1. Introduction
The rapid global economic development has led to higher per capita incomes [
1], which are often associated with increased consumption of animal-based foods [
2], particularly fish, thereby driving growth in aquaculture. Aquaculture is crucial for global food security, especially in the context of the United Nations’ zero hunger goal [
3]. However, to remain sustainable, aquaculture must minimize its environmental impact.
Adopting circular economy principles is one strategy for sustainable aquaculture growth. But climate change threatens food security [
4], with predictions suggesting a decline in aquaculture production due to its effects [
5]. Climate change, along with geopolitical instability, could also disrupt feed ingredients’ availability, creating bottlenecks in production. Thus, exploring alternative feed ingredients from diverse sources, such as byproducts from forestry [
6,
7], food compost [
8], and agriculture [
9], is essential for resilience and sustainability.
Historically, extruded pellets for salmonids were made using fish meal and oil [
3]. However, capture fisheries have plateaued over the past 30 years, with many stocks overfished [
10]. In contrast, aquaculture is rapidly growing [
10], which will increase the demand for aquafeed [
11]. While plant-based ingredients like soy and rapeseed have been used to ease pressure on fisheries, the ethical concerns surrounding plant proteins that humans could consume must also be considered [
7]. Developing novel, sustainable ingredients should take into account not only environmental impacts (such as carbon footprint and biodiversity) but also societal costs (such as ethical sourcing and land use) to avoid shifting one problem to another.
Fungal protein sources hold promise for overcoming the challenges described above, due to several advantages over conventional protein sources. Firstly, they can be produced on a variety of organic substrates that are not suitable for human consumption [
7]. Additionally, they have a high crude protein content, with some yeast species containing approximately 50% crude protein, comparable to the 34% to 42% found in soybeans [
12,
13,
14]. Fungi are also sustainable and resource-efficient compared to fish meal [
15,
16,
17]. They can be tailored to produce different amino acid compositions and nutrients, based on their substrates and growing conditions. Fungi offer a wide variety of species options based on specific needs. Lastly, fungal components can provide health benefits due to their immune-stimulating properties, in addition to their nutritional effects [
18].
Despite their benefits, fungi have limitations that restrict their use in feed production today, such as strong cell walls that reduce their digestibility in fish [
19]. Downstream processing to improve digestibility adds costs and should be implemented only when necessary. To address the cost concerns, it is crucial to explore different side-streams and substrates for microbial biomass production, and to identify the ideal substrate–fungi combination that requires minimal modification for use as a feed ingredient in aquaculture.
Among the various types of fungi currently being evaluated as feed ingredients, filamentous fungi emerge as particularly interesting candidates, in part due to their ability to valorize side-streams. Several important criteria must be considered when evaluating filamentous fungi as potential protein sources for salmonid feed. First, the fungi must be safe for human consumption, meaning that they are non-toxic and non-infectious. Additionally, it is crucial that they are produced from non-food sources and have a high protein content with an amino acid composition similar to that of fish meal. Moreover, the ingredients should have high digestibility and must not negatively impact the physical parameters of the feed. The physical quality of a fish feed pellet can affect feeding behavior, which, in turn, influences fish health, environmental impact, and economic losses [
20]. Pellets that sink too quickly may not give fish enough time to consume them, potentially leading to economic losses and environmental pollution from uneaten feed. Pellets with low water stability can lead to abdominal distension syndrome and oil belching [
20,
21,
22]. Additionally, during storage, handling, and transportation, pellets can become crushed, resulting in further losses. The broken feed particles may be too small for fish to consume or might float instead of sinking, which can also contribute to feed waste.
The microbial ingredients tested in this study include
Paecilomyces variotii,
Aspergillus oryzae,
Rhizopus oligosporus, and
Rhizopus delemar.
