Imidacloprid Exposure Induced Impaired Intestinal Immune Function in Procambarus clarkii: Involvement of Oxidative Stress, Inflammatory Response, and Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. A Preliminary Experiment of Acute Toxicity
2.2. Acute Toxicity Formal Experiment
2.3. Serum Biochemical and Immune Indexes
2.4. Intestinal Antioxidant Enzyme Activity
2.5. Tissue RNA Extraction and Real-Time PCR Analysis
2.6. Data Processing and Statistical Analysis
3. Results
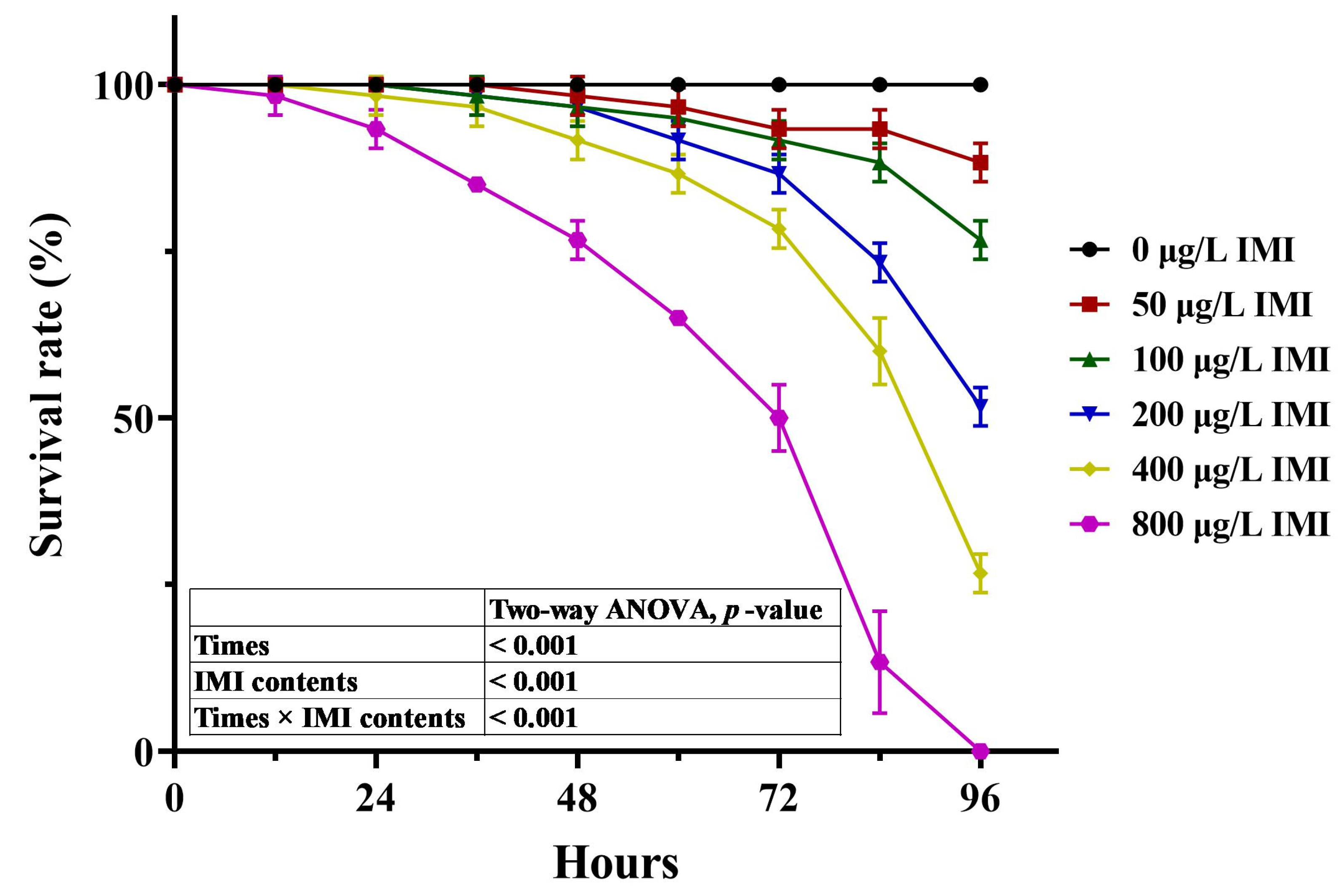
3.1. A Preliminary Experiment of Acute Toxicity Pre-Experiment
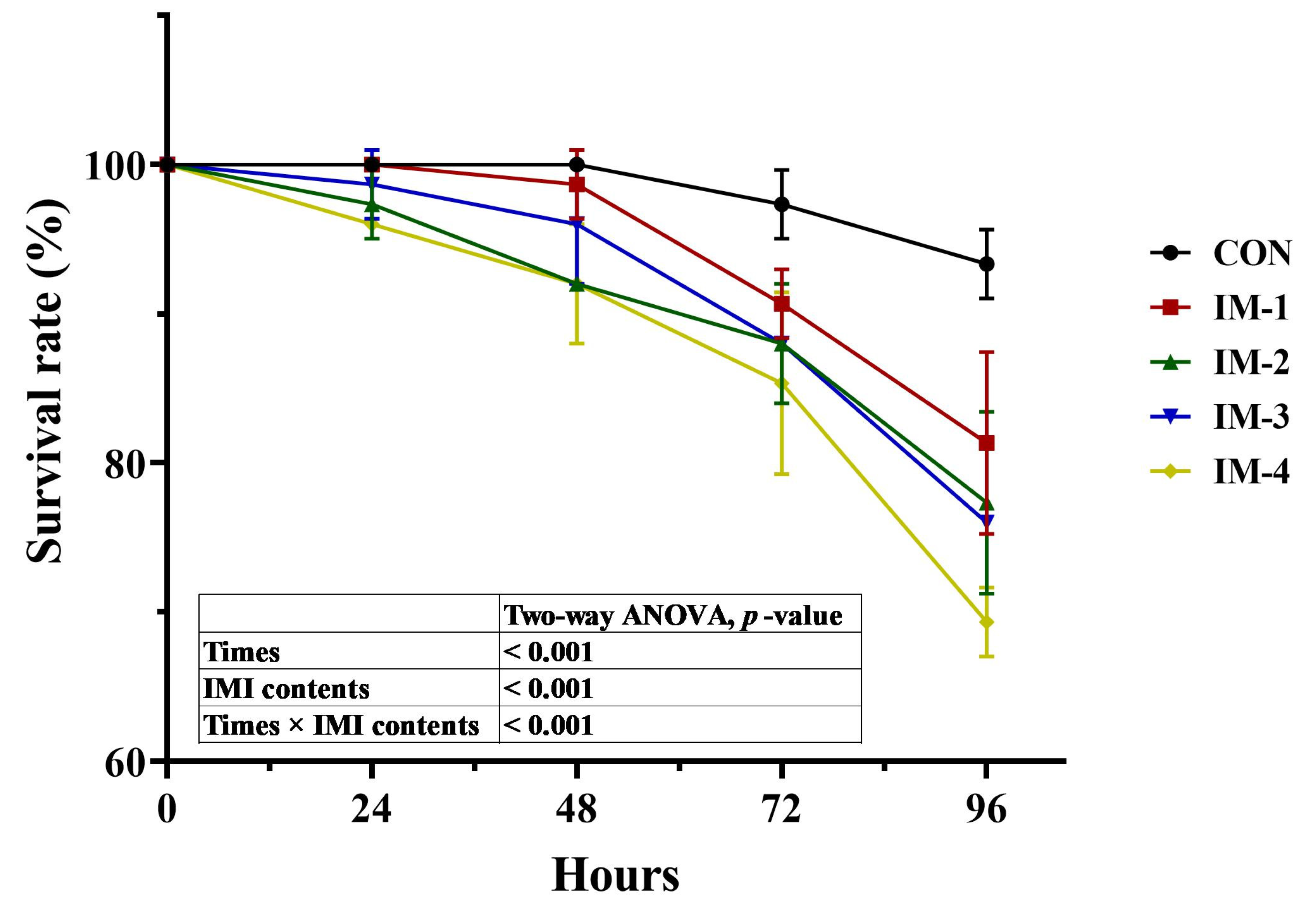
3.2. Acute Toxicity Formal Experiment
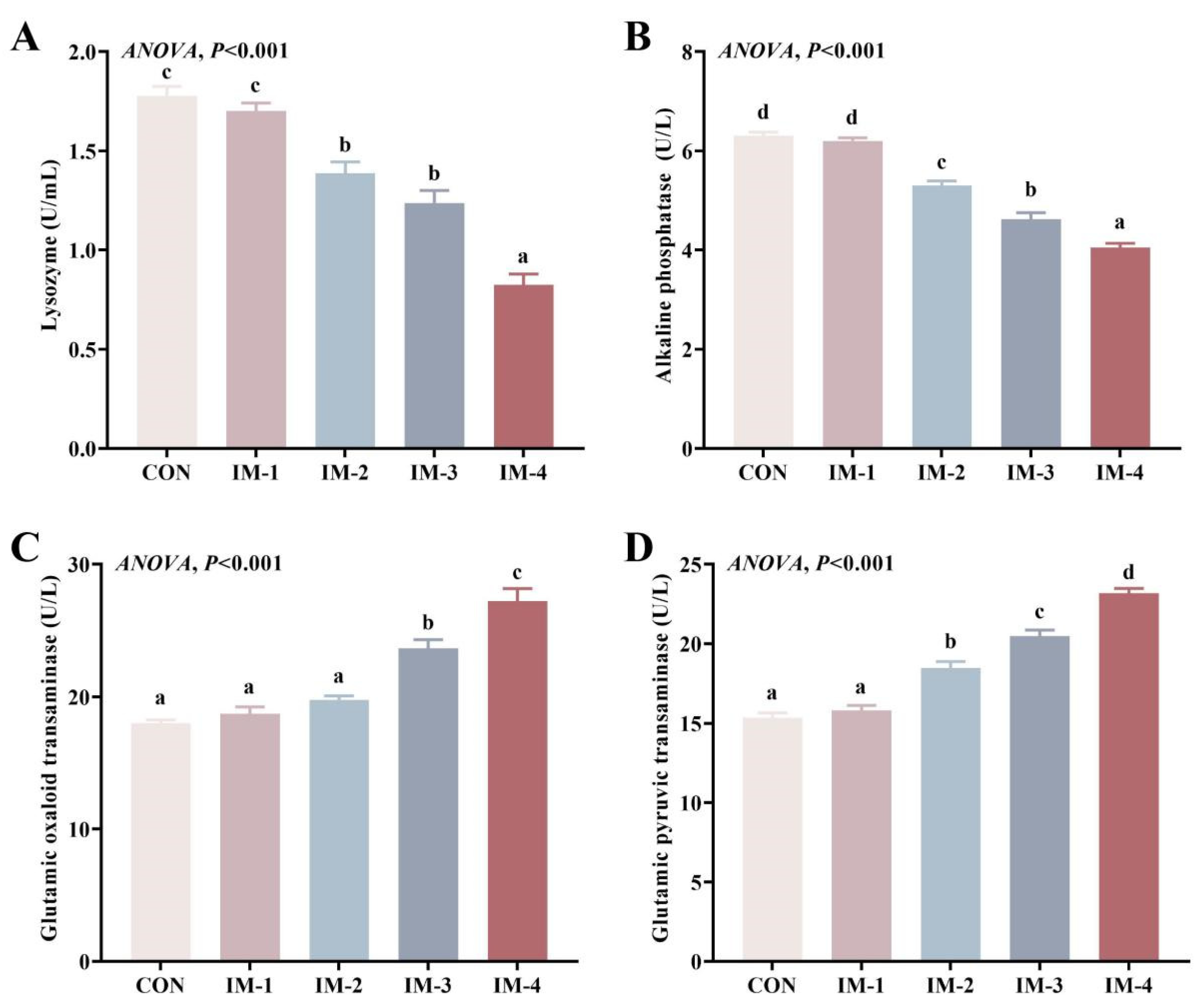
3.3. Hemolymph Biochemical and Immune Indexes
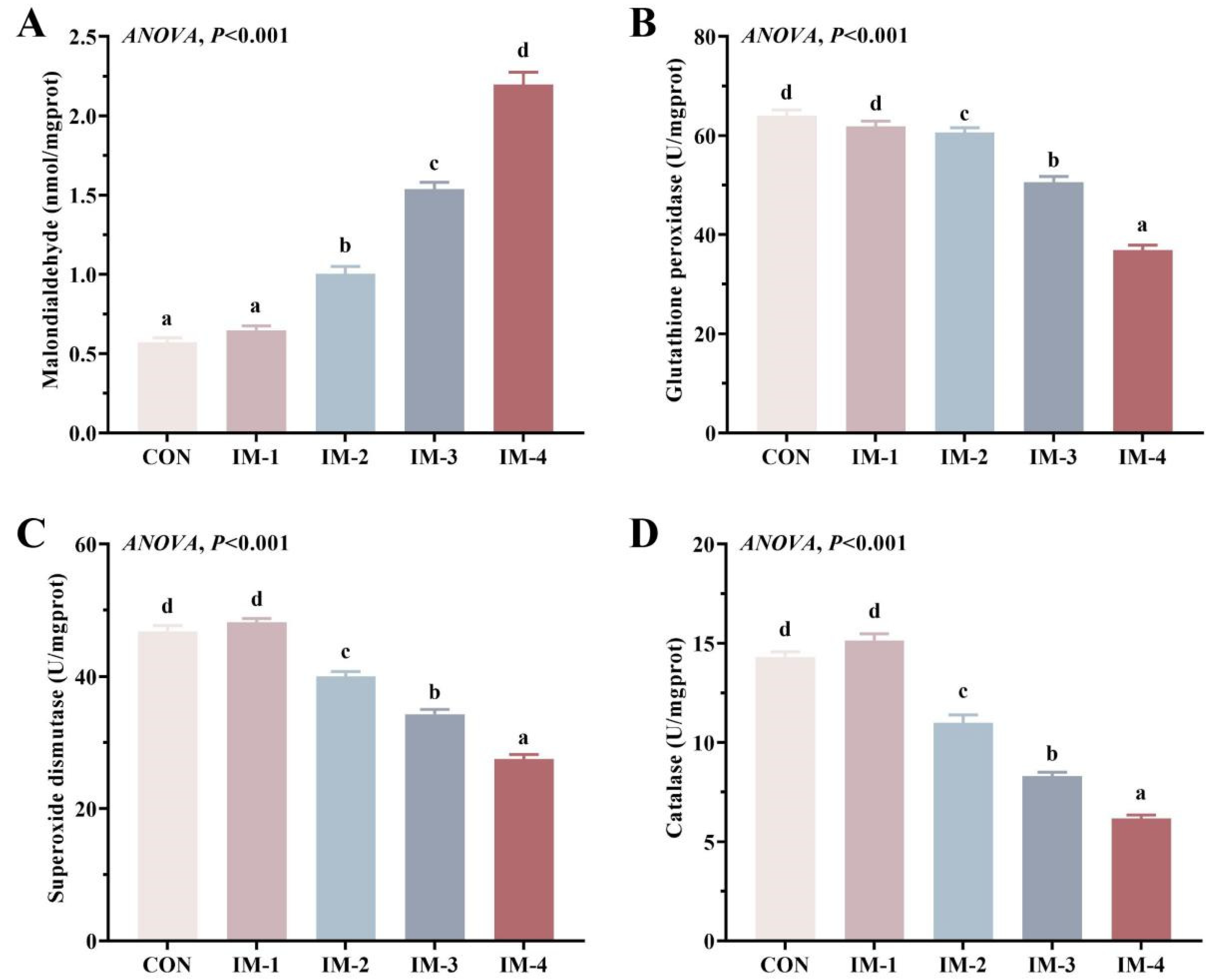
3.4. Intestinal Antioxidant Enzyme Activity
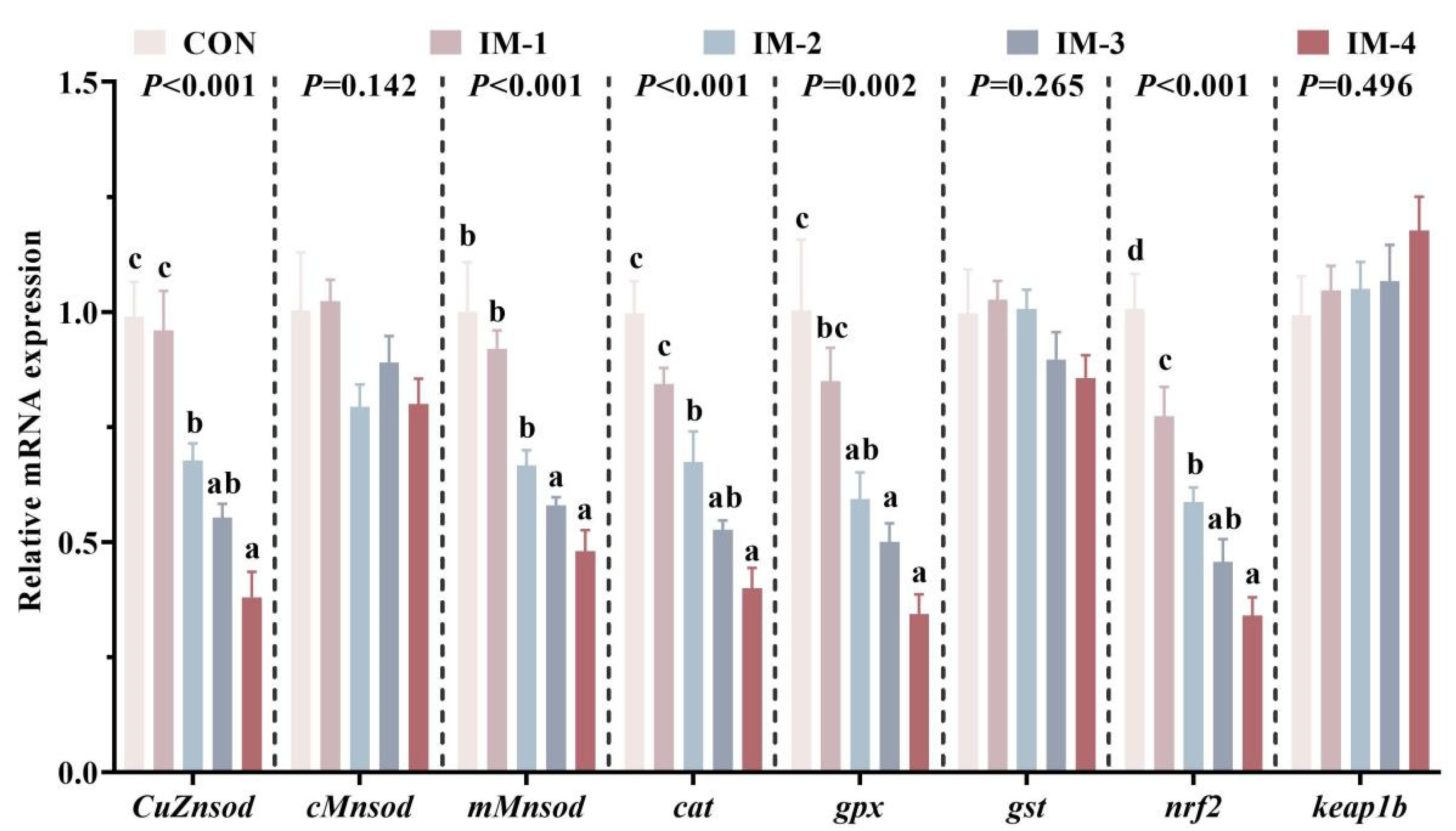
3.5. Expression of Intestinal Antioxidant-Related Genes
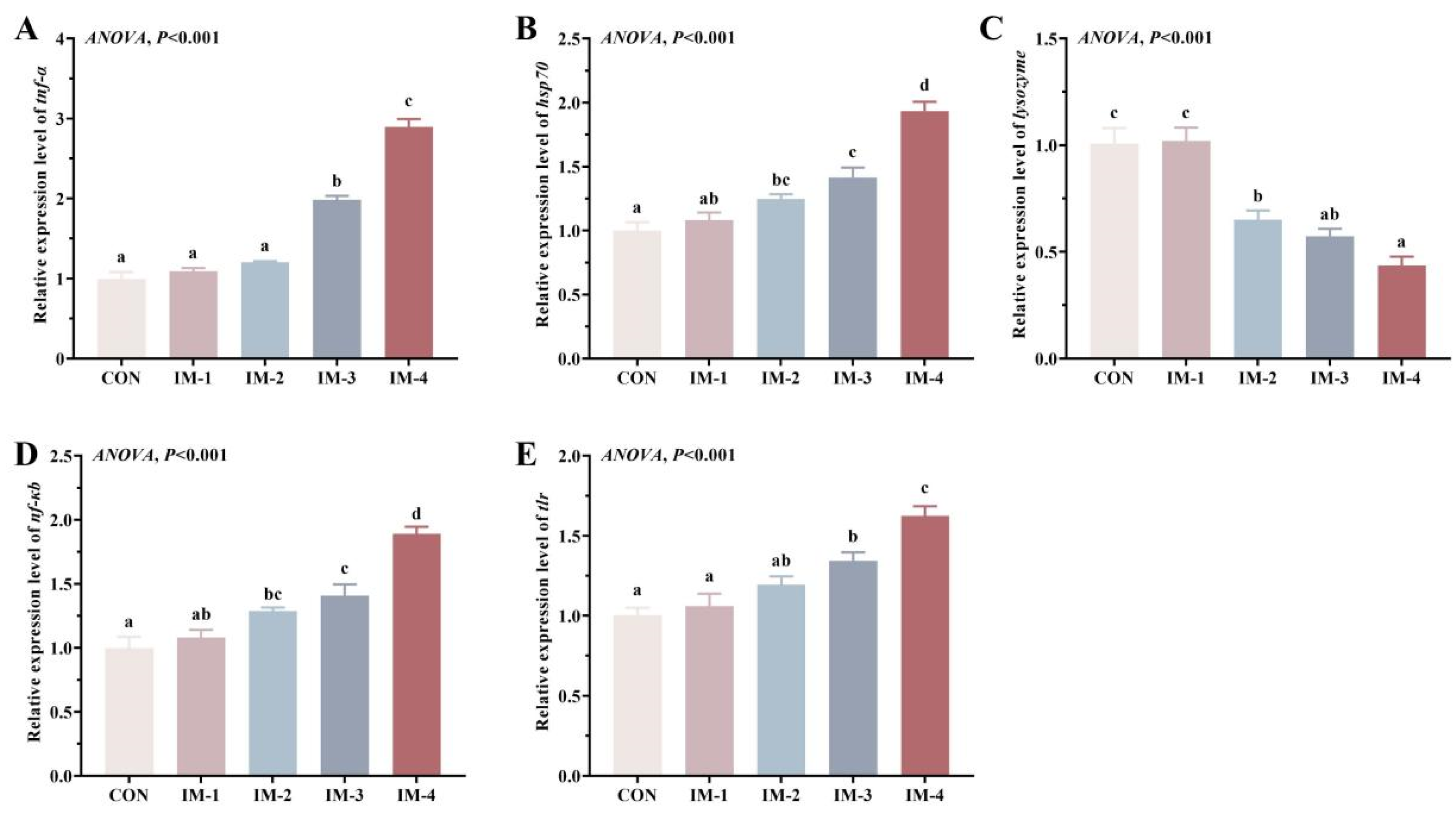
3.6. Expression of Intestinal Immune-Related Genes
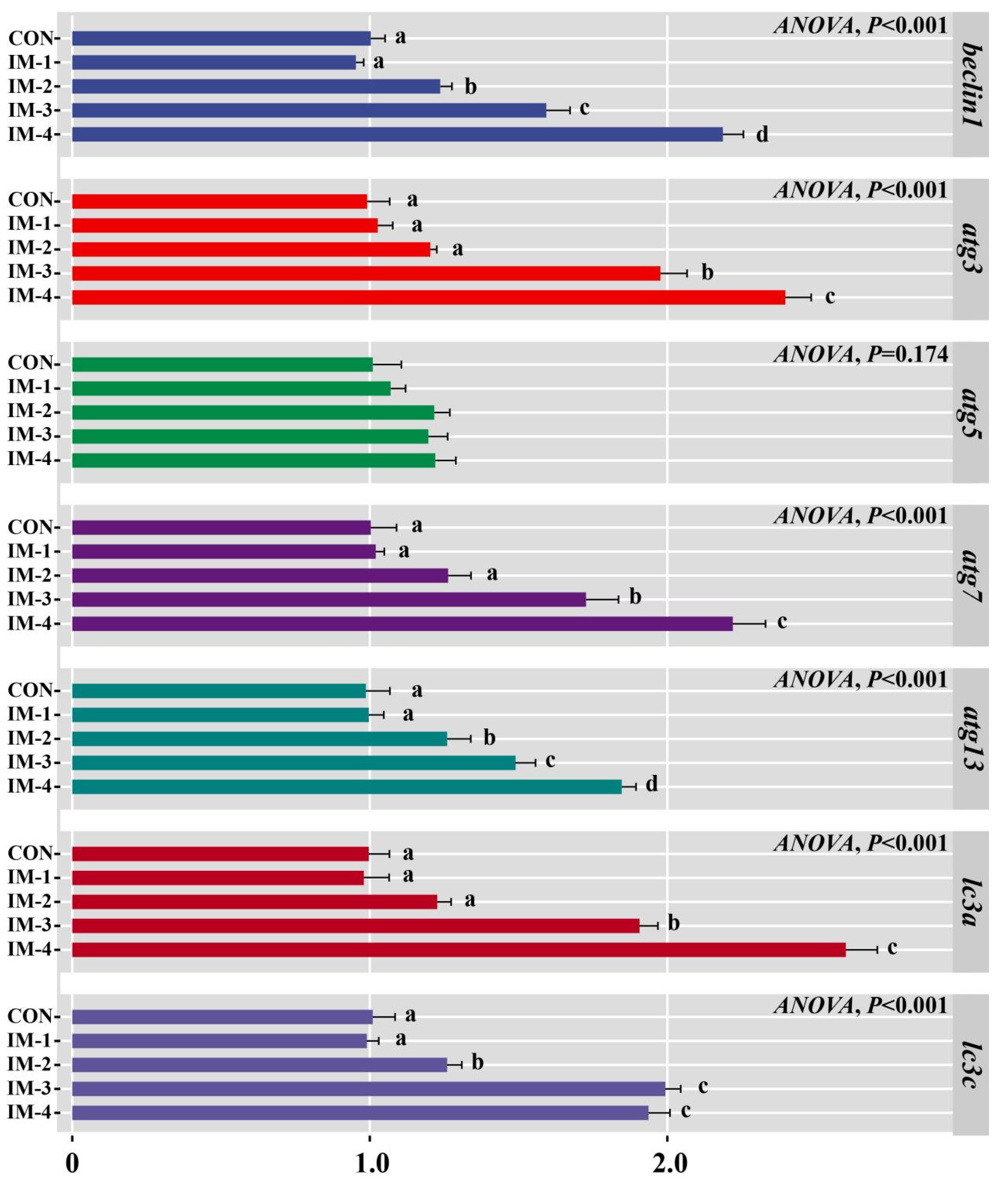
3.7. Expression of Intestinal Autophagy-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oficialdegui, F.J.; Clavero, M.; Sánchez, M.I.; Green, A.J.; Boyero, L.; Michot, T.C.; Klose, K.; Kawai, T.; Lejeusne, C. Unravelling the global invasion routes of a worldwide invader, the red swamp crayfish (Procambarus clarkii). Freshw. Biol. 2019, 64, 1382–1400. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Chen, C.; Feng, Y.; Ren, N.; Sun, K. Effects of cadmium on intestinal histology and microbiota in freshwater crayfish (Procambarus clarkii). Chemosphere 2020, 242, 125105. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Tomizawa, M.; Zhang, N.; Durkin, K.A.; Olmstead, M.M.; Casida, J.E. The neonicotinoid electronegative pharmacophore plays the crucial role in the high affinity and selectivity for the Drosophila nicotinic receptor: An anomaly for the nicotinoid cation—π interaction model. Biochemistry 2003, 42, 7819–7827. [Google Scholar] [CrossRef]
