Study on the Role and Pathological and Immune Responses of Silver Nanoparticles Against Two Aeromonas salmonicida subsp. salmonicida Strains at Different Virulence Levels in Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Methods and Materials
2.1. Fish, Bacteria and AgNPs
2.2. In Vitro Experiments of Bacterial Culture with AgNPs
2.3. Transmission Electron Microscopy (TEM) of Bacterial Shapes
2.4. In Vivo Experiments of AgNPs in Fish with Bacterial Challenge
2.5. Histopathological Analyses
2.6. Immune Gene Transcription (RT-qPCR)
2.7. Statistical Analysis
3. Results
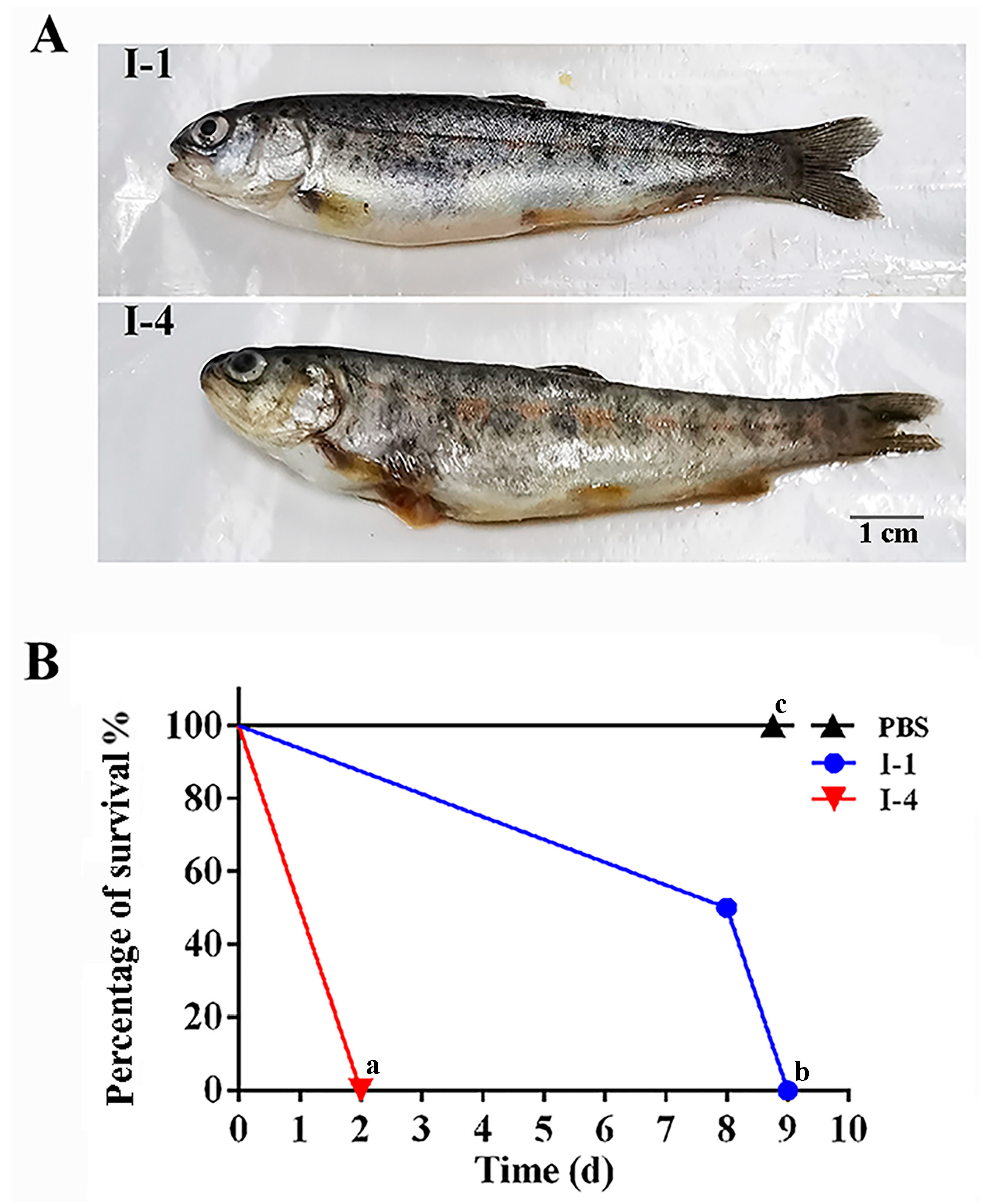
3.1. Characteristic of A. salmonicida I-1 and I-4
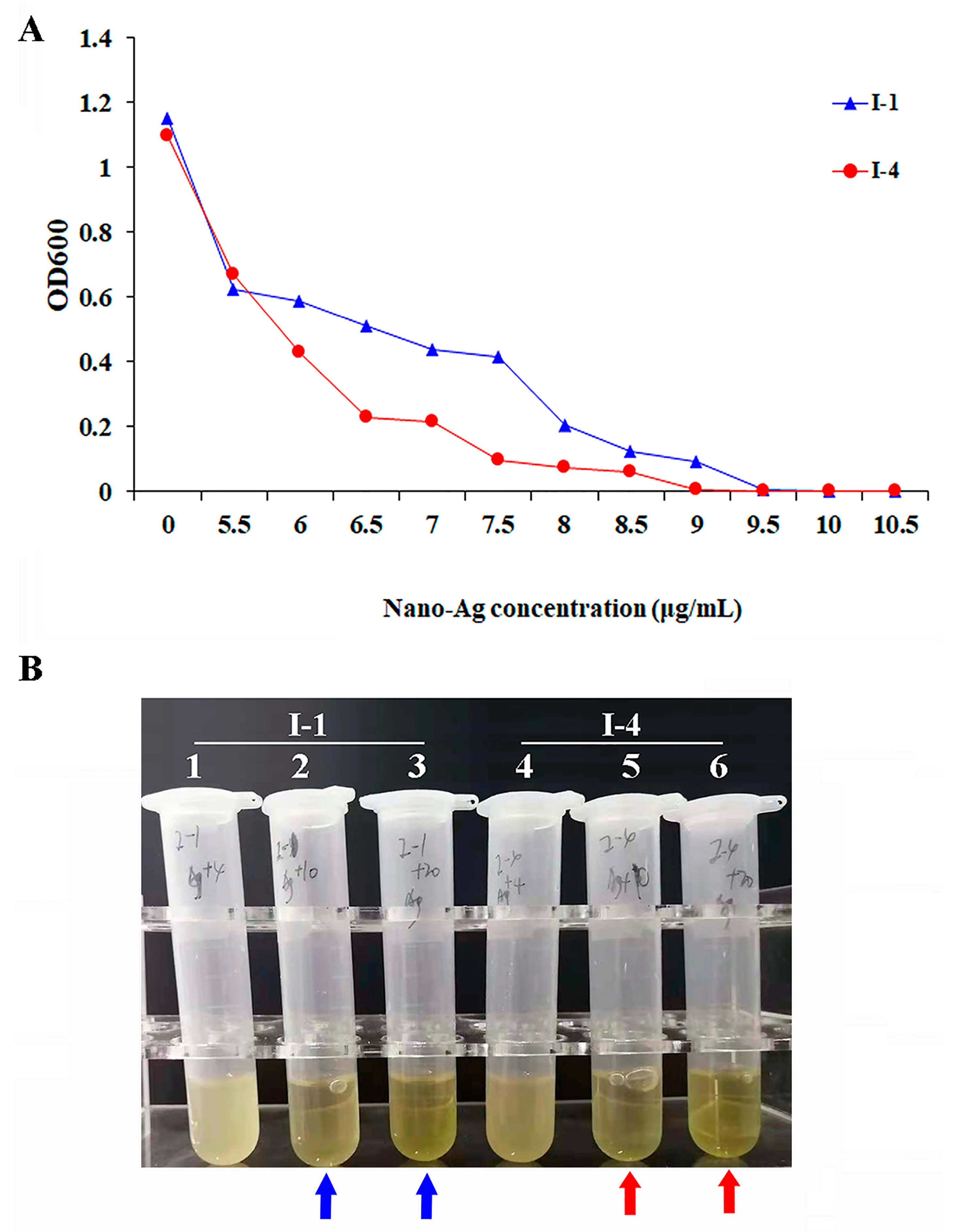
3.2. Antibacterial Effect of AgNPs
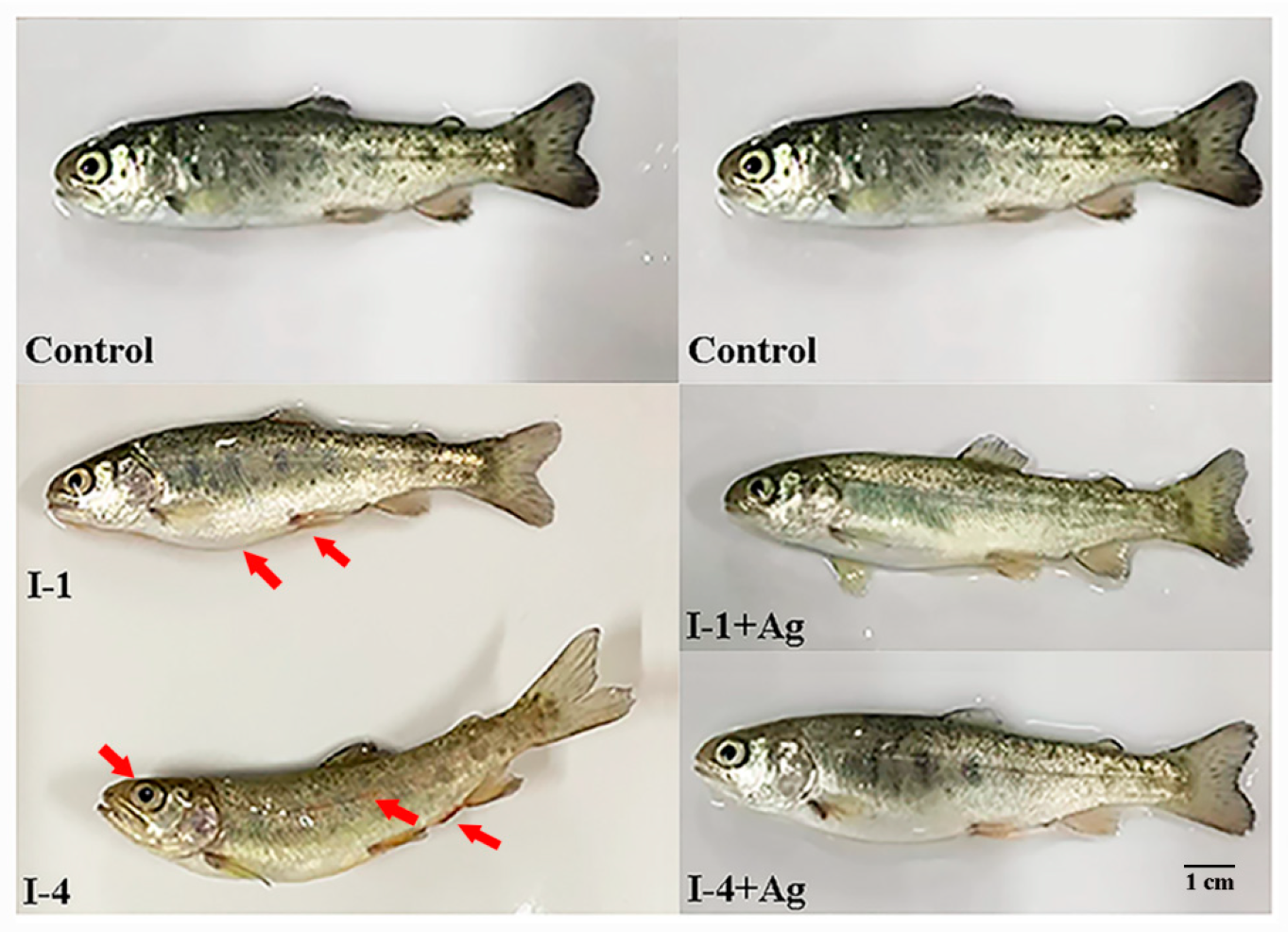
3.3. Influence of AgNPs on the Fish Against I-1 and I-4
3.4. Histopathological Changes
3.5. Gene Expression Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lund, V.; Mikkelsen, H.; Schrøder, M.B. Comparison of atypical furunculosis vaccines in spotted wolffish (Anarhicas minor O.) and Atlantic halibut (Hippoglossus hippoglossus L.). Vaccine 2008, 26, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Sun, G.; Li, X.; Liu, Y. Comparative transcriptome analysis reveals the mechanism of β-glucan in protecting rainbow trout (Oncorhynchus mykiss) from Aeromonas salmonicida infection. Fish. Shellfish Immunol. 2020, 98, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.N.; Wang, Q.; Wang, Y.F.; Tao, Y.; Zheng, C.L.; Wang, M.H.; Che, M.Y.; Cui, Z.H.; Li, X.L.; Zhang, Q.; et al. Isolation and identification of vapA-absent Aeromonas salmonicida in diseased snakehead Channa argus in China. Int. Microbiol. 2024, 27, 1137–1150. [Google Scholar] [CrossRef]
- Marana, M.H.; Jørgensen, L.V.; Skov, J.; Chettri, J.K.; Holm Mattsson, A.; Dalsgaard, I.; Kania, P.W.; Buchmann, K. Subunit vaccine candidates against Aeromonas salmonicida in rainbow trout Oncorhynchus mykiss. PLoS ONE 2017, 12, e0171944. [Google Scholar] [CrossRef]
- Marana, M.H.; Skov, J.; Chettri, J.K.; Krossøy, B.; Dalsgaard, I.; Kania, P.W.; Buchmann, K. Positive correlation between Aeromonas salmonicida vaccine antigen concentration and protection in vaccinated rainbow trout Oncorhynchus mykiss evaluated by a tail fin infection model. J. Fish Dis. 2017, 40, 507–516. [Google Scholar] [CrossRef]