P. variotii (PEKILO
®) was originally used in the 1960s and 1970s to produce mycoprotein biomass from Finnish paper pulp waste. Today, PEKILO
® production is focused on optimizing the valorization of various side-streams and sustainable substrates, leveraging its well-studied properties and the existing infrastructure for large-scale production [
23].
A. oryzae,
R. oligosporus, and
R. delemar can be cultivated on industrial side-streams such as spent sulfite liquor or ethanol stillage while exhibiting high crude protein content, making them potential alternative protein sources for rainbow trout (
Oncorhynchus mykiss) [
24,
25,
26]. Additionally, Karimi S. et al. [
27] observed that the nutritional composition of several filamentous fungi, such as
A. oryzae grown on a pure substrate, is comparable to that of fish meal.
To the best of our knowledge, there are a limited number of studies analyzing the digestibility of selected multicellular fungi in rainbow trout. The digestibility of the ingredients can differ even among related species like Atlantic salmon (
Salmo salar) and rainbow trout [
28]. Hence, digestibility trials with the specific species are required to identify the correct digestibility values. The objective of this study is to evaluate four multicellular fungi derived from industrial side-streams for their suitability as feed ingredients for rainbow trout, assessing the ingredient nutrient composition, digestibility, and pellet quality parameters.
4. Discussion
There is a growing interest in using microbial ingredients, particularly those produced through the valorization of waste streams, as protein sources in aquaculture diets. However, safety, digestibility, palatability, and scalability of production remain significant barriers to incorporating microbial ingredients into fish feed. In this study, we evaluated four different microbial ingredients, grown on various waste streams, for their potential as feed ingredients in the diet of farmed rainbow trout. A digestibility trial was conducted to assess the quality and suitability of these ingredients as alternative feed sources. Studies including nutrient ADs (%) of multicellular fungi are quite scarce. To the best of our knowledge, this is one of the first studies to describe and compare the ADs (%) of various multicellular fungi in rainbow trout.
The different dietary treatments did not result in any statistically significant differences in weight gain percentage among the fish. This is in agreement with the findings of Vidakovic et al. [
39], where high inclusion levels of fungal biomass did not result in varying growth. Regardless, Dahlberg [
40] has shown differences in weight gain percentage even after 4–5 weeks of feeding. The lack of significant differences in weight gain % can be viewed as a positive outcome in the context of this study. However, considering the brief duration of the feeding trial, the lack of significant differences in growth is not surprising. Future research should focus on long-term growth performance trials utilizing the same ingredients within nutritionally balanced diets to gain a more comprehensive understanding of their effects. In addition, the FCR values were all below 1, indicating good conversion levels. RO had a significantly higher FCR than the other diets, indicating that the nutrients may not have been used as optimally as in the other diets. The total feed intake (
Table 4) across all test diets did not differ significantly from the control. This indicates that, even at inclusion levels of 30%, the test ingredients did not contain compounds that adversely impacted feed palatability.
The crude protein (CP) content of the ingredients in this study ranged from 41% to 63%, with RO having a CP of 48.7%, slightly higher than the 47.9% reported by Langeland M. et al. [
16]. The CP value for PEKILO
® in the present study was slightly higher, at 66.7%, compared to the results of Hooft, J.M. et al. [
23], at 62.5%. In contrast, the CP values of AO (44.1%) and RD (49.3%) in this study were lower compared to the ranges of 48.6% to 53.7% for AO and 48.6% to 53.2% for RD reported by Karimi S. et al. [
9]. This variation in CP content could be attributed to differences in the substrates used, the extraction methods, the fungal strains, or the cultivation parameters [
41,
42,
43].
