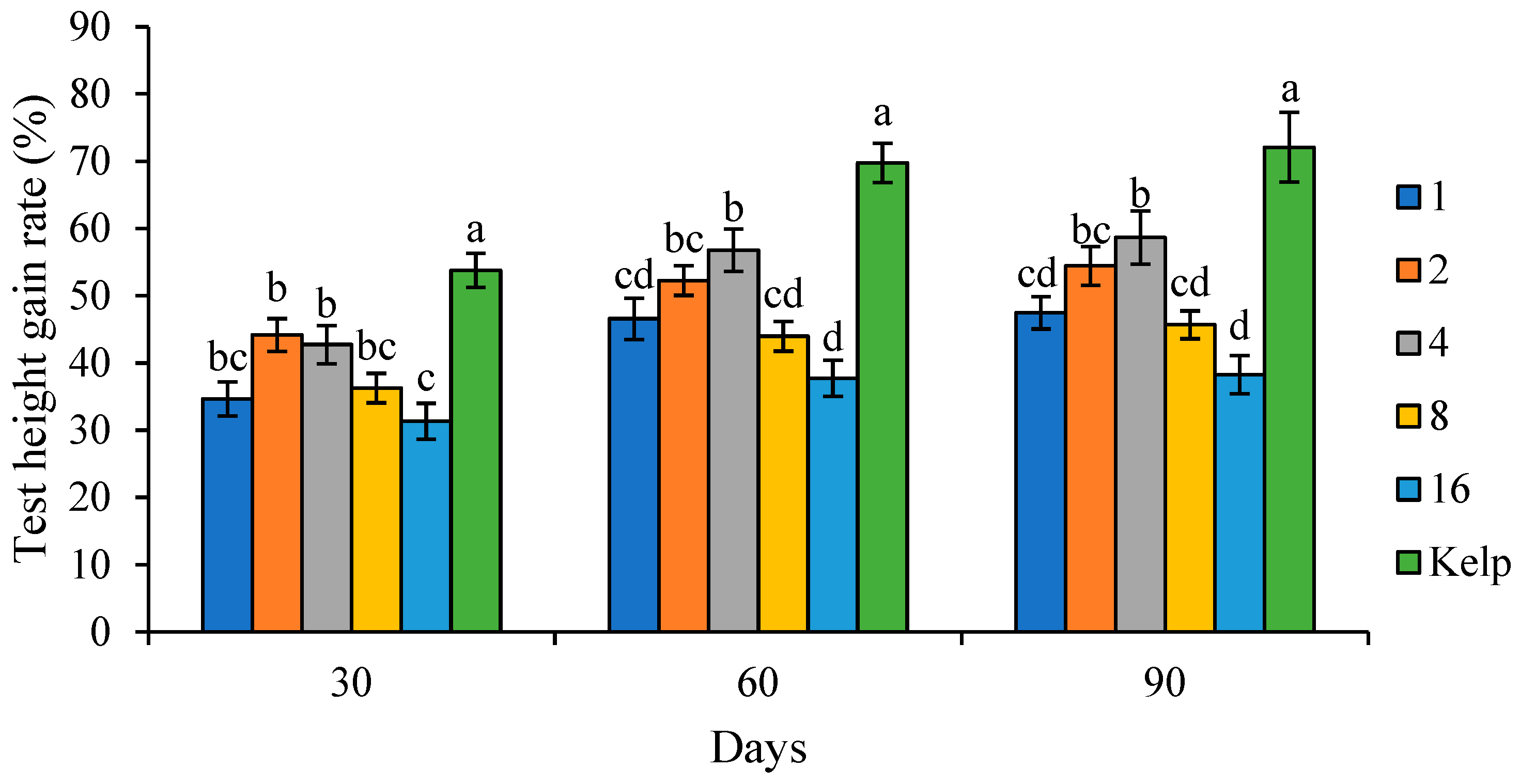1. Introduction
The sea urchin (
Strongylocentrotus intermedius), as a member of the family
Strongylocentridae, is naturally distributed in northern Japan and Russia [
1,
2,
3,
4,
5,
6]. Sea urchin gonads are commonly accepted for their delicious taste and rich long-chain polyunsaturated fatty acids, which can prevent most cardiovascular, neurodegenerative, and proinflammatory disorders [
7,
8,
9,
10,
11,
12,
13]. The worldwide overfishing of sea urchins, coupled with habitat destruction and global climate change, has led to the severe decline of wild resources [
1,
2,
8,
14,
15,
16]. During recent decades, aquaculture has proven to be effective in alleviating the fishing pressure on their wild populations. Different from most fish and crustaceans,
S. intermedius need three years or even more time to become a market-sized aquatic product. From this background, producing large-sized
S. intermedius juveniles (test diameter ≥ 20 mm) is extremely important for shortening the period and reducing the risk of farming of this species [
2]. The shell of sea urchins includes test (the main part of the shell) and spines [
17]. Rapid and coordinated growth of the test height and test diameter is important for producing high-quality juveniles. However, imbalanced growth between the test diameter and height of juvenile
S. intermedius is usually observed when they are fed formulated feeds [
18]. Thus, it is imperative to elucidate the relationship between nutrients and test growth of
