Identification and Characterization of Myxobolus pronini (Cnidaria: Myxozoa) from Gibel Carp Carassius auratus gibelio and Goldfish C. auratus: New Fish Host, Infection Site, and Geographic Distribution in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Collection
2.2. Morphological Examination
2.3. DNA Extraction, Amplification and Sequencing
2.4. Secondary Structure Prediction of SSU rRNA Sequence
2.5. Phylogenetic Analyses
3. Results
3.1. Plasmodia of Myxobolus pronini
3.2. Spores of Myxobolus pronini
3.3. Sequence and Phylogenetic Analyses
- Taxonomic summary:
- Host: Gibel carp Carassius auratus gibelio, goldfish C. auratus.
- Host size: 13.3–17.3 cm in total length (gibel carp), 6.6–12.5 cm in total length (goldfish).
- Site of infection: Intestine (gibel carp), liver (gibel carp), gallbladder (gibel carp), abdominal cavity (gibel carp, goldfish).
- Prevalence: 33.3% (20/60, gibel carp), 4.5% (1/22, goldfish).
- Locality: Lake Weishan Wetland (gibel carp), Chengyang Fish Market in Qingdao City (goldfish), Shandong Province, China.
- Sample preservation: Accession no. MTR202205201, MTR202303121.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holzer, A.S.; Bartošová–Sojková, P.; Born–Torrijos, A.; Lövy, A.; Hartigan, A.; Fiala, I. The joint evolution of the Myxozoa and their alternate hosts: A cnidarian recipe for success and vast biodiversity. Mol. Ecol. 2018, 27, 1651–1666. [Google Scholar] [CrossRef]
- Kayal, E.; Bentlage, B.; Sabrina Pankey, M.; Ohdera, A.H.; Medina, M.; Plachetzki, D.C.; Collins, A.G.; Ryan, J.F. Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evol. Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Okamura, B.; Hartigan, A.; Naldoni, J. Extensive uncharted biodiversity: The parasite dimension. Integr. Comp. Biol. 2018, 58, 1132–1145. [Google Scholar] [CrossRef]