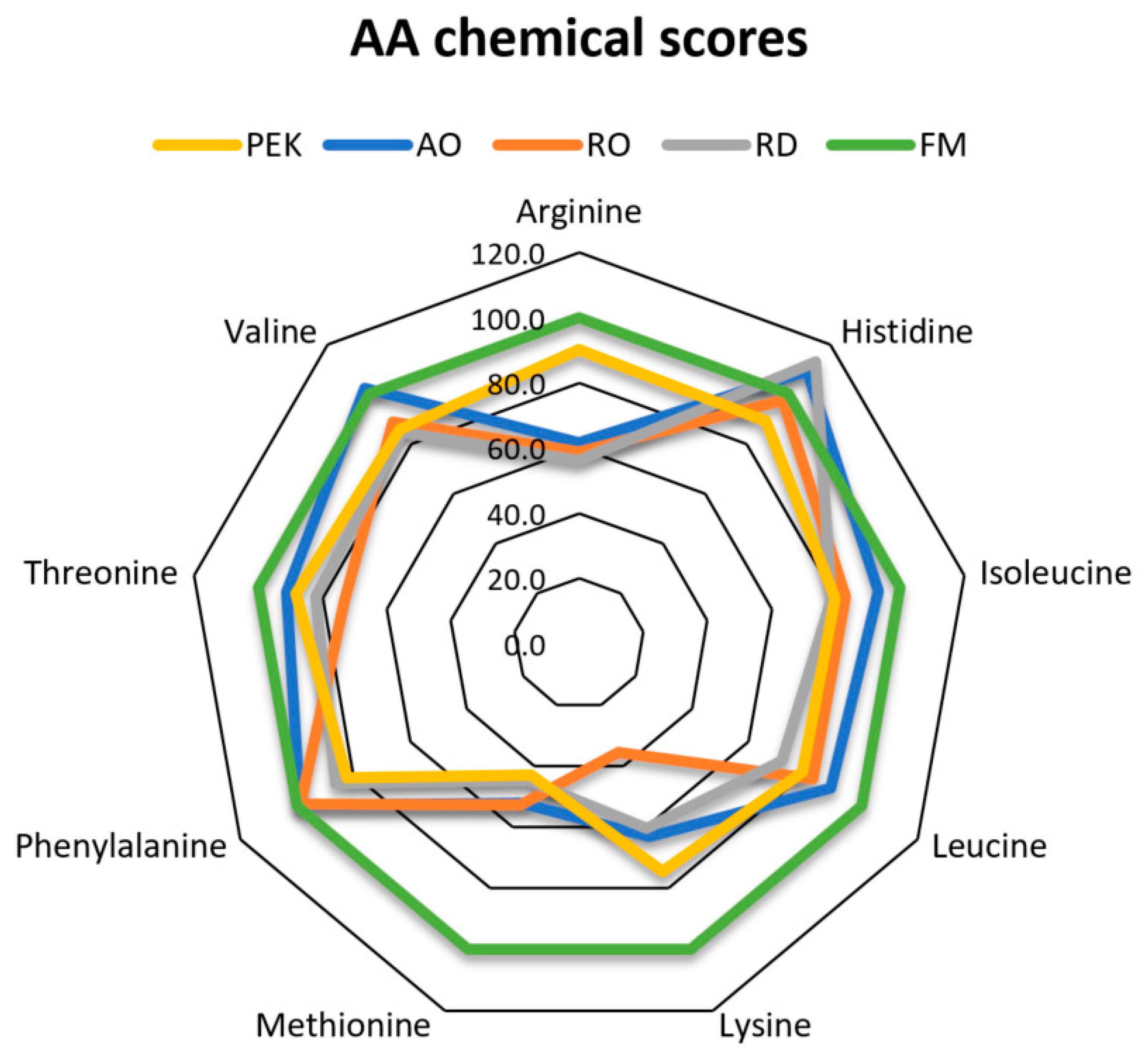
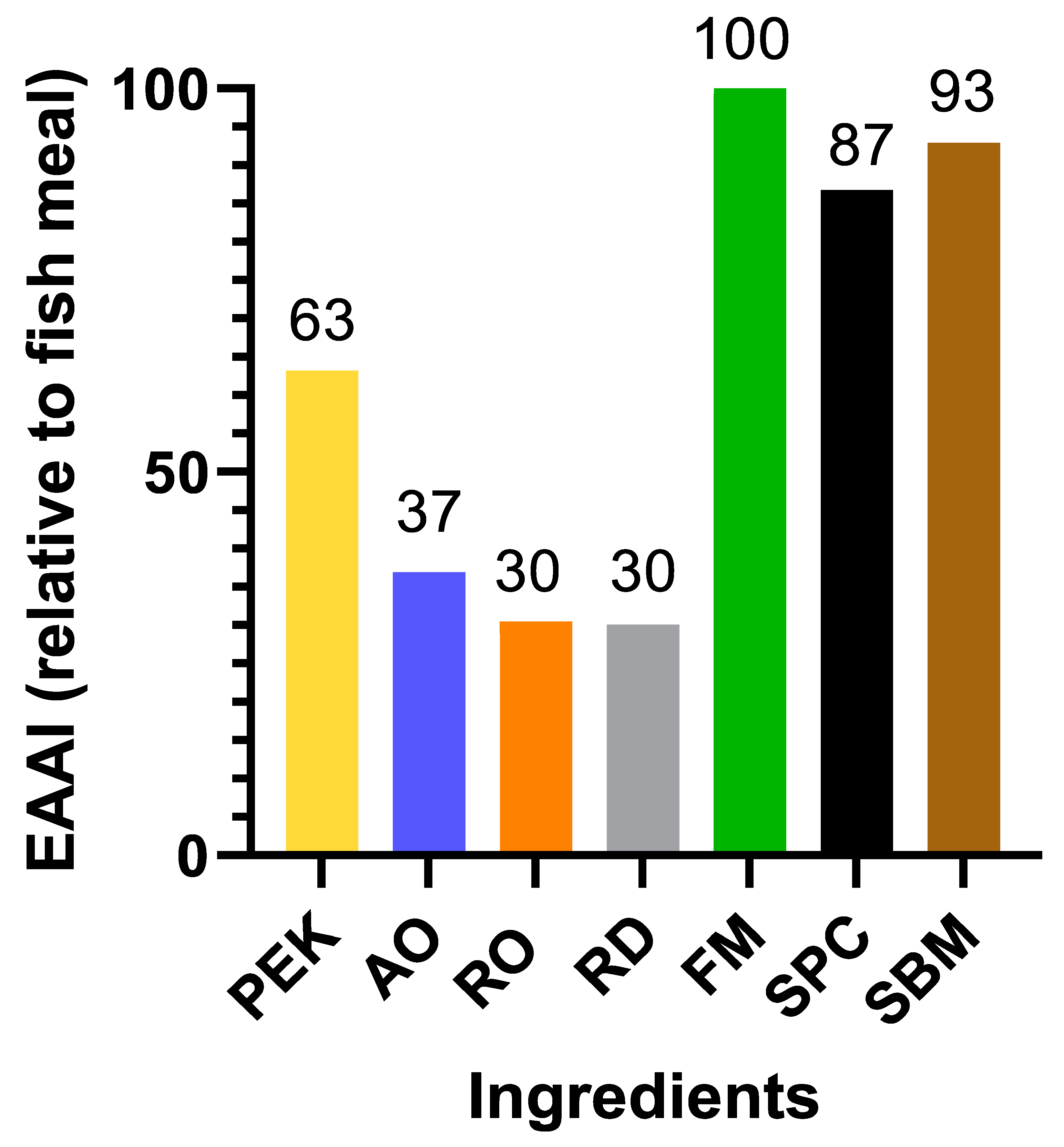
The amino acid CS and EAAI (
Figure 1) indicate that PEK has the most favorable amino acid composition among the different ingredients tested in this trial. Methionine and lysine are the major limiting amino acids in salmonid feeds [
44]. The lower chemical score of methionine for all tested ingredients indicates a major drawback when using these fungal proteins to replace fish meal and soy, as it limits their use at higher inclusion levels in diets for rainbow trout. Karimi et al. [
27] observed that the methionine composition of the multicellular fungi derived from pure cultures was lower than that of fish meal, although the CS and EAAI were not calculated. For PEK, the CS of lysine is above 100, indicating higher lysine composition in its crude protein compared with fish meal. The chemical score for lysine is below 50% for RO, indicating that lysine could be a limiting amino acid. Karimi et al. [
27] observed that the lysine levels of the multicellular fungi grown on pure streams were the about the same as those of fish meal. The EAAI value of SBM, when fish meal was used as a reference diet, was 93%, which is almost the same as described in an earlier study by Agboola et al. [
