Analysis of the Polyculture Model of the Bivalves Anadara broughtonii and Chlamys farreri in Suspension Cages in Shallow Seas
Abstract
1. Introduction
2. Materials and Methods
2.1. The Conditions of the Marine Area
2.2. The Arrangement of Rafts
2.3. Seed Selection and the Role of the Hanging Water Layer
2.4. Comparative Analysis of Polyculture Specifications
2.5. Comparative Experiment on Polyculture Ratio
2.6. Data Collection and Processing
3. Results
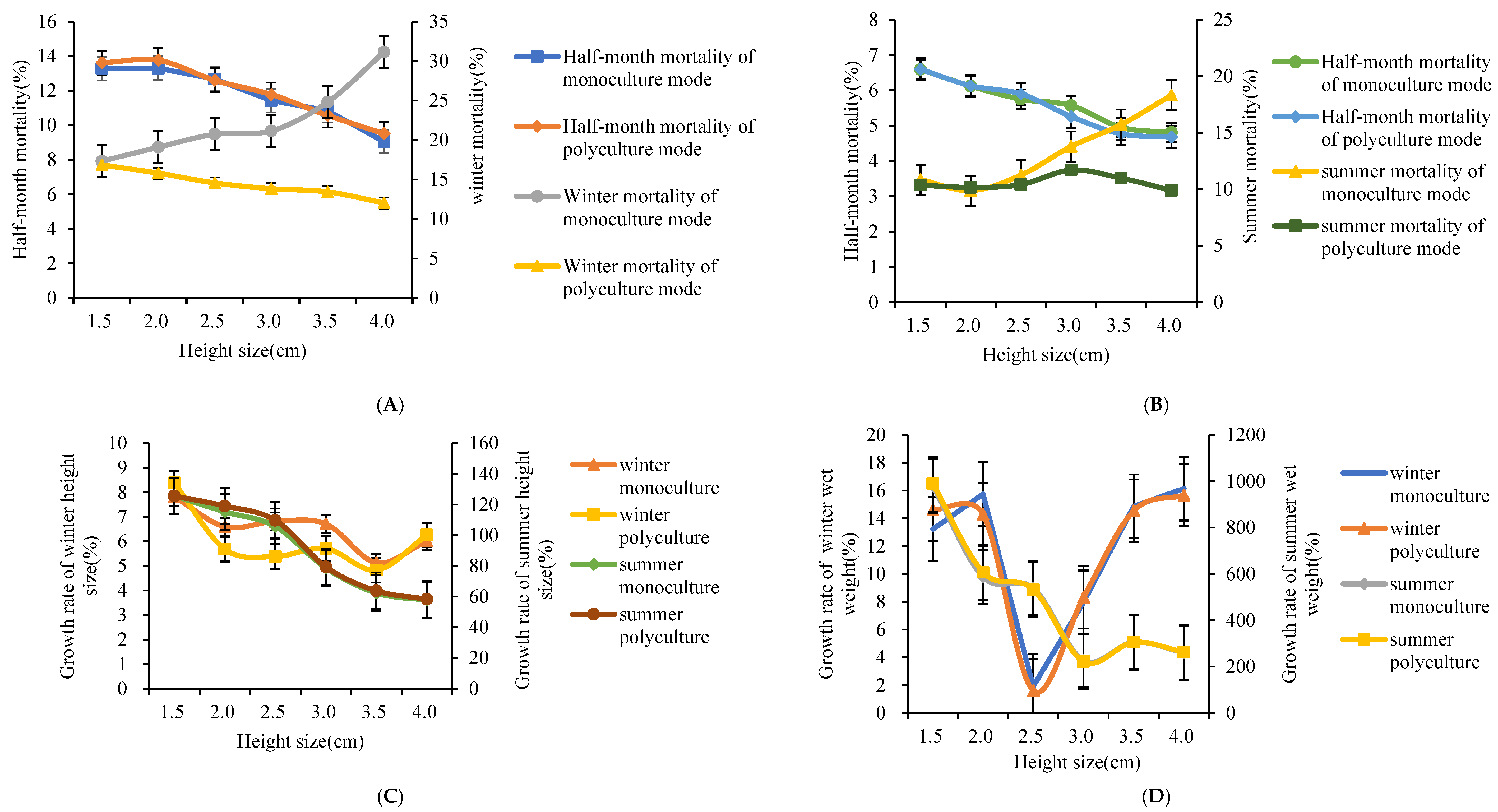
3.1. Analysis of Different Size Specifications in A. broughtonii
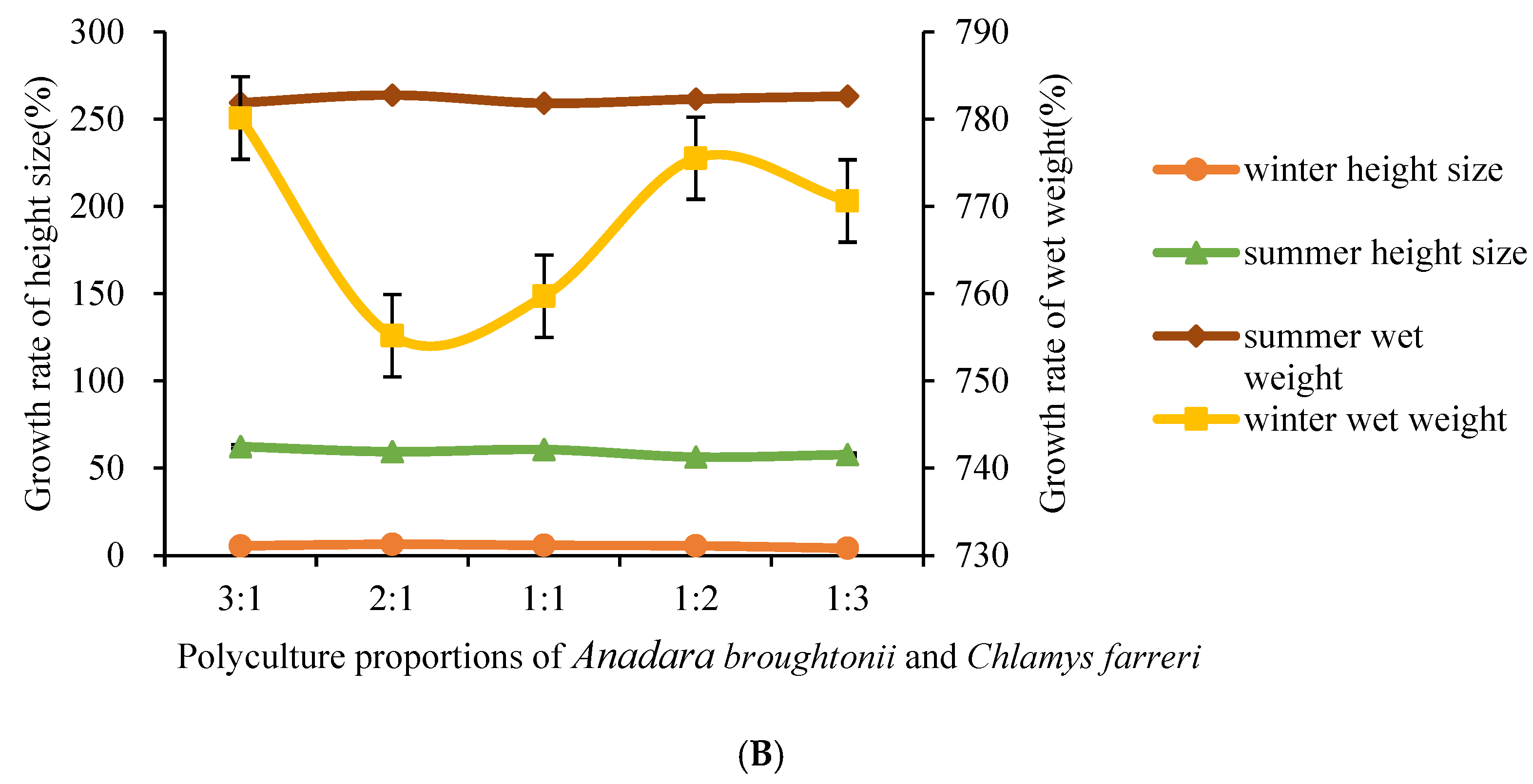
3.2. Analysis of Various Proportions of A. broughtsnii
3.3. Analysis of the Growth and Survival of Chlamys farreri
3.4. Comparison of Breeding Efficiency
4. Discussion
4.1. The Relationship between Height, Proportion, and Growth Survival in Cultured Shellfish
4.2. Analysis of Different Aquaculture Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, H.Y.; Park, J.Y. Ten new highly polymorphic microsatellite loci in the blood clam Scapharca broughtonii. Mol. Ecol. 2005, 5, 896–898. [Google Scholar] [CrossRef]
- Bai, C.-M.; Xin, L.-S.; Rosani, U.; Wu, B.; Wang, Q.-C.; Duan, X.-K.; Liu, Z.-H.; Wang, C.-M. Chromosomal-level assembly of the blood clam, Scapharca (Anadara) broughtonii, using long sequence reads and Hi-C. Gigascience 2019, 8, 67. [Google Scholar] [CrossRef]
- Sugiura, D.; Katayama, S.; Sasa, S.; Sasaki, K. Age and growth of the ark shell Scapharca broughtonii (Bivalvia, Arcidae) in Japanese waters. J. Shellfish Res. 2014, 33, 315–324. [Google Scholar] [CrossRef]
- Liu, Y.; Kurokawa, T.; Sekino, M.; Toru, T.; Kazuhito, W. Tandem repeat arrays in the mitochondrial genome as a tool for detecting genetic differences among the ark shell Scapharca broughtonii. Mar. Ecol. 2014, 35, 273–280. [Google Scholar] [CrossRef]
