Effects of Micro- and Macroalgae-Supplemented Diets on Growth and Muscle Fibrillar Constitution of Gilthead Seabream, Sparus aurata L., in the Final On-Growing Phase
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Rearing Conditions
- -
- Group C1: fish fed with standard algae-free feed containing 15% fishmeal (FM) and 10% fish oil (FO) throughout the experiment (87 days).
- -
- Group C2: fed with an algae-free diet, low in FM (5%) and FO (5%) and rich in vegetable sources (soybean meal, wheat flour and gluten, soybean oil and rapeseed oil), throughout the experiment (87 days).
- -
- Group C2-R: fed for 38 days with diet C2 and then transferred for 49 days to a diet containing 10% of a mixture of raw microalgae (Chlorella vulgaris, Nannochloropsis gaditana, Arthrospira platensis, Schizochytrium sp., and Dunaliella salina) and 2% of the macroalga Alaria esculenta, replacing the 5% FM, 0.7% FO, and 6.4% terrestrial vegetables of the standard C1 diet.
- -
- Group C2-H: like C2-R, but the algal biomass (10% microalgae and 2% macroalgae) was subjected to fibrolytic enzymatic hydrolysis with cellulase and β-glucanase activities to cause the rupture of cell walls and promote the assimilation of intracellular nutrients. Proteolytic enzymes (endo- and exopeptidases) (Novozymes®, DK) were also used to convert high molecular weight proteins into low-molecular-weight hydrolysates. The enzyme cocktail was added to the microalgae biomass under agitation.
- -
- Group C2-O: after 38 days on diet C2, the group was switched for an additional 49 days to a diet lacking FM and FO, supplemented with the above-mentioned raw microalgae mixture at 10% replacing the FM and terrestrial vegetables of the standard C1 diet. Also, 7% algae oil was added to this diet to replace the 7% FO of the standard C1 diet.
| Ingredients | C1 | C2 | C2-R | C2-H | C2-O |
|---|---|---|---|---|---|
| Fishmeal LT94 1 | 15.00 | 5.00 | 10.00 | 10.00 | |
| Poultry meal 2 | 5.00 | ||||
| Lysine 3 | 0.80 | 1.60 | 0.80 | 0.80 | 1.60 |
