Transcriptomic Analysis of Endocrine System Responses in Zebrafish Embryos Following Exposure to Environmentally Relevant Concentrations of Arsenate
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Maintenance and Embryos Acquisition
2.2. Arsenic Exposure
2.3. Hormone Measurement
2.4. Transcriptomic Analysis
2.5. RNA Isolation and cDNA Synthesis RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.6. Statistical Analysis
3. Results
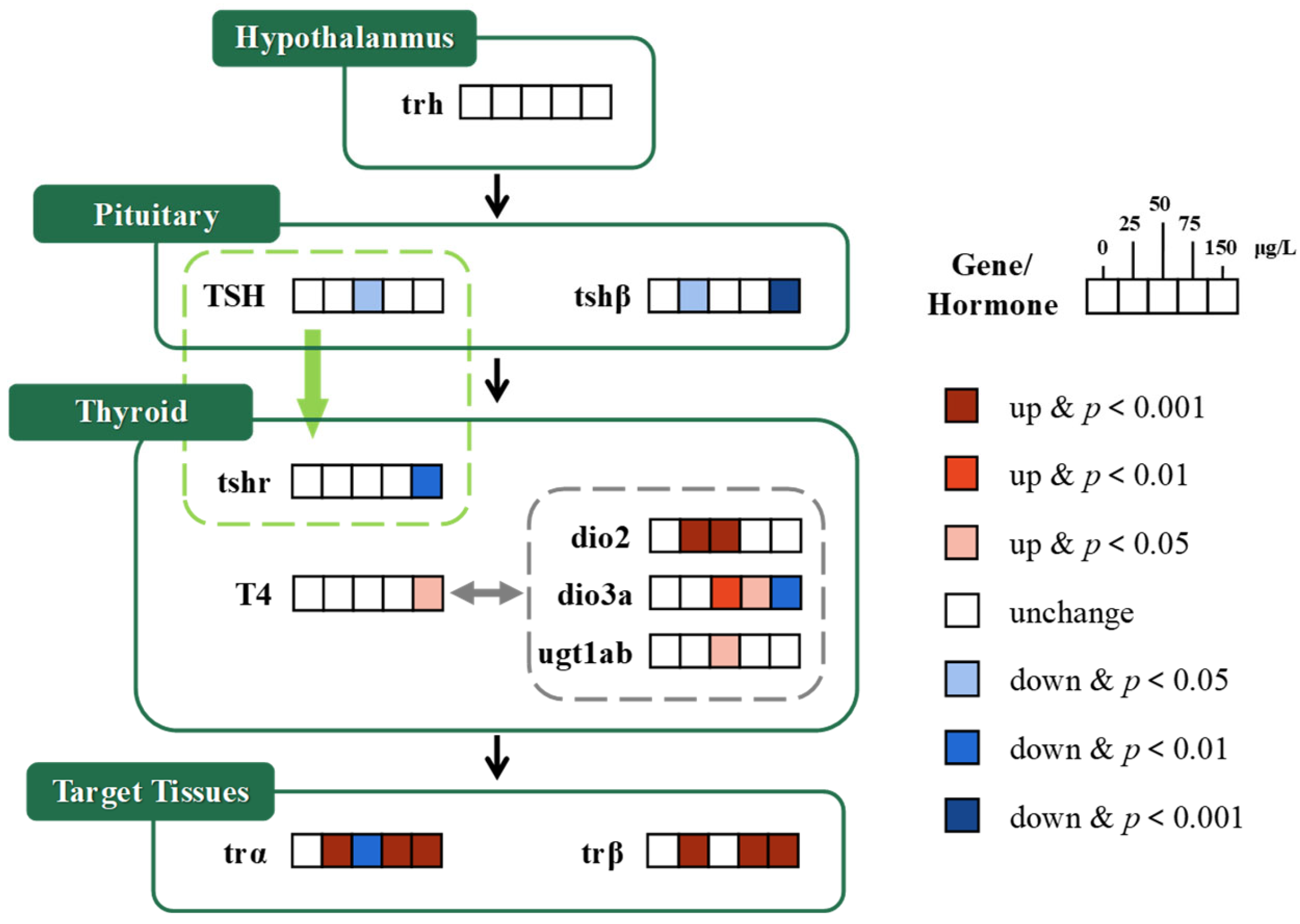
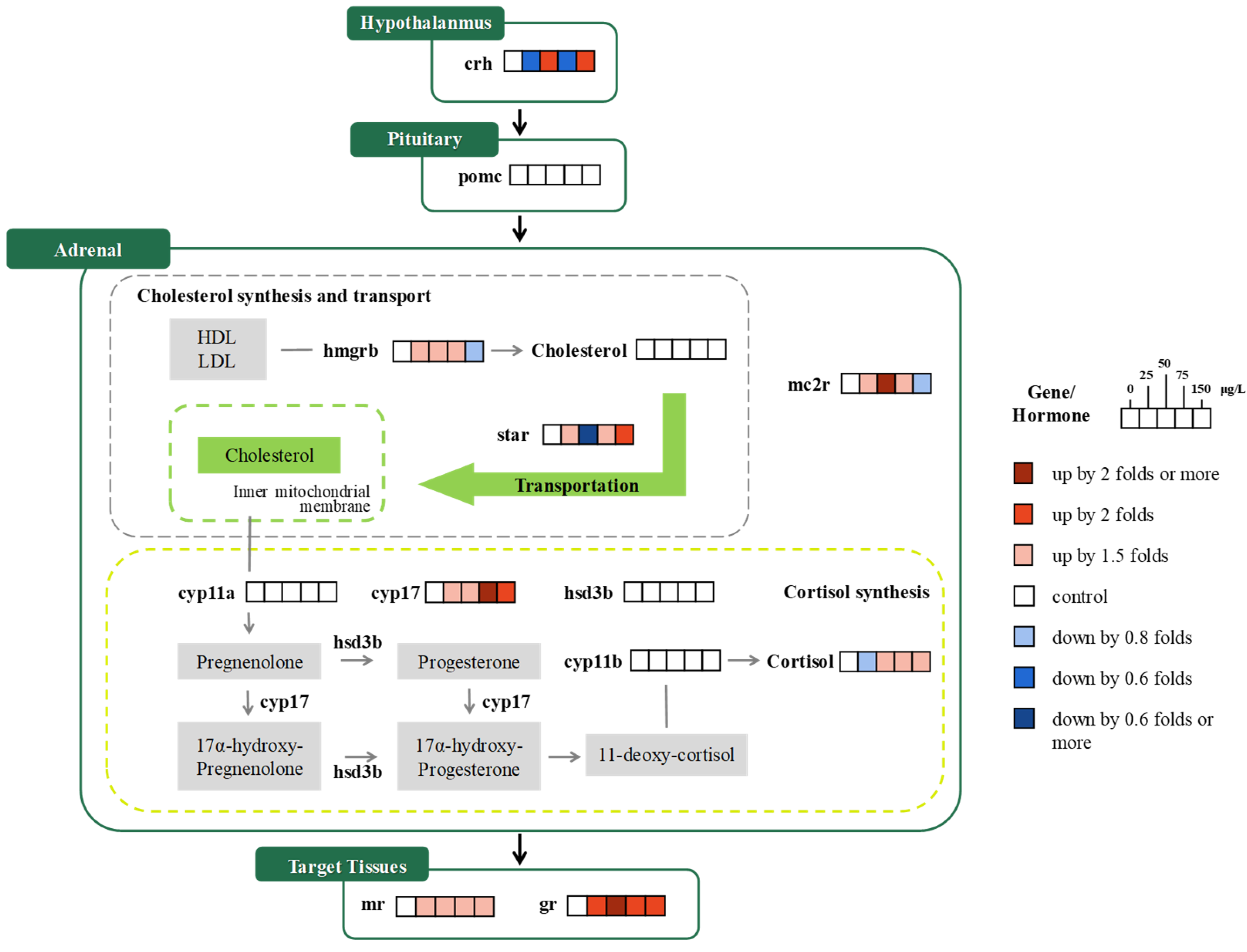
3.1. Hormone
3.1.1. HPT Axis
3.1.2. HPA Axis
3.1.3. HPG Axis
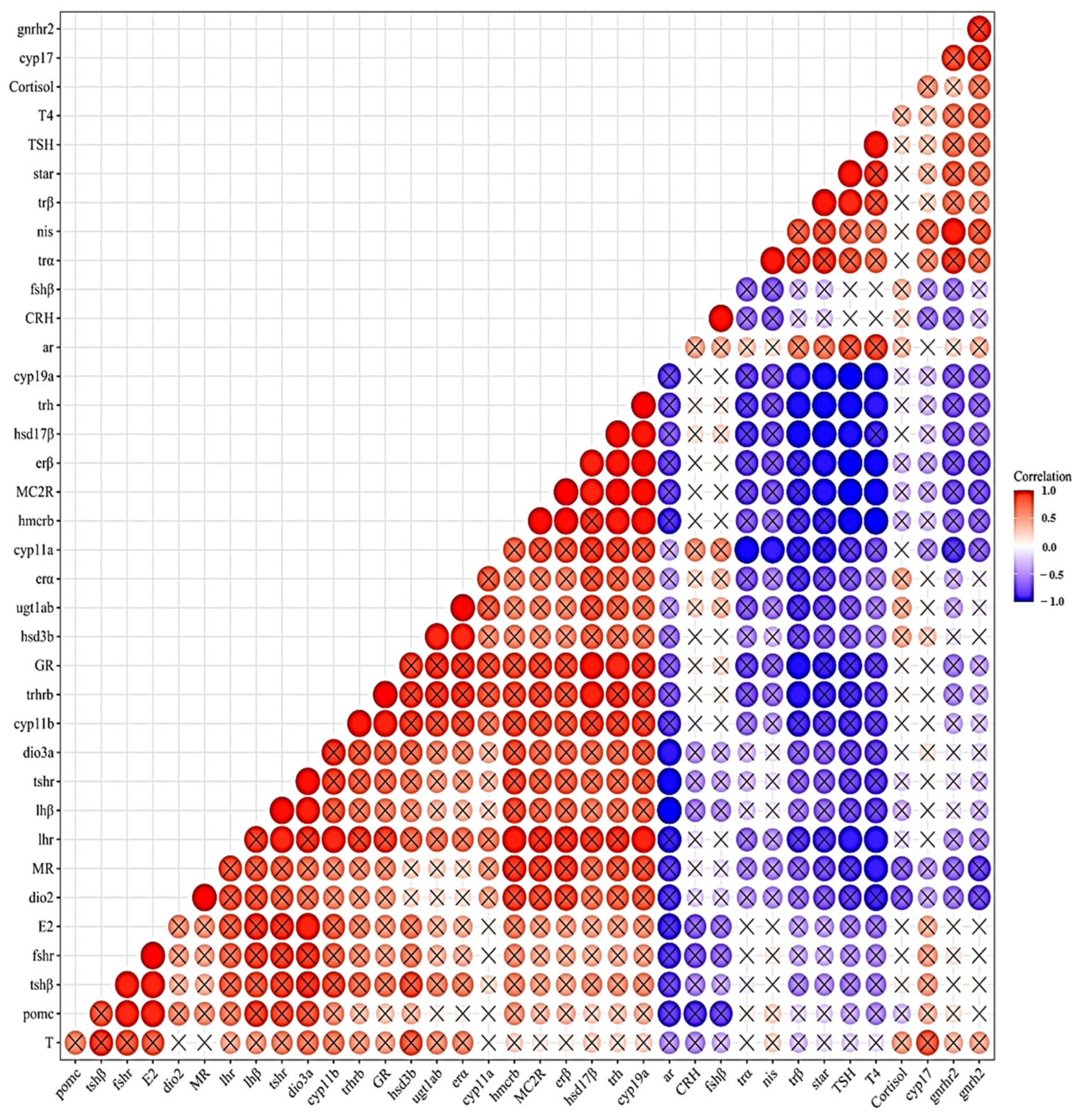
3.2. Gene
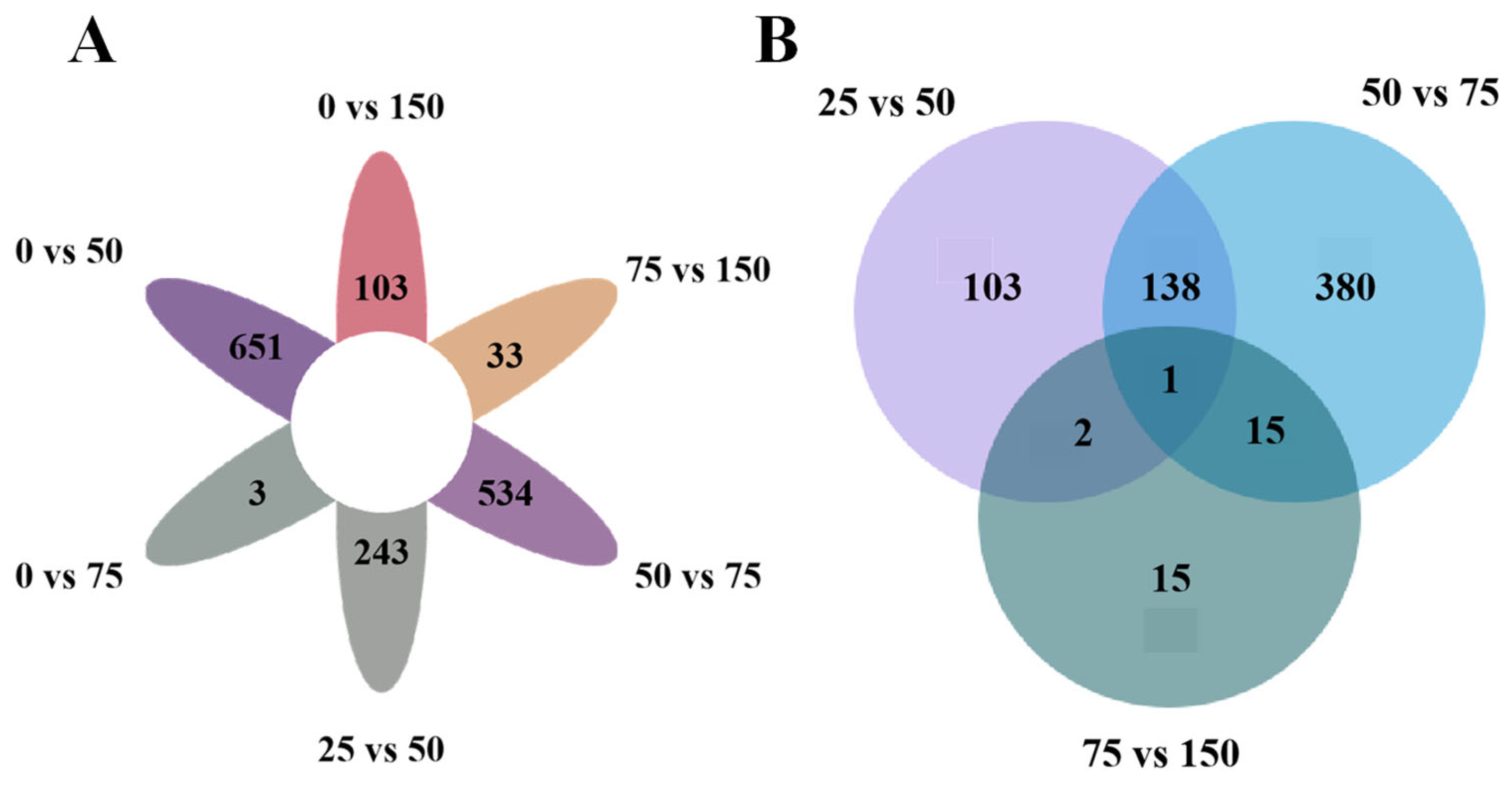
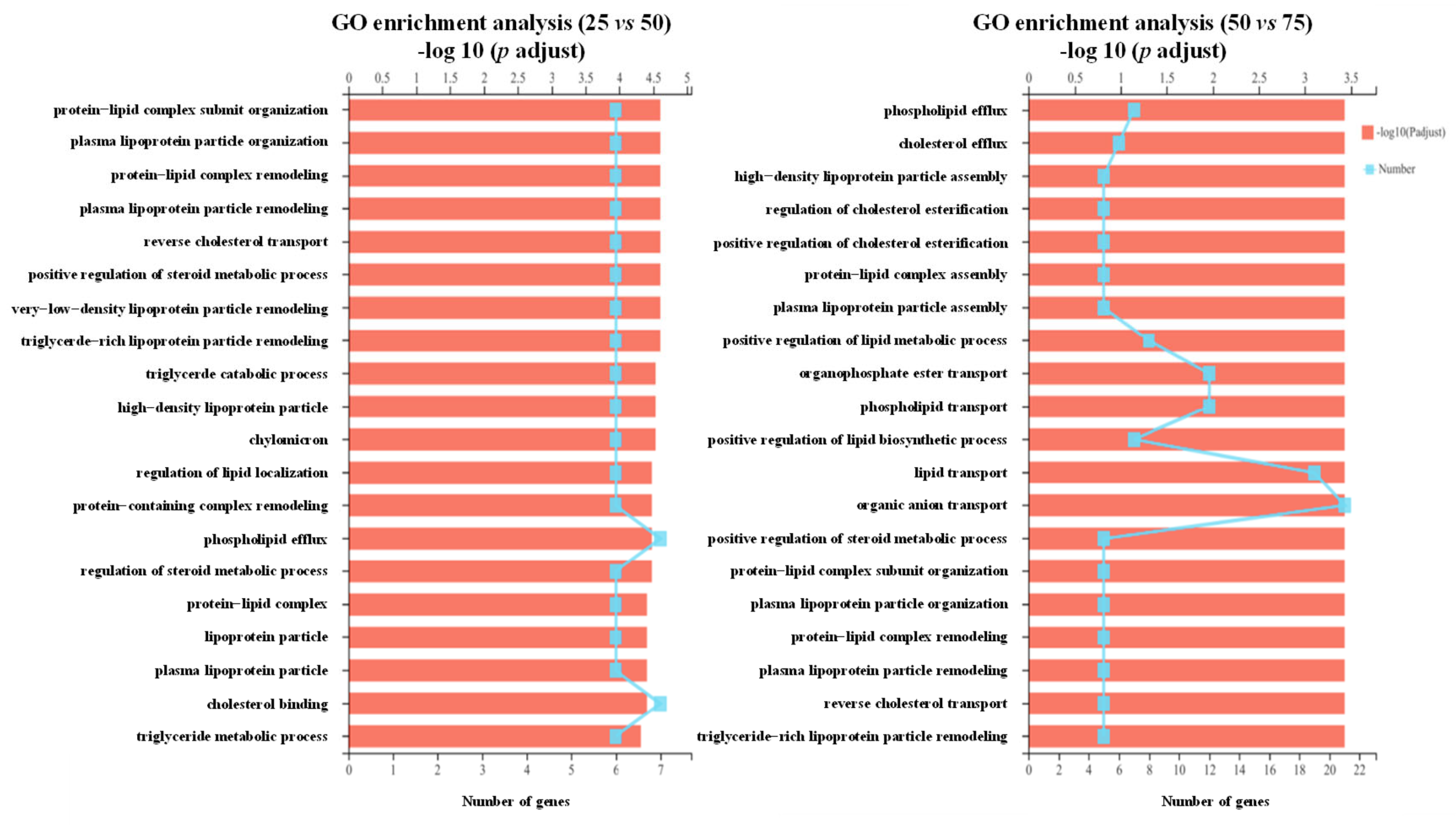
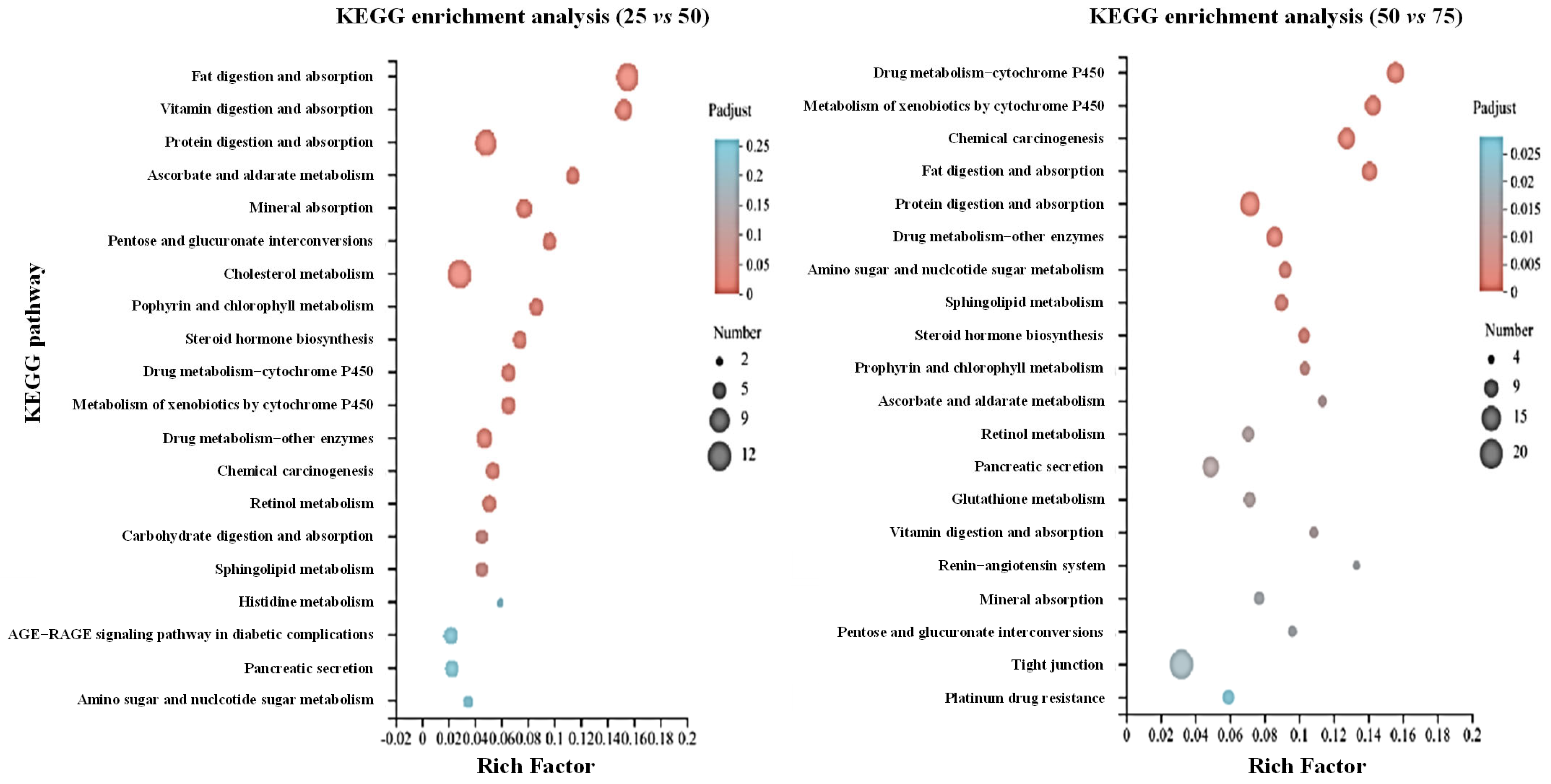
3.3. Transcriptome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.-J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Guha Mazumder, D.N. 6-Health Effects Chronic Arsenic Toxicity. In Handbook of Arsenic Toxicology; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2015; pp. 137–177. [Google Scholar] [CrossRef]
- Li, C.; Kang, S.; Zhang, Q.; Gao, S.; Sharma, C.M. Heavy metals in sediments of the Yarlung Tsangbo and its connection with the arsenic problem in the Ganges–Brahmaputra Basin. Environ. Geochem. Health 2011, 33, 23–32. [Google Scholar] [CrossRef]
- Weiske, A.; Schaller, J.; Hegewald, T.; Kranz, U.; Feger, K.-H.; Werner, I.; Dudel, E.G. Changes in catchment conditions lead to enhanced remobilization of arsenic in a water reservoir. Sci. Total Environ. 2013, 449, 63–70. [Google Scholar] [CrossRef]