- Kagabu, S. Studies on the synthesis and insecticidal activity of neonicotinoid compounds. J. Pestic. Sci. 1996, 21, 231–239. [Google Scholar] [CrossRef]
- Yamamoto, I.; Yabuta, G.; Tomizawa, M. Molecular mechanism for selective toxicity of nicotinoids and neonicotinoids. J. Pestic. Sci. 1995, 20, 33–40. [Google Scholar] [CrossRef]
- Shao, X.; Liu, Z.; Xu, X.; Li, Z.; Qian, X. Overall status of neonicotinoid insecticides in China: Production, application and innovation. J. Pestic. Sci. 2013, 38, 1–9. [Google Scholar] [CrossRef]
- Martelli, F.; Zhongyuan, Z.; Wang, J.; Wong, C.O.; Karagas, N.E.; Roessner, U.; Roessner, U.; Rupasinghe, T.; Venkatachalam, K.; Perry, T.; et al. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 25840–25850. [Google Scholar] [CrossRef]
- Tišler, T.; Jemec, A.; Mozetič, B.; Trebše, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Li, S.Q.; Yang, Q.H.; He, X.G.; Liu, Y.T.; Dong, J.; Yang, Y.B.; Xu, N.; Ai, X.H.; Liu, S.C. Enrichment and residual elimination of imidacloprid in integrated rice and Procambarus clarkii breeding model. J. Huazhong Agric. Univ. 2021, 40, 105–111. [Google Scholar]
- Xia, J.; Jin, C.; Pan, Z.; Sun, L.; Fu, Z.; Jin, Y. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. Sci. Total Environ. 2018, 631, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Limbu, S.M.; Shen, M.; Zhai, W.; Qiao, F.; He, A.; Du, Z.; Zhang, M. Environmental concentrations of antibiotics impair zebrafish gut health. Environ. Pollut. 2018, 235, 245–254. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Shan, Y.; Yan, S.; Hong, X.; Zha, J.; Qin, J. Effect of imidacloprid on the behavior, antioxidant system, multixenobiotic resistance, and histopathology of Asian freshwater clams (Corbicula fluminea). Aquat. Toxicol. 2020, 218, 105333. [Google Scholar] [CrossRef]
- Luo, T.; Wang, X.; Jin, Y. Low concentrations of imidacloprid exposure induced gut toxicity in adult zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108972. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y.; Xie, K.; Zhang, J.; Wang, Y.; Hu, Y.; Zhong, L. Sanguinarine improves intestinal health in grass carp fed high-fat diets: Involvement of antioxidant, physical and immune barrier, and intestinal microbiota. Antioxidants 2023, 12, 1366. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Zhong, H.; Zhang, J.; Che, C.; Fu, G.; Hu, Y.; Mai, K. Taurine supplements in high-fat diets improve survival of juvenile Monopterus albus by reducing lipid deposition and intestinal damage. Aquaculture 2022, 547, 737431. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Liu, Y.; Xu, S.; Dai, J.; Zhang, Y.; Hu, Y. Dietary sanguinarine supplementation recovers the decrease in muscle quality and nutrient composition induced by high-fat diets of grass carp (Ctenopharyngodon idella). Anim. Nutr. 2024, 17, 208–219. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Insecticides mode of action in relation to their toxicity to non-target organisms. J. Environ. Anal. Toxicol. 2012, 4, S4-002. [Google Scholar]
- Wang, X.; Li, E.; Xiong, Z.; Chen, K.; Yu, N.; Du, Z.; Chen, L. Low salinity decreases the tolerance to two pesticides, beta-cypermethrin and acephate, of white-leg shrimp, Litopenaeus vannamei. J. Aquac. Res. Dev. 2013, 4, 2. [Google Scholar] [CrossRef]