12]. However, in that study, the tested ingredients had higher EAAI values, ranging from 67 to 79, compared to the lower range of 30 to 63 observed in this study. This would indicate that, compared with multicellular fungi, yeasts might have an amino acid composition that is better suited for salmonids. However, filamentous fungi can still be advantageous compared to single-celled fungi, owing to their crude protein content and the ease with which they can be separated from their culture medium on a large scale. The deficiency of the amino acids in commercial feeds can be counteracted by adding synthetic amino acids at an additional cost. However, the amino acid composition and content in fungi can be altered to a degree through substrate optimization [
45,
46,
47]. Theoretically, it is possible to develop multicellular fungi with improved methionine content, thus enhancing their potential inclusion levels in fish feed without compromising nutritional quality.
Hardy et al. [
48] defined an ingredient as protein-rich if it contains more than 35% crude protein content. Rainbow trout diets require a digestible protein content of 38% [
48]. Hence, ingredients added as protein sources need to have a protein content significantly higher than that, due to the presence of other ingredients that might not have a high enough protein content and may have other roles in the feed. The ingredients used in this experiment have protein contents in the range of 44 to 66.8%. PEKILO
® has a crude protein content of 66.8%. This is much higher than the crude protein content of the other alternative feed ingredients, such as multicellular fungi, which have a crude protein content of around 51% [
9]. Yeast biomass contains crude protein contents ranging from 40 to 55% [
12], while commonly used feed ingredients such as fish meal and soy protein concentrate have protein contents ranging from around 62% to 70% (NRC 2011). The fat content of the ingredient is not a major concern, as the necessary fats are typically added later in the form of oils. However, fats can influence the extrusion process. The physical quality of novel aquafeed ingredients is of high importance for successful extrusion, since it affects the feed’s ability to maintain its shape, texture, and nutritional quality during processing. Ingredients that do not meet physical quality standards can lead to issues such as poor pellet durability, inconsistent feeding behavior, and nutrient leaching [
19,
49].
As natural lubricants, high fat levels can cause instability during extrusion [
50,
51], making it important to keep their concentrations as low as possible. Except for AO, which has 12.46% fat, all of the other ingredients have relatively low fat contents. This is also reflected in the expansion rate, where AO has a significantly lower expansion rate (%) during extrusion compared with the control and PEK diet pellets.