- Barrett, P.M.; Hull, E.A.; King, C.E.; Burkart, K.; Ott, K.A.; Ryan, J.N.; Gawel, J.E.; Neumann, R.B. Increased exposure of plankton to arsenic in contaminated weakly-stratified lakes. Sci. Total Environ. 2018, 625, 1606–1614. [Google Scholar] [CrossRef]
- Sun, H.; Lü, K.; Minter, E.J.A.; Chen, Y.; Yang, Z.; Montagnes, D.J.S. Combined effects of ammonia and microcystin on survival, growth, antioxidant responses, and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae. J. Hazard. Mater. 2012, 221–222, 213–219. [Google Scholar] [CrossRef]
- Sun, H.; Wang, W.; Li, J.; Yang, Z. Growth, oxidative stress responses, and gene transcription of juvenile bighead carp (Hypophthalmichthys nobilis) under chronic-term exposure of ammonia. Environ. Toxicol. Chem. 2014, 33, 1726–1731. [Google Scholar] [CrossRef]
- Sun, H.-J.; Zhang, J.-Y.; Wang, Q.; Zhu, E.; Chen, W.; Lin, H.; Chen, J.; Hong, H. Environmentally relevant concentrations of arsenite induces developmental toxicity and oxidative responses in the early life stage of zebrafish. Environ. Pollut. 2019, 254, 134250. [Google Scholar] [CrossRef]
- Dong, W.-Q.; Sun, H.-J.; Zhang, Y.; Lin, H.-J.; Chen, J.-R.; Hong, H.-C. Impact on growth, oxidative stress, and apoptosis-related gene transcription of zebrafish after exposure to low concentration of arsenite. Chemosphere 2018, 211, 648–652. [Google Scholar] [CrossRef]
- Nayak, A.S.; Lage, C.R.; Kim, C.H. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio). Toxicol. Sci. 2007, 98, 118–124. [Google Scholar] [CrossRef]