- Diamanka, A.; Loch, T.P.; Cipriano, R.C.; Winters, A.D.; Faisal, M. Infection of sea lamprey with an unusual strain of Aeromonas salmonicida. J. Wildl. Dis. 2014, 50, 159. [Google Scholar] [CrossRef]
- Brietzke, A.; Korytář, T.; Jaros, J.; Köllner, B.; Goldammer, T.; Seyfert, H.M.; Rebl, A. Aeromonas salmonicida infection only moderately regulates expression of factors contributing to Toll-Like receptor signaling but massively activates the cellular and humoral branches of innate immunity in rainbow trout (Oncorhynchus mykiss). J. Immunol. Res. 2015, 2015, 901015. [Google Scholar] [CrossRef]
- Alghabshi, A.; Austin, B.; Crumlish, M. Aeromonas salmonicida isolated from wild and farmed fish and invertebrates in Oman. Int. Aquat. Res. 2018, 10, 145. [Google Scholar] [CrossRef]
- Bhat, R.A.H.; Thakuria, D.; Dubey, M.K.; Tandel, R.S.; Sharma, P.; Khangembam, V.C.; Dash, P.; Tripathi, G.; Sarman, D. Lethal dose and histopathological alterations induced by Aeromonas salmonicida in experimentally challenged common carp, Cyprinus carpio. Microb. Pathog. 2021, 158, 105110. [Google Scholar] [CrossRef]
- Al-Mokaddem, A.K.; Abdel-moneam, D.A.; Ibrahim, R.A.; Saleh, M.; Shaalan, M. Molecular identification, histopathological analysis and immunohistochemical characterization of non-pigmented Aeromonas salmonicida subsp. salmonicida in Mugil carinatus (Valenciennes, 1836). Aquac. Rep. 2022, 24, 101103. [Google Scholar] [CrossRef]
- Dalsgaard, I.; Madsen, L. Bacterial pathogens in rainbow trout, Oncorhynchus mykiss (Walbaum), reared at Danish freshwater farms. J. Fish Dis. 2000, 23, 199–209. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish, 5th ed.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Vanden Bergh, P.; Frey, J. Aeromonas salmonicida subsp. salmonicida in the light of its type-three secretion system. Microb. Biotechnol. 2014, 7, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Hayatgheib, N.; Fournel, C.; Calvez, S.; Pouliquen, H.; Moreau, E. In vitro antimicrobial effect of various commercial essential oils and their chemical constituents on Aeromonas salmonicida subsp. salmonicida. J. Appl. Microbiol. 