Morphological Trait Correlations and Nutrient Compositions of the Japanese Moon Scallop Ylistrum japonicum in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Measurement of Quality Traits and Body Measurements
2.3. Detection of Nutritional Components of Adductor Muscle
2.4. Data Analysis
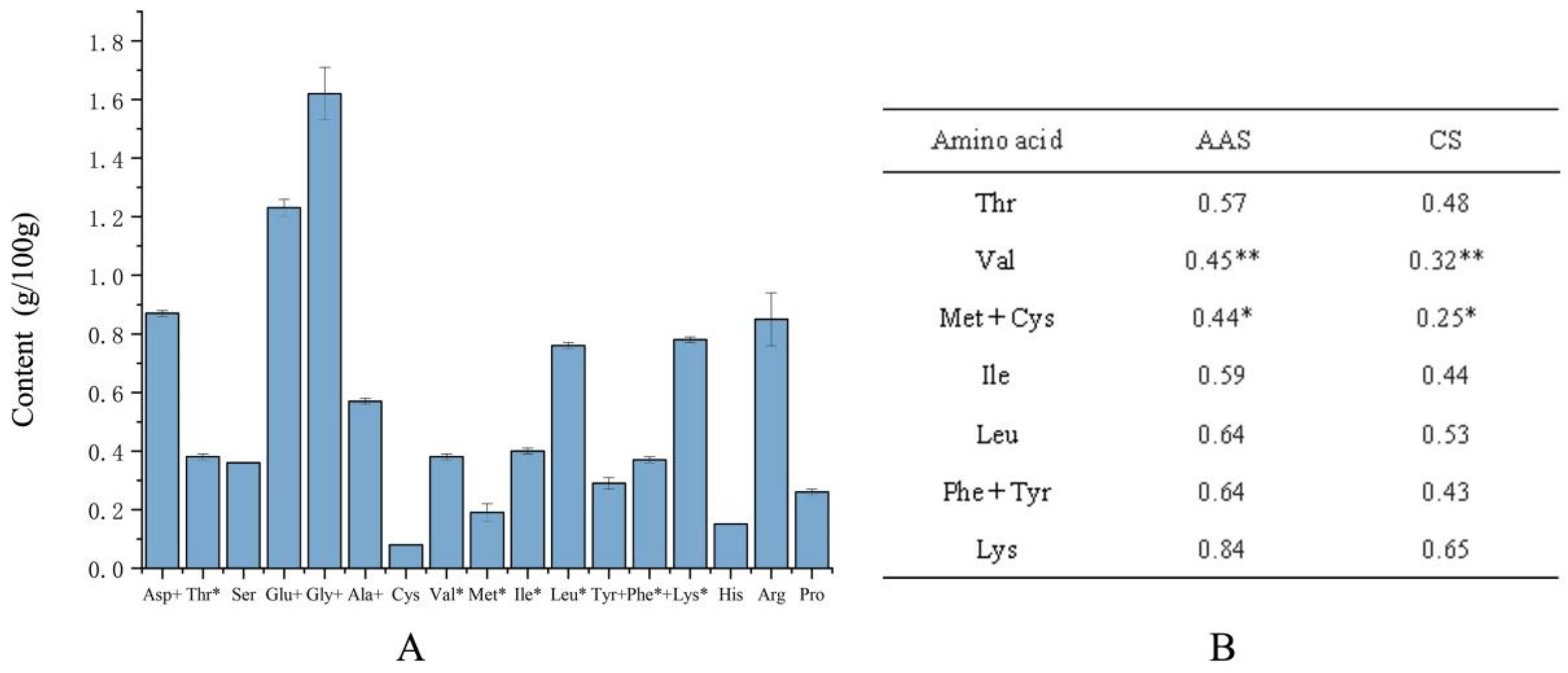
amino acid content in the FAO scoring model
content in the egg
(%)/crude protein content in fresh sample (%) × 6.25 × 1000
3. Results
3.1. Summary Statistics of Measured Traits
3.2. Correlation Analysis of Measured Traits
3.3. Path Analysis and Decision Coefficient Analysis of Morphological Traits to Quality Traits
3.4. Establishment of the Multiple Linear Regression Equation
- (1)
- WW = −94.659 + 0.336SL + 6.885SW (r2 = 0.974);
- (2)
- FW = −34.118 + 0.795SW-0.151SH (r2 = 0.942);
- (3)
- GW = −4.492 + 0.319SW (r2 = 0.681);
- (4)
- AW = −12.514 + 0.076SL + 0.687SW (r2 = 0.929).
- (1)
- WW = −87.48 + 0.517SL + 7.350SW (r2 = 0.969);
- (2)
- FW = −34.255 + 2.568SW (r2 = 0.955);
- (3)
- GW = −4.972 + 0.398SW (r2 = 0.729);
- (4)
- AW = −13.356 + 0.065SL + 0.861SW (r2 = 0.915).