- Okamura, B.; Gruhl, A.; Bartholomew, J.L. Myxozoan Evolution, Ecology and Development; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Hoffman, G.L. Myxobolus cerebralis, a worldwide cause of salmonid whirling disease. J. Aquat. Anim. Health 1990, 2, 30–37. [Google Scholar] [CrossRef]
- Hallett, S.L.; Bartholomew, J.L. Myxobolus cerebralis and Ceratomyxa shasta. In Fish Parasites: Pathobiology and Protection; CAB International: Oxfordshire, UK, 2012; pp. 141–172. [Google Scholar]
- Liu, Y.; Whipps, C.M.; Gu, Z.M.; Zeng, C.; Huang, M.J. Myxobolus honghuensis n. sp. (Myxosporea: Bivalvulida) parasitizing the pharynx of allogynogenetic gibel carp Carassius auratus gibelio (Bloch) from Honghu Lake, China. Parasitol. Res. 2012, 110, 1331–1336. [Google Scholar] [CrossRef]
- Chen, Q.L.; Ma, C.L. Fauna Sinica. Myxozoa. Myxosporea; Science Press: Beijing, China, 1998. [Google Scholar]
- Liu, Y.; Whipps, C.M.; Nie, P.; Gu, Z.M. Myxobolus oralis sp. n. (Myxosporea: Bivalvulida) infecting the palate in the mouth of gibel carp Carassius auratus gibelio (Cypriniformes: Cyprinidae). Folia Parasitol. 2014, 61, 505–511. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yao, X.; Zhou, F.; Yang, C.Z.; Liu, Y. Identification of Thelohanellus pseudonikolskii n. sp. and Myxobolus koi Kudo, 1920 from goldfish Carassius auratus. Aquacult. Rep. 2022, 24, 101167. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Z.M.; Luo, Y.L. Some additional data to the occurrence, morphology and validity of Myxobolus turpisrotundus Zhang, 2009 (Myxozoa: Myxosporea). Parasitol. Res. 2010, 107, 67–73. [Google Scholar] [CrossRef]
- Batueva, M.D.D.; Pan, X.Y.; Zhang, J.Y.; Liu, X.H.; Wei, W.; Liu, Y. Morphological, histological and molecular characterization of Myxidium cf. rhodei infecting the kidney of Rutilus rutilus. Dis. Aquat. Organ. 2020, 141, 39–46. [Google Scholar] [CrossRef]