- Kumar, A.; Kesari, V.P.; Khan, P.K. Fish micronucleus assay to assess genotoxic potential of arsenic at its guideline exposure in aquatic environment. Biometals 2013, 26, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.; Brix, K.V.; Amlund, H.; Lundebye, A.-K.; Hogstrand, C.; Bury, N.R. Natural Arsenic Contaminated Diets Perturb Reproduction in Fish. Environ. Sci. Technol. 2008, 42, 5354–5360. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Li, H.-B.; Xiang, P.; Zhang, X.; Ma, L.Q. Short-term exposure of arsenite disrupted thyroid endocrine system and altered gene transcription in the HPT axis in zebrafish. Environ. Pollut. 2015, 205, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.Q.; Huy, B.T.; Van Tan, L.; Phuong, N.T.K. Lead and Arsenic Accumulation and Its Effects on Plasma Cortisol Levels in Oreochromis sp. Bull. Environ. Contam. Toxicol. 2017, 99, 187–193. [Google Scholar] [CrossRef]
- Sun, H.J.; Xiang, P.; Luo, J.; Hong, H.; Lin, H.; Li, H.-B.; Ma, L.Q. Mechanisms of arsenic disruption on gonadal, adrenal and thyroid endocrine systems in humans: A review. Environ. Int. 2016, 95, 61–68. [Google Scholar] [CrossRef]
- Sobanska, M.; Scholz, S.; Nyman, A.-M.; Cesnaitis, R.; Gutierrez Alonso, S.; Klüver, N.; Kühne, R.; Tyle, H.; de Knecht, J.; Dang, Z.; et al. Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH). Environ. Toxicol. Chem. 2018, 37, 657–670. [Google Scholar] [CrossRef]
- Nüßer, L.K.; Skulovich, O.; Hartmann, S.; Seiler, T.-B.; Cofalla, C.; Schuettrumpf, H.; Hollert, H.; Salomons, E.; Ostfeld, A. A sensitive biomarker for the detection of aquatic contamination based on behavioral assays using zebrafish larvae. Ecotoxicol. Environ. Saf. 2016, 133, 271–280. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Y.; Wu, C.; Ma, L.Q.; Guan, D.; Hong, H.; Yu, H.; Lin, H.; Huang, X.; Gao, P. Dihalogenated nitrophenols in drinking water: Prevalence, resistance to household treatment, and cardiotoxic impact on zebrafish embryo. Eco-Environ. Health 2024, 3, 183–191. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Korevaar, T.I.M.; Medici, M.; Visser, T.J.; Peeters, R.P. Thyroid disease in pregnancy: New insights in diagnosis and clinical management. Nat. Rev. Endocrinol. 2017, 13, 610–622. [Google Scholar] [CrossRef]
- Lei, L.; Qiao, K.; Guo, Y.; Han, J.; Zhou, B. Titanium dioxide nanoparticles enhanced thyroid endocrine disruption of pentachlorophenol rather than neurobehavioral defects in zebrafish larvae. Chemosphere 2020, 249, 126536. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, J.-C. The immune responses and expression of metallothionein (MT) gene and heat shock protein 70 (HSP 70) in juvenile rockfish, Sebastes schlegelii, exposed to waterborne arsenic (As3+). Environ. Toxicol. Pharmacol. 2016, 47, 136–141. [Google Scholar] [CrossRef]
- Lee, J.; Park, N.-Y.; Kho, Y.; Ji, K. Effects of 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) Exposure on Reproduction and Endocrine System of Zebrafish. Environ. Sci. Technol. 2018, 52, 1506–1513. [Google Scholar] [CrossRef]
- Heijlen, M.; Houbrechts, A.M.; Bagci, E.; Van Herck, S.L.J.; Kersseboom, S.; Esguerra, C.V.; Blust, R.; Visser, T.J.; Knapen, D.; Darras, V.M. Knockdown of Type 3 Iodothyronine Deiodinase Severely Perturbs Both Embryonic and Early Larval Development in Zebrafish. Endocrinology 2014, 155, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Walter, K.M.; Miller, G.W.; Chen, X.; Yaghoobi, B.; Puschner, B.; Lein, P.J. Effects of thyroid hormone disruption on the ontogenetic expression of thyroid hormone signaling genes in developing zebrafish (Danio rerio). Gen. Comp. Endocrinol. 2019, 272, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Power, D.M.; Llewellyn, L.; Faustino, M.; Nowell, M.A.; Björnsson, B.T.; Einarsdottir, I.E.; Canario, A.V.M.; Sweeney, G.E. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 447–459. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, L.; Yang, L.; Zhou, B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat. Toxicol. 2012, 110–111, 141–148. [Google Scholar] [CrossRef]
- Mu, X.; Wang, K.; Chai, T.; Zhu, L.; Yang, Y.; Zhang, J.; Pang, S.; Wang, C.; Li, X. Sex specific response in cholesterol level in zebrafish (Danio rerio) after long-term exposure of difenoconazole. Environ. Pollut. 2015, 197, 278–286. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, D.; Dang, Y.; Yu, L.; Liu, C.; Wang, J. Reproduction impairment and endocrine disruption in adult zebrafish (Danio rerio) after waterborne exposure to TBOEP. Aquat. Toxicol. 2017, 182, 163–171. [Google Scholar] [CrossRef]
- Bacila, I.; Cunliffe, V.T.; Krone, N.P. Interrenal development and function in zebrafish. Mol. Cell. Endocrinol. 2021, 535, 111372. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H.; Li, D.; Zhan, M.; Hou, L.; Dong, W.; Luo, Y.; Xie, L. The effects of norethindrone on the ontogeny of gene expression along the hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes in zebrafish (Danio rerio). Sci. Total Environ. 