- Lu, Y.P.; Liu, J.H.; Zhang, X.X.; Xu, C.; Zheng, P.H.; Li, J.T.; Li, J.J.; Wang, D.M.; Xian, J.A.; Zhang, Z.L. Integration of transcriptome, gut microbiota, and physiology reveals toxic responses of the red claw crayfish (Cherax quadricarinatus) to imidacloprid. J. Hazard. Mater. 2024, 470, 134293. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Huang, Y.; Wu, S.; Yang, X.; Dong, Y.; Xu, D.; Huang, Z. Effects of imidacloprid on the oxidative stress, detoxification and gut microbiota of Chinese mitten crab, Eriocheir sinensis. Sci. Total Environ. 2020, 729, 138276. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Han, F.; Huang, K.; Zhang, J.; Qin, J.G.; Chen, L.; Li, E. Impact of imidacloprid exposure on the biochemical responses, transcriptome, gut microbiota and growth performance of the Pacific white shrimp Litopenaeus vannamei. J. Hazard. Mater. 2022, 424, 127513. [Google Scholar] [CrossRef]
- Rose, K.V.S.; Joseph, A. Acute toxicity of imidacloprid to various life stages of the giant freshwater prawn Macrobrachium rosenbergii, de Man, 1879. Saudi J. Life Sci. 2020, 5, 298–308. [Google Scholar] [CrossRef]
- Stoner, A.W. Assessing stress and predicting mortality in economically significant crustaceans. Rev. Fish. Sci. 2012, 20, 111–135. [Google Scholar] [CrossRef]
- Sladkova, S.V.; Kholodkevich, S.V. Total protein in hemolymph of crawfish Pontastacus leptodactylus as a parameter of the functional state of animals and a biomarker of quality of habitat. J. Evol. Biochem. Physiol. 2011, 47, 160–167. [Google Scholar] [CrossRef]
- Xia, X.; Xia, X.; Huo, W.; Dong, H.; Zhang, L.; Chang, Z. Toxic effects of imidacloprid on adult loach (Misgurnus anguillicaudatus). Environ. Toxicol. Pharmacol. 2016, 45, 132–139. [Google Scholar] [CrossRef]