- Molnár, K.; Székely, C.; Hallett, S.L.; Atkinson, S.D. Some remarks on the occurrence, host–specificity and validity of Myxobolus rotundus Nemeczek, 1911 (Myxozoa: Myxosporea). Syst. Parasitol. 2009, 72, 71–79. [Google Scholar] [CrossRef]
- Huang, M.J.; Liu, Y.; Jia, L.; Zhai, Y.H.; Deng, Q.; Gu, Z.M. Supplementary studies on Myxobolus tsangwuensis Chen, 1954 (Myxozoa: Myxosporea) infecting the gills of common carp Cyprinus carpio (L.): Molecular and histological data. Acta Parasitol. 2014, 59, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Baiko, D.; Lisnerová, M.; Bartošová–Sojková, P.; Holzer, A.S.; Blabolil, P.; Schabuss, M.; Fiala, I. Solving the Myxidium rhodei (Myxozoa) puzzle: Insights into its phylogeny and host specificity in Cypriniformes. Parasite 2024, 31, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whipps, C.M.; Liu, W.S.; Zeng, L.B.; Gu, Z.M. Supplemental diagnosis of a myxozoan parasite from common carp Cyprinus carpio: Synonymy of Thelohanellus xinyangensis with Thelohanellus kitauei. Vet. Parasitol. 2011, 178, 355–359. [Google Scholar] [CrossRef]
- Liu, Y.; Whipps, C.M.; Gu, Z.M.; Huang, M.J.; He, C.; Yang, H.L.; Molnár, K. Myxobolus musseliusae (Myxozoa: Myxobolidae) from the gills of common carp Cyprinus carpio and revision of Myxobolus dispar recorded in China. Parasitol. Res. 2013, 112, 289–296. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, Y.H.; Liu, Y.; Gu, Z.M. Myxobolus pseudowulii sp. n. (Myxozoa: Myxosporea), a new skin parasite of yellow catfish Tachysurus fulvidraco (Richardson) and redescription of Myxobolus voremkhai (Akhmerov, 1960). Folia Parasitol. 2017, 64, 030. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, J.T.; Zhou, M.; Sato, H.; Zhang, J.Y. Morphological and molecular characterization of a new freshwater Ceratomyxa species (Cnidaria: Myxozoa) from the yellow catfish, Trachysurus fulvidraco in China. Parasitol. Int. 2023, 97, 102778. [Google Scholar] [CrossRef]