2020, 129, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Tripathi, P.H.; Pandey, A.; Ciji, A. β-glucan modulates non-specific immune gene expression, thermal tolerance and elicits disease resistance in endangered Tor putitora fry challenged with Aeromonas salmonicida. Fish Shellfish Immunol. 2021, 119, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, K.A.; Wang, T.; Russell, K.S.; Tubbs, L.; Ben Arous, J.; Secombes, C.J. Montanide™ ISA 763A VG and ISA 761 VG induce different immune pathway responses in rainbow trout (Oncorhynchus mykiss) when used as adjuvant for an Aeromonas salmonicida bacterin. Fish Shellfish Immunol. 2021, 114, 171–183. [Google Scholar] [CrossRef]
- Vincent, A.T.; Paquet, V.E.; Bernatchez, A.; Tremblay, D.M.; Moineau, S.; Charette, S.J. Characterization and diversity of phages infecting Aeromonas salmonicida subsp. salmonicida. Sci. Rep. 2017, 7, 7054. [Google Scholar] [CrossRef]
- Lim, J.; Hong, S. Characterization of Aeromonas salmonicida and A. sobria isolated from cultured salmonid fish in Korea and development of a vaccine against furunculosis. J. Fish Dis. 2020, 43, 609–620. [Google Scholar] [CrossRef]
- Gao, X.Y.; Liu, Y.; Miao, L.L.; Li, E.W.; Sun, G.X.; Liu, Y.; Liu, Z.P. Characterization and mechanism of anti-Aeromonas salmonicida activity of a marine probiotic strain, Bacillus velezensis V4. Appl. Microbiol. Biotechnol. 2017, 101, 3759–3768. [Google Scholar] [CrossRef]
- Cornet, V.; Khuyen, T.D.; Mandiki, S.N.M.; Betoulle, S.; Bossier, P.; Reyes-López, F.E.; Tort, L.; Kestemont, P. GAS1: A new β-Glucan immunostimulant candidate to increase rainbow trout (Oncorhynchus mykiss) resistance to bacterial infections with Aeromonas salmonicida achromogenes. Front. Immunol. 2021, 12, 693613. [Google Scholar] [CrossRef]
- Yonar, M.E.; Mişe Yonar, S.; İspir, Ü.; Ural, M.Ş. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish Immunol. 2019, 89, 83–90. [Google Scholar] [CrossRef]
- Schultz, A.G.; Ong, K.J.; MacCormack, T.; Ma, G.; Veinot, J.G.; Goss, G.G. Silver nanoparticles inhibit sodium uptake in juvenile rainbow trout (Oncorhynchus mykiss). Environ. Sci. Technol. 2012, 46, 10295–10301. [Google Scholar] [CrossRef] [PubMed]