S. intermedius.
Proteins, as the most expensive energy substances, will generate nitrogen-containing waste, which has detrimental effects on the growth and health of aquatic animals. Carbohydrates and lipids possess the advantages of low costs and nitrogen-free excrement [
19,
20]. Carbohydrates are primarily utilized as the energy sources for the daily activities and gametogenesis of sea urchins, while lipids mainly provide essential fatty acids for the normal growth and development of sea urchins [
21,
22]. Sea urchins, as marine omnivorous invertebrates, can normally accept starch- and cellulose-rich foods such as maize, spinach, pumpkin, and kelp, and consume a certain amount of lipid-rich foods such as fish and shellfish [
23,
24]. To date, the requirements for carbohydrates and lipids have been reported in several sea urchin species. Juvenile sea urchin
Lytechinus variegatus (initial body weight, IBW, of approximately 3.95 g) fed diets containing 18% carbohydrates showed a significantly higher weight gain rate (WGR) than those fed diets containing 12% carbohydrates [
25]. Juvenile
L. variegatus (IBW of approximately 5.60 g) fed diets with 19% carbohydrates had higher WGR and gonadosomatic index (GSI) values than those fed diets with 26% and 38% carbohydrates [
22]. As for lipid requirements, it was found that formulated feeds with above 9% lipids negatively influenced the WGR of juvenile
L. variegatus (IBW of approximately 0.09 g) [
26]. Diets with 5–6% lipids are optimal for achieving the highest WGR of juvenile
L. variegatus (IBW of approximately 0.07–0.10 g) [
27]. Formulated feeds with 6% lipids resulted in the highest GSI of adult
Paracentrotus lividus (IBW of approximately 35 g) [
21].
It is understood that an inseparable bond exists between lipids and carbohydrates, and an imbalance can adversely impact the feed conversion and somatic growth of aquatic animals [
19,
28,
29,
30]. Since lipids and carbohydrates are crucial for bone growth and development, it is essential to ascertain a suitable carbohydrate-to-lipid ratio (C/L) to achieve fast and coordinated test growth of sea urchins. A previous study found that juvenile
L. variegatus fed dry feeds with C/L2 exhibited markedly lower final test diameters than that of individuals fed dry feeds with C/L2.56–6.88 [
26]. It was also shown that Chinese longsnout catfish (
Leiocassis longirostris) fed diets with C/L1.98 can maintain healthy skeleton growth, but those fed diets with C/L5.07 displayed skeletal malformations [
31]. A previous study suggested that abalone
Haliotis discus hannai fed formulated feeds with C/L10.06 showed significantly greater daily increments in shell length than those with C/L12.94 and C/L4.78 [
32]. Furthermore, extremely high C/L could result in an insufficient intake of docohexaenic acid and α-tocopherol, which increases the occurrence possibility of axial and cranial skeletal deformities of fish by delaying the process of early biomineralization [
33].
Thus, this study was performed to investigate the effects of different C/L on feed intake, somatic growth, and test characteristics of S. intermedius juveniles. It was expected to precisely quantify the optimal C/L for achieving the fastest and most coordinated test growth of juvenile S. intermedius. These findings can provide a reference for formulating feeds suitable for the effective and efficient production of juvenile S. intermedius with healthy body shapes, reflected by coordinated test height/test diameter, especially under intensive farming patterns.
2. Materials and Methods
2.1. Ethics Statement
The Institution Ethics Committee deemed it unnecessary to grant authorization for this research on S. intermedius because it is classified as an invertebrate. Nevertheless, all necessary precautions were taken to minimize potential harm to the animals and acknowledge their essential contribution to this research.
2.2. Experimental Diets and Feeding Experiment
Fresh
Saccharina japonica (control diet) (crude protein 8.16%, crude lipid 0.4%) and five formulated feeds were prepared. The kelp was collected from March to June, and the age was 4–7 months. As described in
Table 1, the five formulated feeds contained different C/L values of 1.04, 2.00, 4.01, 8.04, and 16.10. All solid ingredients were pulverized and sifted through a 200 μm sieve. Next, the powders in each formulated feed were blended evenly. Subsequently, oil was added and blended with the mixture above. After 35% water was added, the feedstuffs were extruded with a machine (DES-TS1280, Dingrun, Jinan, China) to make pellets. Finally, the feeds were dried (50 °C), cooled, sealed, and stored in a freezer (−20 °C).
S. intermedius juveniles were obtained from a local breeding farm. Following a 9-day domestication period, 270 healthy S. intermedius juveniles of similar size (initial weight: 1.19 ± 0.01 g, initial test height: 6.65 ± 0.03 mm, initial test diameter: 13.96 ± 0.05 mm) were selected and randomly allocated to 18 floating cages (height: 38 cm, diameter: 20 cm). All the cages were placed in a tank (500 L), and each cage held 15 S. intermedius juveniles. Petri dishes were used to prevent food from falling out of the cages. Each diet was randomly dispensed into three cages of juvenile S. intermedius.
S. intermedius juveniles were hand-fed until they showed obvious satiation. The feeding frequency was twice (10:00 and 19:30) daily. Every two days, 60% of the volume of seawater in the tank was changed. During the 90 d feeding experiment, the water temperature was 15.7 ± 4.1 °C, the pH was 8.1 ± 0.1, the salinity was 31 ± 0.8‰, and the DO concentration was maintained above 7.8 mg L−1 using an aerator.
2.3. Sampling
After each feeding, the residual feeds were collected, dried, and weighed to calculate the feed intake (FI) and feed conversion ratio (FCR). Then, the digestive tracts and gonads of nine sea urchins in each cage were individually dissected and weighed to calculate the digestive tract index (DTI) and GSI, respectively. The average values of the DTI and GSI of sea urchins from each cage were calculated for subsequent statistical analysis. After that, an appropriate amount of the gonads was put into Bonn’s reagent for 24 h before they were used for subsequent histological analysis.