- Nishida, K.; Ishimura, T.; Suzuki, A.; Sasaki, T. Seasonal changes in the shell microstructure of the bloody clam, Scapharca broughtonii (Mollusca: Bivalvia: Arcidae). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 363–364, 99–108. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Kim, E.; Yang, S.-M.; Kim, H.-Y. Rapid on-site identification for three Arcidae species (Anadara kagoshimensis, Tegillarca granosa, and Anadara broughtonii) using ultrafast PCR combined with direct DNA extraction. Foods 2022, 11, 2449. [Google Scholar] [CrossRef]
- Tabakaeva, O.V.; Piekoszewski, W.; Kalenik, T.K.; Maximova, S.N.; Tabakaev, A.V.; Poleshyk, D.V.; Proniewicz, L. Antiradical Activity of Hydrolysates and Extracts from Mollusk A. broughtonii and Practical Application to the Stabilization of Lipids. Foods 2020, 9, 304. [Google Scholar] [CrossRef]
- Tanaka, T.; Aranishi, F. Comparative genetic characterization of ark shell Scapharca broughtonii in Northeast Asia. J. Shellfish Res. 2016, 35, 421–427. [Google Scholar] [CrossRef]
- Wang, W.; Wu, B.; Liu, Z.; Sun, X.; Zhou, L.; Xu, W.; Yu, T.; Zheng, Y.; Zhang, S. Comprehensive analysis on the regulation of differentially expressed of mRNA and ncRNA in different ovarian stages of ark shell Scapharca broughtonii. BMC Genom. 2023, 24, 563. [Google Scholar] [CrossRef]
- Li, R.; Li, Q.; Wang, C. Sibship reconstruction and effective population size estimation in mass spawning ark shell, Scapharca broughtonii based on microsatellite analysis. Genes Genom. 2013, 35, 703–708. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, D.; Wu, B.; Sun, X.; Liu, Z.; Zhao, F.; Lv, Z.; Yang, A.; Zhao, Q.; Zhang, G.; et al. Ark shell Scapharca broughtonii hemocyte response against Vibrio anguillarum challenge. Fish Shellfish Immunol. 2018, 84, 304–311. [Google Scholar] [CrossRef]
- Ruhua, Z.; Yanqing, J.; Kun, Y.; Liu, L.; Zhang, H.; Liu, Y. Genome-wide SNP-based diversity analysis and phylogeographic inference in the ark shell (Anadara broughtonii). Fish. Res. 2024, 270, 106892. [Google Scholar]
- Yihang, W.; Yingqiu, Z.; Jianyu, D.; Zhang, X. Two-sided effects of prolonged hypoxia and sulfide exposure on juvenile ark shells (Anadara broughtonii). Mar. Environ. Res. 2021, 169, 105326. [Google Scholar]
