Heat Shock Factors in the European Eel: Gene Characterization and Expression Response to Different Environmental Conditions and to Induced Sexual Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of HSF Sequences
2.1.1. Gene Search for HSF Sequences
2.1.2. Phylogenetic Analyses of the HSF Family in Vertebrates
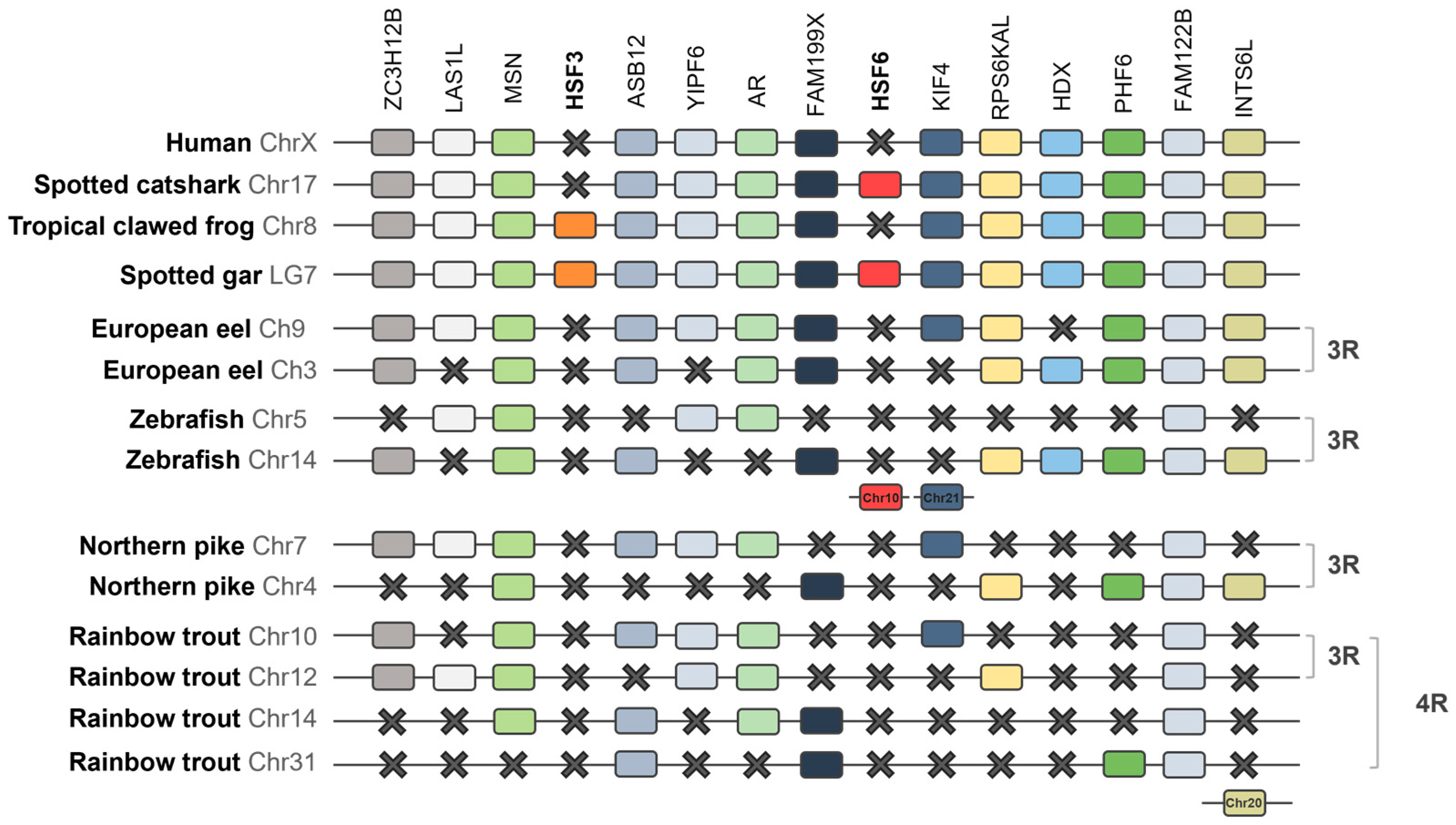
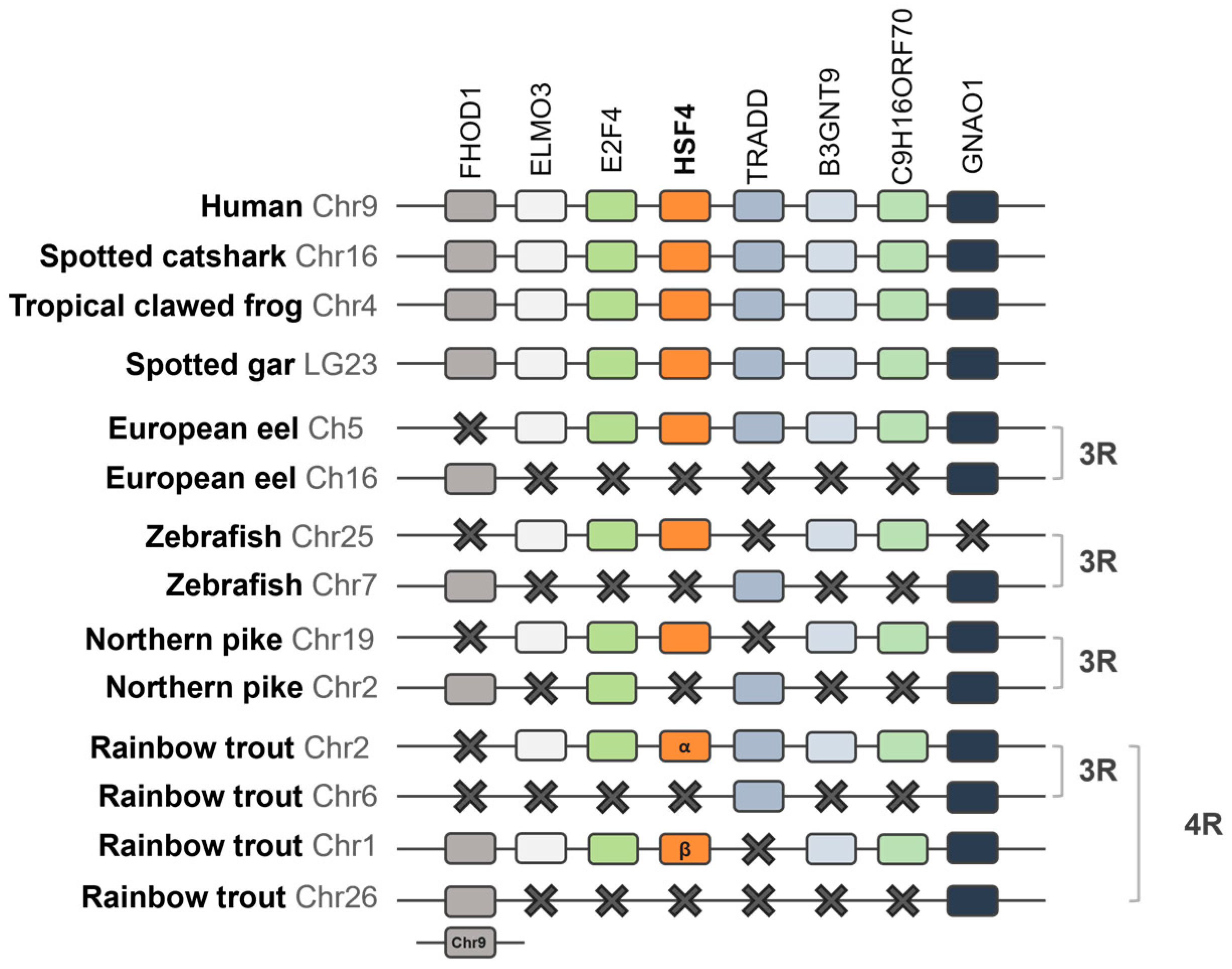
2.1.3. Synteny Analysis of the HSF Family in Vertebrates
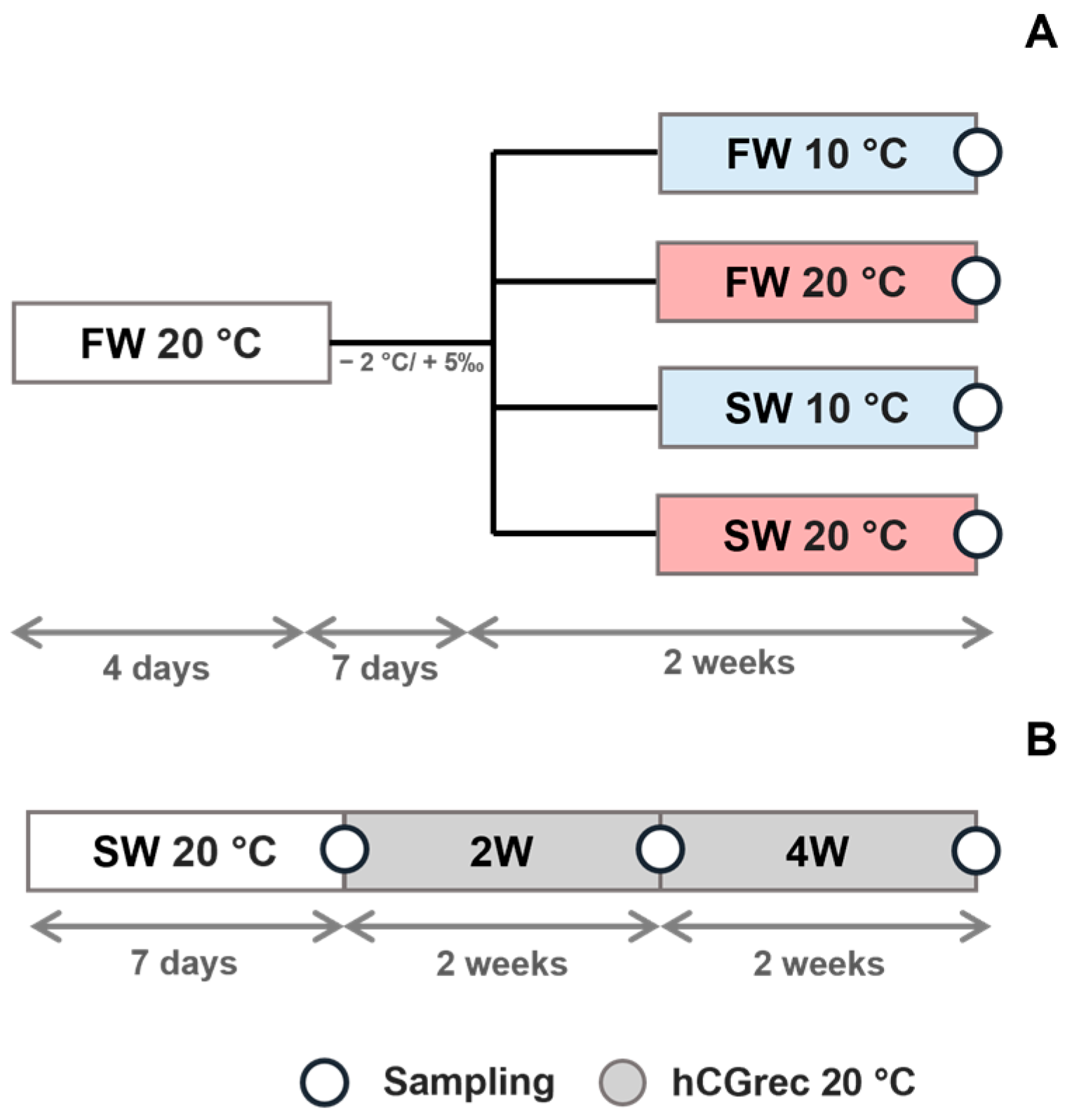
2.2. Experimental Design
2.2.1. Fish Maintenance and Handling
2.2.2. Expression of HSF Genes in Different Tissues from the Male European Eel
2.2.3. Expression of HSF Genes in the European Eel Testis Under Different Temperature and Salinity Conditions
2.2.4. Expression of HSF Genes in the European Eel Testis During Spermatogenesis Induced with Hormonal Maturation Treatment
2.2.5. Samplings
2.3. Gene Expression Analyses by Quantitative Real-Time PCR
2.3.1. RNA Extraction and cDNA Synthesis
2.3.2. Primer and Reference Genes
2.3.3. Gene Expression Analysis
2.4. Testis Histology
2.5. Statistical Analysis
3. Results
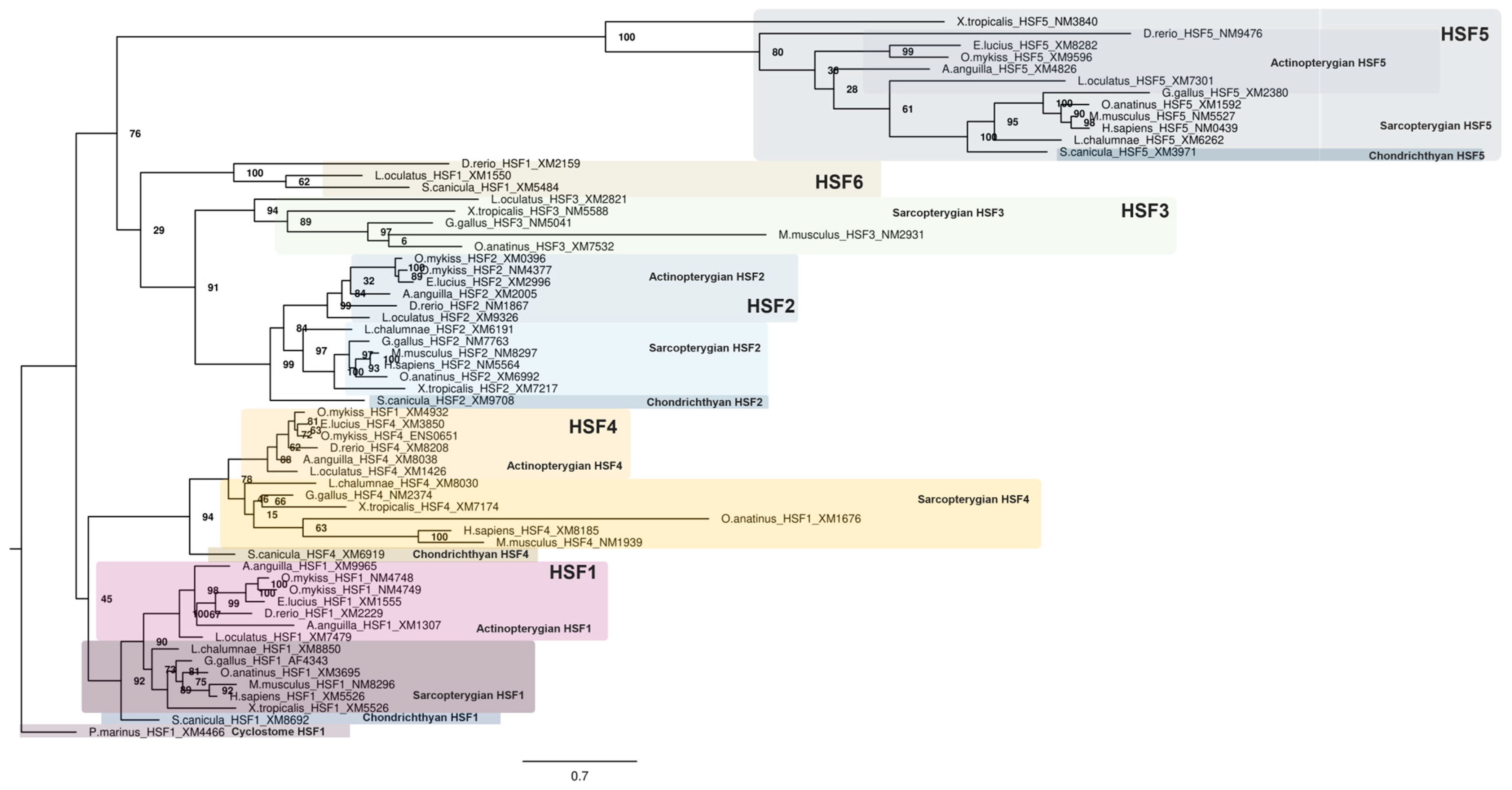
3.1. Phylogenetic Analyses of the HSF Family in Vertebrates
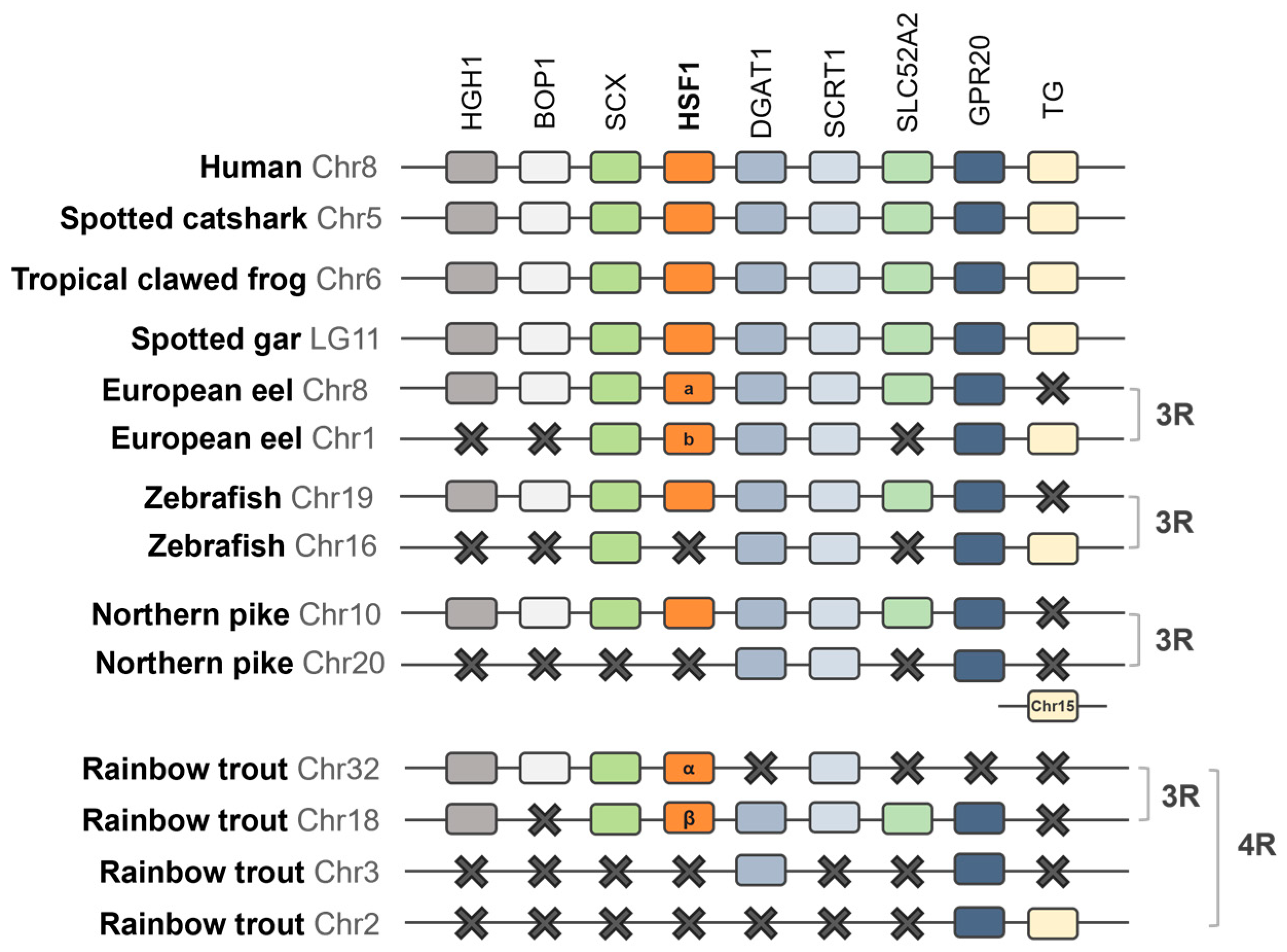
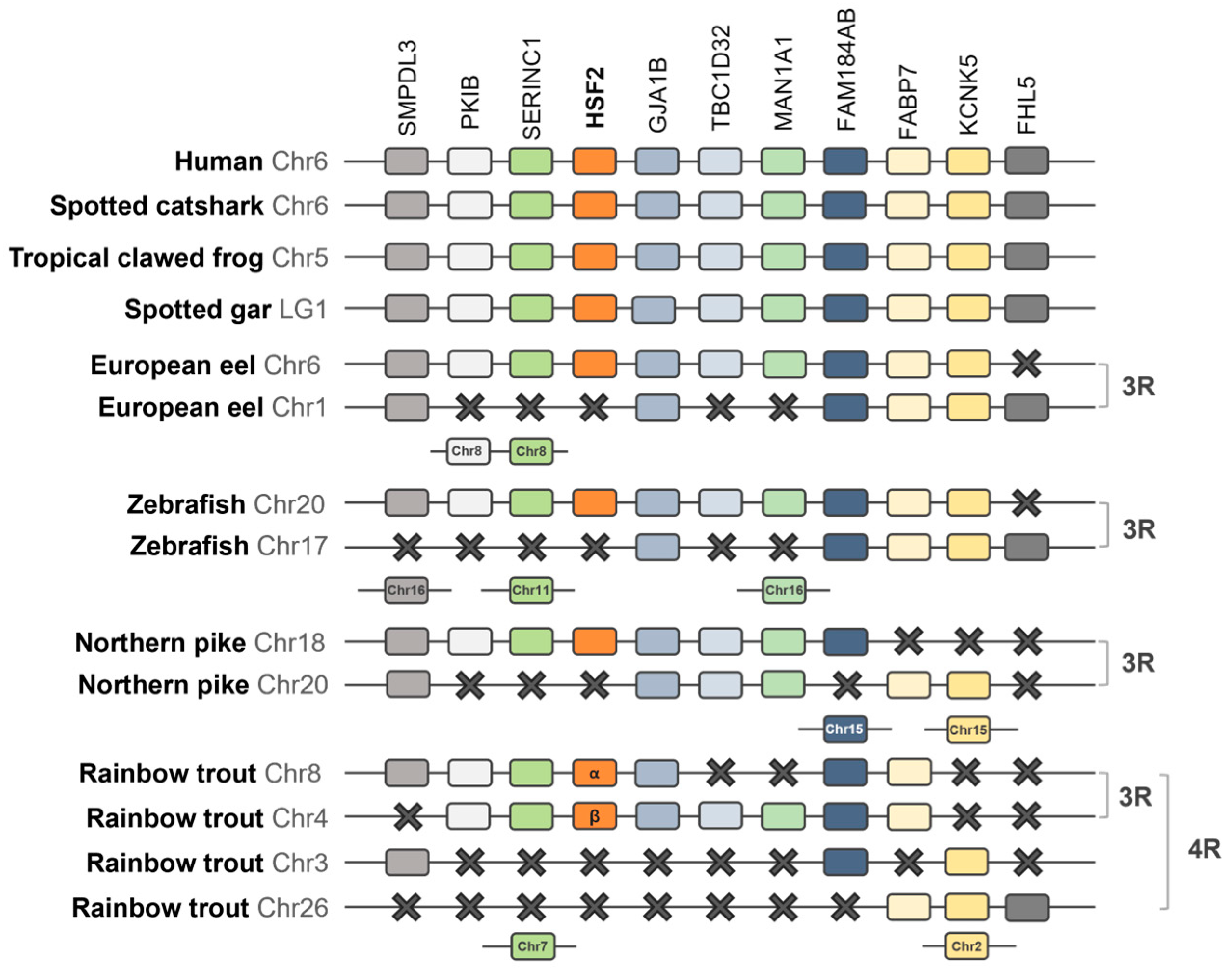
3.2. Synteny Analysis of the HSF Family in Vertebrates
3.3. Expression of HSF Genes in Different Tissues of the Male European Eel
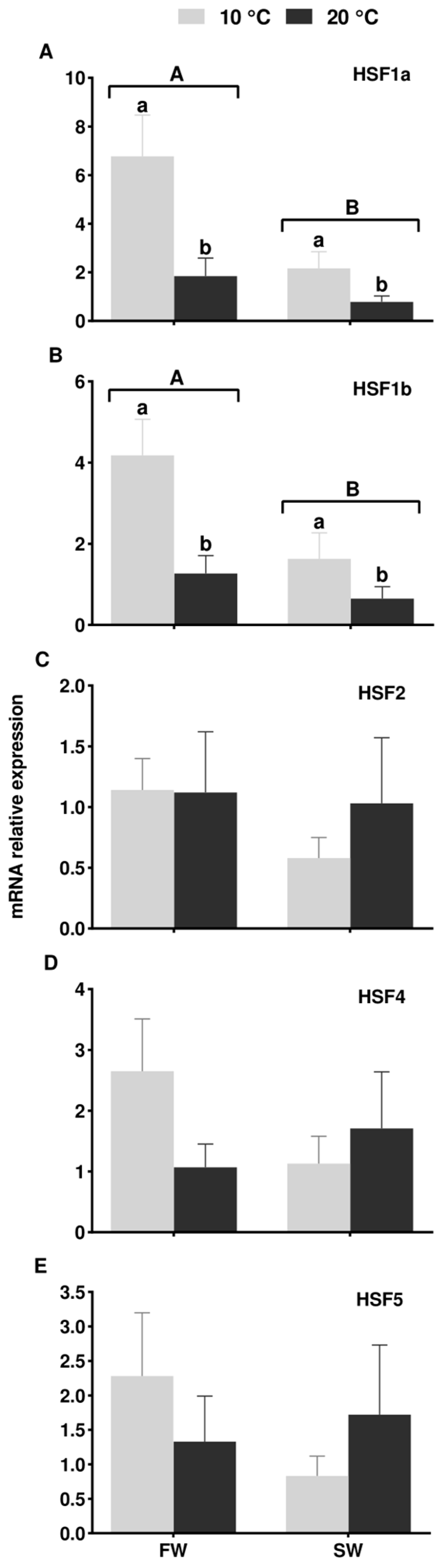
3.4. Expression of HSF Genes in the European Eel Testis Under Different Temperature and Salinity Conditions
3.5. Expression of HSF Genes in the European Eel Testis During Spermatogenesis Induced by Hormonal Maturation
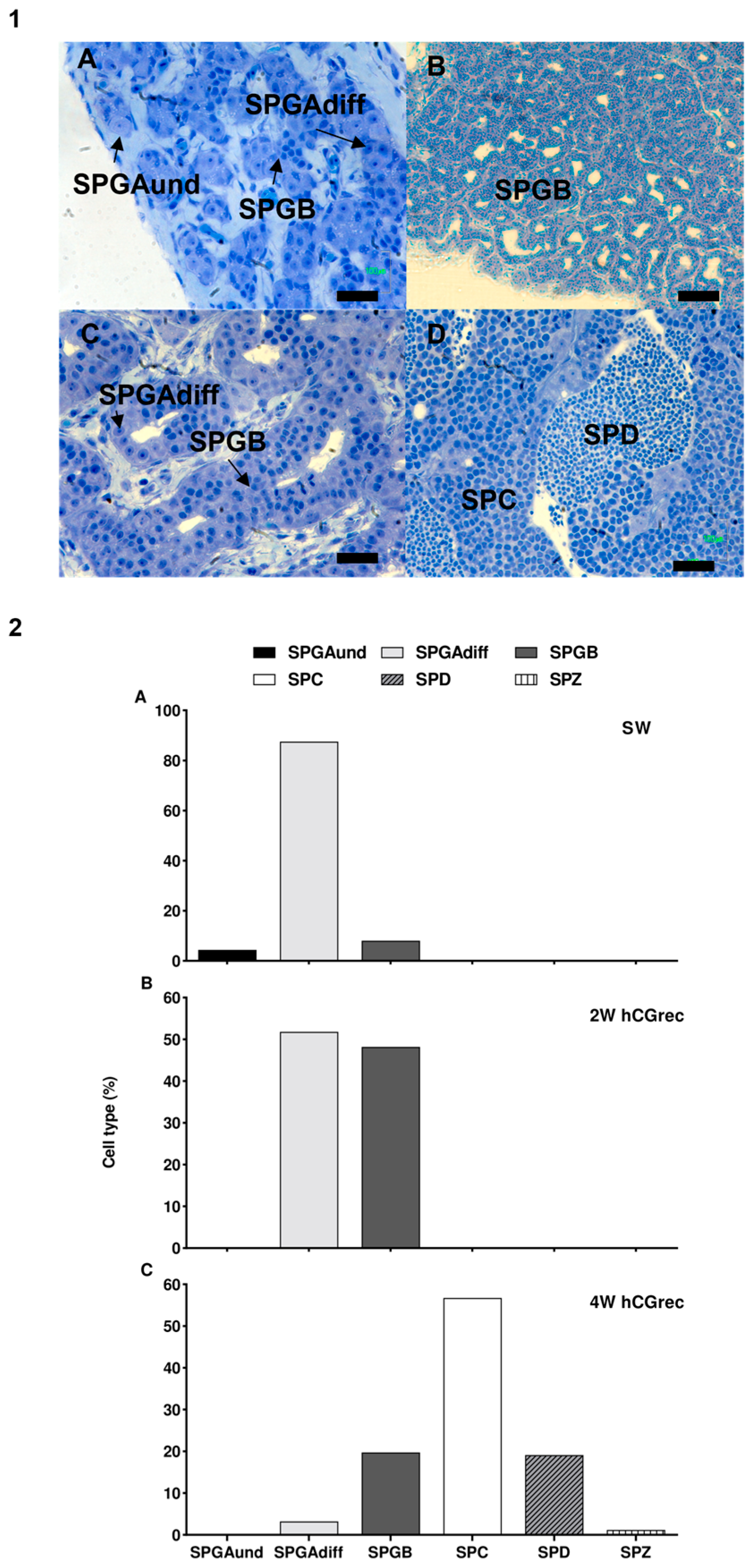
3.6. Testis Histology
4. Discussion
4.1. Evolution of the HSF Family and Discovery of HSF6
4.2. Expression of HSF Genes in Different Tissues from the Male European Eel
4.3. Expression of HSF Genes in the European Eel Testis Under Different Temperature and Salinity Conditions
