1. Introduction
Length and weight are two basic biological indicators for fish individuals and their populations, and they reflect the physiological status and population structure of fish species. They are also important indexes showing life-history traits of fish [
1,
2]. The length–weight relationships (LWRs) are fundamental in fish life-history research, allowing for the calculation of weight from length, or vice versa, and thereby determining population biomass [
3,
4,
5]. The LWRs provide information on fish growth patterns, facilitate population production evaluation, and enable biometric and morphological comparisons at different levels [
6,
7]. In fisheries research, LWRs are considered an integral part of fish stock assessment models and used as a fundamental tool in resource assessment and management [
6,
8,
9]. Many studies have shown that the length–weight relationship of fishes is affected by factors such as sex, season, geography, and environmental conditions, leading to different heterogeneity of parameter values in the relationship [
2,
7,
8].
The power function with the expression of BW =
a SL
b has been demonstrated to be the most reasonable mathematical expression describing the LWRs of fish [
8,
10]. The parameter “
b”, which is defined as an allometric factor, can effectively reflect the growth pattern of fish and can also reflect the environmental quality of the fish habitat indirectly [
7,
11]. However, many factors, such as sex, developmental phases, stomach fullness, season, and environmental conditions, will affect the relationship between length and weight, which leads to different heterogeneity of parameter values in the relationship [
12,
13,
14,
15]. Thus, to increase the reliability of the description of fish growth patterns based on the average
b value, these factors should be considered.
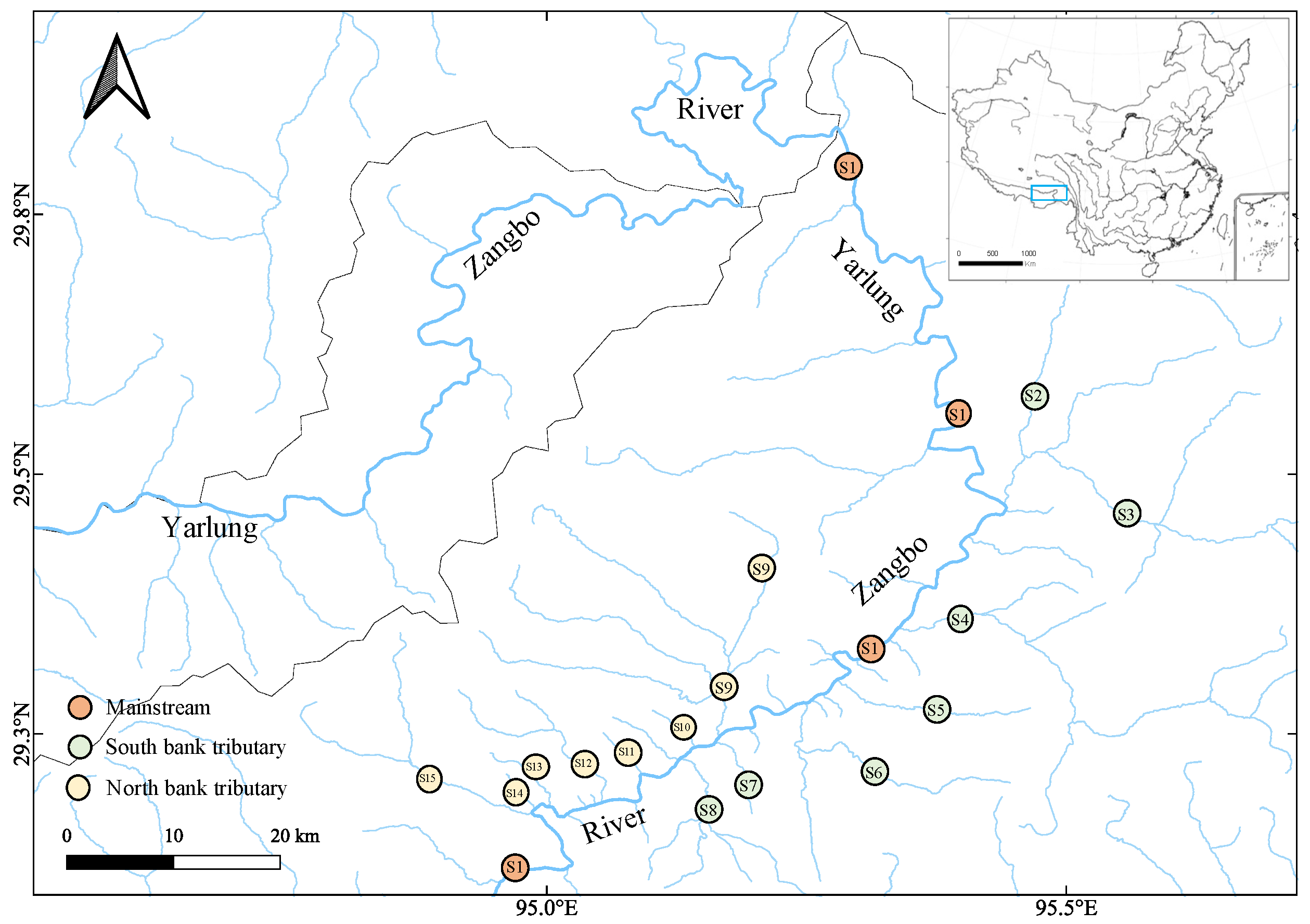
The Yarlung Zangbo River is located in the highest Qinghai–Tibet Plateau. The unique and diverse natural environment in the Qinghai–Tibet Plateau breeds the unique biodiversity in the river [
16,
17,
18]. The lower reaches of this river are one of the most important biodiversity hotspots worldwide, being extremely rich in biodiversity [
19]. The indigenous fish fauna of the lower reaches of the river are mainly
Schizothorax integrilabiatus (Wu et al., 1992),
Schizothorax curvilabiatus (Wu & Tsao, 1992), and
Schizothorax molesworthi (Chaudhuri, 1913), which belong to Schizothoracinae;
Garra tibetana Gong, Deng, Wang & Liu, 2018 that belong to Labeoninae; and
Aborichthys kempi Chaudhuri, 1913 and
Nemacheilus subfuscus (McClelland, 1839) that are affiliated with Nemacheilidae. There are many fish species of Sisoridae, including
Glyptosternum maculatum (Regan, 1905),
Pseudecheneis sulcata (McClelland, 1842),
Parachiloglanis hodgarti (Hora, 1923),
Glyptothorax annandalei Hora, 1923,
Glyptothorax cavia (Hamilton, 1822),
Glyptothorax gracilis (Günther, 1864),
Exostoma labiatum (McClelland, 1842),