- Xin, L.; Li, C.; Bai, C.; Wang, C. Ostreid Herpesvirus1 infects specific hemocytes in ark clam Scapharca broughtonii. Viruses 2018, 10, 529. [Google Scholar] [CrossRef]
- Xin, L.; Huang, B.; Bai, C.; Wang, C. Validation of housekeeping genes for quantitative mRNA expression analysis in OsHV-1 infected ark clam, Scapharca broughtonii. J. Invertebr. Pathol. 2018, 155, 44–51. [Google Scholar] [CrossRef]
- Xin, L.; Wei, Z.; Bai, C.; Chen, H.; Huang, B.; Wang, C. Influence of temperature on the pathogenicity of Ostreid herpesvirus-1 in ark clam, Scapharca broughtonii. J. Invertebr. Pathol. 2020, 169, 107299. [Google Scholar] [CrossRef]
- Wang, Q.C.; Bai, C.M.; Zhang, T.W.; Wang, C.-M.; Qiu, Z.-X.; Huang, J. Pathogenicity of Ostreid herpesvirus-1 to Scapharca broughtonii. J. Fish. China 2016, 40, 468–474. [Google Scholar]
- Bai, C.-M.; Rosani, U.; Xin, L.-S.; Li, G.-Y.; Li, C.; Wang, Q.-C.; Wang, C.-M. Dual transcriptomic analysis of Ostreid herpesvirus 1 infected Scapharca broughtonii with an emphasis on viral anti-apoptosis activities and host oxidative bursts. Fish Shellfish Immunol. 2018, 82, 554–564. [Google Scholar] [CrossRef]
- Li, Y.; Xue, S.Y.; Li, J.Q. Effect of Cu2+ stress on physiology biochemistry and histopathological structure of Scapharca broughtonii. J. Fish. China 2018, 42, 1531–1540. [Google Scholar]
- Li, Y. Scapharca broughtonii to Cu2+ and Ocean Acidification Stress; Shanghai Ocean University: Shanghai, China, 2018. [Google Scholar]
- Wang, W.M.; Zhang, T.W.; Liu, G.X.; Chen, H.; Tang, L.; Mao, X. Effects of Elevated Seawater pCO2 on the Burrowing Ability and Three Enzymes of Scapharca broughtonii (Bivalvia: Arcidae) Juvenile. Period. Ocean. Univ. China 2018, 48, 19–24. [Google Scholar]
- Nishida, K.; Hayashi, M.; Yamamoto, Y.; Irie, T.; Watanabe, Y.; Kishida, C.; Nojiri, Y.; Sato, M.; Ishimura, T.; Suzuki, A. Effects of elevated CO2 on shell 13C and 18O content and growth rates in the clam Scapharca broughtonii. Geochim. Cosmochim. Acta 2018, 235, 246–261. [Google Scholar] [CrossRef]