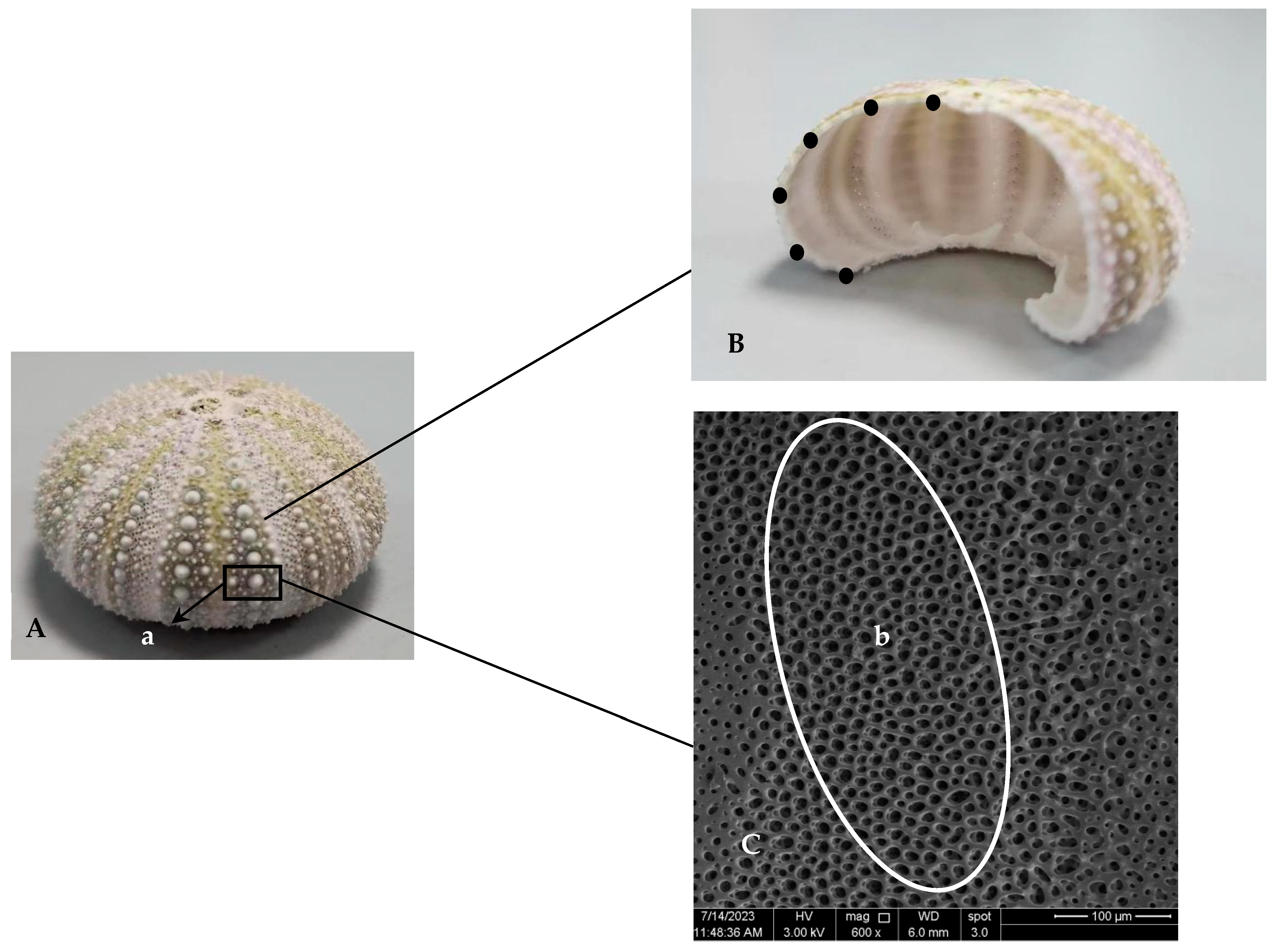
The shells and lanterns of nine S. intermedius juveniles in each cage were individually weighed and recorded to calculate shell index (SI) and lantern index (LI), respectively. After that, the average values of SI and LI of sea urchins from each cage were calculated for subsequent statistical analysis. Three out of nine sea urchin shells were randomly selected and submersed in 1% NaClO solution for 12 h. Then, the spines on the primary tubercles were collected for the measurement of spine length, and the sea urchin shells were gently rubbed to make the other spines fall off. After that, the tests were cleaned and resubjected to 1% NaClO solution for 12 h. The tests were flushed with distilled water after they were removed from the NaClO solution. Next, the tests were dried in an oven (70 °C). An interambulacral plate was used to measure the test thickness. The equatorial part of the interambulacral plate was taken for scanning electron microscopy observation. Similarly, three of the remaining six sea urchin shells were randomly selected and processed into tests, which were subsequently dried, ground to a fine powder, and then stored in sealed bags for subsequent assays of calcium and magnesium contents.