2020, 747, 141554. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, S.; Zheng, X.; Zhu, Y.; Ma, Z.; Liu, C.; Hecker, M.; Saunders, D.M.V.; Giesy, J.P.; Zhang, X.; et al. Bioaccumulation, Biotransformation, and Toxicity of BDE-47, 6-OH-BDE-47, and 6-MeO-BDE-47 in Early Life-Stages of Zebrafish (Danio rerio). Environ. Sci. Technol. 2015, 49, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.W.; de França, L.R.; Lareyre, J.-J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Miyake, H.; Miura, C.; Kamei, H.; Aida, K.; Miura, T. Follicle-Stimulating Hormone Induces Spermatogenesis Mediated by Androgen Production in Japanese Eel, Anguilla japonica1. Biol. Reprod. 2007, 77, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Hinfray, N.; Baudiffier, D.; Leal, M.C.; Porcher, J.-M.; Aït-Aïssa, S.; Le Gac, F.; Schulz, R.W.; Brion, F. Characterization of testicular expression of P450 17α-hydroxylase, 17,20-lyase in zebrafish and its perturbation by the pharmaceutical fungicide clotrimazole. Gen. Comp. Endocrinol. 2011, 174, 309–317. [Google Scholar] [CrossRef]
- Kallivretaki, E.; Eggen, R.; Neuhauss, S.; Alberti, M.; Kausch, U.; Segner, H. Aromatase in zebrafish: A potential target for endocrine disrupting chemicals. Mar. Environ. Res. 2006, 62, S187–S190. [Google Scholar] [CrossRef]


| Gene Name | Forward Primers (5′-3′) | Reverse Primers (5′-3′) | NCBI Accession |
|---|---|---|---|
| β-actin | atggatgaggaaatcgctgcc | ctccctgatgtctgggtcgtc | AF057040.1 |
| dio2 | ttataagccagctgccggtc | caccgtaggctatgttggca | NM_212789.4 |
| dio3a | cgcgtacggagcttacttcg | tcacggagaatacaggtgcg | NM_001256003.1 |