- Wu, L.; Wu, B.; Liu, Z.; Yu, T.; Sun, X.; Zhou, L.; Zheng, Y.; Wang, Z. Effects of hypoxic preconditioning on the physiological and biochemical characteristics of Scapharca broughtonii under hypoxia stress. Prog. Fish. Sci. 2023, 44, 98–106. [Google Scholar]
- Xin, B.; Wu, B.; Zhou, L.; Zengguang, Y.; Xiujun, S.; Jiteng, T.; Zhihong, L.; Aiguo, Y. Characters of main measurable traits of Scapharca broughtonii in 4 different geographical populations. Mar. Fish. 2021, 43, 652–660. [Google Scholar]
- Zhao, D.; Zhou, L.Q.; Wu, B.; Xiujun, S.; Feng, Z.; Aiguo, Y.; Zhihong, L.; Qing, Z.; Gaowei, Z.; Xi, C. Response of lysozyme activity to Vibrio anguillarum infection in different tissues of Scapharca broughtonii. J. Fish. China 2020, 44, 480–486. [Google Scholar]
- Huang, B.W. Studies on the Influence of OsHV-1 Infection on the Iron Metabolism of Scapharca broughtonii; Tianjin Agricultural University: Tianjin, China, 2018. [Google Scholar]
- Bai, C.M.; Wang, Q.C.; Morga, B.; Shi, J.; Wang, C.-M. Experimental infection of adult Scapharca broughtonii with Ostreid herpesvirus SB strain. J. Invertebr. Pathol. 2017, 143, 79–82. [Google Scholar] [CrossRef]
- Noh, E.S.; Kim, Y.K.; Park, J.Y.; An, C.M.; Kang, J.H. Genetic differentiation and phylogenetic relationships among Granulated ark (Anadara granosa), Bloody clam (A. subcrenata) and Ark shell (A. broughtonii) inferred from mitochondrial gene sequences. Korean Soc. Fish. Aquac. Sci. Aquac. Div. Acad. Conf. 2015, 10, 302. [Google Scholar]
- Xie, X.; Teng, W.M.; Li, H.L.; Shouwei, Z.; Shangkun, D.; Zuoan, Y.; Xiangfeng, L.; Qingzhi, W. Advances in biology and breeding technology of ark shell Scapharca broughtonii: A review. J. Dalian Ocean. Univ. 2020, 35, 930–938. [Google Scholar]
- Yao, H.W.; Guo, J.; Jing, N.N. Culture status and research progress on geneticdiversity of Scapharca broughtonii. Hebei Fish. 2010, 5, 45–47. [Google Scholar]
- Xie, X.; Teng, W.; Sun, X.; Liang, M.; Du, S.; Zhu, S.; Liu, X.; Nie, H.; Wang, Q. Transcriptomic analysis of the ark shell Scapharca kagoshimensis: De novo assembly and identification of genes and pathways involved growth. Aquac. Rep. 2020, 18, 100522. [Google Scholar] [CrossRef]