2.4. Gonad Histological Analysis
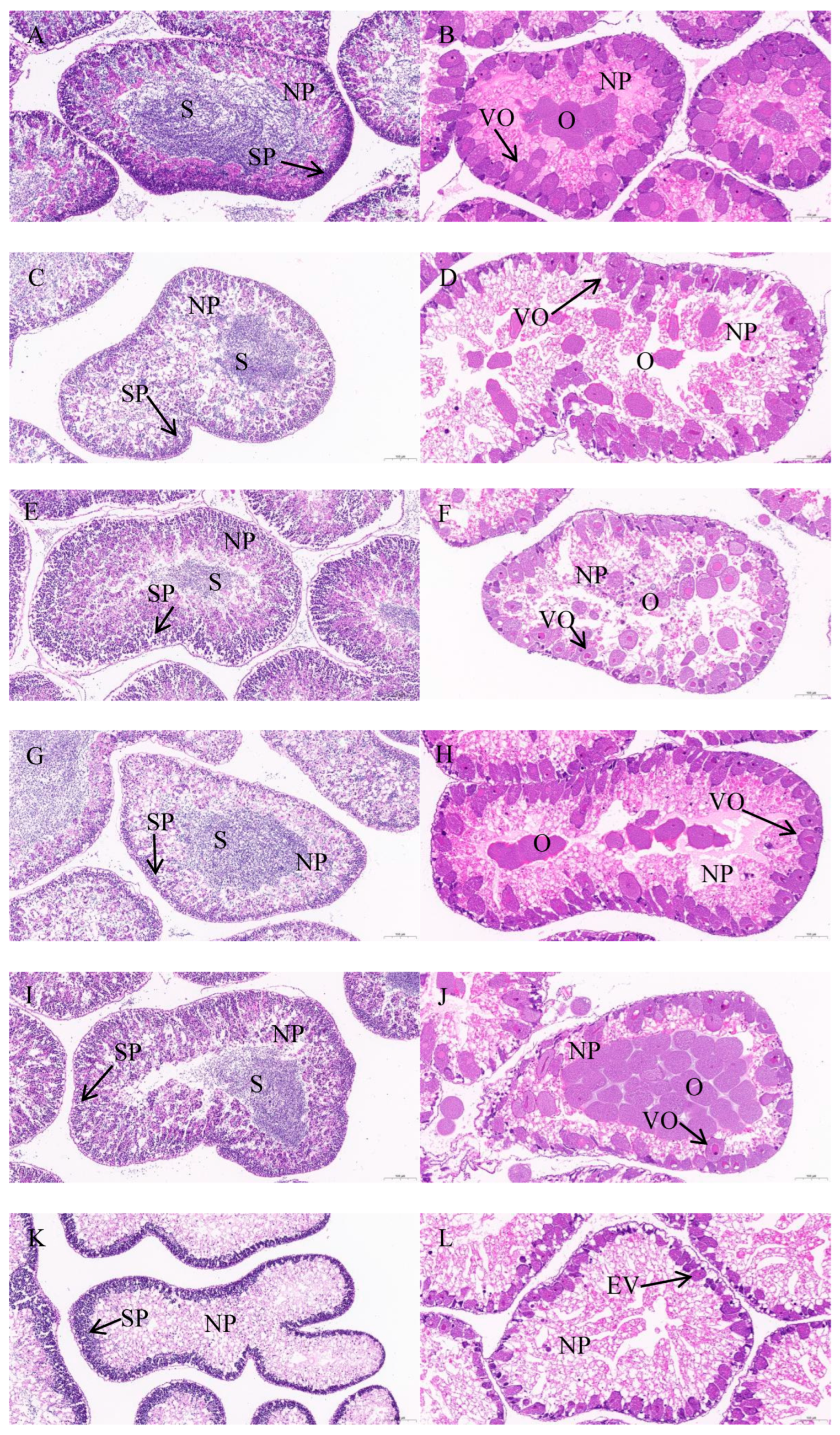
Since Di et al. [
1] described the detailed procedures, we just briefly outline the operation steps. After the gonads of
S. intermedius were fixed, dehydrated, and embedded in paraffin, 4 μm slices were taken with a microtome (RM2016, Leica, Nussloch, Germany). Subsequently, the dewaxed sections were stained using a dye (hematoxylin and eosin). Finally, the microstructure of the gonads was observed under a microscope. The maturity stages (stages I–VI) of gonads were identified according to Zhang et al. [
13]. Stage I is the recovery stage characterized by primary gametes and nutritive phagocytes; stage II is the growing stage characterized by large quantities of primary gametes and nutritive phagocytes; stage III is the premature stage characterized by a reduced number of nutritive phagocytes and increased gametes at all developmental stages; stage IV is the mature stage characterized by few nutritive phagocytes and clusters of mature gametes; stage V is the spawning stage characterized by depletion of nutritive phagocytes and loosely packed gametes; and stage VI is the spent stage characterized by no gametes.
2.5. Measurement of Spine Length and Test Thickness
The six longest spines of each sea urchin were selected, measured with a digital calliper (MarCal 16 EWR, Marh GmbH, Esslingen, Germany), and averaged to record the spine length.
Six points were taken to divide the interambulacral plate into five equally spaced segments from the mouth end to the back end. The thickness of each sea urchin was measured at six points with a digital caliper to calculate the average thickness (
Figure 1).
