Portable Smoking Ovens: What Are the PAH Levels in Grilled and Smoked Rainbow Trout?
Abstract
1. Introduction
2. Materials and Methods
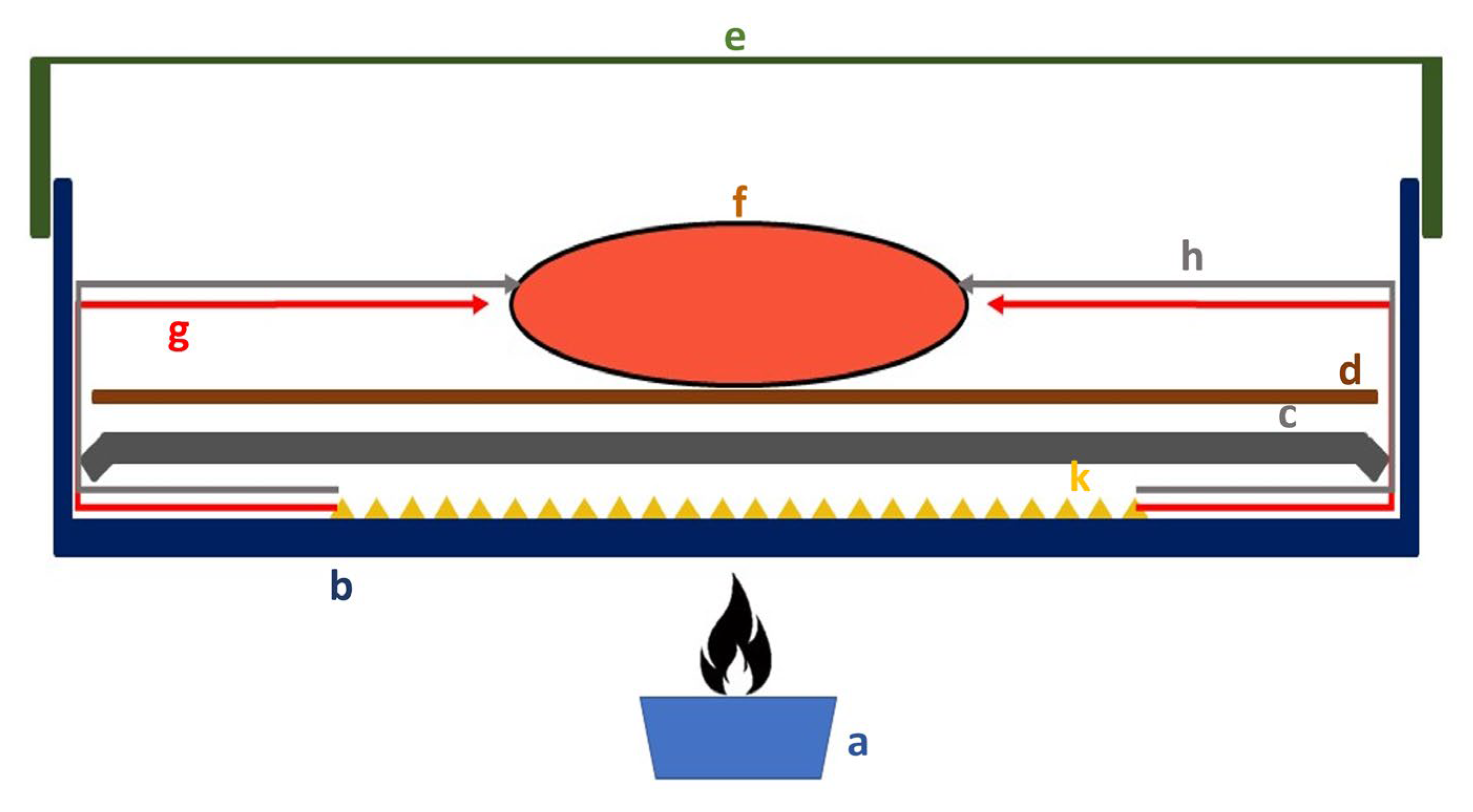
2.1. Fish and Experimental Protocol
2.2. Physicochemical and Polycyclic Aromatic Hydrocarbons (PAHs) Analysis
2.3. Statistical Analysis
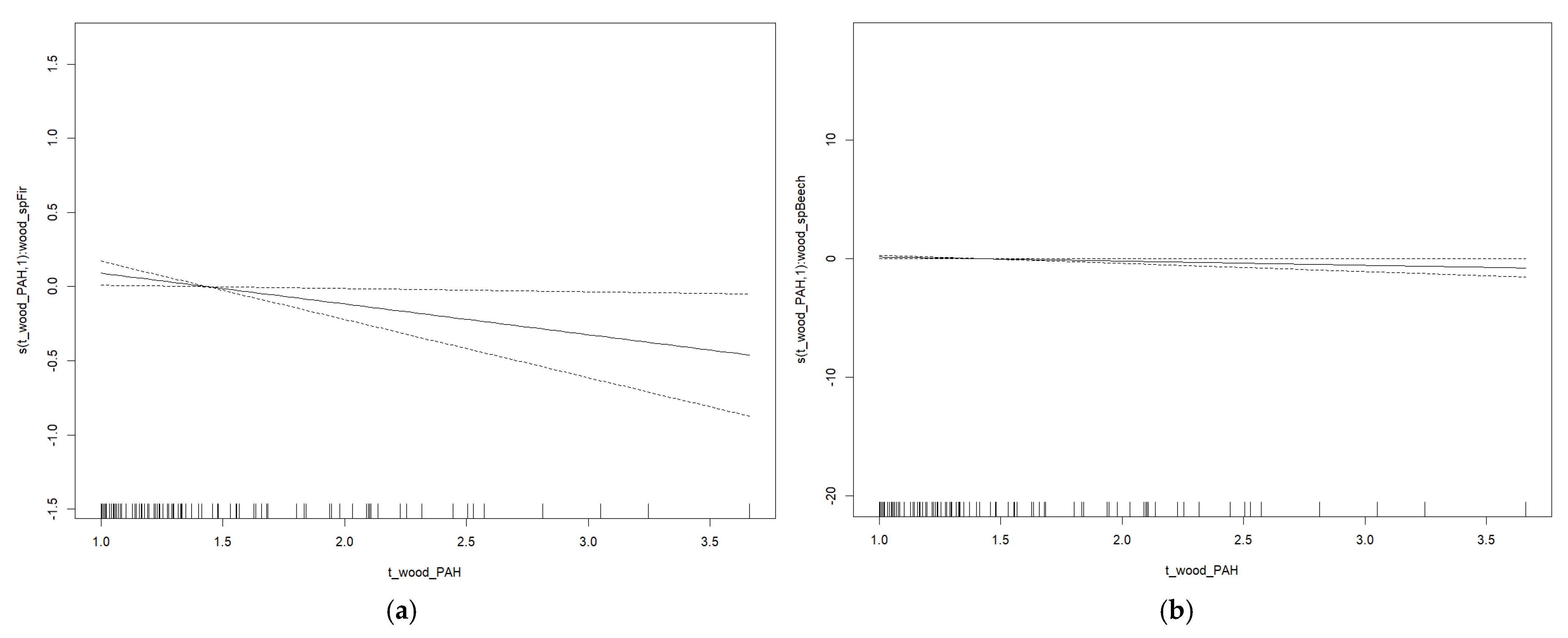