4.4. Expression of HSF Genes in the European Eel Testis During Hormonal Maturation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakai, A. (Ed.) Proteostasis and adaptation to high temperature stress. In Heat Shock Factor; Springer: Tokyo, Japan, 2016; pp. 3–30. [Google Scholar]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Kovács, D.; Kovács, M.; Ahmed, S.; Barna, J. Functional diversification of heat shock factors. Biol. Futur. 2022, 73, 427–439. [Google Scholar] [CrossRef]
- Takii, R.; Fujimoto, M. Structure and function of the HSF family members. In Heat Shock Factor; Nakai, A., Ed.; Springer: Tokyo, Japan, 2016; pp. 31–50. [Google Scholar]
- Swan, C.L.; Evans, T.G.; Sylvain, N.; Krone, P.H. Zebrafish HSF4: A novel protein that shares features of both HSF1 and HSF4 of mammals. Cell Stress Chaperones 2012, 17, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hao, X.; Liu, K.; Feng, B.; Zhang, Z.; Tang, L.; Mhaboob, S.; Shao, C. Early response to heat stress in Chinese tongue sole (Cynoglossus semilaevis): Performance of different sexes, candidate genes and networks. BMC Genom. 2020, 21, 745. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Tan, S.; Zhou, T.; Liu, Y.; Tian, C.; Bao, L.; Xing, D.; Su, B.; Wang, J.; et al. Genomic imprinting-like monoallelic paternal expression determines sex of channel catfish. Sci. Adv. 2022, 8, eadc8786. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, L.; Li, W.; Wu, J.; Wang, D. Gata2a mutation causes progressive microphthalmia and blindness in Nile tilapia. Int. J. Mol. Sci. 2023, 24, 3567. [Google Scholar] [CrossRef] [PubMed]
- Saju, J.; Hossain, M.; Liew, W.; Pradhan, A.; Thevasagayam, N.; Tan, L.; Anand, A.; Olsson, P.; Orban, L. Heat shock factor 5 is essential for spermatogenesis in zebrafish. Cell Rep. 2018, 25, 3252–3261.e4. [Google Scholar] [CrossRef] [PubMed]
- Hemati, A.; Modarressi, M.H.; Kolivand, S.; Azarnia, M. Heat shock factor 5 is essential for spermatogenesis in mice: Detected by a new monoclonal antibody. Iran. J. Basic Med. Sci. 2020, 23, 293–297. [Google Scholar] [PubMed]