- (1)
- WW = −99.078 + 0.280SL + 6.657SW (r2 = 0.978);
- (2)
- FW = −35.811 + 2.637SW (r2 = 0.947);
- (3)
- GW = −4.051 + 0.34SW (r2 = 0.664);
- (4)
- AW = −12.37 + 0.129SL + 0.642SW (r2 = 0.948).
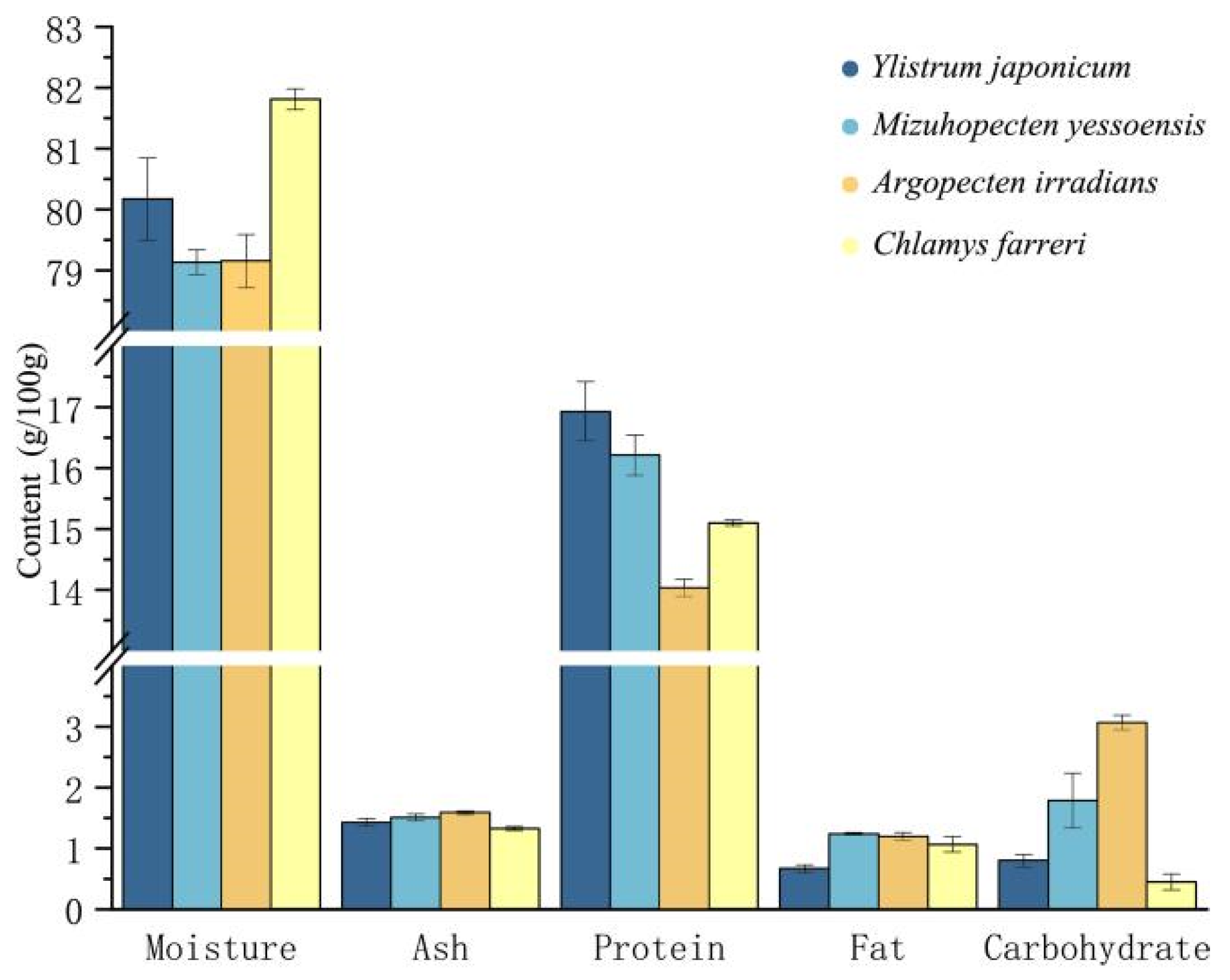
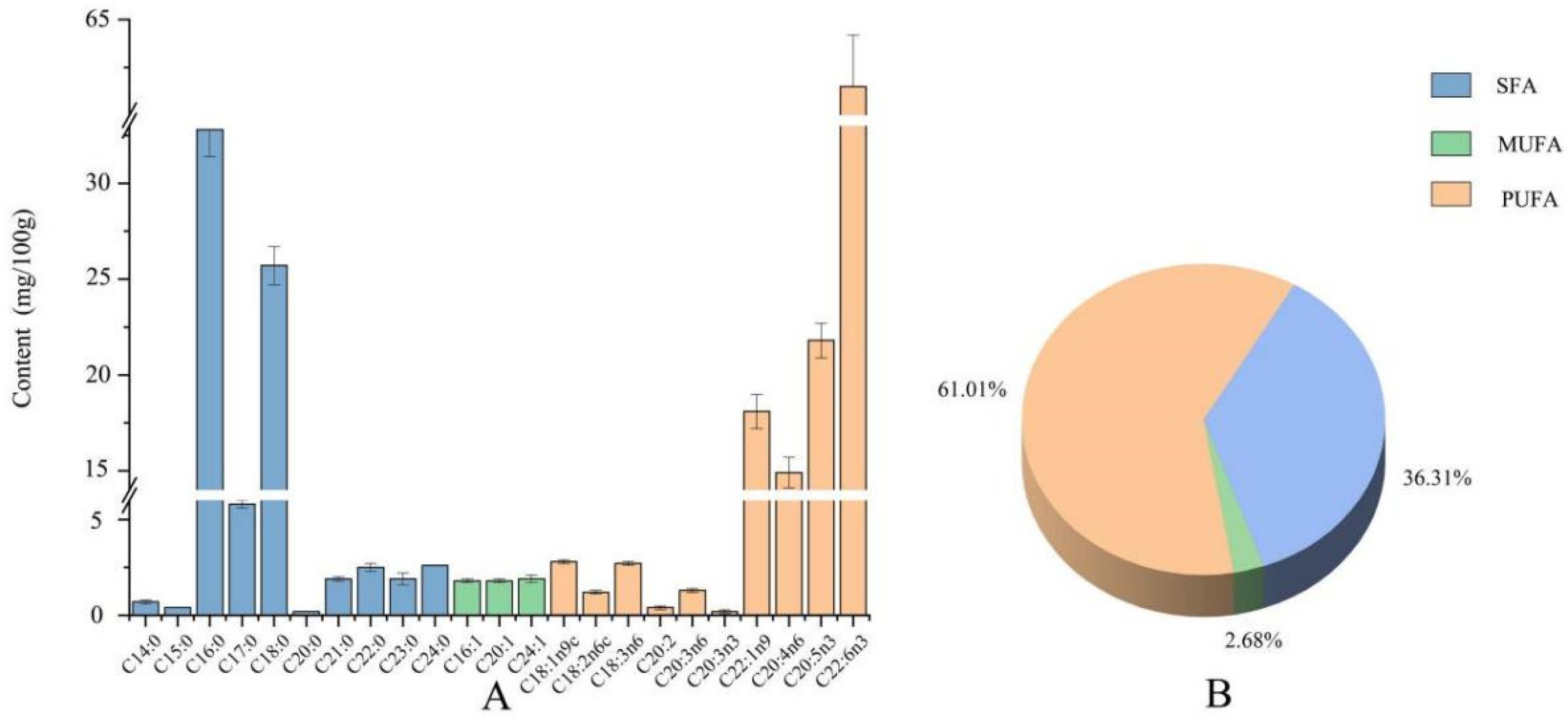
3.5. Nutrient Composition Analysis of Adductor Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, W.J.; Liang, G.Y. A proliminary study on artificial breeding of Amussium japonica (Gmelin). Trans. Oceanol. Limnol. 1989, 04, 86–93. (In Chinese) [Google Scholar] [CrossRef]
- Ye, W.J.; Liang, G.Y. A preliminary observation on the ecology of Amussium japonica (Gmelin). Chin. J. Zool. 1990, 25, 5–7. (In Chinese) [Google Scholar]
- Pal-Won, S.; Ee-Yung, C. Gonadal development, first sexual maturity and sex ratio of the sun and moon scallop Amusium japonicum japonicum on the coastal waters of Jejudo, Korea. Dev. Reprod. 2005, 9, 95–103. [Google Scholar]
- Zhang, S.P. Atlas of Marine Mollusks of China; China Ocean Press: Beijing, China, 2008; p. 295. (In Chinese) [Google Scholar]
- Liu, J.Y. Checklist of Marine Biota of China Seas; China Science Press: Beijing, China, 2008; p. 558. (In Chinese) [Google Scholar]
- Hu, L. Species diversity and geographical distribution of marine, benthic, shell-bearing mollusks on the coast and adjacent area of Pingtan Island, Fujian Province. Biodivers. Sci. 2021, 29, 1403–1410. (In Chinese) [Google Scholar] [CrossRef]
- Pal-Won, S.; Ee-Yung, C. Annual reproductive cycle and size at first sexual maturity of the sun and moon scallop Amusium Japonlcum Japonicum (Gmelin, 1791) (Bivalvia: Pectinidae) in the coastal waters of Jejudo Korea. Malacologia 2009, 51, 119–129. [Google Scholar] [CrossRef]
- Pal-Won, S.; Dong-Soo, H.; Sum, R.; Dae-Soo, C. Studies on the age and growth fo sun and moon scallop, Amusium japonicum japonicum. J. Aquac. 1996, 9, 409–417. [Google Scholar]
- Wang, X.; Yang, Z.; Jiang, L.; Li, Z.; Dong, X.P.; Sui, M.; Yin, C.; Shen, X.; Zhao, A.Q.; Hu, J.; et al. Assessment of germplasm resource and detection of genomic signature under artificial selection of Zhikong scallop (Chlamys farreri). Aquaculture 2023, 574, 739730. [Google Scholar] [CrossRef]
- Gong, X.; Chang, J.; Zhang, Y.; Li, D.; Xia, N.; Wang, J.; Sun, Z. Structural changes of the interface material of scallop adductor under ultra-high pressure. Processes 2023, 11, 521. [Google Scholar] [CrossRef]