- Delavari, N.M.; Gharaei, A.; Mirdar, H.J.; Davari, A.; Rastiannasab, A. Modulatory effect of dietary copper nanoparticles and vitamin C supplementations on growth performance, hematological and immune parameters, oxidative status, histology, and disease resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2022, 48, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Lemus, F.; Moraga, R.; Smith, C.T.; Aguayo, P.; Sánchez-Alonzo, K.; García-Cancino, A.; Valenzuela, A.; Campos, V.L. Selenium Nanoparticle-Enriched and Potential Probiotic, Lactiplantibacillus plantarum S14 Strain, a Diet Supplement Beneficial for Rainbow Trout. Biology 2022, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Kulikouskaya, V.; Nikalaichuk, V.; Hileuskaya, K.; Ladutska, A.; Grigoryan, K.; Kozerozhets, I.; Hovsepyan, V.; Sargsyan, M.; Sidarenka, A. Alginate coated biogenic silver nanoparticles for the treatment of Pseudomonas infections in rainbow trout. Int. J. Biol. Macromol. 2023, 251, 126302. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Z.; Quan, J.; Li, L.; Zhao, G.; Lu, J. Protective effects of different concentrations of selenium nanoparticles on rainbow trout (Oncorhynchus mykiss) primary hepatocytes under heat stress. Ecotoxicol. Environ. Saf. 2022, 230, 113121. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar]
- Suchomel, P.; Kvitek, L.; Panacek, A.; Prucek, R.; Hrbac, J.; Vecerova, R.; Zboril, R. Comparative study of antimicrobial activity of AgBr and Ag nanoparticles (NPs). PLoS ONE 2015, 10, e0119202. [Google Scholar] [CrossRef]
- Sarmast, M.K.; Salehi, H. Silver nanoparticles: An influential element in plant nanobiotechnology. Mol. Biotechnol. 2016, 58, 441–449. [Google Scholar] [CrossRef]
- Clark, N.J.; Shaw, B.J.; Handy, R.D. Low hazard of silver nanoparticles and silver nitrate to the haematopoietic system of rainbow trout. Ecotoxicol. Environ. Saf. 2018, 152, 121–131. [Google Scholar] [CrossRef]
- Saadh, M.J. Silver nanoparticles inhibit goatpox virus replication. Arch. Virol. 2023, 168, 32. [Google Scholar] [CrossRef]