| trhrb | cttcctccaggacgcttacc | ctgccttattggcctgagca | NM_001114688.1 |
| nis | gccacagatttctgacacgc | aagactggaacagcccgatg | BC134942.1 |
| trα | ctatgaacagcacatccgacaagag | cacaccacacacggctcatc | BC096778.1 |
| trβ | tgggagatgatacgggttgt | ataggtgccgatccaatgtc | XM_068215030.1 |
| ugt1ab | gcctctctgctccacaagtt | atccactggcatgacaagca | NM_213422.2 |
| tshr | gctccttgatgtgtccgaat | cgggcagtcaggttacaaat | NM_001145763.2 |
| tshβ | gcagatcctcacttcacctacc | gcacaggtttggagcatctca | BC163605.1 |
| crh | tttagtcgaaccgcagccaa | cgacaaccacgtgcagattc | EU052232.1 |
| star | gaacaagctctccggacctg | gcccttgttgcacatagcac | NM_131663.1 |
| hmgrb | ctgggataccgtctggaagc | caagagctgaagagtccggg | NM_001014292.3 |
| pomc | gaggggagtgaggatgttgtgt | tcggagggaggctgtagatg | AY125332.2 |
| hsd3b | gtgctccgacccttcctaac | ctggcacgtttaaccaacagg | NM_001386297.1 |
| gr | ttctacgttgctgacgatgc | ccggtgttctcctgtttgat | EF567112.1 |
| mr | attgggcctagtgcaaaatg | tctctgtttggctcggtctt | EF567113.1 |
| mc2r | ctccgttctcccttcatctg | gcagatccttgaagctgagg | NM_180971.1 |
| cyp11a | gaggggtggactcggttactt | gcaatacgagcggctgagat | NM_152953.2 |
| cyp11b | ctgggccacacatcgagag | agcgaacggcagaaatcc | BC155806.1 |
| cyp17 | agcactcgtgatgtcggttt | tagattccccctgtcgctga | NM_212806.3 |
| erα | agcatccagcctgtaatggga | agttgacagaggagctgatgc | KF275027.1 |
| erβ | tgtcaagcggcctattctgg | ttcgaaggccgatgctactg | AF516874.1 |
| ar | atctgtgcgctagcaggaat | caactgcgagtggaaagtca | NM_001083123.1 |
| gnrh2 | acctcaagagaagacgtgcc | caggataccagccgtgagac | NM_181439.4 |
| gnrhr2 | tgtcgtgttgtccataccgc | gcagctgcactttgttggac | NM_001144979.1 |