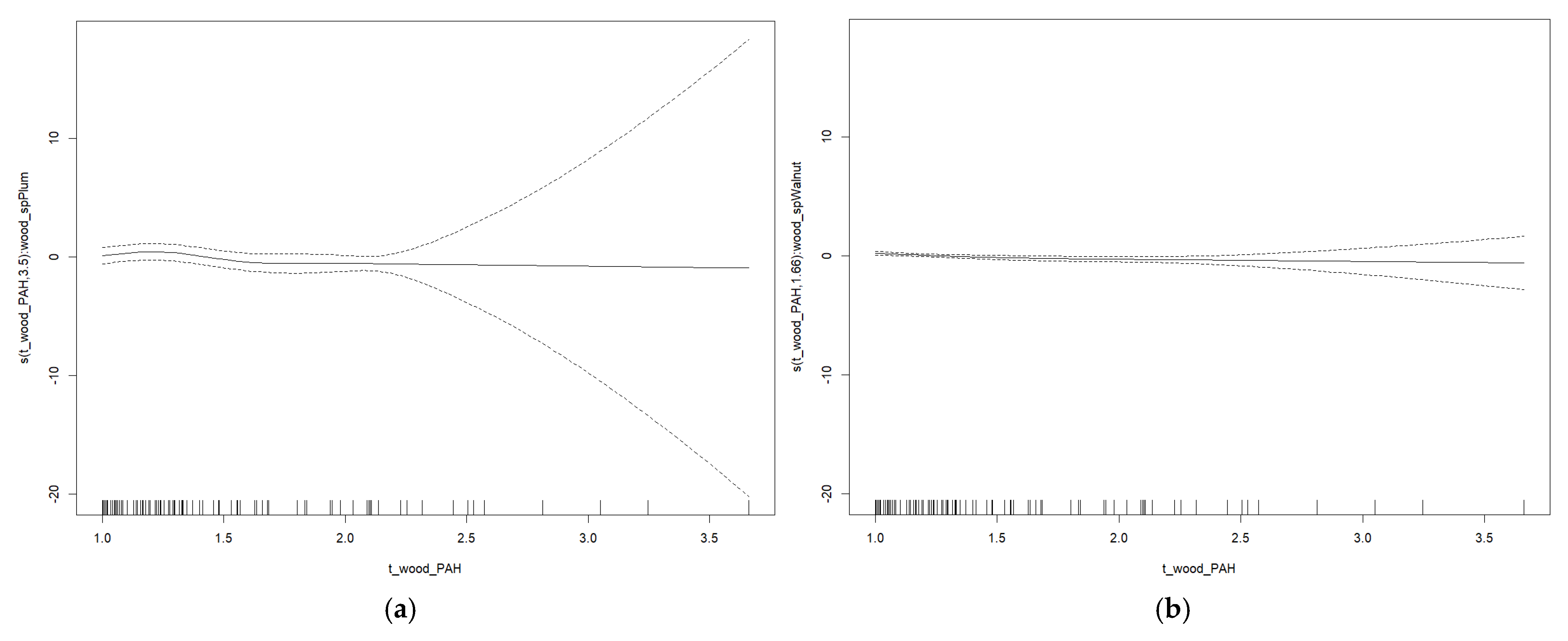
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Shang, H.; Tong, H.; Nehlich, O.; Liu, W.; Zhao, C.; Yu, J.; Wang, C.; Trinkaus, E.; Richards, M.P. Stable isotope dietary analysis of the Tianyuan 1 early modern human. Proc. Natl. Acad. Sci. USA 2009, 106, 10971. [Google Scholar] [CrossRef]
- Braun, D.R.; Harris, J.W.K.; Levin, N.E.; McCoy, J.T.; Herries, A.I.R.; Bamford, M.K.; Bishop, L.C.; Richmond, B.G.; Kibunjia, M. Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proc. Natl. Acad. Sci. USA 2010, 107, 10002–10007. [Google Scholar] [CrossRef]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture. Towards Blue Transformation. 2024. Available online: www.fao.org (accessed on 10 October 2024).