- Newkirk, G.F. Review of the genetics and the potential for selective breeding of commercially important bivalves. Aquaculture 1980, 19, 209–228. [Google Scholar] [CrossRef]
- Li, A.; Li, J.; Liu, L.; Xue, S.; Zhu, L.; Mao, Y. Path analysis of body weight and shell morphological traits in two Pacific abalone (Haliotis discus hannai) strains. Aquac. Int. 2024, 32, 2493–2505. [Google Scholar] [CrossRef]
- Du, M.R.; Wang, B.; Zhang, J.H.; Fang, J.G. Correlation and path analysis on shell length and shell height to wet weight of Chlamys farreri at one-year old. Chin. Agric. Sci. Bull. 2012, 28, 136–139. (In Chinese) [Google Scholar]
- Xiao, L.Y.; Ma, G.F.; Guo, W.X.; Yan, X.W.; Yang, F.; Zhang, G.F. Correlation and path analysis to quantitative traits of Mactra chinensis in different sexes. Chin. Agric. Sci. Bull. 2012, 28, 115–119. [Google Scholar]
- Zheng, G.C.; Wu, H.Y.; Guo, M.M.; Zhao, C.X.; Fu, S.L.; Tan, Z.J. The relationship between morphometric traits and bodyweight of Yesso scallop Patinopecten yessoensis. Chin. Fish. Qual. Stand. 2016, 6, 1–6. (In Chinese) [Google Scholar]
- Xu, X.Y.; Hu, L.P.; He, J.B.; Jiang, L.M.; Yang, T.; Shi, W.K. Effects of morphometric traits of Argopecten irradians on its quality traits. Trans. Oceanol. Limnol. 2023, 45, 73–78. (In Chinese) [Google Scholar] [CrossRef]
- Yu, D.; Chen, W.; Teng, W.M.; Xie, X.; Yu, Z.A.; Lin, D.C. Correlation and path analysis between morphological traits and body weight of scallop Placopecten Magellanicus. Fish. Sci. 2023, 42, 496–501. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, C.N.; Yu, T.; Zheng, Y.; Li, B.; Wang, X.; Cai, Z.; Wang, X.M.; Ren, L.Q.; Xu, S.; Wu, Y.P.; et al. Correlation and path analysis of traits of male and female chlamys farreri with different shell colors. J. Fish Sci. China 2023, 30, 268–283. [Google Scholar]
- Shin, J.M.; Hwang, Y.O.; Tu, O.J.; Jo, H.B.; Kim, J.H.; Chae, Y.Z.; Rhu, K.H.; Park, S.K. Comparison of Different Methods to Quantify Fat Classes in Bakery Products. Food Chem. 2013, 136, 703–709. [Google Scholar] [CrossRef]
- Consultation, J.F.W.E. Protein Quality Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy, 1991; Volume 51, pp. 1–66. [Google Scholar]
- Salimon, J.; Omar, T.A.; Salih, N. An Accurate and Reliable Method for Identification and Quantification of Fatty Acids and Trans Fatty Acids in Food Fats Samples Using Gas Chromatography. Arab. J. Chem. 2017, 10, 1875–1882. [Google Scholar] [CrossRef]