| fshβ | ctccacgaaactcccgcagat | tggtgtcgattgtgacgcag | NM_205624.1 |
| fshr | gcaggctaacctgacctacc | accagatcagaacacgcagg | AY278107.1 |
| lhβ | tgagaccattaacctgcccg | gtccgaggtctagtatgcgg | NM_205622.2 |
| lhr | acgcgttgtatcaacttcaagc | gatctccggacactcgaaaca | AY424302.1 |
| 17βhsd | tcgtcttgactgggacttgc | tttcgaaagctgcccatttcc | NM_200136.1 |
| cyp19a | tccagccctgtggaatgaag | tgtagctccacacgcattgt | NM_131154.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Zhang, D.; Ding, L.; Zhou, H.; Hou, Y.; Hong, H.; Sun, H.; Yu, X. Transcriptomic Analysis of Endocrine System Responses in Zebrafish Embryos Following Exposure to Environmentally Relevant Concentrations of Arsenate. Fishes 2025, 10, 97. https://doi.org/10.3390/fishes10030097
Li T, Zhang D, Ding L, Zhou H, Hou Y, Hong H, Sun H, Yu X. Transcriptomic Analysis of Endocrine System Responses in Zebrafish Embryos Following Exposure to Environmentally Relevant Concentrations of Arsenate. Fishes. 2025; 10(3):97. https://doi.org/10.3390/fishes10030097
Chicago/Turabian StyleLi, Tao, Di Zhang, Liang Ding, Hongyan Zhou, Yizhong Hou, Huachang Hong, Hongjie Sun, and Xinwei Yu. 2025. "Transcriptomic Analysis of Endocrine System Responses in Zebrafish Embryos Following Exposure to Environmentally Relevant Concentrations of Arsenate" Fishes 10, no. 3: 97. https://doi.org/10.3390/fishes10030097
APA StyleLi, T., Zhang, D., Ding, L., Zhou, H., Hou, Y., Hong, H., Sun, H., & Yu, X. (2025). Transcriptomic Analysis of Endocrine System Responses in Zebrafish Embryos Following Exposure to Environmentally Relevant Concentrations of Arsenate. Fishes, 10(3), 97. https://doi.org/10.3390/fishes10030097