- Speranza, B.; Racioppo, A.; Bevilacqua, A.; Buzzo, V.; Marigliano, P.; Mocerino, E.; Scognamiglio, R.; Corbo, M.R.; Scognamiglio, G.; Sinigaglia, M. Innovative Preservation Methods Improving the Quality and Safety of Fish Products: Beneficial Effects and Limits. Foods 2021, 10, 2854. [Google Scholar] [CrossRef] [PubMed]
- Saediman, H.; Merlina, J.; Rianse, I.S.; Taridala, S.A.A.; Rosmawaty, R. Economic returns and constraints of traditional fish smoking in North Buton District of Southeast Sulawesi. IOP Conf. Ser. Earth Environ. Sci. 2021, 782, 022049. [Google Scholar] [CrossRef]
- Kitts, D.D.; Pratap-Singh, A.; Singh, A.; Chen, X.; Wang, S. A risk-benefit analysis of First Nation’s traditional smoked fish processing. Foods 2023, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Sava, A.; Uiuiu, P.; Raducu, C.; Cocan, D.; Constantinescu, R.; Lațiu, C.; Coroian, A.; Ihuț, A.; Mireșan, V. Meat quality of traditionally smoked trout from Trecătoarea Ursului salmonid farm, Brașov County. Sci. Papers. Ser. D Anim. Sci. 2020, 63, 427–432. [Google Scholar]
- Uiuiu, P.; Sava, A.; Raducu, C.; Constantinescu, R.; Lațiu, C.; Craioveanu, C.; Ihuț, A.; Muntean, G.-C.; Papuc, T.; Mireșan, V.; et al. Quality assessment of traditional smoked Arctic char, Salvelinus alpinus (Linnaeus, 1758). Sci. Papers. Ser. D Anim. Sci. 2024, 67, 812–817. [Google Scholar]
- Messina, C.M.; Arena, R.; Ficano, G.; La Barbera, L.; Morghese, M.; Santulli, A. Combination of freezing, low sodium brine, and cold smoking on the quality and shelf-life of sea bass (Dicentrarchus labrax L.) fillets as a strategy to innovate the market of aquaculture products. Animals 2021, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Iko Afé, O.H.; Kpoclou, Y.E.; Douny, C.; Anihouvi, V.B.; Igout, A.; Mahillon, J.; Hounhouigan, D.J.; Scippo, M.-L. Chemical hazards in smoked meat and fish. Food Sci. Nutr. 2021, 9, 6903–6922. [Google Scholar] [CrossRef]
- Onyemaechi, E.C.; Ndudi, O.J.; Okaliwe, E.F. Assessment of Polycyclic Aromatic Hydrocarbons (Pahs) in Hardwood, Palmwood and Softwood-Smoked Fish. Int. J. Ecotoxicol. Ecobiol. 2018, 2, 178–181. [Google Scholar] [CrossRef]
- Siskos, I.; Zotos, A.; Taylor, K.D.A. The effect of drying, pressure and processing time on the quality of liquid-smoked trout (Salmo gairdnerii) fillets. J. Sci. Food Agric. 2005, 85, 2054–2060. [Google Scholar] [CrossRef]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef] [PubMed]
- Malarut, J.; Vangnai, K. Influence of wood types on quality and carcinogenic polycyclic aromatic hydrocarbons (PAHs) of smoked sausages. Food Control 2018, 85, 98–106. [Google Scholar] [CrossRef]
- Sava, A.; Uiuiu, P.; Lațiu, C.; Cocan, D.; Muntean, G.-C.; Papuc, T.; Ihuț, A.; Răducu, C.; Becze, A.; Craioveanu, C.; et al. PAHs, Physicochemical and Microbiological Analyses of Trout Processed by Traditional Smoking, in Different Types of Packaging. Fishes 2023, 8, 424. [Google Scholar] [CrossRef]
- Savin, R.-L.; Ladoși, D.; Ladoși, I.; Păpuc, T.; Becze, A.; Cadar, O.; Torök, I.; Simedru, D.; Mariș, Ș.C.; Coroian, A. Influence of Fish Species and Wood Type on Polycyclic Aromatic Hydrocarbons Contamination in Smoked Fish Meat. Foods 2024, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, R.; Cheng, L.; Zhu, W.; Li, S.; Chen, X. Mechanisms underlying the deterioration of fish quality after harvest and methods of preservation. Food Control 2022, 135, 108805. [Google Scholar] [CrossRef]
- European Commission. EUMOFA (European Market Observatory for Fisheries and Aquaculture Products). In Country Analyses; 2020 Edition; Publications Office of the European Union: Luxembourg, 2021; 59p, ISBN 978-92-76-28896-1. [Google Scholar]
- Kraemer, E.O.; Stamm, A.J. Mohr’s Method for the Determination of Silver and Halogens in other than Neutral Solutions. J. Am. Chem. Soc. 1924, 46, 2707–2709. [Google Scholar]
- Masithoh, R.E.; Amanah, H.Z.; Cho, B.K. Application of Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) Spectroscopy Coupled with Wavelength Selection for Fast Discrimination of Similar Color of Tuber Flours. Indones. J. Chem. 2020, 20, 680–687. [Google Scholar] [CrossRef]
- SR EN ISO 17993/2006; Water Quality. Determination of 15 Polycyclic Aromatic Hydrocarbons [PAH) in Water by HPLC with Fluorescence Detection after Liquid-Liquid Extraction. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2024; Available online: http://www.rstudio.com/ (accessed on 18 December 2024).