- Kowalska-Góralska, M.; Senze, M.; Łuczyńska, J.; Czyż, K. Effects of the ionic and nanoparticle forms of Cu and Ag on these metals’ bioaccumulation in the eggs and fry of rainbow trout (Oncorhynchus mykiss W.). Int. J. Environ. Res. Public Health 2020, 17, 6392. [Google Scholar] [CrossRef] [PubMed]
- Vaseeharan, B.; Ramasamy, P.; Chen, J.C. Antibacterial activity of silver nanoparticles (AgNps) synthesized by tea leaf extracts against pathogenic Vibrio harveyi and its protective efficacy on juvenile Feneropenaeus indicus. Lett. Appl. Microbiol. 2010, 50, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Chandran, N.; Ramesh, S.; Shanmugam, R. Synthesis of silver nanoparticles using Azadirachta indica and Syzygium aromaticum extract and its antibacterial action against Enterococcus faecalis: An in vitro study. Cureus 2024, 16, e65044. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, M.I.; El-Mahdy, M.M.; Theiner, S.; El-Matbouli, M.; Saleh, M. In vitro assessment of the antimicrobial activity of silver and zinc oxide nanoparticles against fish pathogens. Acta Vet. Scand. 2017, 59, 49. [Google Scholar] [CrossRef]
- Antony, J.J.; Nivedheetha, M.; Siva, D.; Pradeepha, G.; Kokilavani, P.; Kalaiselvi, S.; Sankarganesh, A.; Balasundaram, A.; Masilamani, V.; Achiraman, S. Antimicrobial activity of Leucas aspera engineered silver nanoparticles against Aeromonas hydrophila in infected Catla catla. Colloids Surf. B Biointerfaces 2013, 109, 20–24. [Google Scholar] [CrossRef]
- Nemati, T.; Johari, S.A.; Sarkheil, M. Will the antimicrobial properties of ZnONPs turn it into a more suitable option than AgNPs for water filtration? Comparative study in the removal of fish pathogen, Aeromonas hydrophila from the culture of juvenile common carp (Cyprinus carpio). Environ. Sci. Pollut. Res. Int. 2019, 26, 30907–30920. [Google Scholar] [CrossRef]
- Ramya, J.R.; Ali, S.; K, T.A.; Vijayalakshmi, R.; Gajendiran, J.; Gnanam, S.; Ramachandran, K. Antimicrobial efficiency against fish pathogens on the green synthesized silver nanoparticles. Microb. Pathog. 2024, 193, 106725. [Google Scholar] [CrossRef]
- Saad, M.F.; Elsayed, M.M.; Khder, M.; Abdelaziz, A.S.; El-Demerdash, A.S. Biocontrol of multidrug resistant pathogens isolated from fish farms using silver nanoparticles combined with hydrogen peroxide insight to its modulatory effect. Sci. Rep. 2024, 14, 7971. [Google Scholar] [CrossRef]