| Metionina 4 | 0.30 | 0.60 | 0.30 | 0.30 | 0.80 |
| Squid meal 5 | 2.00 | 0.25 | 2.00 | 2.00 | |
| Fish meal hydrolysate CPSP90 6 | 1.00 | 0.25 | 1.00 | 1.00 | |
| Krill meal 7 | 1.00 | 0.25 | 1.00 | 1.00 | |
| Blood meal 8 | 2.00 | ||||
| Tenebrio molitor meal 9 | 5.00 | ||||
| Hermetia illucens meal 10 | 5.00 | ||||
| Microalgal blend 11 | 10.00 | 10.00 | 10.00 | ||
| Alaria esculenta meal 12 | 2.00 | 2.00 | |||
| Wheat gluten 13 | 16.00 | 18.00 | 16.00 | 16.00 | 15.00 |
| Soybean meal 14 | 16.00 | 24.00 | 15.00 | 15.00 | 8.30 |
| Soybean protein concentrate 15 | 11.00 | 17.00 | 11.10 | 11.10 | 8.20 |
| Pea protein concentrate 16 | 5.00 | ||||
| Fish oil 17 | 10.00 | 5.00 | 9.30 | 9.30 | |
| Algal oil 18 | 7.00 | ||||
| Soybean and rapeseed oil 19 | 4.00 | 10.50 | 4.00 | 4.00 | 5.90 |
| Soybean lecithin 20 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Wheat meal 21 | 18.80 | 13.45 | 13.40 | 13.40 | 17.10 |
| Choline chloride 22 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Betaine 23 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin and mineral premix 24 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Vitamin C 25 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Proximate composition | |||||
| Crude protein | 47.8 | 47.9 | 47.1 | 47.0 | 47.7 |
| Crude lipid | 18.2 | 18.4 | 18.8 | 18.8 | 18.6 |
| Ash | 7.1 | 5.8 | 8.4 | 8.4 | 6.4 |
| Carbohydrates | 27.0 | 27.9 | 25.7 | 25.8 | 27.3 |
| Moisture | 4.7 | 4.8 | 4.8 | 4.7 | 4.7 |
2.2. Algal Biomass Pre-Treatment
2.3. Experimental Diets
2.4. Sampling Points
2.5. Body Parameters, Conversion Rates, Specific Growth Rate and Survival
2.6. Muscle Growth
2.7. Statistical Analysis
3. Results
3.1. Day 38 of the Experiment
3.2. Day 87 of the Experiment
3.3. Survival
4. Discussion
5. Conclusions
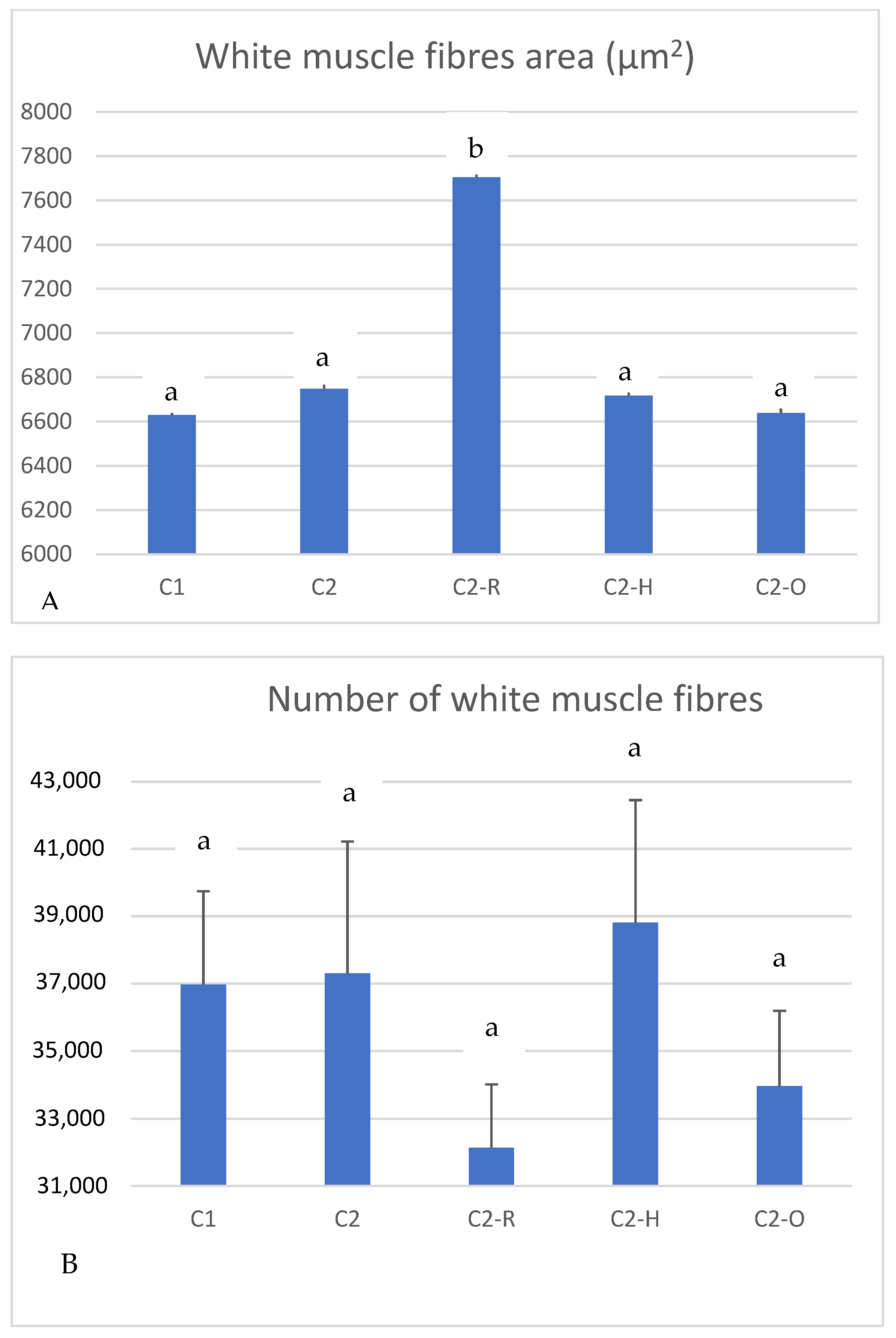
- The C2-R diet produced higher body weight values than the other diets. Likewise, the C2-R group showed the highest values of fibrillar hypertrophy.