- Yang, F.; Huang, X.; Zhang, C.; Zhang, M.; Huang, C.; Yang, H. Amino Acid Composition and Nutritional Value Evaluation of Chinese Chestnut (Castanea mollis sima Blume) and Its Protein Subunit. RSC Adv. 2018, 8, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Bai, Z.; Fu, L.; Zhang, G.; Li, J. Genetic analysis of early growth traits of the triangle shell mussel, Hyriopsis Cumingii, as an insight for potential genetic improvement to pearl quality and yield. Aquac. Int. 2012, 20, 927–933. [Google Scholar] [CrossRef]
- Seyoum, M.; Alamerew, S.; Bantte, K. Genetic variability, heritability, correlation coefficient and path analysis for yield and yield related traits in upland rice (Oryza sativa L.). J. Plant Sci. 2012, 7, 13–22. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, H.Y.; Ma, C.Y.; Li, S.J.; Liu, Y.X.; Qiao, Z.G.; Ma, L.B. Characteristics of growth traits and their effects on body weight of G1 individuals in the mud crab (Scylla paramamosain). Genet. Mol. Res. 2014, 13, 6050–6059. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, C.Y.; Chen, W.; Ma, H.Y.; Zhang, H.; Meng, Y.Y.; Ni, Y.; Ma, L.B. Optimization of selective breeding through analysis of morphological traits in chinese sea bass (Lateolabrax maculatus). Genet Mol. Res. 2016, 15, gmr.15038285. [Google Scholar] [CrossRef]
- Yu, L.; Yin, W.; Han, S.; Zhao, T.; Hao, Z.; Yin, D.; Zhan, Y.; Chang, Y. Morphological trait correlations, gonadal development characteristics and pleopod nutrient compositions of the whelk Volutharpa perryi. Fishes 2024, 9, 72. [Google Scholar] [CrossRef]
- Cao, S.M.; Wang, H.; Chen, W.; Liang, W.F.; Wang, J.; Zou, J.W. Analysis, evaluation and comparison of nutritive composition in rock scallop Crassadoma gigantean with three chinese scallops. J. Dalian Ocean Univ. 2016, 31, 544–550. (In Chinese) [Google Scholar] [CrossRef]
- Loukovitis, D.; Sarropoulou, E.; Batargias, C.; Apostolidis, A.P.; Kotoulas, G.; Tsigenopoulos, C.S.; Chatziplis, D. Quantitative trait loci for body growth and sex determination in the hermaphrodite teleost fish Sparus aurata L. Anim. Genet. 2012, 43, 753–759. [Google Scholar] [CrossRef]
- Tanyaros, S.; Tarangkoon, W. Variability in larval period, post-setting growth and survival of the oyster Crassostrea Belcheri produced by gamete stripping method. Agric. Nat. Resour. 2016, 50, 295–298. [Google Scholar] [CrossRef]
- Muto, N.; Kawasaki, T.; Kakioka, R.; Nagano, A.J.; Shimizu, Y.; Inose, S.; Shimizu, Y.; Takahashi, H. Genetic architectures of postmating isolation and morphology of two highly diverged rockfishes (genus Sebastes). J. Hered. 2023, 114, 231–245. [Google Scholar] [CrossRef]