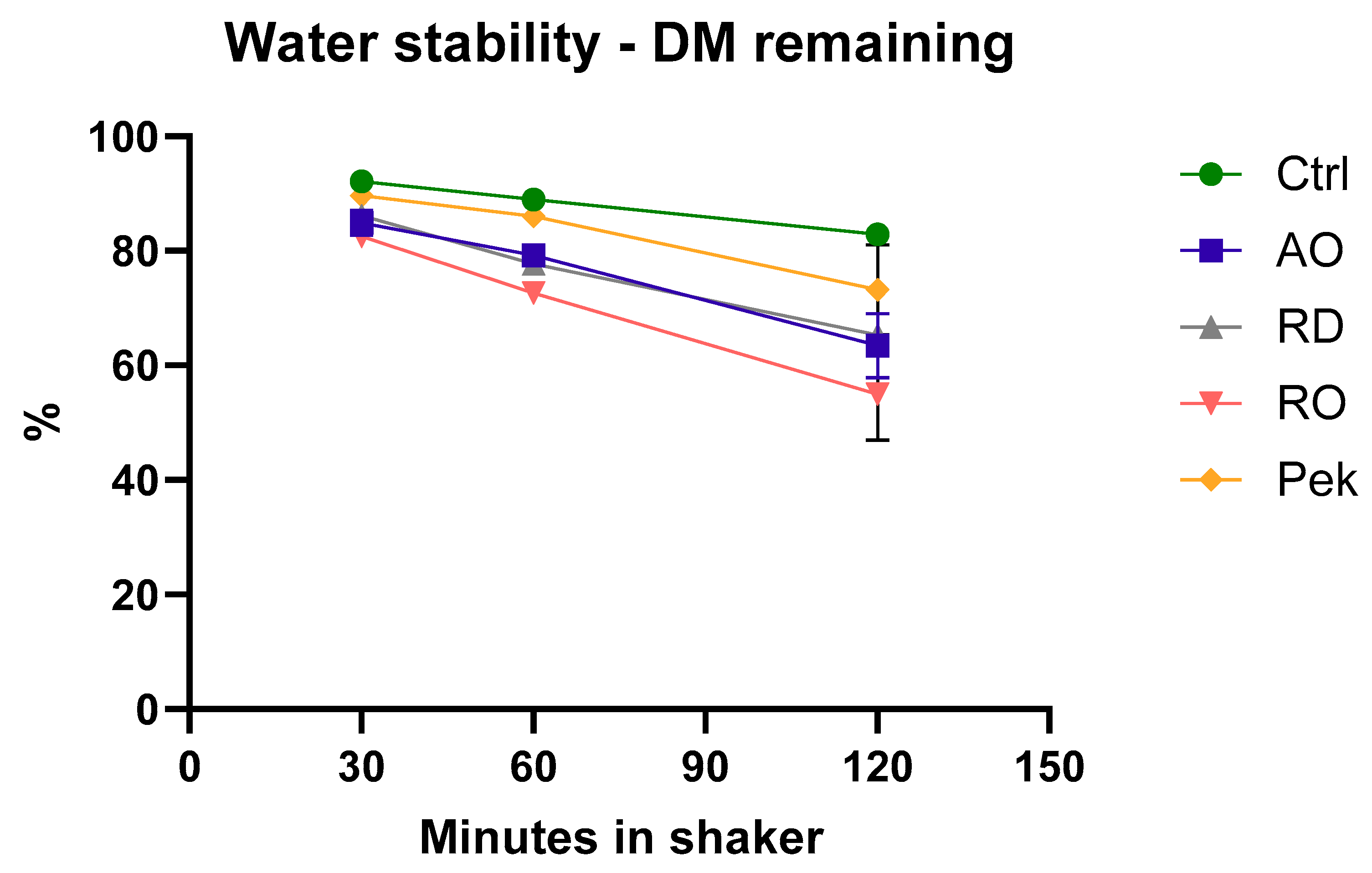
High water stability is preferred in rainbow trout feeds, as low feed stability, combined with certain environmental factors, can lead to issues like oil belching and abdominal distention syndrome [
20,
21,
22]. The water stability of a diet is influenced by the composition of its ingredients [
20,
52]. Among the tested diets, the one containing PEK showed the highest water stability, as well as the highest AD
CP. This finding aligns with those of previous studies, which suggest that increased feed stability is associated with higher AD
CP and AD
CF [
22].