- Barutc, A.R.; Frit, A.J.; McCor, R.P.; Nick, J.A.; Asla, M. Heat shock factor 5 establishes the male germ-line meiotic sex chromosome inactivation through regulation of Smarca4. Heliyon 2023, 9, e15194. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, H.; He, C.; Shou, C.; Han, Z. Heat shock protein 70 (Hsp70) and heat shock transcription factor (HSF) gene families in Cynoglossus semilaevis: Genome-wide identification and correlation analysis in response to low salinity stress. Mar. Freshw. Res. 2021, 72, 1132–1141. [Google Scholar] [CrossRef]
- Himanen, S.V.; Puustinen, M.C.; Da Silva, A.J.; Vihervaara, A.; Sistonen, L. HSFs drive transcription of distinct genes and enhancers during oxidative stress and heat shock. Nucleic Acids Res. 2022, 50, 6102–6115. [Google Scholar] [CrossRef] [PubMed]
- Izu, H.; Inouye, S.; Fujimoto, M.; Shiraishi, K.; Naito, K.; Nakai, A. Heat shock transcription factor 1 is involved in quality-control mechanisms in male germ cells. Biol. Reprod. 2004, 70, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, N.; Inouye, S.; Fujimoto, M.; Tanaka, Y.; Izu, H.; Takaki, E.; Ichikawa, H.; Rho, J.; Nakai, A. A novel HSF1-mediated death pathway that is suppressed by heat shock proteins. EMBO J. 2006, 25, 4773–4783. [Google Scholar] [CrossRef] [PubMed]
- Korfanty, J.; Stokowy, T.; Widlak, P.; Gogler-Piglowska, A.; Handschuh, L.; Podkowiński, J.; Vydra, N.; Naumowicz, A.; Toma-Jonik, A.; Widlak, W. Crosstalk between HSF1 and HSF2 during the heat shock response in mouse testes. Int. J. Biochem. Cell Biol. 2014, 57, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, J.; Moskophidis, D.; Mivechi, N.F. Targeted disruption of the heat shock transcription factor (HSF)-2 gene results in increased embryonic lethality, neuronal defects, and reduced spermatogenesis. Genesis 2003, 36, 48–61. [Google Scholar] [CrossRef]