- Norman, R.; Crumlish, M.; Stetkiewicz, S. The importance of fisheries and aquaculture production for nutrition and food security. Rev. Sci. Tech. (Int. Off. Epizoot.) 2019, 38, 395–407. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, W.; Tang, R.; Li, L.; Refaey, M.M.; Li, D. Thermally processed diet greatly affects profiles of amino acids rather than fatty acids in the muscle of carnivorous Silurus meridionalis. Food Chem. 2018, 256, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Thilagavathi, M.; Christy, P.D.A. Nutritional value of marine bivalve, Donax variabilis (Linnaeus 1758) from Porayar coastal area, Nagapattinam District Tamil Nadu India. Pramana Res. J. 2019, 9, 812–819. [Google Scholar]
- Xie, R.T.; Amenyogbe, E.; Chen, G.; Huang, J. Effects of feed fat level on growth performance, body composition and serum biochemical indices of hybrid grouper (Epinephelus fuscoguttatus × Epinephelus polyphekadion). Aquaculture 2021, 530, 735813. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Wang, Y.; Zhang, D.C.; Su, T.F.; Wu, K.C. Analysis and evaluation of nutritional components of Amusium Pleuronecte. Mar. Sci. 2011, 35, 87–91. (In Chinese) [Google Scholar]
- Ren, H.; Huang, H.; Yang, N.; Wu, D.P.; Yang, R. Analysis and evaluation of nutritional components in edible part of Chlamys nobilis. Food Ind. 2015, 36, 279–282. (In Chinese) [Google Scholar]
- Wright, A.C.; Fan, Y.; Baker, G. Nutritional value and food safety of bivalve molluscan shellfish. J. Shellfish Res. 2018, 37, 695–708. [Google Scholar] [CrossRef]
- Gunarathne, R.; Guan, X.; Feng, T.; Zhao, Y.; Lu, J. L-lysine dietary supplementation for childhood and adolescent growth: Promises and precautions. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, J.; Yu, J.; Dou, J.; Ning, Y.; Qi, B.; Li, Y. Effects of l-arginine/l-lysine modifications on the protein structure, binding interactions, and functional properties of soy protein hydrolysate. Food Hydrocoll. 2024, 146, 109319. [Google Scholar] [CrossRef]
- Zhu, W.L.; Zhang, C.X.; Tan, K.S.; Wang, B.P.; Huang, R.H.; Wen, J.H.; Xu, B.; Liu, X.; Lichu, L.; Zheng, H. Variation of lipids and fatty acids in noble scallop Chlamys nobilis under low temperature stress. Aquaculture 2022, 554, 738121. [Google Scholar] [CrossRef]