- Yoloğlu, E.; Uçkun, M.; Alkan Uçkun, A.; Barım Öz, Ö. Biochemical responses of crayfish exposed to imidacloprid: Assessment of oxidative stress, osmoregulatory response and neurotoxicity. Chem. Ecol. 2025, 41, 50–68. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.G.; Das, K.; Agrawal, P.K.; Paital, B. Modulation of physiological oxidative stress and antioxidant status by abiotic factors especially salinity in aquatic organisms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108971. [Google Scholar] [CrossRef] [PubMed]
- Frías-Espericueta, M.G.; Bautista-Covarrubias, J.C.; Osuna-Martínez, C.C.; Delgado-Alvarez, C.; Bojórquez, C.; Aguilar-Juárez, M.; Roos-Muñoz, S.; Osuna-López, I.; Páez-Osuna, F. Metals and oxidative stress in aquatic decapod crustaceans: A review with special reference to shrimp and crabs. Aquat. Toxicol. 2022, 242, 106024. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.E.D.; Pérez, M.R.; Acayaba, R.D.A.; Raimundo, C.C.M.; Martinez, C.B. DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the Neotropical fish Prochilodus lineatus. Chemosphere 2018, 195, 125–134. [Google Scholar] [CrossRef]
- Özdemir, S.; Altun, S.; Arslan, H. Imidacloprid exposure cause the histopathological changes, activation of TNF-α, iNOS, 8-OHdG biomarkers, and alteration of caspase 3, iNOS, CYP1A, MT1 gene expression levels in common carp (Cyprinus carpio L.). Toxicol. Rep. 2018, 5, 125–133. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Wang, S.; Wu, H.; Xu, S. Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish. Immunol. 2022, 120, 674–685. [Google Scholar] [CrossRef]