- Cai, X.Y. Filter-Feeding Effect of Anadara broughtonii and Comparison Study of Food Sources in Different Proliferation and Culturing Modes; Ocean University of China: Qingdao, China, 2015; Volume 7. [Google Scholar]
- Kim, B.J.; Kim, C.H.; Hong, S.; Park, J.I. Mixed Aquaculture of Anadara broughtonii and A. kagoshimensis (Bivalvia: Arcidae) in Jinju Bay on the South Coast of the Korean Peninsula. J. Coast. Res. 2018, 85, 341–345. [Google Scholar] [CrossRef]
- Zhao, C.N.; Ren, L.; Xu, S.; Wu, Y.; Han, H.; Li, B.; Zheng, Y.; Chen, Y.; Wang, X.; Cai, S.; et al. Experiment research of the cage culture and bottom sowing culture of Scapharca broughtonii in Bohai Gulf. Mar. Sci. 2017, 41, 15–24. [Google Scholar]
- Wang, B.; Han, L.M. A Study on the Basic Situation and Pattern of Shellfish Breeding in China. J. Ocean. Univ. China 2017, 3, 5–12. [Google Scholar]
- Chen, X.Z. Multi-trophic level integrated culture technology model. China Fish. 2020, 10, 76–78. [Google Scholar]
- Wang, M.Z.; Chen, X.L. Preliminary research on several polyculture models of shellfishes. Fish. Sci. 2006, 10, 520–523. [Google Scholar]
- Clements, C.J.; Comeau, A.L. Nitrogen removal potential of shellfish aquaculture harvests in eastern Canada: A comparison of culture methods. Aquac. Rep. 2019, 13, 100183. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Li, G.; Zhao, M.; Li, W. The suitability assessment on site selection for bottom-seeding scallop culture based on analytic hierarchy process. J. Oceanol. Limnol. 2024, 42, 647–663. [Google Scholar] [CrossRef]
- Ge, C.Z.; Zhang, F.; Xu, B.D. Phosphorus accumulation in mudflats in bottom-sowing culture for Manila clam Ruditapes philippinarum zone. Indian J. Geo-Mar. Sci. 2017, 46, 1320–1326. [Google Scholar]
- Wang, W.; Wang, Y.; Li, Y.; Song, Y.; Liu, G.; Yin, Y.; Cai, Y. Effects of physical disturbance of sediment on the cycling of mercury in coastal regions. Sci. Total Environ. 2022, 838, 156298. [Google Scholar] [CrossRef]