- Åkerfelt, M.; Trouillet, D.; Mezger, V.; Sistonen, L. Heat shock factors at a crossroad between stress and development. Ann. N. Y. Acad. Sci. 2008, 1113, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell. Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ying, Z.; Jin, X.; Tu, N.; Zhang, Y.; Phillips, M.; Moskophidis, D.; Mivechi, N.F. Essential requirement for both HSF1 and HSF2 transcriptional activity in spermatogenesis and male fertility. Genesis 2004, 38, 66–80. [Google Scholar] [CrossRef]
- Fujimoto, M.; Hayashida, N.; Katoh, T.; Oshima, K.; Shinkawa, T.; Prakasama, R.; Tan, K.; Inouye, S.; Takii, R.; Nakai, A. A novel mouse HSF3 has the potential to activate nonclassical heat-shock genes during heat shock. Mol. Biol. Cell 2010, 21, 106–116. [Google Scholar] [CrossRef]
- Takii, R.; Fujimoto, M.; Matsuura, Y.; Wu, F.; Oshibe, N.; Takaki, E.; Katiyar, A.; Akashi, H.; Makino, T.; Kawata, M.; et al. HSF1 and HSF3 cooperatively regulate the heat shock response in lizards. PLoS ONE 2017, 12, e0180776. [Google Scholar] [CrossRef]
- Fujimoto, M.; Izu, H.; Seki, K.; Fukuda, K.; Nishida, T.; Yamada, S.; Kato, K.; Yonemura, S.; Inouye, S.; Nakai, A. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004, 23, 4297–4306. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Nakai, A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010, 277, 4112–4125. [Google Scholar] [CrossRef]
- Wright, R.; Piper, A.; Aarestrup, K.; Azevedo, J.; Cowan, G.; Don, A.; Gollock, M.; Ramallo, S.; Velterop, R.; Walker, A.; et al. First direct evidence of adult European eels migrating to their breeding place in the Sargasso Sea. Sci. Rep. 2022, 12, 15362. [Google Scholar]
- Righton, D.; Westerberg, H.; Feunteun, E.; Økland, F.; Gargan, P.; Amilhat, E.; Metcalfe, J.; Lobon-Cervia, J.; Sjöberg, N.; Simon, J.; et al. Empirical observations of the spawning migration of European eels: The long and dangerous road to the Sargasso Sea. Sci. Adv. 2016, 2, e1501694. [Google Scholar] [CrossRef]