2.6. Analysis of the Test Microstructure
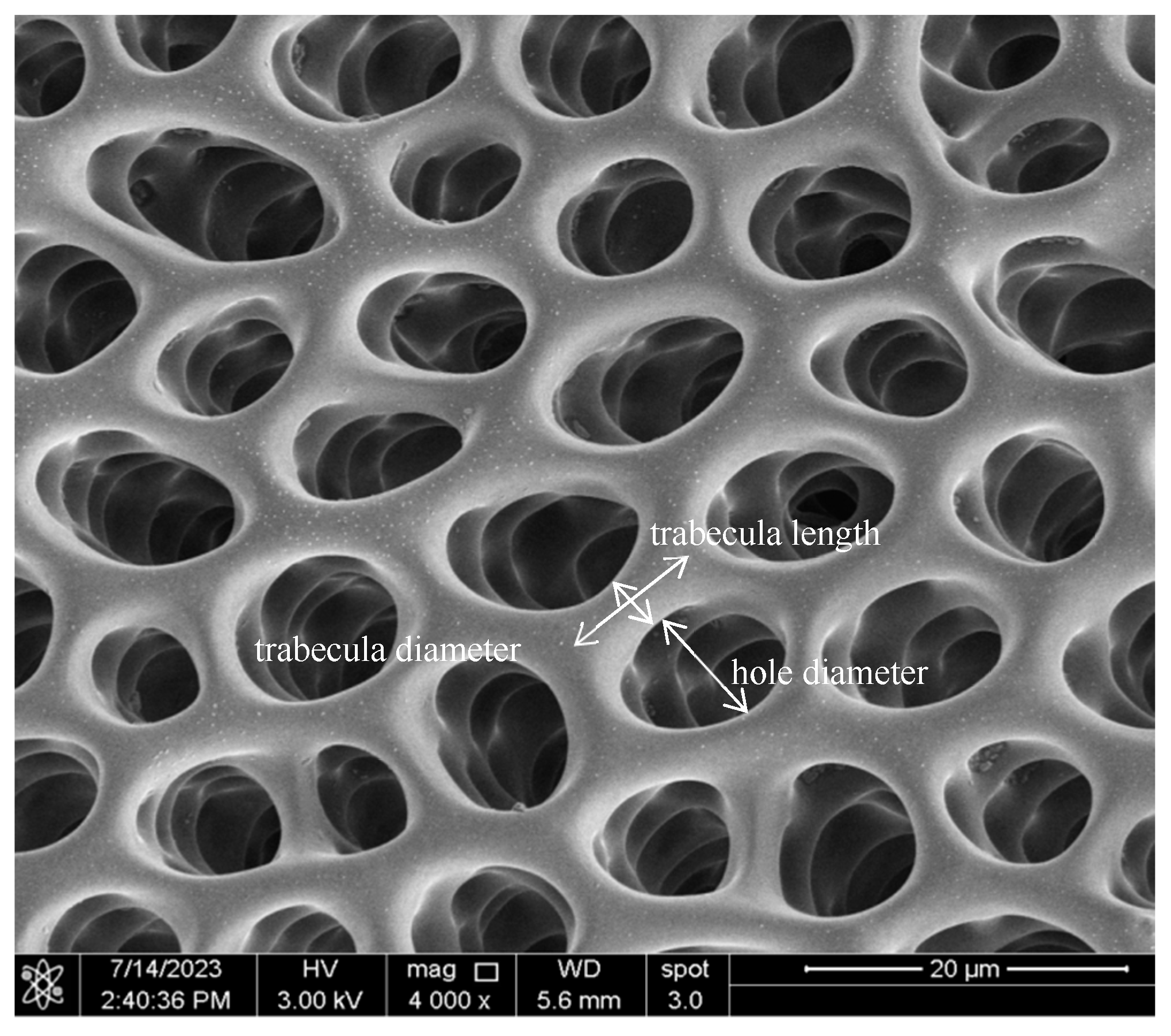
The microstructure of the sea urchins was observed using a field emission scanning electron microscope (Nova Nano SEM 650, FEI, Hillsboro, OR, USA). Due to the poor conductivity of the sample, an ion-sputtering instrument (ETD-800, Vision Precision Instruments, Beijing, China) was used to coat the sample with platinum, and then the micromorphology of the outer and inner surfaces of the sample was observed (
Figure 1). After microstructure images were captured, twenty holes were randomly chosen for the measurement of hole diameter and trabecula length and diameter (
Figure 2), which were measured using an image analysis software (‘Fiji is Just ImageJ’ software, ver. 1.53t,
http://fiji.sc/Fiji, accessed on 1 July 2024).
2.7. Analysis of Test Calcium and Magnesium Concentrations
Single-element standard solutions of calcium and magnesium (1000 µg mL−1) were quantitatively diluted with 5% nitric acid and 2% nitric acid to prepare a series of standard solutions with concentrations of 1, 5, 10, 50, 100, and 200 µg L−1. The series of standard solutions were tested by inductively coupled plasma-optical emission spectrometry (ICP-OES) (Optima 8000, Perkin Elmer, Waltham, MA, USA) to construct standard curves.
Subsequently, the weighed samples were put into a digestion tank with 5 mL of 5% nitric acid. Then, the digestion tank was put into a microwave digestion apparatus (TOPEX, Yiyao, Shanghai, China). After that, the samples were assayed by ICP-OES following the instructions. Finally, the calcium and magnesium contents of the samples were quantified based on the standard curves.
2.8. Calculations and Statistical Analysis
Survival rate (SR, %) = final sea urchin number/initial sea urchin number × 100.
FI (mg ind−1 day−1) = daily food consumption of sea urchins/final sea urchin number.
FCR = total food consumption/(final sea urchin wet weight − initial sea urchin wet weight).