- Bao, J.; Li, X.; Xing, Y.; Feng, C.; Jiang, H. Effects of hypoxia on immune responses and carbohydrate metabolism in the Chinese mitten crab, Eriocheir sinensis. Aquac. Res. 2020, 51, 2735–2744. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, X.; Zhou, B.; Sun, T.; Li, X. Physicochemical properties and antibacterial mechanism of TP microcapsules/LZM-PVA gradual sustained-release composite coatings. Prog. Org. Coat. 2020, 146, 105740. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, J.; Liu, H.; Gong, C.; Huang, X.; Hu, Y.; Liu, Q.; He, Z.; Zhang Xi Yang, S.; Rahimnejad, S. Yinchenhao Decoction ameliorates the high-carbohydrate diet induced suppression of immune response in largemouth bass (Micropterus salmoides). Fish Shellfish. Immunol. 2022, 125, 141–151. [Google Scholar] [CrossRef]
- Carmody, R.J.; Chen, Y.H. Nuclear factor-kappaB: Activation and regulation during toll-like receptor signaling. Cell. Mol. Immunol. 2007, 4, 31–41. [Google Scholar]
- Ceciliani, F.; Giordano, A.; Spagnolo, V. The systemic reaction during inflammation: The acute-phase proteins. Protein Pept. Lett. 2002, 9, 211–223. [Google Scholar] [CrossRef]
- Borges, T.J.; Wieten, L.; Herwijnen, M.J.; Broere, F.; Zee, R.; Bonorino, C.; Eden, W. The anti-inflammatory mechanisms of Hsp70. Front. Immunol. 2012, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Nawaz, A.; Rehman, M.S.; Ali, M.A.; Dilshad, S.M.; Yang, C. Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim. Nutr. 2019, 5, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Opferman, J.T.; Korsmeyer, S.J. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 2003, 4, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.J.; Simon, A.K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 2019, 19, 170–183. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D.J.C.D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Noda, N.N.; Inagaki, F. Mechanisms of autophagy. Annu. Rev. Biophys. 