- Farkas, J.; Christian, P.; Gallego-Urrea, J.A.; Roos, N.; Hassellöv, M.; Tollefsen, K.E.; Thomas, K.V. Uptake and effects of manufactured silver nanoparticles in rainbow trout (Oncorhynchus mykiss) gill cells. Aquat. Toxicol. 2011, 101, 117–125. [Google Scholar] [CrossRef]
- Massarsky, A.; Labarre, J.; Trudeau, V.L.; Moon, T.W. Silver nanoparticles stimulate glycogenolysis in rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 2014, 147, 68–75. [Google Scholar] [CrossRef]
- Ali, I.; Khan, S.; Shah, K.; Haroon; Kalimullah; Bian, L. Microscopic analysis of plant-mediated silver nanoparticle toxicity in rainbow trout fish (Oncorhynchus mykiss). Microsc. Res. Tech. 2021, 84, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, M.M.; Devassy, R.P.; El-Hefnawy, M.E.; Al-Goul, S.T.; Orif, M.I.; El-Newehy, M.H. Facile synthesis, characterization, and antimicrobial assessment of a silver/montmorillonite nanocomposite as an effective antiseptic against foodborne pathogens for promising food protection. Molecules 2023, 28, 3699. [Google Scholar] [CrossRef] [PubMed]
- Thepbandit, W.; Papathoti, N.K.; Hoang, N.H.; Siriwong, S.; Sangpueak, R.; Saengchan, C.; Laemchiab, K.; Kiddeejing, D.; Tonpho, K.; Buensanteai, K. Bio-synthesis and characterization of silver nanoparticles from Trichoderma species against cassava root rot disease. Sci. Rep. 2024, 14, 12535. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, M.; El-Mahdy, M.; Theiner, S.; Dinhopl, N.; El-Matbouli, M.; Saleh, M. Silver nanoparticles: Their role as antibacterial agent against Aeromonas salmonicida subsp. salmonicida in rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci. 2018, 119, 196–204. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abdel-Aziz, M.S. Preparation of polystyrene nanocomposites based on silver nanoparticles using marine bacterium for packaging. Polym.-Plast. Technol. Eng. 2013, 52, 607–613. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; He, W.; Li, D.; Meng, Y.; Feng, Q. Silver nanoparticles: In vivo toxicity in zebrafish embryos and a comparison to silver nitrate. J. Nanopart Res. 2016, 18, 222. [Google Scholar] [CrossRef]