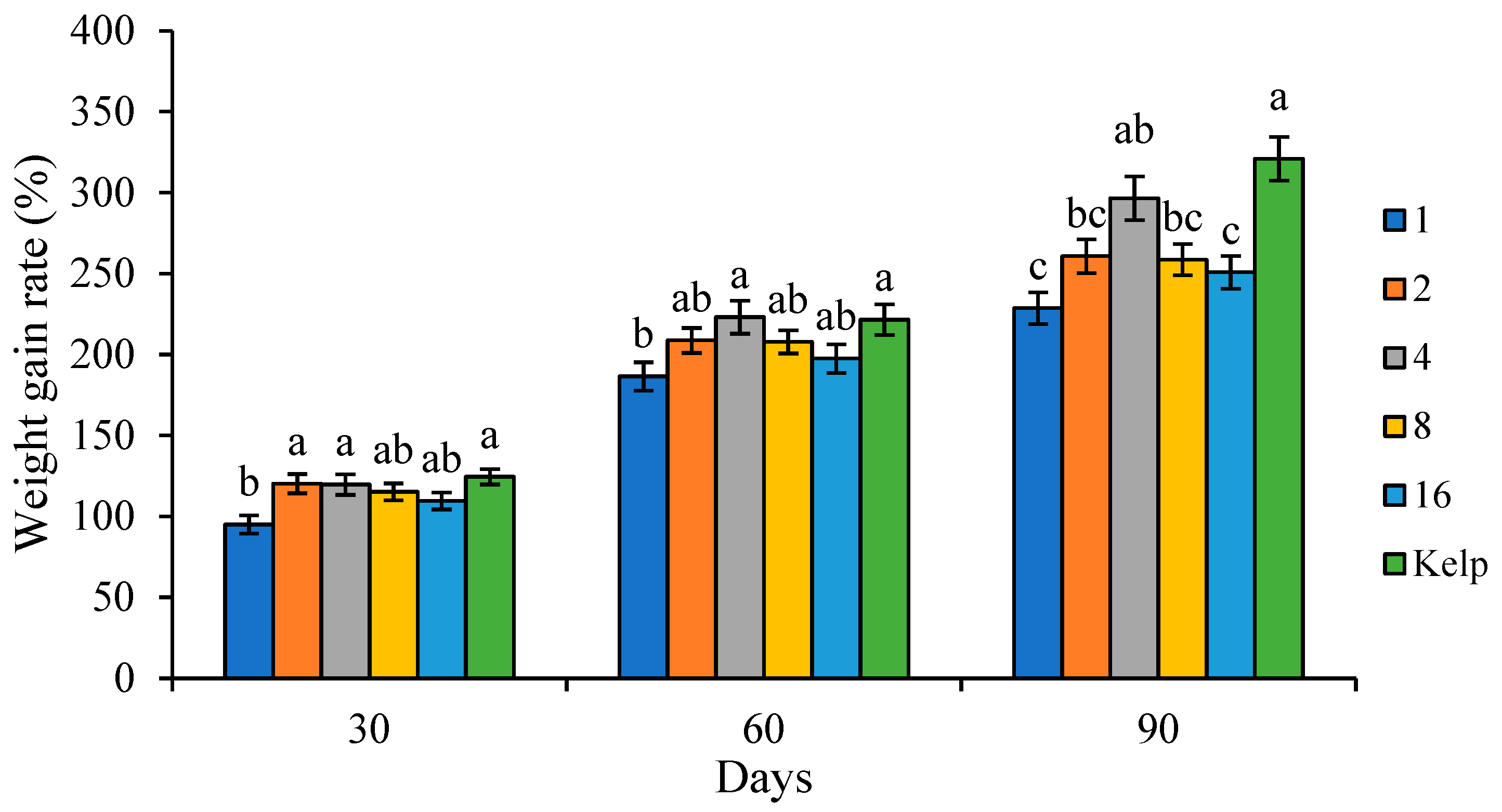
WGR (%) = (final sea urchin wet weight − initial sea urchin wet weight)/initial sea urchin wet weight × 100.
Test diameter gain rate (TDGR, %) = (final sea urchin test diameter − initial sea urchin test diameter)/initial sea urchin test diameter × 100.
Test height gain rate (THGR, %) = (final sea urchin test height − initial sea urchin test height)/initial sea urchin test height × 100.
GSI (%) = final gonad wet weight/final sea urchin wet weight × 100.
DTI (%) = final digestive tract wet weight/final sea urchin wet weight × 100.
SI (%) = final shell wet weight/final sea urchin wet weight × 100.
LI (%) = final lantern wet weight/final sea urchin wet weight × 100.
After testing for normality and heterogeneity, all data were analyzed using SPSS 22.0 software, and a one-way analysis of variance was used to analyze the significance (p < 0.05) of differences among dietary groups. When a significant difference was found, differences between dietary groups were compared using the Tukey method. The statistical results were denoted as means ± S.E.M.
4. Discussion
Protein is an essential macronutrient that supports growth, maintenance, and reproduction by supplying the necessary amino acids to sea urchins [
34,
35,
36]. However, proteins, as one of the most expensive nutrients in formulated feeds, should be mainly used for body growth instead of energy supply [
37]. Lipids and carbohydrates are generally utilized as inexpensive non-protein energy substances for the preparation of feeds [
19,
20]. Appropriate C/L values could improve feed utilization, while low or high dietary C/L may depress growth performance and feed utilization. The WGR is traditionally used as an indicator of sea urchin somatic growth in sea urchins and other aquatic animals. In this study, juvenile
S. intermedius fed C/L4 showed higher WGR than those fed low or high C/L groups. This optimal diet C/L (4) was slightly higher than that (approximately 3) reported for juvenile and adult
L. variegatus [
21,
36]. These inconsistent findings could be attributed to different sizes and growth stages of sea urchins. However, previous researchers also found that the optimal diet C/L value was approximately 4 for blunt snout bream (
Megalobrama amblycephala) and redclaw crayfish (
Cherax quadricarinatus) [
37,
38]. The optimal diet C/L was below 2 for yellowfin seabream (
Sparus latus), yellow croaker (
Larmichthys crocea), orange-spotted grouper (
Epinephelus coioides), and tilapia (
Oreochromis niloticus) [
39,
40,
41,
42]. However, the optimal diet C/L was above 4 for grass carp (
Ctenopharyngodon idella), turbot (
Scophthalmus maximus), and
H.
discus hannai [
32,
43,
44]. However, the differences in the optimal diet C/L could be attributed to differences in the diet habits of these aquatic animals. It is well known that carnivorous fish exhibit a superior capability to utilize lipids and have poorer tolerance to high-carbohydrate diets than herbivorous and omnivorous fish. As an omnivorous invertebrate species,
S. intermedius can readily accept starch and cellulose-rich foods such as kelp, pumpkin, and maize and need a certain amount of lipid-rich foods such as fish and shellfish [
23,
24].