- Mei, X.; Ou, Z.Q. Research progress of the physiological function and mechanism of two kinds of fatty acid (EPA and DHA) in the fish oil of deep sea. Food Sci. 2005, 26, 522–526. (In Chinese) [Google Scholar]
- Prato, E.; Fanelli, G.; Parlapiano, I.; Biandolino, F. Bioactive fatty acids in seafood from ionian sea and relation to dietary recommendations. Int. J. Food Sci. Nutr. 2020, 71, 693–705. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]

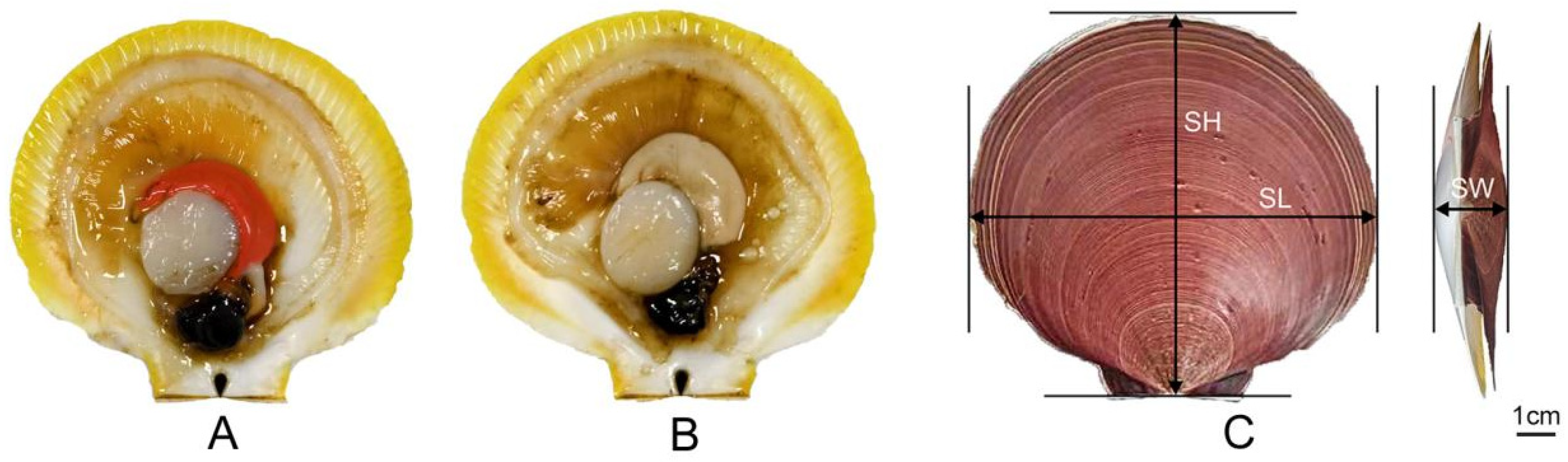
| Individual | Parameter | SL/mm | SW/mm | SH/mm | WW/g | FW/g | GW/g | AW/g |
|---|---|---|---|---|---|---|---|---|
| All individuals | Mean | 100.60 | 20.57 | 97.37 | 79.63 | 27.68 | 1.83 | 10.56 |
| SD | 4.42 | 1.09 | 3.95 | 18.08 | 6.62 | 0.60 | 2.42 | |
| CV/% | 4.39 | 5.29 | 4.05 | 22.70 | 23.90 | 32.78 | 22.92 | |
| P (K-S) | 0.51 | 0.41 | 0.54 | 0.44 | 0.42 | 0.12 | 0.35 | |
| Female individuals | Mean | 101.04 | 20.74 | 97.16 | 80.87 * | 28.53 * | 2.00 * | 10.75 |
| SD | 4.51 | 1.12 | 4.00 | 18.40 | 6.82 | 0.61 | 2.58 | |
| CV/% | 4.46 | 5.40 | 4.12 | 22.80 | 23.90 | 30.50 | 24.00 | |
| P (K-S) | 0.47 | 0.42 | 0.61 | 0.41 | 0.49 | 0.14 | 0.41 | |
| Male individuals | Mean | 100.21 | 20.42 | 97.56 | 78.49 * | 26.91 * | 1.67 * | 10.38 |
| SD | 4.41 | 1.08 | 3.96 | 18.04 | 6.52 | 0.59 | 2.30 | |
| CV/% | 4.40 | 5.29 | 4.06 | 22.98 | 24.23 | 35.33 | 22.16 | |
| P (K-S) | 0.46 | 0.37 | 0.64 | 0.51 | 0.47 | 0.11 | 0.34 |
| Individual | Character | SW | SH | WW | FW | GW | AW |
|---|---|---|---|---|---|---|---|
| All individuals | SL | 0.972 ** | 0.987 ** | 0.967 ** | 0.952 ** | 0.796 ** | 0.952 ** |
| SW | 1 | 0.958 ** | 0.986 ** | 0.969 ** | 0.817 ** | 0.960 ** | |
| SH | 1 | 0.953 ** | 0.934 ** | 0.767 ** | 0.940 ** | ||
| WW | 1 | 0.987 ** | 0.841 ** | 0.979 ** | |||
| FW | 1 | 0.889 ** | 0.965 ** | ||||
| GW | 1 | 0.786 ** | |||||
| Female individuals | SL | 0.970 ** | 0.990 ** | 0.962 ** | 0.956 ** | 0.823 ** | 0.938 ** |
| SW | 1 | 0.968 ** | 0.984 ** | 0.978 ** | 0.853 ** | 0.955 ** | |
| SH | 1 | 0.956 ** | 0.957 ** | 0.823 ** | 0.941 ** | ||
| WW | 1 | 0.988 ** | 0.852 ** | 0.981 ** | |||
| FW | 1 | 0.880 ** | 0.976 ** | ||||
| GW | 1 | 0.848 ** | |||||
| Male individuals | SL | 0.975 ** | 0.985 ** | 0.973 ** | 0.954 ** | 0.784 ** | 0.964 ** |
| SW | 1 | 0.951 ** | 0.987 ** | 0.972 ** | 0.814 ** | 0.970 ** | |
| SH | 1 | 0.953 ** | 0.937 ** | 0.770 ** | 0.940 ** | ||
| WW | 1 | 0.988 ** | 0.805 ** | 0.982 ** | |||
| FW | 1 | 0.862 ** | 0.964 ** | ||||
| GW | 1 | 0.731 ** |
