patrycky Antunes Souza, Matheus Philip Santos Amorim, Lívia de Assis Porto, Paulo Edson Camilo Mol de Oliveira, Carina Alves Santos, Gean Paulo Andrade Reis, Ronald Kennedy Luz, and Gisele Cristina Favero. 2025. "Effect of Feeding Frequency and Restriction on the Growth Performance, Physiology, and Intestinal Histomorphometry of Colossoma macropomum in a Recirculating Aquaculture System" Fishes 10, no. 4: 148. https://doi.org/10.3390/fishes10040148
APA StyleAssis, Y. P. A. S., Amorim, M. P. S., de Assis Porto, L., de Oliveira, P. E. C. M., Santos, C. A., Reis, G. P. A., Luz, R. K., & Favero, G. C. (2025). Effect of Feeding Frequency and Restriction on the Growth Performance, Physiology, and Intestinal Histomorphometry of Colossoma macropomum in a Recirculating Aquaculture System. Fishes, 10(4), 148. https://doi.org/10.3390/fishes10040148