- Liaqat, F.; Hanif, U.; Bahadur, S.; Faheem, M.; Rasool, S.; Gulzar, S.; Zaman, W.; Urooj, Z.; Shaheen, S.; Munir, M. Comparative evaluation of the toxicological effect of silver salt (AgNO3) and silver nanoparticles on Cyprinus carpio synthesized by chemicals and marine algae using scanning electron microscopy. Microsc. Res. Tech. 2021, 84, 1531–1541. [Google Scholar] [CrossRef]
- Bird, S.; Tafalla, C. Teleost Chemokines and Their Receptors. Biology 2015, 4, 756–784. [Google Scholar] [CrossRef]
- Syahputra, K.; Kania, P.W.; Al-Jubury, A.; Marnis, H.; Setyawan, A.C.; Buchmann, K. Differential immune gene response in gills, skin, and spleen of rainbow trout Oncorhynchus mykiss infected by Ichthyophthirius multifiliis. PLoS ONE 2019, 14, e0218630. [Google Scholar] [CrossRef] [PubMed]
- You, S.L.; Jiang, X.X.; Zhang, G.R.; Ji, W.; Ma, X.F.; Zhou, X.; Wei, K.J. Molecular characterization of nine TRAF genes in Yellow catfish (Pelteobagrus fulvidraco) and their expression profiling in response to Edwardsiella ictaluri infection. Int. J. Mol. Sci. 2023, 24, 8363. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, Y.; Chen, Z.; Zou, P.; Zhu, Y.; Zhang, J.; Zhang, Z.; Wang, Y. Members of the TRAF gene family in Octopus sinensis and their response to PGN, poly I:C, and Vibrio parahaemolyticus. Fish Shellfish Immunol. 2024, 154, 109905. [Google Scholar] [CrossRef] [PubMed]
- Skadberg, E.S.S. Inflammatory Effects in Zebrafish (Danio rerio) Exposed to Gold Nanoparticles. Master’s Thesis, University of Oslo, Oslo, Norway, 2015. [Google Scholar]
- Opal, S.M.; Wherry, J.C.; Grint, P. Interleukin-10: Potential benefits and possible risks in clinical infectious diseases. Clin. Infect. Dis. 1998, 271, 497–507. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Wang, T.; Holland, J.W.; Bols, N.; Secombes, C.J. Cloning and expression of the first nonmammalian interleukin-11 gene in rainbow trout Oncorhynchus mykiss. FEBS J. 2005, 272, 1136–1147. [Google Scholar] [CrossRef]





| Gene Names | Genebank. No | Sequences (5′-3’) |
|---|---|---|
| EF1α-F | AF498320 | TCCTCTTGGTCGTTTCGCTG |
| EF1α-R | ACCCGAGGGACATCCTGTG | |