In this study, the downregulated growth of juvenile
S. intermedius fed high C/L could be due to lipid deficiency. An insufficient supply of essential fatty acids leads to the slow growth of sea urchins [
45]. In low-C/L formulated feed groups, excessively high lipid content can result in low feed utilization efficiency, which restricts the intake of protein and other nutrients required for maximum growth [
46]. A high-lipid diet can lead to lipid deposition and lipid peroxidation, which can cause a slowdown in body growth [
47,
48]. Low dietary carbohydrate levels also appeared to be inadequate for the growth of
L. variegatus in the low-C/L formulated feed groups [
21,
36]. The energy provided by low carbohydrates in the formulated feed is insufficient to sustain the normal growth of sea urchins, and some proteins and lipids will be consumed for energy supply instead of tissue growth. This can not only lead to the waste of proteins and lipids but can also induce the metabolic burden of proteins and lipids, which is not conducive to the somatic growth of sea urchins. Previous studies considered that the energy supplied by dietary carbohydrates is prone to use for test growth rather than for gonad growth in
Psammechinus miliaris [
49,
50]. However, it should be noted that the mass of gonads was not excluded when calculating the WGR of juvenile sea urchins in previous studies. Thus, the optimal C/L requirement was usually underestimated by using WGR to assess the somatic growth of sea urchin juveniles.
Sea urchin gonads are both the nutrient storage organ and the sex organ responsible for gametogenesis [
51]. In general, sea urchins allocate fewer nutrients and less energy to somatic growth when they enter the gametogenesis stage [
52]. In the current research,
S. intermedius juveniles fed diets with C/L4 had the highest GSI. Similarly, a previous study found that juvenile
L. variegatus fed diets with C/L3 had higher gonad weight than those with C/L6 [
22]. Chinese sturgeon (
Acipenser sinensis) fed high lipid levels were observed to develop their ovaries from stage II to stage III or IV in low-C/L formulated feed groups [
38,
53]. In high-C/L formulated feed groups, the higher proportion of diet carbohydrates can arouse the sexual maturation of rainbow trout (
Oncorhynchus mykiss) [
54]. In this research, the gonads of juvenile
S. intermedius fed kelp remained at stage II, but some of the gonads of juvenile
S. intermedius fed formulated feeds with different C/L ratios entered stage III. There were fewer ova and spermatozoa in moderate C/L (2 and 4) groups than in low or high C/L (1, 8, and 16) groups. Under the circumstances of sexual maturation, more energy will be allocated to gonad development and gametes production than somatic growth. Thus, moderate C/L (2 and 4) can, to some extent, hinder early sexual maturation, reduce the substances and energy used for gametes production, and better promote the somatic growth of sea urchin juveniles.
Coordinated and rapid growth is important for producing high-quality sea urchin seeds. The test diameter was commonly used to assess the somatic growth of sea urchins [
24,
55,
56], but the height of sea urchin test was generally neglected. Lately, the uncoordinated test growth in height and diameter of juvenile
S. intermedius has drawn increased attention. Juvenile
L. variegatus fed dry feeds with 33% proteins exhibited slightly lower TH/TD than those of individuals fed 9% and 15% proteins [
57]. In this experiment, the TH/TD and test microstructure of
S. intermedius juveniles fed natural food kelp were regarded as the standard and normal values. Generally, the TH/TD tended to decrease as the C/L increased. This indicated that juvenile
S. intermedius fed diets with extremely high C/L showed signs of skeletal malformations. This was supported by the findings of Tan et al. [
31], which showed that skeletal malformations of
L. longirostris occurred in the groups with C/L5.07 rather than C/L1.98 [
31]. This could be due to the insufficient intake of essential fatty acids and lipid-soluble vitamins such as α-tocopherol, which have been found to be critical for biomineralization and thus decrease the occurrence of axial and cranial skeletal deformities [
33]. Most sea urchin species have natural cryptic behavior, and kelp can provide shelter for sea urchins. However, based on our observations,
S. intermedius juveniles fed dry feeds tend to be more sensitive and often seek refuge under Petri dishes. This behavior may inhibit the growth in test height of juveniles. Therefore, special culture systems with more shelters should be designed for the coordinated growth of sea urchin juveniles fed dry feeds. In this study, the juvenile
S. intermedius fed kelp showed higher WGR and test growth rates than those fed formulated feeds. Sea urchins showed a stronger preference for natural foods like kelp and achieved better growth performances. Therefore, it is crucial to improve the palatability and attraction of formulated feeds in the future.