- Zhao, X.J.; Xu, L.W.; Ren, S.S.; Wei, Q.; Yin, Q.; Yang, Y.J.; Zhang, J.Y.; Xiang, J.G.; Yu, J.B.; Li, D.L.; et al. Myxobolus dumerilii sp. n. (Myxozoa: Myxobolidae) infecting the brain of Chinese longsnout catfish Tachysurus dumerili (Bleeker) in China. Syst. Parasitol. 2023, 100, 715–723. [Google Scholar] [CrossRef]
- Lom, J.; Arthur, J.R. A guideline for preparation of species descriptions in Myxosporea. J. Fish. Dis. 1989, 12, 151–156. [Google Scholar] [CrossRef]
- Barta, J.R.; Martin, D.S.; Liberator, P.A.; Dashkevicz, M.; Anderson, J.W.; Feigner, S.D.; Elbrecht, A.; Perkins–Barrow, A.; Jenkins, M.C.; Danforth, H.D.; et al. Phylogenetic relationships among eight eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 1997, 83, 262–271. [Google Scholar] [CrossRef]
- Fiala, I. The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. Int. J. Parasitol. 2006, 36, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user–friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Holzer, A.S.; Wootten, R.; Sommerville, C. The secondary structure of the unusually long 18S ribosomal RNA of the myxozoan Sphaerospora truttae and structural evolutionary trends in the Myxozoa. Int. J. Parasitol. 2007, 37, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Gonzalez, P.; Labarère, J. Phylogenetic relationships of Pleurotus species according to the sequence and secondary structure of the mitochondrial small–subunit rRNA V4, V6 and V9 domains. Microbiology 2000, 146, 209–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, S.G.; Jung, H.S. Phylogenetic analysis of Ganoderma based on nearly complete mitochondrial smallsubunit ribosomal DNA sequences. Mycologia 2004, 96, 742–755. [Google Scholar] [CrossRef] [PubMed]