| CK10-F | CA361535 | ATTGCCAAGATCCTCTTCTGTGTTC |
| CK10-R | CCTGAGGCTGGTAACCTATGACAAC | |
| TNF-α-F | AJ277604 | AGCATGGAAGACCGTCAACGAT |
| TNF-α-R | ACCCTCTAAATGGATGGCTGCTT | |
| TRAF1-F | XM021605353 | CCAGGTATGGATTCAAGGTGTG |
| TRAF1-R | TTTGGAAGGATGAGGAGGTTAGAT | |
| IL-10-F | AB118099 | CGACTTTAAATCTCCCATCGAC |
| IL-10-R | GCATTGGACGATCTCTTTCTTC | |
| IL-1β-F | AJ223954 | GGAGAGGTTAAAGGGTGGCGA |
| IL-1β-R | TGCCGACTCCAACTCCAACA | |
| IL-11-F | AJ535687 | CCCTGCCACTAATGAACAACA |
| IL-11-R | GCAGGGTGGTGGTGTTTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zheng, C.; Wang, Y.; Dang, Y.; Li, R.; Tao, Y.; Yang, Y.; Sun, X.; Song, Z.; Sun, P.; et al. Study on the Role and Pathological and Immune Responses of Silver Nanoparticles Against Two Aeromonas salmonicida subsp. salmonicida Strains at Different Virulence Levels in Rainbow Trout (Oncorhynchus mykiss). Fishes 2025, 10, 29. https://doi.org/10.3390/fishes10010029
Guo Y, Zheng C, Wang Y, Dang Y, Li R, Tao Y, Yang Y, Sun X, Song Z, Sun P, et al. Study on the Role and Pathological and Immune Responses of Silver Nanoparticles Against Two Aeromonas salmonicida subsp. salmonicida Strains at Different Virulence Levels in Rainbow Trout (Oncorhynchus mykiss). Fishes. 2025; 10(1):29. https://doi.org/10.3390/fishes10010029
Chicago/Turabian StyleGuo, Yunqiang, Chaoli Zheng, Yingfei Wang, Yongji Dang, Ruiyuan Li, Ye Tao, Yucheng Yang, Xiaofeng Sun, Zekun Song, Pengcheng Sun, and et al. 2025. "Study on the Role and Pathological and Immune Responses of Silver Nanoparticles Against Two Aeromonas salmonicida subsp. salmonicida Strains at Different Virulence Levels in Rainbow Trout (Oncorhynchus mykiss)" Fishes 10, no. 1: 29. https://doi.org/10.3390/fishes10010029
APA StyleGuo, Y., Zheng, C., Wang, Y., Dang, Y., Li, R., Tao, Y., Yang, Y., Sun, X., Song, Z., Sun, P., Zhang, Q., Qian, D., Ren, W., Cao, X., Wang, B., Xu, M., Jiang, B., Li, Y., Sun, Q., ... Sun, Y. (2025). Study on the Role and Pathological and Immune Responses of Silver Nanoparticles Against Two Aeromonas salmonicida subsp. salmonicida Strains at Different Virulence Levels in Rainbow Trout (Oncorhynchus mykiss). Fishes, 10(1), 29. https://doi.org/10.3390/fishes10010029