| Individuals | Qualitative Trait | Morphological Trait | Relative Coefficient | Direct Effect | Indirect Effect | |||
|---|---|---|---|---|---|---|---|---|
| SL | SW | SH | Σ | |||||
| All individuals | WW | SL | 0.967 ** | 0.164 | —— | 0.808 | −0.005 | 0.803 |
| SW | 0.986 ** | 0.831 | 0.159 | —— | −0.005 | 0.154 | ||
| SH | 0.953 ** | −0.005 | 0.162 | 0.796 | —— | 0.958 | ||
| FW | SL | 0.952 ** | 0.356 | —— | 0.773 | −0.177 | 0.596 | |
| SW | 0.969 ** | 0.795 | 0.346 | —— | −0.171 | 0.175 | ||
| SH | 0.934 ** | −0.179 | 0.351 | 0.762 | —— | 1.113 | ||
| SW | SL | 0.796 ** | 0.705 | —— | 0.794 | −0.663 | 0.131 | |
| SW | 0.817 ** | 0.776 | 0.685 | —— | −0.644 | 0.621 | ||
| SH | 0.767 ** | −0.672 | 0.696 | 0.743 | —— | 1.439 | ||
| AW | SL | 0.952 ** | 0.296 | —— | 0.612 | 0.043 | 0.655 | |
| SW | 0.960 ** | 0.630 | 0.288 | —— | 0.042 | 0.330 | ||
| SH | 0.940 ** | 0.044 | 0.292 | 0.604 | —— | 0.896 | ||
| Female individuals | WW | SL | 0.962 ** | 0.254 | —— | 0.868 | −0.161 | 0.707 |
| SW | 0.984 ** | 0.895 | 0.246 | —— | −0.158 | 0.088 | ||
| SH | 0.956 ** | −0.163 | 0.251 | 0.866 | —— | 1.117 | ||
| FW | SL | 0.956 ** | 0.006 | —— | 0.834 | 0.114 | 0.948 | |
| SW | 0.978 ** | 0.860 | 0.006 | —— | 0.111 | 0.117 | ||
| SH | 0.957 ** | 0.115 | 0.006 | 0.832 | —— | 0.838 | ||
| SW | SL | 0.823 ** | 0.014 | —— | 0.943 | −0.135 | 0.808 | |
| SW | 0.853 ** | 0.972 | 0.014 | —— | −0.132 | −0.118 | ||
| SH | 0.823 ** | −0.136 | 0.014 | 0.941 | —— | 0.955 | ||
| AW | SL | 0.922 ** | −0.005 | —— | 0.727 | 0.216 | 0.943 | |
| SW | 0.925 ** | 0.749 | −0.005 | —— | 0.211 | 0.206 | ||
| SH | 0.897 ** | 0.218 | −0.005 | 0.725 | —— | 0.720 | ||
| Male individuals | WW | SL | 0.973 ** | 0.137 | —— | 0.776 | 0.060 | 0.836 |
| SW | 0.987 ** | 0.796 | 0.134 | —— | 0.058 | 0.192 | ||
| SH | 0.953 ** | 0.061 | 0.135 | 0.757 | —— | 0.892 | ||
| FW | SL | 0.954 ** | −0.074 | —— | 0.852 | 0.176 | 1.028 | |
| SW | 0.972 ** | 0.874 | −0.072 | —— | 0.170 | 0.098 | ||
| SH | 0.937 ** | 0.179 | −0.073 | 0.831 | —— | 0.758 | ||
| SW | SL | 0.784 ** | −0.406 | —— | 0.967 | 0.225 | 1.192 | |
| SW | 0.814 ** | 0.992 | −0.396 | —— | 0.217 | −0.179 | ||
| SH | 0.770 ** | 0.228 | −0.400 | 0.943 | —— | 0.543 | ||
| AW | SL | 0.964 ** | 0.494 | —— | 0.585 | −0.115 | 0.470 | |
| SW | 0.970 ** | 0.600 | 0.482 | —— | −0.111 | 0.371 | ||
| SH | 0.940 ** | −0.117 | 0.487 | 0.571 | —— | 1.058 | ||
| Individuals | Qualitative Trait | Morphological Trait | Coefficient of Determination | |||
|---|---|---|---|---|---|---|