- Diets enriched with hydrolysed algae showed a tendency to generate a higher number of fibres in the fish muscles than diets with raw algae biomass, although this was not significant.
- Diets supplemented with algal biomass (microalgae and macroalgae) were able to reverse the negative effect of a diet low or lacking in FM and FO and rich in vegetable ingredients in terms of feed conversion rate and weight gain.
- Our data point to algal biomass-containing diets as good alternatives to diets rich in plant ingredients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.; Leng, Y.; Lu, Q. The application of microalgae biomass and bio-products as aquafeed for aquaculture. Algal Res. 2021, 60, 102541. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Ceccotti, C. Fish Health and Fillet Quality Following Substitution of Fishmeal (FM) and Fish Oil (FO) with Vegetable Meal (VM) and Oil (VO) in the Modern Aquaculture. Ph.D. Thesis, University of Insubria, Varese, Italy, 2015; 124p. Available online: https://hdl.handle.net/11383/2090635 (accessed on 20 April 2022).
- Ponis, E.; Robert, R.; Parisi, G. Nutritional value of fresh and concentrated algal diets for larval and juvenile Pacific oysters (Crassostrea gigas). Aquaculture 2003, 221, 491–505. [Google Scholar] [CrossRef]
- Hussein, E.; Dabrowski, K.; El-Saidy, D.; Lee, B.J. Enhancing the growth of Nile tilapia larvae/juveniles by replacing plant (gluten) protein with algae protein. Aquac. Res. 2012, 44, 937–949. [Google Scholar] [CrossRef]
- Rincón, D.; Velásquez, H.; Dávila, M.; Semprun, A.; Morales, E.; Hernández, J. Substitution levels of fish meal by Arthrospira (=Spirulina) maxima meal in experimental diets for red tilapia fingerlings (Oreochromis sp.). Rev. Colomb. Cienc. Pecu. 2012, 25, 430–437. [Google Scholar] [CrossRef]
- Kim, S.; Rahimnejad, S.; Kim, K.; Lee, K. Partial replacement of fish meal with Spirulina pacifica in diets for Parrot fish (Oplegnathus fasciatus). Turk. J. Fish. Aquat. Sci. 2013, 13, 197–204. [Google Scholar] [CrossRef]
- Khanzadeh, M.; Esmaeili Fereidouni, A.; Seifi Berenjestanaki, S. Effects of partial replacement of fish meal with Spirulina platensis meal in practical diets on growth, survival, body composition, and reproductive performance of three-spot gourami (Trichopodustri chopterus) (Pallas, 1770). Aquac. Int. 2016, 24, 69–84. [Google Scholar] [CrossRef]
- Pérez-Velázquez, M.; Gatlin III, D.M.; González-Félix, M.L.; García-Ortega, A. Partial replacement of fishmeal and fish oil by algal meals in diets of red drum Sciaenopsocellatus. Aquaculture 2018, 487, 41–50. [Google Scholar] [CrossRef]
- Roohani, A.M.; Abedian Kenari, A.; Fallahi Kapoorchali, M.; Borani, M.S.; Zoriezahra, S.J.; Smiley, A.H.; Esmaeili, M.; Rombenso, A.N. Effect of spirulina Spirulina platensis as a complementary ingredient to reduce dietary fish meal on the growth performance, whole-body composition, fatty acid and amino acid profiles, and pigmentation of Caspian brown trout (Salmo trutta caspius) juveniles. Aquac. Nutr. 2019, 25, 633–645. [Google Scholar]