2015, 44, 101–122. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Keulers, T.G.; Vooijs, M.A.; Rouschop, K.M. LC3/GABARAP family proteins: Autophagy-(un) related functions. FASEB J. 2016, 30, 3961–3978. [Google Scholar] [CrossRef]
- Li, X.; Yao, Y.; Wang, J.; Shen, Z.; Jiang, Z.; Xu, S. Eucalyptol relieves imidacloprid-induced autophagy through the miR-451/Cab39/AMPK axis in Ctenopharyngodon idellus kidney cells. Aquat. Toxicol. 2022, 249, 106204. [Google Scholar] [CrossRef]
- Eriksson, I.; Ward, L.J.; Vainikka, L.; Sultana, N.; Leanderson, P.; Flodin, U.; Li, W.; Yuan, X.M. Imidacloprid induces lysosomal dysfunction and cell death in human astrocytes and fibroblasts—Environmental implication of a clinical case report. Cells 2023, 12, 2772. [Google Scholar] [CrossRef]

| Gene | Forward Primer Sequence (5′→3′) | Reverse Primer Sequence (5′→3′) |
|---|---|---|
| CuZnsod | ACGGTGTATGGGCTGACTC | CCGATGTAAGACTGGGACG |
| cMnsod | CTGCAGCCAGTGTTGGAGTGAA | AAGGGAATCAGACCGTGAGTGATC |
| mMnsod | CCTGACCTGCCTTATGATTACA | TCTCCTCCATCTGGTGACAAA |
| cat | TTTCCCGTCTTTCATTCACAC | AAAATAAGAAAGTGGCTTGGTGT |
| gpx | CCGCTCTTCACCTTCTTG | GCGAGTGTATGGCTTACC |
| gst | ACCTGCCATATTACATTGAC | CCTTATCTTCTCTTGCTCTG |
| nrf2 | CATCTTCCAAAGCCTCCGA | GGGGTCGTCTTTGCCTCTA |
| keap1b | CCCCAAGAACTAAACCTCGG | GCGAATAGCCTTGGGAGC |
| tnf-α | CACCTTTCATCCCCTTCCAT | AATGCAGATGATAAAGCCCG |
| hsp70 | GGTGTTGGTGGGAGGGTCTA | GGCTCGCTCTCCCTCATACAC |
| lysozyme | GGACGTCCTCAGGAAAGGTG | TTGTTAGTAGCGGCCGTGTT |
| nf-κb | TAGTGCGTGATGATGGGTCTT | GCTGATTATGGAGGCAGAAAA |
| beclin1 | AATCTGGCTCACTGTAAAGGAA | ACAACCAATCAGGAGGAAACT |
| atg3 | TGTTGTGAGGAGCGTCTGC | TTGCCCTTGACCGTATTGA |
| atg5 | ACTGGATAGTGCCATTTTAGAGGG | GCAGAAGATCGGGAGATTTTAC |