- De Rijk, P.; Wuyts, J.; De Wachter, R. RnaViz 2: An improved representation of RNA secondary structure. Bioinformatics 2003, 19, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ–TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Nylander, J.A.; Ronquist, F.; Huelsenbeck, J.P.; Nieves–Aldrey, J.L. Bayesian phylogenetic analysis of combined data. Syst. Biol. 2004, 53, 47–67. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Batueva, M.D.; Zhao, Y.L.; Zhang, J.Y.; Zhang, Q.Q.; Li, T.T.; Li, A.H. Morphological and molecular characterisation of Myxobolus pronini n. sp. (Myxozoa: Myxobolidae) from the abdominal cavity and visceral serous membranes of the gibel carp Carassius auratus gibelio (Bloch) in Russia and China. Parasit. Vectors 2016, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Studies on Integrative Taxonomy and Molecular Phylogeny of Some Species of Myxobolus. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2023. [Google Scholar]
- Urawa, S.; Freeman, M.A.; Johnson, S.C.; Jones, S.R.M.; Yokoyama, H. Geographical variation in spore morphology, gene sequences, and host specificity of Myxobolus arcticus (Myxozoa) infecting salmonid nerve tissues. Dis. Aquat. Organ. 2011, 96, 229–237. [Google Scholar] [CrossRef]
- Guo, Q.X.; Huang, M.J.; Liu, Y.; Zhang, X.P.; Gu, Z.M. Morphological plasticity in Myxobolus Bütschli, 1882: A taxonomic dilemma case and renaming of a parasite species of the common carp. Parasit. Vectors 2018, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, J.Y.; Yao, X.; Zhang, K.J.; Wang, Q.Y.; Pan, X.Y.; Zhang, J.Y. Intraspecific morphological variation in myxosporeans: High pleomorphic myxospores in the same plasmodium of Myxobolus drjagini (Akhmerov, 1954). Folia Parasitol. 2022, 69, 006. [Google Scholar] [CrossRef]