| SL | SW | SH | In Total | |||
| All individuals | WW | SL | 0.027 | 0.265 | −0.002 | 0.974 |
| SW | —— | 0.691 | −0.008 | |||
| SH | —— | —— | 0.001 | |||
| FW | SL | 0.127 | 0.550 | −0.126 | 0.942 | |
| SW | —— | 0.632 | −0.273 | |||
| SH | —— | —— | 0.032 | |||
| GW | SL | 0.497 | 1.064 | −0.935 | 0.681 | |
| SW | —— | 0.602 | −0.999 | |||
| SH | —— | —— | 0.452 | |||
| AW | SL | 0.088 | 0.363 | 0.026 | 0.929 | |
| SW | —— | 0.397 | 0.053 | |||
| SH | —— | —— | 0.002 | |||
| Female individuals | WW | SL | 0.065 | 0.441 | −0.082 | 0.969 |
| SW | —— | 0.801 | −0.283 | |||
| SH | —— | —— | 0.027 | |||
| FW | SL | 0.000 | 0.010 | 0.001 | 0.955 | |
| SW | —— | 0.740 | 0.191 | |||
| SH | —— | —— | 0.013 | |||
| GW | SL | 0.000 | 0.026 | −0.004 | 0.729 | |
| SW | —— | 0.945 | −0.256 | |||
| SH | —— | —— | 0.018 | |||
| AW | SL | 0.000 | −0.007 | −0.002 | 0.915 | |
| SW | —— | 0.561 | 0.316 | |||
| SH | —— | —— | 0.047 | |||
| Male individuals | WW | SL | 0.019 | 0.213 | 0.016 | 0.978 |
| SW | —— | 0.634 | 0.092 | |||
| SH | —— | —— | 0.004 | |||
| FW | SL | 0.005 | −0.126 | −0.026 | 0.947 | |
| SW | —— | 0.764 | 0.298 | |||
| SH | —— | —— | 0.032 | |||
| GW | SL | 0.165 | −0.785 | −0.182 | 0.664 | |
| SW | —— | 0.984 | 0.430 | |||
| SH | —— | —— | 0.052 | |||
| AW | SL | 0.244 | 0.578 | −0.114 | 0.948 | |
| SW | —— | 0.360 | −0.134 | |||
| SH | —— | —— | 0.014 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Han, Y.; Jia, M.; Cai, L.; Zhao, B.; Chang, Y.; Tian, Y. Morphological Trait Correlations and Nutrient Compositions of the Japanese Moon Scallop Ylistrum japonicum in China. Fishes 2025, 10, 45. https://doi.org/10.3390/fishes10020045
Xie Y, Han Y, Jia M, Cai L, Zhao B, Chang Y, Tian Y. Morphological Trait Correlations and Nutrient Compositions of the Japanese Moon Scallop Ylistrum japonicum in China. Fishes. 2025; 10(2):45. https://doi.org/10.3390/fishes10020045
Chicago/Turabian StyleXie, Yaoyu, Yida Han, Menghao Jia, Linxuan Cai, Bin Zhao, Yaqing Chang, and Ying Tian. 2025. "Morphological Trait Correlations and Nutrient Compositions of the Japanese Moon Scallop Ylistrum japonicum in China" Fishes 10, no. 2: 45. https://doi.org/10.3390/fishes10020045
APA StyleXie, Y., Han, Y., Jia, M., Cai, L., Zhao, B., Chang, Y., & Tian, Y. (2025). Morphological Trait Correlations and Nutrient Compositions of the Japanese Moon Scallop Ylistrum japonicum in China. Fishes, 10(2), 45. https://doi.org/10.3390/fishes10020045