| atg7 | CTGCTTGGGTGTTACTTC | ACATCAGCGTCATTCAGG |
| atg13 | GTCAGCAAACATTCGGAGGG | AGTGGGGCGACAACTGGTAC |
| lc3a | AGGCACCTCCCTCTTCTGG | CTGGAGACGCCGCCTTAT |
| lc3c | GAGCGATACGCCAAAGAAAC | GGATGGTGACGAACTGGGAC |
| β-actin | GAGGTTGCTGCCCTGGTT | TAGCGGGAGTGTTGAAAG |
| Reagent | Volume | Final Concentration |
|---|---|---|
| SYBR Green qPCR Mix | 10 μL | 1× |
| Forward primer sequence (10 μM) | 0.4 μL | 0.2 μM |
| Reverse primer sequence (10 μM) | 0.4 μL | 0.2 μM |
| DNA template | × μL | 10~200 ng/20 μL |
| Nuclease-free water | To 20 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Shi, Y.; Xie, K.; Zhong, L.; Hu, Y.; Chen, K. Imidacloprid Exposure Induced Impaired Intestinal Immune Function in Procambarus clarkii: Involvement of Oxidative Stress, Inflammatory Response, and Autophagy. Fishes 2025, 10, 131. https://doi.org/10.3390/fishes10030131
Li Z, Shi Y, Xie K, Zhong L, Hu Y, Chen K. Imidacloprid Exposure Induced Impaired Intestinal Immune Function in Procambarus clarkii: Involvement of Oxidative Stress, Inflammatory Response, and Autophagy. Fishes. 2025; 10(3):131. https://doi.org/10.3390/fishes10030131
Chicago/Turabian StyleLi, Zhaolin, Yong Shi, Kai Xie, Lei Zhong, Yi Hu, and Kaijian Chen. 2025. "Imidacloprid Exposure Induced Impaired Intestinal Immune Function in Procambarus clarkii: Involvement of Oxidative Stress, Inflammatory Response, and Autophagy" Fishes 10, no. 3: 131. https://doi.org/10.3390/fishes10030131
APA StyleLi, Z., Shi, Y., Xie, K., Zhong, L., Hu, Y., & Chen, K. (2025). Imidacloprid Exposure Induced Impaired Intestinal Immune Function in Procambarus clarkii: Involvement of Oxidative Stress, Inflammatory Response, and Autophagy. Fishes, 10(3), 131. https://doi.org/10.3390/fishes10030131