- Whipps, C.M.; Diggles, B.K. Kudoa alliaria in flesh of Argentinian Hoki Macruronus magellanicus (Gadiformes; Merlucciidae). Dis. Aquat. Organ. 2006, 69, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.A.; Atkinson, S.D.; Whipps, C.M.; Kent, M.L. Molecular and morphological analysis of Myxobolus spp. of salmonid fishes with the description of Myxobolus species. J. Parasitol. 2008, 94, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Li, N.N.; Tang, F.H.; Dong, J.L. Remarks on the validity of Myxobolus ampullicapsulatus and Myxobolus honghuensis (Myxozoa: Myxosporea) based on SSU rDNA sequences. Parasitol. Res. 2013, 112, 3817–3823. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ding, P.; Zhang, C.H.; Sun, R.H.; Yang, C.Z.; Liu, Y. Redescription of Myxobolus ampullicapsulatus and comparison with molecular marker of M. honghuensis. J. Fish. China 2024, 48, 182–192. [Google Scholar]
- Guo, R.; Zhang, S.P.; Jiang, X.B.; Wang, W.; Wang, H.C.; Cai, L.M.; Yang, X.Q. Molecular identification and phylogenetic analysis of Myxobolus honghuensis found in pharynx of goldfish Carassius auratus. J. Fish. Res. 2021, 43, 61–66. [Google Scholar]
- Li, D.; Zhai, Y.H.; Gu, Z.M.; Liu, Y. Development of a multiplex PCR method for the simultaneous detection of four myxosporeans infecting gibel carp Carassius auratus gibelio. Dis. Aquat. Organ. 2017, 124, 31–39. [Google Scholar] [CrossRef] [PubMed]
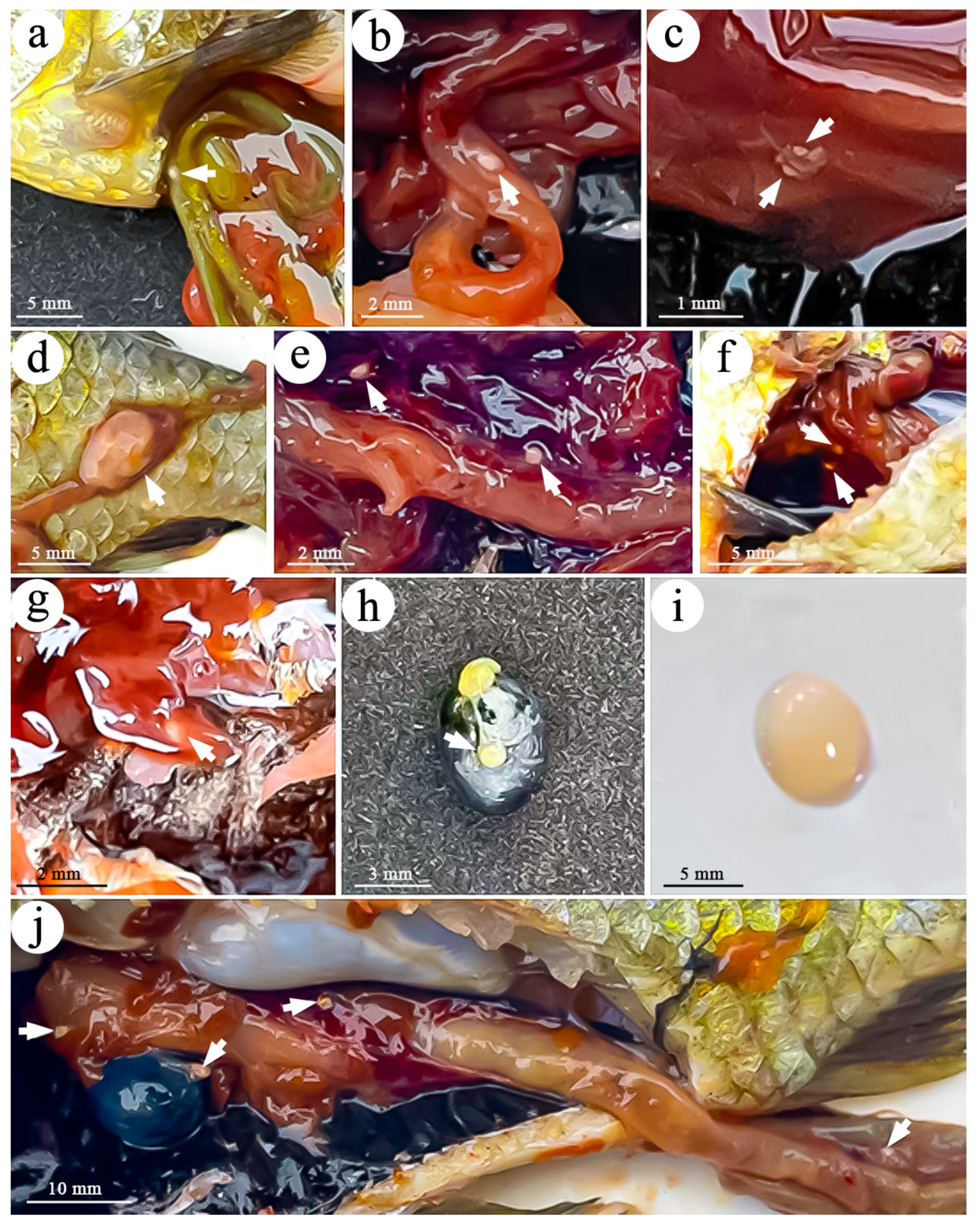
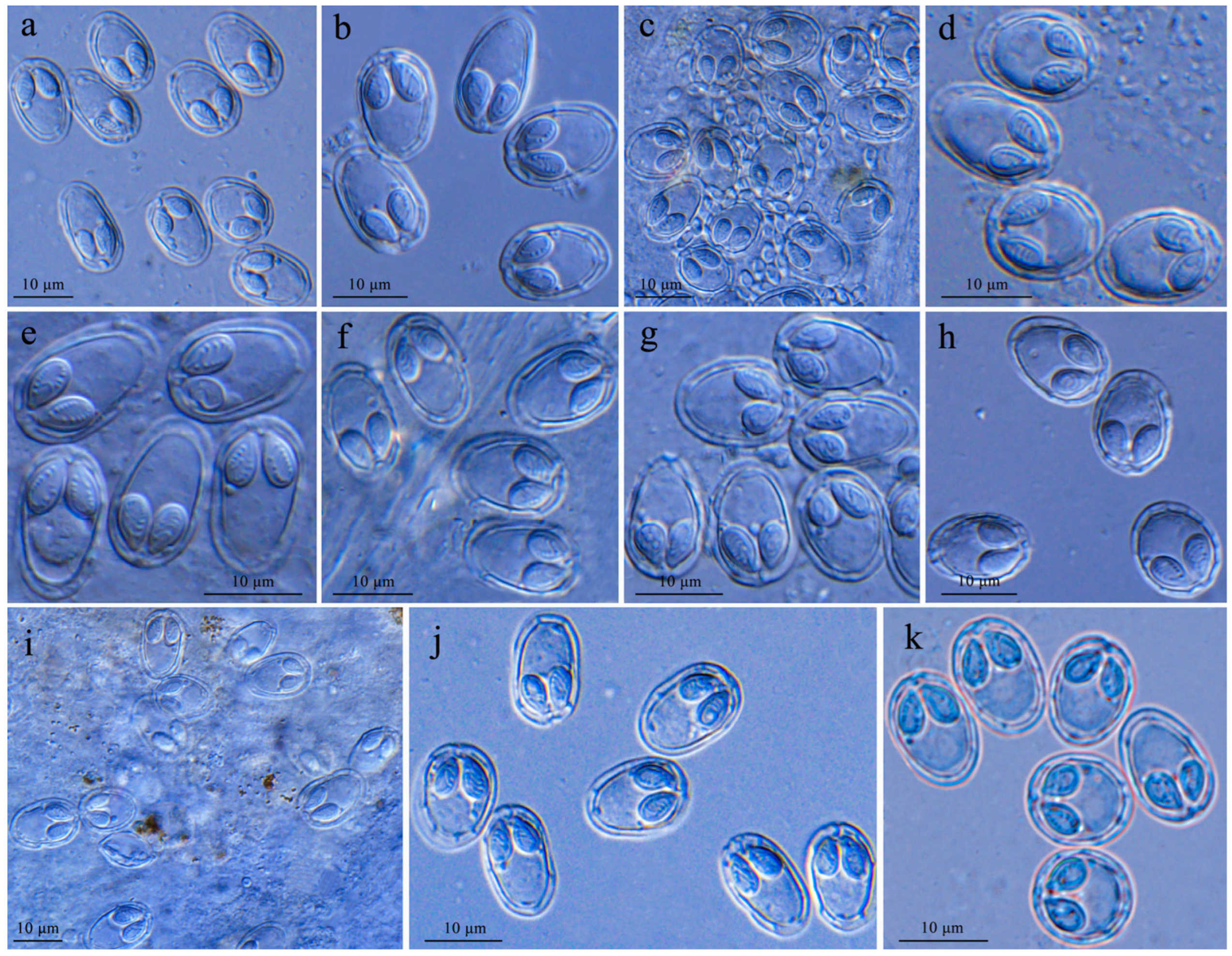

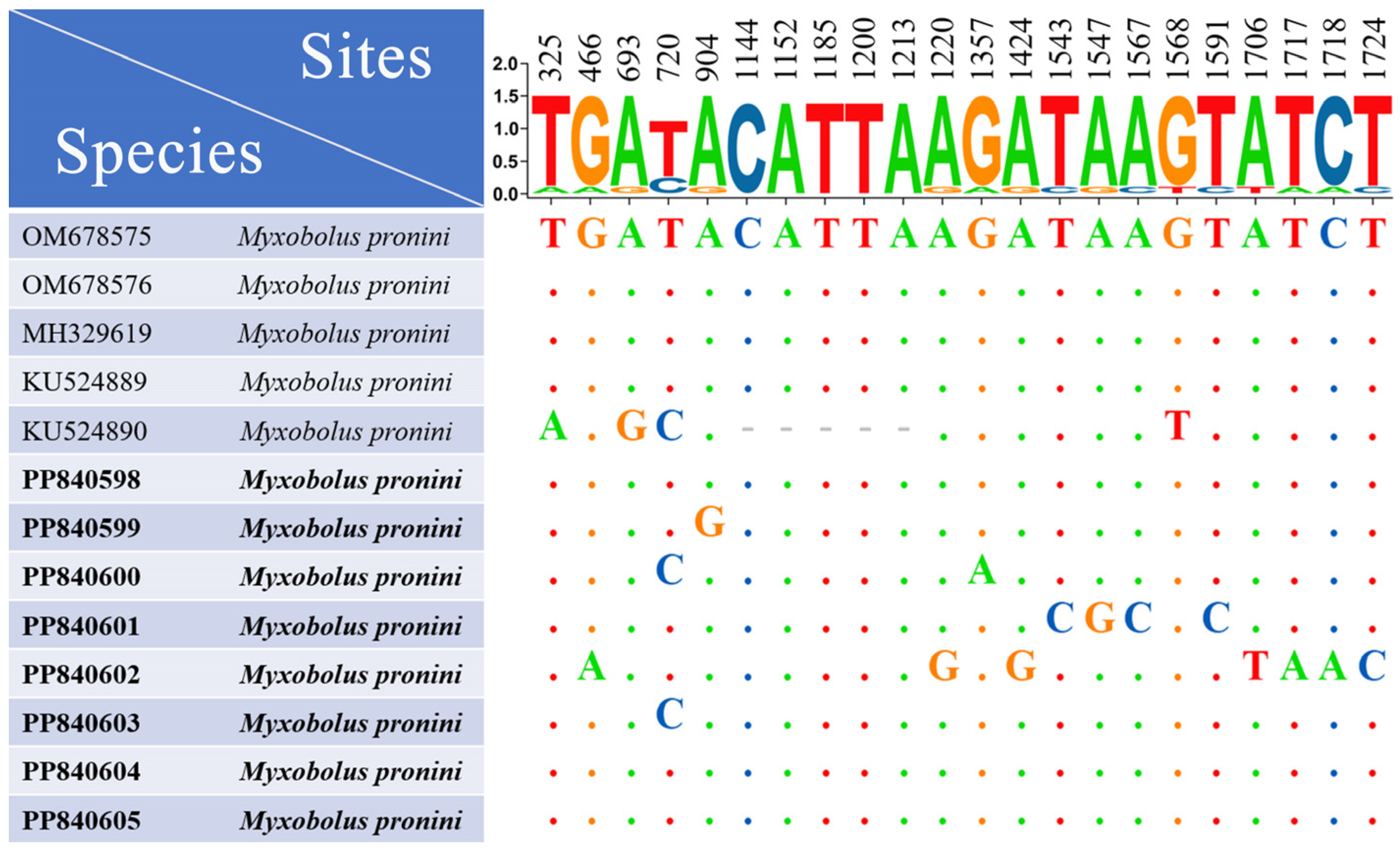

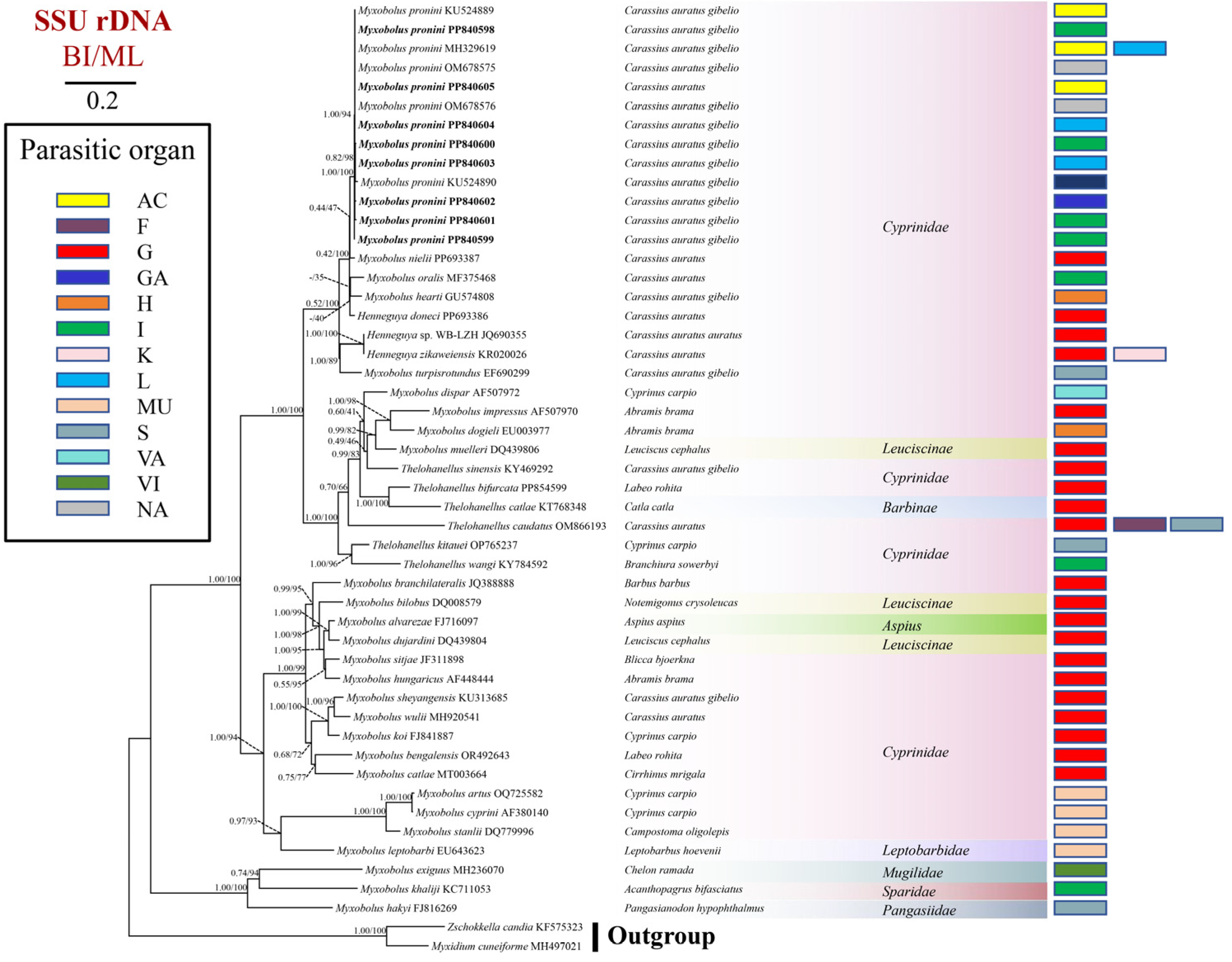
| SL | SW | PCL | PCW | SI | Host | Source | Accession Number |
|---|---|---|---|---|---|---|---|
| 15.1 ± 0.3 (14.3–16.2) | 10.1 ± 0.1 (9.6–10.8) | 5.4 ± 0.6 (4.3–6.7) | 3.1 ± 0.1 (2.2–3.6) | Abdominal cavity | C. auratus gibelio | [35] | KU524889 |