- Enyidi, U.D. Chlorella vulgaris as protein source in the diets of african catfish Clarias gariepinus. Fishes 2017, 2, 17. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lee, S.-M.; Park, H.-G.; Choi, J. Effects of Dietary Inclusion of Chlorella vulgaris on Growth, Blood Biochemical Parameters, and Antioxidant Enzyme Activity in Olive Flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2017, 48, 103–112. [Google Scholar] [CrossRef]
- Carneiro, W.F.; Castro, T.F.D.; Orlando, T.M.; Meurer, F.; Paula, D.A.d.J.; Virote, B.d.C.R.; Vianna, A.R.d.C.B.; Murgas, L.D.S. Replacing fish meal by Chlorella sp. meal: Effects on zebrafish growth, reproductive performance, biochemical parameters and digestive enzymes. Aquaculture 2020, 528, 735612. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, F.; Liao, K.; Xiao, Y.; Chen, S.; Lu, Q.; Li, J.; Zhou, W. Microalgae for nutrient recycling from food waste to aquaculture as feed substitute: A promising pathway to eco-friendly development. J. Chem. Technol. Biotechnol. 2021, 96, 2496–2508. [Google Scholar] [CrossRef]
- Becker, W. Microalgae in human and animal nutrition. In Handbook of Microalgal Culture; Blackwell Publishing Ltd.: Oxford, UK, 2003; pp. 312–351. [Google Scholar] [CrossRef]
- Kiron, V. Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Forster, I.P.; Dominy, W.G. Effects of supplementing two species of marine algae or their fractions to a formulated diet on growth, survival and composition of shrimp (Litopenaeus vannamei). Aquaculture 2009, 292, 237–243. [Google Scholar] [CrossRef]
- Nayak, S.; Khozin-Goldberg, I.; Cohen, G.; Zilberg, D. Dietary supplementation with ω6 LC-PUFA-rich algae modulates zebrafish immune function and improves resistance to streptococcal infection. Front. Immunol. 2018, 9, 1960. [Google Scholar] [CrossRef]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. Replacement of fish oil with Thraustochytrid Schizochytrium sp. L oil in Atlantic salmon par (Salmo salar L.) diets. Comp. Biochem. Physiol. A 2007, 148, 382–392. [Google Scholar] [CrossRef]
- Katerina, K.; Berge, G.M.; Turid, M.; Aleksei, K.; Grete, B.; Trine, Y.; Mats, C.; John, S.; Bente, R. Microalgal Schizochytrium limacinum biomass improves growth and filet quality when used long-term as a replacement for fish oil, in modern Salmon diets. Front. Mar. Sci. 2020, 7, 57. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, H.; Song, Z.; Ma, J.; Li, B.; Liu, X.; Zhang, S.; Wang, J.; Zhang, L. Effects of dietary fish oil replacement by microalgae raw materials on growth performance, body composition and fatty acid profile of juvenile olive flounder, Paralichthys olivaceus. Aquac. Nutr. 2014, 20, 646–665. [Google Scholar] [CrossRef]