- Chen, B.H. Analysis, Formation and Inhibition of Polycyclic Aromatic Hydrocarbons in Foods. An Overview. J. Food Drug Anal. 1997, 5, 25–42. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The occurrence of 16 EPA PAHs in food—A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef]
- Abraha, B.; Admassu, H.; Mahmud, A.; Tsighe, N.; Shui, X.W.; Fang, Y. Effect of processing methods on nutritional and physico-chemical composition of fish: A review. Food Process. Technol. 2018, 6, 376–382. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Oyewole, O.B.; Obadina, O.; Adeniran, O.E.; Oyedele, H.A.; Olugbile, A.; Omemu, A.M. Effect of smoking methods on microbial safety, polycyclic aromatic hydrocarbon, and heavy metal concentrations of traditional smoked fish from Lagos State, Nigeria. J. Culin. Sci. Technol 2016, 14, 91–106. [Google Scholar] [CrossRef]
- Aksun Tümerkan, E.T. Investigations of the Polycyclic Aromatic Hydrocarbon and Elemental Profile of Smoked Fish. Molecules 2022, 27, 7015. [Google Scholar] [CrossRef] [PubMed]
- Sonego, E.; Bhattarai, B.; Duedahl-Olesen, L. Detection of Nitrated, Oxygenated and Hydrogenated Polycyclic Aromatic Compounds in Smoked Fish and Meat Products. Foods 2022, 11, 2446. [Google Scholar] [CrossRef]
- Purcaro, G.; Moret, S.; Conte, L.S. Overview on polycyclic aromatic hydrocarbons: Occurrence, legislation and innovative determination in foods. Talanta 2013, 105, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, M.; Luhtasela, U.; Kostamo, P.; Ritvanen, T.; Peltonen, K.; Jestoi, M. Critical Effects of Smoking Parameters on the Levels of Polycyclic Aromatic Hydrocarbons in Traditionally Smoked Fish and Meat Products in Finland. J. Chem. 2018, 2018, 2160958. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and REPEALING Regulation (EC) No 1881/2006; The Official Journal of the European Union: Brussels, Belgium, 2023; L119/103, 66; pp. 103–157. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Di Domenico, A.; Fernández-Cruz, M.L.; Fink-Gremmels, J.; Fürst, P.; Galli, C.; et al. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Polycyclic Aromatic Hydrocarbons in Food. EFSA J. 2008, 724, 1–114. [Google Scholar]
- Racovita, R.C.; Secuianu, C.; Israel-Roming, F. Quantification and risk assessment of carcinogenic polycyclic aromatic hydrocarbons in retail smoked fish and smoked cheeses. Food Control 2020, 121, 107586. [Google Scholar] [CrossRef]