| 14.7 ± 0.2 (13.8–15.6) | 9.6 ± 0.7 (9.0–13.3) | 5.3 ± 0.2 (4.8–5.6) | 3.0 ± 0.1 (2.9–3.4) | Visceral serous membranes of liver | C. auratus gibelio | [35] | KU524890 |
| 13.86 ± 0.8 (12.2–15.39) | 9.83 ± 0.5 (8.57–10.51) | 5.69 ± 0.42 (4.8–6.47) | 3.36 ± 0.21 (2.79–3.73) | Abdominal cavity, gallbladder | C. auratus gibelio | [36] | MH329619 |
| 16.8 ± 0.4 (16.0–17.9) | 9.5 ± 0.4 (8.7–10.2) | 6.0 ± 0.3 (5.6–6.8) | 3.4 ± 0.2 (3.1–3.8) | Intestine | C. auratus gibelio | Present research | PP840598 |
| 15.2 ± 0.5 (14.4–16.4) | 9.5 ± 0.5 (8.5–10.5) | 5.8 ± 0.4 (5.0–6.4) | 3.4 ± 0.2 (3.1–3.8) | Intestine | C. auratus gibelio | Present research | PP840599 |
| 16.5 ± 0.2 (16.1–16.9) | 10.6 ± 0.4 (9.9–11.2) | 5.8 ± 0.3 (5.3–6.3) | 3.6 ± 0.2 (3.3–3.9) | Intestine | C. auratus gibelio | Present research | PP840600 |
| 14.1 ± 0.4 (13.3–15.0) | 9.5 ± 0.5 (8.8–10.2) | 4.9 ± 0.4 (4.0–5.5) | 3.3 ± 0.3 (2.7–3.9) | Intestine | C. auratus gibelio | Present research | PP840601 |
| 13.0 ± 0.8 (11.4–14.3) | 9.5 ± 0.5 (8.3–10.6) | 5.3 ± 0.5 (4.4–6.3) | 3.1 ± 0.4 (2.3–3.9) | Intestine | C. auratus gibelio | Present research | - |
| 13.6 ± 0.5 (12.2–14.4) | 9.2 ± 0.5 (8.4–10.3) | 5.1 ± 0.3 (4.6–5.6) | 3.3 ± 0.2 (2.8–3.7) | Intestine | C. auratus gibelio | Present research | - |
| 15.0 ± 0.8 (13.6–16.5) | 9.4 ± 0.5 (8.0–10.4) | 5.8 ± 0.3 (5.0–6.3) | 3.2 ± 0.2 (2.9–3.5) | Gallbladder wall | C. auratus gibelio | Present research | PP840602 |
| 15.0 ± 0.3 (14.5–15.6) | 10.2 ± 0.3 (9.6–10.7) | 5.9 ± 0.2 (5.4–6.4) | 3.4 ± 0.2 (3.1–4.0) | Liver | C. auratus gibelio | Present research | PP840603 |
| 13.5 ± 0.4 (12.9–14.6) | 9.7 ± 0.4 (8.8–11.0) | 5.4 ± 0.3 (4.8–6.1) | 3.3 ± 0.2 (3.0–3.6) | Liver | C. auratus gibelio | Present research | PP840604 |