- Atalah, E.; Cruz, C.M.H.; Izquierdo, M.S.; Rosenlund, G.; Caballero, M.J.; Valencia, A.; Robaina, L. Two microalgae Crypthecodinium cohnii and Phaeodactylum tricornutum as alternative source of essential fatty acids in starter feeds for sea bream (Sparus aurata). Aquaculture 2007, 270, 178–185. [Google Scholar] [CrossRef]
- Ganuza, E.; Benítez-Santana, T.; Atalah, E.; Vega-Orellana, O.; Ganga, R.; Izquierdo, M.S. Crypthecodinium cohnii and Schizochytrium sp. as potential substitutes to fisheries-derived oils from seabream (Sparus aurata) microdiets. Aquaculture 2008, 277, 109–116. [Google Scholar] [CrossRef]
- Eryalcin, K.M.; Roo, J.; Saleh, R.; Atalah, E.; Benítez, R.; Betancor, M.; Hernández-Cruz, C.M.; Izquierdo, M.S. Fish oil replacement by different microalgal products in microdiets for early weaning of gilthead sea bream (Sparus aurata, L.). Aquac. Res. 2013, 44, 819–828. [Google Scholar] [CrossRef]
- Weatherley, A.H.; Gill, H.S.; Lobo, A.F. Recruitment and maximal diameter of axial muscle fibres in teleosts and their relationship to somatic growth and ultimate size. J. Fish Biol. 1998, 33, 851–859. [Google Scholar] [CrossRef]
- Fauconneau, B.; Andre, S.; Chmaitilly, J.; Le Bail, P.Y.; Krieg, F.; Kaushik, S.J. Control of skeletal muscle fibres and adipose cells in the flesh of rainbow trout. J. Fish Biol. 1997, 50, 296–314. [Google Scholar] [CrossRef]
- Hatae, K.; Yoshimatsu, F.; Matsumoto, J.J. Role of muscle fibres in contributing firmness of cooked fish. J. Food Sci. 1990, 55, 693–696. [Google Scholar] [CrossRef]
- Periago, M.J.; Ayala, M.D.; López-Albors, O.; Abdel, I.; Martínez, C.; García-Alcázar, A.; Ros, G.; Gil, F. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture 2005, 249, 175–188. [Google Scholar] [CrossRef]
- Knutsen, H.R.; Johnsen, I.H.; Keizer, S.; Sorensen, M.; Roques, J.A.C.; Heden, I.; Sundell, K. Fish welfare, fast muscle cellularity, fatty acid and body-composition of juvenile spotted wolffish (Anarhichas minor) fed a combination of plant proteins and microalgae (Nannochloropsis oceanica). Aquaculture 2019, 506, 212–223. [Google Scholar] [CrossRef]
- Ayala, M.D.; Chaves-Pozo, E.; Sáez, M.I.; Galafaz, A.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Effect on muscle cellularity of diet supplementation with Nannochloropsis gaditana microalgae in the final fattening phase of gilthead seabream culture up to commercial size. Fishes 2023, 8, 532. [Google Scholar] [CrossRef]
- Sáez, M.J.; Galafat, A.; Suárez, M.D.; Chaves-Pozo, E.; Arizcun, M.; Ayala, M.D.; Alarcón, F.J.; Martínez, T.F. Effects of raw and hydrolysed Nannochloropsis gaditana biomass included at low level in finishing diets for gilthead seabream (Sparus aurata) on filet quality and shelf life. J. Appl. Phycol. 2023, 35, 1163–1181. [Google Scholar] [CrossRef]