The water stability index (WSI) of PEK was not statistically different from that of the control diet, even after 60 min of exposure, which can be considered to be a positive outcome. This minimizes the risk of the digestibility-related issues mentioned above and suggests that the feed is more likely to remain intact when consumed by the fish. Additionally, Hooft et al. [
23] observed that including the microbial ingredient PEKILO
® enhanced the water stability. However, in our study, the inclusion of microbial ingredients actually reduced the feed stability. One possible explanation for this discrepancy could be the differences in inclusion levels and ingredient composition. Since the diets in our study were formulated using a 70:30 formula, the levels of starch, which is an important binding agent, may have varied, affecting the overall stability of the feed.
The digestibility of dietary dry matter AD
DM (
Table 6) was significantly higher in the control than in the other diets. PEK had the second-highest, whereas AO, RD, and RO had statistically the same AD
DM. The ingredient dry matter AD
DM (
Table 5) of RD and RO was 23.6% and 24.1%, respectively. This lower digestibility may be attributed to the presence of indigestible components such as cell walls in the microbial biomass, or to residual substrates from the fermentation process [
53,
54]. At the same time, feces collection through stripping may lead to an underestimation of AD [
55]. Previous studies have shown that the method of feces collection may lead to under- or overestimation of AD [
55,
56]. In addition, repeated handling has been shown to depress the feed intake and growth in rainbow trout [
57], and this may raise concerns in relation to the potential effects on digestibility. However, despite this, the AD
CP for the control diet in our study was found to be 91.2%, which is in agreement with other studies in salmonids, where fish-meal-based diets’ digestibility usually ranged from 82.7 to 92.1% [
16,
39,
54]. The digestibility of proteins can be negatively affected by cell walls present in fungi [
12,
58,
59]. The sum of the crude protein, crude fat, and ash contents for AO, RO, RD, and PEK was 642, 537, 638, and 802 g/kg DM, respectively. This indicates that the carbohydrate fractions, and possibly the fiber fractions, of AO, RO, and RD are much higher. This is further supported by the lower pellet expansion in the diets with RO, RD, and AO. Hansen et al. [
60] have observed that expansion decreased with the increase in non-soluble polysaccharide (NSP) inclusion. This could be one of the reasons for the differing protein digestibility among the different multicellular fungi observed in the present trial. Further studies on the cell wall composition could shed light on any possible correlations.
Comparing the AD
AA for methionine and lysine (
Table 5), PEK demonstrated better quality compared to the other tested ingredients. A higher AD reflects a greater amino acid absorption, reducing the proportion that is excreted undigested and enhancing nutrient utilization. The AD
CP varies among diets, likely due to differences between species, but also in substrate composition, extraction methods, or cultivation parameters. The composition of fungal cell walls is highly dynamic and is influenced by environmental conditions and processing methods. Factors such as temperature, pH, osmotic pressure, and nutrient availability affect the cell wall structure, enzyme activities, and digestibility [
61,
62,
63,
64,
65]. These parameters regulate the biosynthesis of key polysaccharides (e.g., chitin, β-glucans, α-glucans) and the expression of remodeling enzymes, impacting fungal growth and downstream applications in biotechnology and feed production. Adjusting the fermentation strategies and environmental conditions can modify the fungal structure and potentially enhance digestibility. Studies on various yeast species have reported AD
DM values ranging from 38% to 53% [
66]. Similarly, the AD
CP values in the same research ranged between 63% and 73%, which are comparable to the AD
CP values observed for AO, RO, and RD in this study, but lower than that of PEK. In salmonids, the AD
CP of inactivated yeast generally falls within the range of 51% to 91% [
16,
67,
68], aligning with the findings of the present study. Improvements in downstream processing have also been shown to enhance AD
CP [
68].
P. variotii, a fungus produced from sour lye, which is a byproduct of textile cellulose production, has shown AD
DM values between 51.5% and 66% in previous studies. The same research reported AD
CP values ranging from 82.6% to 88.4% and AD
CF values between 72.8% and 99.8% [
40]. These results are consistent with the findings for PEKILO
® in the current study, indicating a potential similarity between the ingredients despite differences in their substrate origins.