| 14.2 ± 0.8 (12.9–15.7) | 9.1 ± 0.3 (8.7–10.0) | 5.1 ± 0.4 (4.1–5.7) | 3.2 ± 0.2 (2.8–3.6) | Liver | C. auratus gibelio | Present research | - |
| 13.3 ± 0.8 (12.0–15.3) | 9.6 ± 0.6 (8.4–10.7) | 4.9 ± 0.4 (4.2–5.6) | 3.1 ± 0.3 (2.6–3.7) | Abdominal cavity | C. auratus gibelio | Present research | - |
| 12.8 ± 0.8 (11.2–14.3) | 9.5 ± 0.6 (8.4–10.5) | 4.8 ± 0.4 (4.2–5.6) | 2.9 ± 0.3 (2.3–3.5) | Abdominal cavity | C. auratus | Present research | PP840605 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Zhang, X.; Ding, P.; Sun, R.; Wang, Z.; Liu, Y. Identification and Characterization of Myxobolus pronini (Cnidaria: Myxozoa) from Gibel Carp Carassius auratus gibelio and Goldfish C. auratus: New Fish Host, Infection Site, and Geographic Distribution in China. Fishes 2025, 10, 61. https://doi.org/10.3390/fishes10020061
Zhou F, Zhang X, Ding P, Sun R, Wang Z, Liu Y. Identification and Characterization of Myxobolus pronini (Cnidaria: Myxozoa) from Gibel Carp Carassius auratus gibelio and Goldfish C. auratus: New Fish Host, Infection Site, and Geographic Distribution in China. Fishes. 2025; 10(2):61. https://doi.org/10.3390/fishes10020061
Chicago/Turabian StyleZhou, Fan, Xiaoyi Zhang, Peng Ding, Ronghua Sun, Zhe Wang, and Yang Liu. 2025. "Identification and Characterization of Myxobolus pronini (Cnidaria: Myxozoa) from Gibel Carp Carassius auratus gibelio and Goldfish C. auratus: New Fish Host, Infection Site, and Geographic Distribution in China" Fishes 10, no. 2: 61. https://doi.org/10.3390/fishes10020061
APA StyleZhou, F., Zhang, X., Ding, P., Sun, R., Wang, Z., & Liu, Y. (2025). Identification and Characterization of Myxobolus pronini (Cnidaria: Myxozoa) from Gibel Carp Carassius auratus gibelio and Goldfish C. auratus: New Fish Host, Infection Site, and Geographic Distribution in China. Fishes, 10(2), 61. https://doi.org/10.3390/fishes10020061