- Asamoah, E.K.; Nunoo, F.K.E.; Addo, S.; Josephine; Nyarko, O.; Hyldig, G. Polycyclic aromatic hydrocarbons (PAHs) in fish smoked using traditional and improved kilns: Levels and human health risk implications through dietary exposure in Ghana. Food Control 2021, 121, 107576. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Christensen, J.; Højgård, A.; Granby, K.; Timm-Heinrich, M. Influence of smoking parameters on the concentration of polycyclic aromatic hydrocarbons (PAHs) in Danish smoked fish. Food Addit. Contam. Part A 2010, 27, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Yebra-Pimentel, I.; Martínez-Carballo, E.; Simal-Gándara, J. Profiling, distribution and levels of carcinogenic polycyclic aromatic hydrocarbons in traditional smoked plant and animal foods. Food Control 2016, 59, 581–590. [Google Scholar] [CrossRef]
- Coroian, A.; Mireşan, V.; Coroian, C.O.; Răducu, C.; Cocan, D.; Andronie, L.; Naghiu, A.; Longodor, A.L.; Constantinescu, R.; Marchiş, Z. The level of polycyclic aromatic hydrocarbons (PAHs) from pork meat depending on the heat treatment applied. Rom. Biotechnol. Lett. 2020, 25, 1601–1606. [Google Scholar] [CrossRef]
- Tiwo, C.T.; Tchoumbougnang, F.; Nganou, E.; Kumar, P.; Nayak, B. Effect of different smoking processes on the nutritional and polycyclic aromatic hydrocarbons composition of smoked Clarias gariepinus and Cyprinus carpio. Food Sci. Nutr. 2019, 7, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H. Dietary fat: A history. Nutr. Rev. 1999, 57, 11–14. [Google Scholar] [CrossRef] [PubMed]

| Chemical Analysis (Whole Fish) % | Beech | Cherry | Sour Cherry | Plum | Walnut | Fir | Willow |
|---|---|---|---|---|---|---|---|
| Total lipids | 8.4 ± 0.9 | 11.7 ± 2.5 | 11.7 ± 1.3 | 7.6 ± 2.8 | 9.1 ± 1.6 | 9.7 ± 1.9 | 11.77 ± 2 |
| Moisture | 64.5 ± 8.4 | 65.7 ± 6.1 | 65.2 ± 10.6 | 61.5 ± 7.9 | 63.1 ± 9.7 | 63.1 ± 7.6 | 64.5 ± 8.7 |
| Protein | 20.1 ± 2.3 | 19.4 ± 2.7 | 20.5 ± 2.2 | 21.9 ± 1.9 | 21.7 ± 3.1 | 21.0 ± 2.1 | 21.6 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uiuiu, P.; Cocan, D.; Lațiu, C.; Constantinescu, R.; Mireșan, V.; Papuc, T.; Savin, R.-L.; Ihuț, A.; Răducu, C.; Becze, A.; et al. Portable Smoking Ovens: What Are the PAH Levels in Grilled and Smoked Rainbow Trout? Fishes 2025, 10, 82. https://doi.org/10.3390/fishes10020082
Uiuiu P, Cocan D, Lațiu C, Constantinescu R, Mireșan V, Papuc T, Savin R-L, Ihuț A, Răducu C, Becze A, et al. Portable Smoking Ovens: What Are the PAH Levels in Grilled and Smoked Rainbow Trout? Fishes. 2025; 10(2):82. https://doi.org/10.3390/fishes10020082
Chicago/Turabian StyleUiuiu, Paul, Daniel Cocan, Călin Lațiu, Radu Constantinescu, Vioara Mireșan, Tudor Papuc, Raul-Lucian Savin, Andrada Ihuț, Camelia Răducu, Anca Becze, and et al. 2025. "Portable Smoking Ovens: What Are the PAH Levels in Grilled and Smoked Rainbow Trout?" Fishes 10, no. 2: 82. https://doi.org/10.3390/fishes10020082
APA StyleUiuiu, P., Cocan, D., Lațiu, C., Constantinescu, R., Mireșan, V., Papuc, T., Savin, R.-L., Ihuț, A., Răducu, C., Becze, A., Craioveanu, C., & Vlaic, B. A. (2025). Portable Smoking Ovens: What Are the PAH Levels in Grilled and Smoked Rainbow Trout? Fishes, 10(2), 82. https://doi.org/10.3390/fishes10020082