- Herranz-Jusdado, J.G.; Kása, E.; Kollár, T.; Gallego, V.; Peñaranda, D.S.; Rozenfeld, C.; Pérez, L.; Horváth, Á.; Asturiano, J.F. Handling and treatment of male European eels (Anguilla anguilla) for hormonal maturation and sperm cryopreservation. JoVE 2018, 131, e56835. [Google Scholar] [CrossRef]
- Asturiano, J.F. Improvements on the reproductive control of the European eel. In Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology; Yoshida, M., Asturiano, J.F., Eds.; Springer Nature: Singapore, 2020; pp. 293–320. [Google Scholar]
- Peñaranda, D.S.; Morini, M.; Tveiten, H.; Vílchez, M.C.; Gallego, V.; Dirks, R.P.; van der Thillart, G.E.; Pérez, L.; Asturiano, J.F. Temperature modulates testis steroidogenesis in European eel. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 197, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, I.; Peñaranda, D.S.; Gallego, V.; Baloche, S.; Nourizadeh-Lillabadi, R.; Tveiten, H.; Dufour, S.; Asturiano, J.F.; Weltzien, F.A.; Pérez, L. Temperature modulates the vitellogenesis progression in European eel. Aquaculture 2014, 434, 38–47. [Google Scholar] [CrossRef]
- Rozenfeld, C.; García-Carpintero, V.; Pérez, L.; Gallego, V.; Herranz-Jusdado, J.G.; Tveiten, H.; Johnsen, H.K.; Fontaine, R.; Weltzien, F.A.; Cañizares, J.; et al. Cold seawater induces early sexual developmental stages in the BPG axis of European eel males. BMC Genom. 2019, 20, 597. [Google Scholar] [CrossRef]
- Henkel, C.V.; Burgerhout, E.; de Wijze, D.L.; Dirks, R.P.; Minegishi, Y.; Jansen, H.J.; Spaink, H.P.; Dufour, S.; Weltzien, F.A.; Tsukamoto, K.; et al. Primitive duplicate Hox clusters in the European eel’s genome. PLoS ONE 2012, 7, e32231. [Google Scholar] [CrossRef] [PubMed]
- Henkel, C.V.; Dirks, R.P.; de Wijze, D.L.; Minegishi, Y.; Aoyama, J.; Jansen, H.J.; Turner, B.; Knudsen, B.; Bundgaard, M.; Hvam, K.L.; et al. First draft genome sequence of the Japanese eel, Anguilla japonica. Gene 2012, 511, 195–201. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliams, H.; Remmert, M.; Sodling, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 539, 7. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.J.; Liem, M.; Jong-Raadsen, A.; Dufour, S.; Weltzien, F.; Swinkels, W.; Koelewijn, A.; Palstra, A.P.; Pelster, B.; Spaink, H.P.; et al. Rapid de novo assembly of the European eel genome from nanopore sequencing reads. Sci. Rep. 2017, 7, 7213. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Abascal, F.; Zardoya, R.; Posada, D. ProtTest: Selection of best-fit models of protein evolution. Bioinformatics 2005, 21, 2104–2105. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.; Peñaranda, D.S.; Vílchez, M.C.; Nourizadeh-Lillabadi, R.; Lafont, A.; Dufour, S.; Asturiano, J.F.; Weltzien, F.A.; Pérez, L. Nuclear and membrane progestin receptors in the European eel: Characterization and expression in vivo through spermatogenesis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 207, 79–92. [Google Scholar] [CrossRef]
- Pflaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Cardoso, E.R.; Nóbrega, R.H.; Batlouni, S.R.; Bogerd, J.; França, L.R.; Schulz, R.W. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with an emphasis on spermatogonial generations. Biol. Reprod. 2009, 81, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, J.; Braasch, I.; Batzel, P.; Cabau, C.; Montfort, J.; Nguyen, T.; Jouanno, E.; Berthelot, C.; Klopp, C.; Journot, L.; et al. Evolution of gene expression after whole-genome duplication: New insights from the spotted gar genome. J. Exp. Zool. B Mol. Dev. Evol. 2017, 328, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Robertson, F.M.; Gundappa, M.K.; Grammes, F.; Hvidsten, T.R.; Redmond, A.K.; Lien, S.; Martin, S. Lineage-specific rediploidization is a mechanism to explain time-lags between genome duplication and evolutionary diversification. Genome Biol. 2017, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Ojima, N.; Yamashita, M. Cloning and characterization of two distinct isoforms of rainbow trout heat shock factor 1. Eur. J. Biochem. 2004, 271, 703–712. [Google Scholar] [CrossRef]