- Ayala, M.D.; Chaves-Pozo, E.; Hermoso, E.; Sáez, M.I.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Influencia de las Microalgas en la Dieta Sobre la Dinámica de Crecimiento Muscular en Postlarvas de Dorada, Sparus aurata L. In Proceedings of the XIX Congreso Nacional de Acuicultura, Las Palmas de Gran Canaria, Spain, 17–19 June 2024; López, J.M.A., Arbelo, F.A.A., Eds.; ECOAQUA Institute: Las Palmas, Spain, 2024. [Google Scholar]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Ayala, M.D.; Balsalobre, N.; Chaves-Pozo, E.; Sáez, M.I.; Galafaz, A.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Long-term effects of a short juvenile feeding period with diets enriched with the microalgae Nannochloropsis gaditana on the subsequent body and muscle growth of gilthead seabream, Sparus aurata L. Animals 2023, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Sarker, P.K.; Kapuscinski, A.R.; Fitzgerald, D.; Greenwood, C.; Nocera, P.; O’Shelski, K.; Lee, B.; Mkulama, A.; Gwynne, D.; et al. Enzyme-treated microalgal co-product diets for rainbow trout aquaculture: Supporting fish growth, phosphorus digestibility, and reducing phosphorus waste emission. Elem. Sci. Anth. 2024, 12, 00119. [Google Scholar] [CrossRef]
- Sáez, M.I.; Galafat, A.; Vizcaíno, A.J.; Chaves-Pozo, E.; Ayala, M.D.; Arizcun, M.; Alarcón, F.J.; Suárez, M.D.; Martínez, T.F. Evaluation of Nannochloropsis gaditana raw and hydrolysed biomass at low inclusion level as dietary functional additive for gilthead seabream (Sparus aurata) juveniles. Aquaculture 2022, 556, 738288. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Folch, J. Simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Camacho-Rodríguez, J.; Macías-Sánchez, M.D.; Cerón-García, M.C.; Alarcón, F.J.; Molina-Grima, E. Microalgae as a potential ingredient for partial fish meal replacement in aquafeeds: Nutrient stability under different storage conditions. J. Appl. Phycol. 2018, 30, 1049–1059. [Google Scholar] [CrossRef]
- Sen Roy, S.; Pal, R. Microalgae in aquaculture: A review with special references to nutritional value and fish dietetics. Proc. Zool. Soc. 2015, 68, 1–8. [Google Scholar]
- Teimouri, M.; Amirkolaie, A.; Yeganeh, S. The effects of dietary supplement of Spirulina platensis on blood carotenoid concentration and fillet colour stability in rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 414–415, 224–228. [Google Scholar] [CrossRef]
- Usher, M.L.; Stickland, N.C.; Thorpe, J.E. Muscle development in Atlantic salmon (Salmo salar) embryos and the effect of temperature on muscle cellularity. J. Fish Biol. 1994, 44, 953–964. [Google Scholar] [CrossRef]
- Seong, T.; Uno, Y.; Kitagima, R.; Kabeya, N.; Haga, Y.; Satoh, S. Microalgae as main ingredient for fish feed: Non-fish meal and non-fish oil diet development for red sea bream, Pagrus major, by blending of microalgae Nannochloropsis, Chlorella and Shizochytrium. Aquac. Res. 2021, 52, 6025–6036. [Google Scholar] [CrossRef]