Exostoma tenuicaudatum Tamang, Sinha & Gurumayum, 2015, etc. [
16,
17]. And in recent years, some new species, such as
Garra dengba Deng, Cao & Zhang, 2018,
Garra tibetana Gong, Deng, Wang & Liu, 2018,
Garra motuoensis Gong, Freyhof, Wang, Liu, Liu, Lin, Jiang & Liu 2018, and
Garra yajiangensis Gong, Freyhof, Wang, Liu, Liu, Lin, Jiang & Liu 2018, have been found successively in the lower reaches [
20,
21,
22,
23]. Most of the fish species are endemic and highly rare.
Additionally, the lower Yarlung Zangbo River area is situated in the transitional zone from the Tibetan Plateau to the Indian peninsula, having a humid subtropical climate with a high-altitude gradient and containing huge hydro-energy [
24]. The future development of water resources, coupled with global climate change, will inevitably have a profound impact on the biodiversity of the region, especially on fish resources [
19,
25]. Recently, influenced by factors such as habitat degradation, overfishing, hydropower development, and biological invasion, the fish resources in the Yarlung Zangbo River have declined dramatically [
26]. Despite this, research on fishes of the lower Yarlung Zangbo River is still limited, and some basic biological information is scarce, which further hinders the fishery conservation efforts and development. Therefore, it is very necessary to explore fish biology, population dynamics, and fish diversity in this area to implement conservation and management measures for the protection of local fish assemblages. This study provides information on the LWRs of six fish species from the lower Yarlung Zangbo River, describing their growth patterns and identifying the spatiotemporal variations of LWRs to expand the biological knowledge of these species and provide basic data for conservation.
4. Discussion
Length–weight relationships (LWRs) of different fishes were different; hence, the differences in parameters were calculated from the relationship [
29]. The variation in this
b value reflected the heterogeneity of growth and related to the body shapes of the respective fish species [
6]. According to the results of this study, the overall mean values of the allometric coefficient
b for the six studied fish species were 2.738 to 3.002, within the expected range of 2.5 to 3.5 [
6]. Strong allometric growth patterns were observed in
S. curvilabiatus,
G. tibetana,
P. hodgarti, and
E. labiatum in this study, indicating that the weight growth of these fishes was in the “different” dimension as the cube of length. Negative allometric growth patterns of
S. curvilabiatus,
G. tibetana, and
P. hodgarti may relate to their relatively elongated body shapes [
16,
17], and the growth in length was faster than that of weight for these fishes accordingly. While the positive allometric growth pattern of
E. labiatum was probably due to a large number of samples (exceeding 75%) being collected in winter and spring, when they were just at the gonad accumulation period [
17], and the growth in weight was faster than that of length for this fish.