- Kim, S.; Chang, Z.; Park, J. Identification, tissue distribution and characterization of two heat shock factors (HSFs) in goldfish (Carassius auratus). Shellfish Immunol. 2015, 43, 375–386. [Google Scholar] [CrossRef]
- Råbergh, C.M.; Airaksinen, S.; Soitamo, A.; Björklund, H.V.; Johansson, T.; Nikinmaa, M.; Sistonen, L. Tissue-specific expression of zebrafish (Danio rerio) heat shock factor 1 mRNAs in response to heat stress. J. Exp. Biol. 2000, 203, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Björk, J.K.; Sandqvist, A.; Elsing, A.N.; Kotaja, N.; Sistonen, L. miR-18, a member of Oncomir-1, targets heat shock transcription factor 2 in spermatogenesis. Development 2010, 137, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Abane, R.; Mezger, V. Roles of heat shock factors in gametogenesis and development. FEBS J. 2010, 277, 4150–4172. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, N.; Kaya, Y.; Rebl, H.; Stüeken, M.; Rebl, A.; Nguinkal, J.A.; Franz, G.P.; Brunner, R.M.; Goldammer, T.; Grunow, B.; et al. Insights into early ontogenesis: Characterization of stress and development key genes of pikeperch (Sander lucioperca) in vivo and in vitro. Fish Physiol. Biochem. 2021, 47, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, L.; Blanes-García, M.; Pérez, L.; Asturiano, J.F.; Morini, M. Superoxidase dismutases (SODs) in the European eel: Gene characterization, expression response to temperature combined with hormonal maturation and possible migratory implications. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 290, 111590. [Google Scholar] [CrossRef] [PubMed]
- Widlak, W.; Vydra, N. The role of heat shock factors in mammalian spermatogenesis. In The Role of Heat Shock Proteins in Reproductive System Development and Function; MacPhee, D., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Le Goff, P.; Michel, D. HSF1 activation occurs at different temperatures in somatic and male germ cells in the poikilotherm rainbow trout. Biochem. Biophys. Res. Commun. 1999, 259, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Zhang, Q.; Lai, Y.; Li, C.; Zhang, Q.; Huang, W.; Duan, Y.; Jiang, Z.; Li, X.; et al. Identification of HSF1 as a novel androgen receptor-regulated gene in mouse Sertoli cells. Mol. Reprod. Dev. 2014, 81, 514–523. [Google Scholar] [CrossRef]