- Carvalho, M.; Izquierdo, M.; Valdés, M.; Montero, D.; Farías, A. Oils Combination with Microalgal Products as a Strategy for Increasing the N-3 Long-Chain Polyunsaturated Fatty Acid Content in Fish Oil-Free Diets for Meagre (Argyrosomus regius). Aquac. Nutr. 2022, 2022, 5275570. [Google Scholar] [CrossRef]
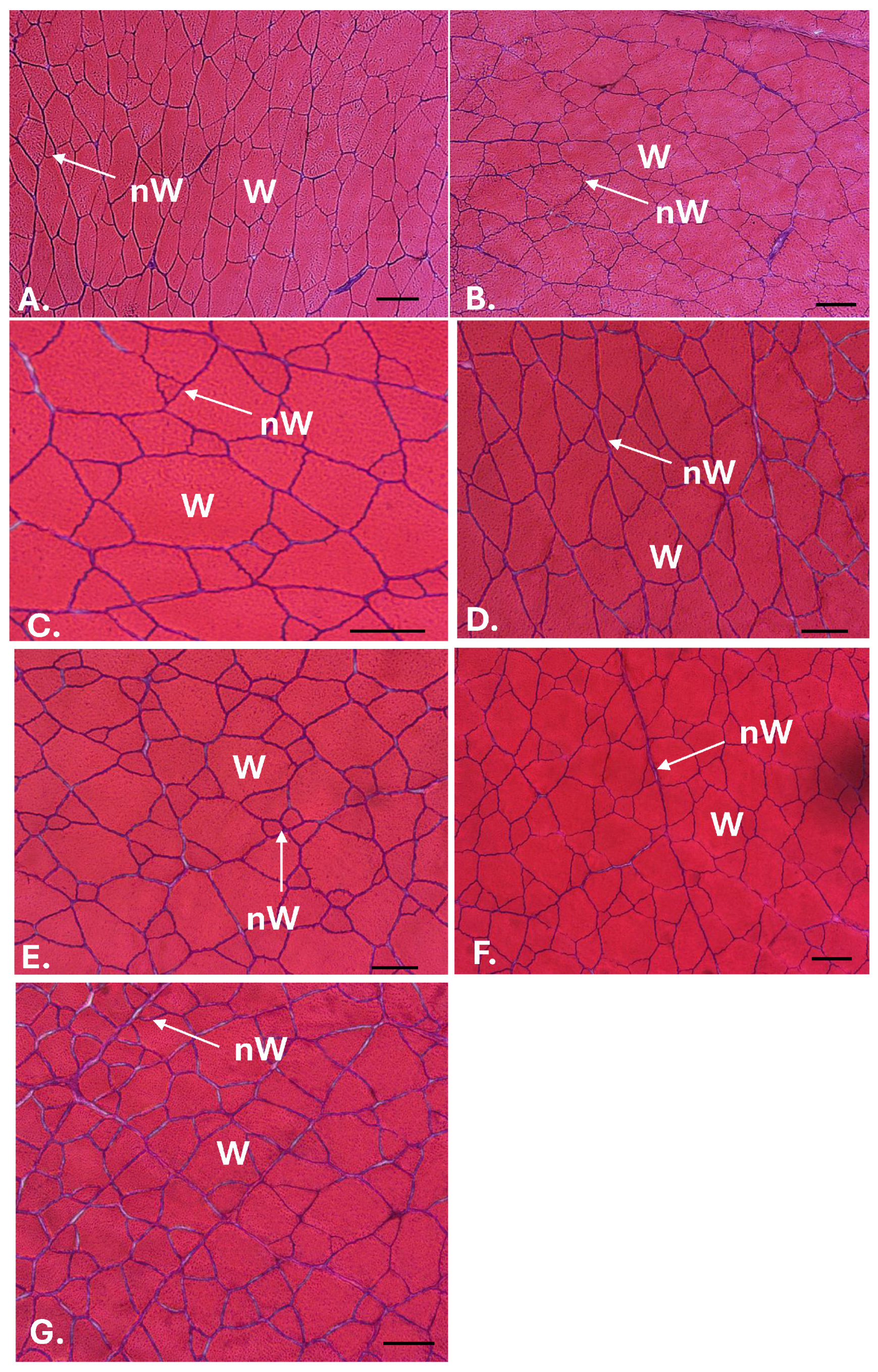
| Day 38 of the Experiment | ||
|---|---|---|
| Groups | C1 | C2 |
| BL (cm) | 27.0 ± 0.43 a | 26.689 ± 0.25 a |
| BW (g) | 330.0 ± 12.67 a | 318 ± 11.35 a |
| B (cm2) | 19.15 ± 0.94 a | 19.52 ± 0.99 a |
| A (μm2) | 7288.0 ± 382.87 a | 6286.22 ± 544.71 a |
| D (μm) | 87.23 ± 2.14 a | 81.74 ± 3.8 a |
| N (×103) | 273.34 ± 25.20 a | 307.57 ± 26.97 a |
| Density | 139.31 ± 8.05 a | 163.37 ± 16.58 a |
| Day 87 of the Experiment | |||||
|---|---|---|---|---|---|
| Groups | C1 | C2 | C2-R | C2-H | C2-O |
| BL (cm) | 28.28 ± 0.17 a | 28.24 ± 0.14 a | 28.8 ± 0.25 a | 28.24 ± 0.19 a | 28.35 ± 0.21 a |
| BW (g) | 378.49 ± 7.68 ab | 355.99 ± 6.88 b | 391.85 ± 6.94 a | 366.83 ± 8.74 ab | 367.22 ± 6.53 ab |
| SGR | 0.99 | 0.77 | 1.51 | 1.00 | 1.00 |
| Conversion rate | 2.5 | 3.1 | 2.3 | 2.7 | 2.7 |
| B (cm2) | 24.06 ± 0.9 a | 24.73 ± 0.1 a | 25.04 ± 0.76 a | 25.26 ± 0.16 a | 22.82 ± 0.96 a |
| D (μm) | 67.76 ± 0.58 a | 66.64 ± 0.64 a | 73.63 ± 0.66 b | 69.44 ± 0.62 a | 66.93 ± 0.61 a |
| Density | 154.30 ± 9.9 a | 153.09 ± 17.0 a | 126.4 ± 4.72 a | 151.80 ± 6.67 a | 152.69 ± 11.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, M.D.; Chaves-Pozo, E.; Sáez, M.I.; Alarcón, F.J.; Martínez, T.F.; Arizcun, M. Effects of Micro- and Macroalgae-Supplemented Diets on Growth and Muscle Fibrillar Constitution of Gilthead Seabream, Sparus aurata L., in the Final On-Growing Phase. Fishes 2025, 10, 262. https://doi.org/10.3390/fishes10060262
Ayala MD, Chaves-Pozo E, Sáez MI, Alarcón FJ, Martínez TF, Arizcun M. Effects of Micro- and Macroalgae-Supplemented Diets on Growth and Muscle Fibrillar Constitution of Gilthead Seabream, Sparus aurata L., in the Final On-Growing Phase. Fishes. 2025; 10(6):262. https://doi.org/10.3390/fishes10060262
Chicago/Turabian StyleAyala, María Dolores, Elena Chaves-Pozo, María Isabel Sáez, Francisco Javier Alarcón, Tomás Francisco Martínez, and Marta Arizcun. 2025. "Effects of Micro- and Macroalgae-Supplemented Diets on Growth and Muscle Fibrillar Constitution of Gilthead Seabream, Sparus aurata L., in the Final On-Growing Phase" Fishes 10, no. 6: 262. https://doi.org/10.3390/fishes10060262
APA StyleAyala, M. D., Chaves-Pozo, E., Sáez, M. I., Alarcón, F. J., Martínez, T. F., & Arizcun, M. (2025). Effects of Micro- and Macroalgae-Supplemented Diets on Growth and Muscle Fibrillar Constitution of Gilthead Seabream, Sparus aurata L., in the Final On-Growing Phase. Fishes, 10(6), 262. https://doi.org/10.3390/fishes10060262