The LWRs of fish were influenced by many factors, such as sex, season, geography, and environmental conditions, thus leading to varying heterogeneity in the parameter values within this relationship [
30,
31,
32]. In the present study, sexual differences in LWRs were detected in
S. molesworthi and
P. sulcata (
Table 4;
Figure 3A,E). Further analysis showed that the significant sexual differences in the LWRs for these two species could be associated with the individual size difference between sexes. The body size of the female
S. molesworthi (
Figure 3A) was larger than that of the male, while the size of the male
P. sulcata was larger than that of the female (
Figure 3E). Sexual differences were also observed in other fish species [
11,
13,
33].
Seasonal variations in the LWRs were observed in many fish species [
2,
11,
30,
34,
35]. These variations may relate to differences in the life history stage, gonad developmental phases, and stomach fullness of fishes at the time [
36,
37]. In the present study, for
S. molesworthi, spring was the fattening season; a large amount of food intake resulted in more weight gain than in other seasons. Additionally, according to the wild investigation, the breeding period of
S. molesworthi lasted from May to October; energy obtained could have been spent on gonad development, not on length and weight. However, the spawning type of this species is not yet clear. As for
S. curvilabiatus, with a breeding time mainly in winter, from December to January of the next year, reproductive activities coupled with food scarcity in winter accounted for the lower weight growth in winter than in spring and autumn, respectively. The seasonal variations in the LWRs for
G. tibetana and
P. hodgarti were probably associated with the gonad developmental phases of the respective fish species. The breeding season for
G. tibetana was concentrated in summer [
17]; when the investigation was conducted in the autumn, many individuals had not recovered from the breeding, leading to less weight growth in autumn. For
P. hodgarti, the reproductive season was mainly in summer [
27], and the gonad accumulation resulted in more weight gain in this season.
Geographical differences in LWRs within species were general in many fishes [
14,
38]. Because the growth of fish is affected by external environmental factors, such as water temperature, dissolved oxygen, water-flow velocity, food resources [
11,
35,
39], and so on. Usually, these factors are spatial variations that would result in variations in growth patterns for fishes in different rivers. In the present study, geographical differences in LWRs for each fish species were observed from the results of comparison among mainstream and tributaries, except for
S. curvilabiatus. The variations among geographical populations could relate to the environmental factors of the respective habitats. Firstly, located in the Qinghai–Tibet Plateau and affected by geographical conditions, there were prominent spatiotemporal variations among water bodies of the lower Yarlung Zangbo River [
40]. This variation could result in spatiotemporal variations of water quality and food supply accordingly [
41,
42], affecting the growth of the fish distributed there and ultimately resulting in within-species geographical differences in LWRs. Additionally, according to geographical location, distances among tributaries on the south bank were farther than that of tributaries on the north bank (
Figure 1), which may cause more environmental heterogeneity among tributaries on the south bank. This is why LWR variations were different between the two banks for
S. molesworthi and
P. sulcata. However, specific environmental variables were not detected in this study; further studies are needed to confirm the environment–growth relationship.
5. Conclusions
The present study provides a basic understanding of the LWRs of six fish species distributed in the lower Yalung Zangbo River, Tibet, China. The LWRs for five species are published herein for the first time for FishBase. New records of maximum standard length for four species and total length for P. sulcatawere were recorded. Sexual differences in LWRs were observed in two fish species: S. molesworthi and P. sulcata. Seasonal differences were observed in five species, except for E. labiatum. And with the exception of S. curvilabiatus, geographical differences in LWRs were observed in all other species. According to the overall mean b values, the growth patterns of S. molesworthi and P. sulcata were isometric, and those of S. curvilabiatus, G. tibetana, and P. hodgarti were negative allometric, while E. labiatum had positive allometric growth. However, other factors that will affect the LWRs and the associated parameters were not evaluated in the present study, such as size ranges, reproductive stage, fishing gears, fishing intensity, and water condition. Further research involving more factors that affect fish growth is required to increase the reliability of the description of fish growth patterns and to provide additional valuable information for local fish conservation and fishery management.
S. molesworthi and S. curvilabiatus are important economic fishes of local fisheries; our results will be useful for future fisheries research evaluating their population dynamics. Additionally, these fishes in the lower Yarlung Zangbo River are important in maintaining local biodiversity and aquatic food webs. We suggest that further studies concentrate on long-term fish resources monitoring; community and population dynamics analysis; and environment–fishery resources relationship evaluations. Based on this research, scientific fishery resource management should be conducted to ensure that the river has sustainable fishery production while supporting important ecological service functions.