| Name | Sequence (5′–3′) | Orientation | Efficiency |
|---|---|---|---|
| HSF1a | GGC CAG TTT TGT CCG ACA GC CGA GCC TGA CGT CCT CAT GT | Forward Reverse | 1.92 |
| HSF1b | CCC CGA GTT CCC CAC CTT AC GGG CGG GGC ATT CTT TTC TG | Forward Reverse | 2.05 |
| HSF2 | AAC ATG GCC AGC TTT GTC AG ATT CCA CTG GTC CAT CAC GT | Forward Reverse | 2.00 |
| HSF4 | TGG ACC CCT TCA ACC CCA AC GCC ACC GTA GAC CTC TGC AT | Forward Reverse | 1.96 |
| HSF5 | TTG GGA TCA CAC TGG CCA GG ACC GGG ACT CAG CTC TAC CT | Forward Reverse | 2.03 |
| ARP | GTG CCA GCT CAG AAC ACT G ACA TCG CTC AAG ACT TCA ATG G | Forward Reverse | 2.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrão, L.; Pérez, L.; Asturiano, J.F.; Morini, M. Heat Shock Factors in the European Eel: Gene Characterization and Expression Response to Different Environmental Conditions and to Induced Sexual Maturation. Fishes 2025, 10, 73. https://doi.org/10.3390/fishes10020073
Ferrão L, Pérez L, Asturiano JF, Morini M. Heat Shock Factors in the European Eel: Gene Characterization and Expression Response to Different Environmental Conditions and to Induced Sexual Maturation. Fishes. 2025; 10(2):73. https://doi.org/10.3390/fishes10020073
Chicago/Turabian StyleFerrão, Leonor, Luz Pérez, Juan F. Asturiano, and Marina Morini. 2025. "Heat Shock Factors in the European Eel: Gene Characterization and Expression Response to Different Environmental Conditions and to Induced Sexual Maturation" Fishes 10, no. 2: 73. https://doi.org/10.3390/fishes10020073
APA StyleFerrão, L., Pérez, L., Asturiano, J. F., & Morini, M. (2025). Heat Shock Factors in the European Eel: Gene Characterization and Expression Response to Different Environmental Conditions and to Induced Sexual Maturation. Fishes, 10(2), 73. https://doi.org/10.3390/fishes10020073