In this experiment, juvenile
S. intermedius fed formulated feeds had shorter spines and thicker tests than those fed kelp. Among the formulated feed groups, juvenile
S. intermedius fed diets with high C/L (8 and 16) showed slightly thicker test than those with C/L1. This could be due to the different shell biomineralization of juvenile
S. intermedius. A previous study showed that juvenile
L. variegatus fed a high-protein diet had a thicker test than that fed a low-protein diet [
57]. In this experiment, more carbohydrates in the high-C/L formulated feeds were used for energy support of juvenile sea urchins, and proteins were saved for thickening the tests. The growth of sea urchin test is characterized by the resorption on the inner surface, the expansion of existing inorganic networks, and the formation of new networks [
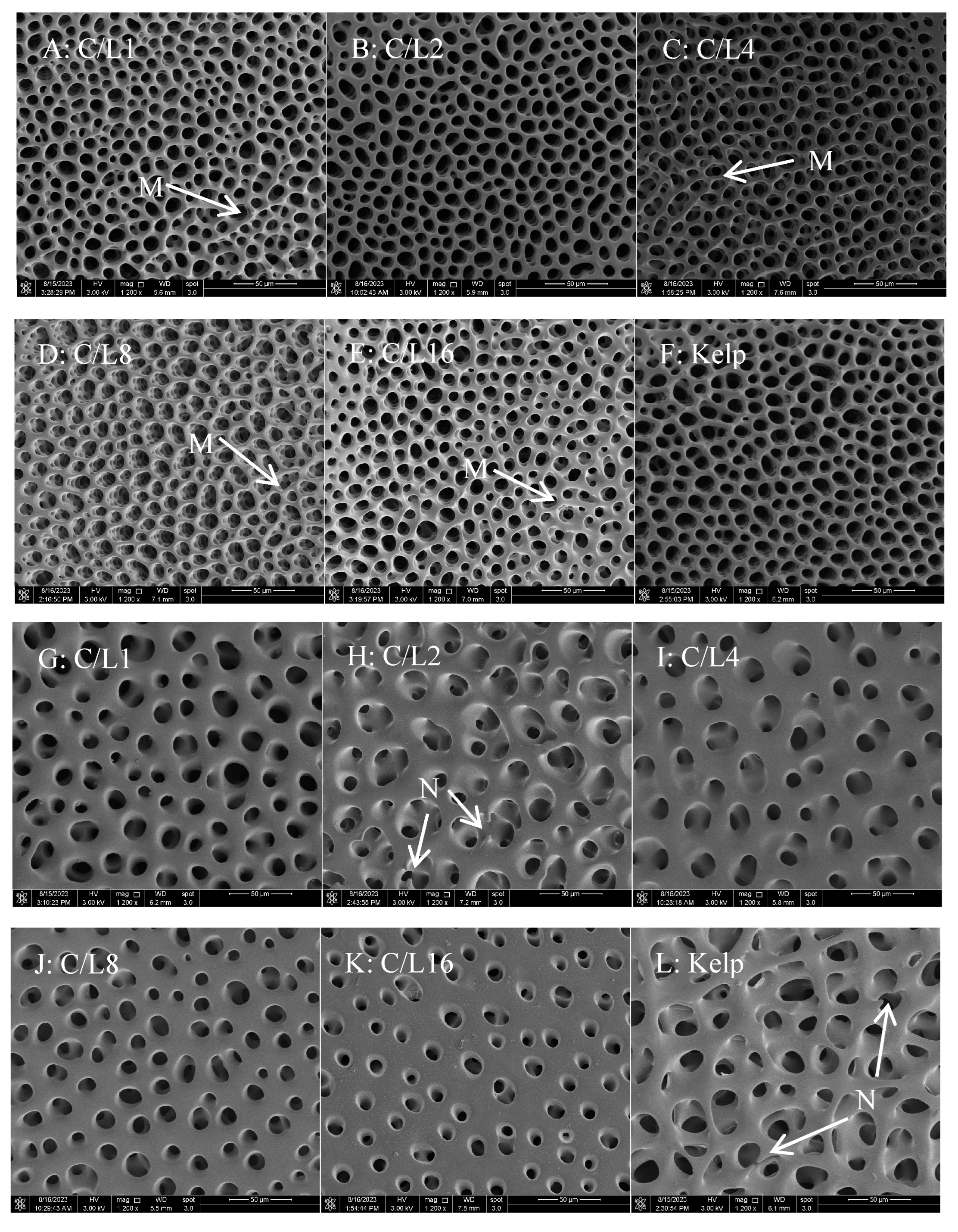
58]. Elements from test resorption are reutilized for calcification in other parts. The test microstructure exhibited numerous trabeculae and holes [
59,
60]. The diameter of the hole and trabecula shows a positive correlation with the longitudinal growth of the equatorial plates, which are eventually reflected in the test height of sea urchins [
61,
62]. This study suggested that
S. intermedius juveniles fed formulated feeds with a moderate level of C/L (2 and 4) had larger holes and longer but thinner trabeculae on the inner and outer surfaces than those fed relatively low or high C/L (1, 8, and 16). A larger hole diameter and thinner test will increase the efficiency of test construction. In this study, dietary C/L had no marked impact on the content of Ca deposited in the test of juvenile
S. intermedius, but the content of Mg deposited in the test of juveniles fed diets with C/L2 was greater than that of individuals fed other formulated feeds. Due to the instability of magnesium calcite, there will be more active calcite deposition and resorption in the test of juvenile
S. intermedius fed C/L2, which can accelerate the growth of the test [
63,
64]. A thinner but larger test suggests faster outer surface expansion and inner surface resorption. In this experiment,
S. intermedius juveniles fed kelp exhibited higher test height and diameter and more obvious resorption on the inner surface than those of individuals fed dry feeds. Similarly, a study indicated that sea urchins with greater growth rates exhibited more pronounced resorption and thinner tests [
63]. Moreover, fast growth can result in a limited supply of minerals required for forming trabeculae in sea urchins, which could drive the formation of thinner tests with thinner trabeculae and larger holes.