- Liu, C.; Liu, G.; Cristiano, S.; Ulgiati, S.; Xu, L.; Yang, Z. Investigating potential ecological benefits from mariculture. Earth’s Future 2024, 12, e2023EF003766. [Google Scholar] [CrossRef]



| Cultured Bivalves | Number of Seedlings | ||||||
|---|---|---|---|---|---|---|---|
| 1.5 cm | 2.0 cm | 2.5 cm | 3.0 cm | 3.5 cm | 4.0 cm | ||
| Polyculture | Anadara broughtonii | 500 | 400 | 300 | 200 | 100 | 50 |
| Chlamys farreri | 500 | 400 | 300 | 200 | 100 | 50 | |
| Monoculture | Anadara broughtonii | 1000 | 800 | 600 | 400 | 100 | 50 |
| Chlamys farreri | 1000 | 800 | 600 | 400 | 100 | 50 | |
| Cultivated Bivalves | Number of Seedlings | ||||
|---|---|---|---|---|---|
| 3:1 | 2:1 | 1:1 | 1:2 | 1:3 | |
| Anadara broughtonii | 75 | 67 | 50 | 33 | 25 |
| Chlamys farreri | 25 | 33 | 50 | 67 | 75 |
| Indicators | The Bottom Sowing Culture of Anadara broughtonii | The Cage Culture of Anadara broughtonii | The Cage Culture of Chlamys farreri | The Polyculture of Anadara broughtonii and Chlamys farreri | |
|---|---|---|---|---|---|
| Anadara broughtonii | Chlamys farreri | ||||
| Survival Rate (%) | 55.60 a | 60.34 a | 86.55 b | 73.36 c | 89.74 b |
| Height (cm) | 4.61 a | 5.42 a | 7.33 b | 5.45 a | 7.46 b |
| Wet Weight (g) | 39.32 a | 47.81 b | 52.46 c | 48.63 bc | 52.91 c |
| Total Weight (Kilograms/Hectare) | 656.35 a | 865.28 b | 1360.57 c | 534.79 ad | 712.09 ab |
| Price of Adult Bivalves (Dollars/Kilograms) | 1.70 a | 2.13 b | 1.42 a | 2.13 b | 1.42 a |
| Unit Input (Dollars/Hectare) | 638.19 a | 992.73 b | 992.73 b | 1063.64 b | |
| Unit Output Value (Dollars/Hectare) | 1116.97 a | 1840.67 b | 1929.59 b | 2147.57 b | |
| Input–output Ratio | 1:1.75 a | 1:1.85 a | 1:1.94 a | 1:2.02 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Ren, L.; Xu, S.; Wu, Y.; Han, H.; Li, B.; Zheng, Y.; Chen, Y.; Wang, X.; Fan, N.; et al. Analysis of the Polyculture Model of the Bivalves Anadara broughtonii and Chlamys farreri in Suspension Cages in Shallow Seas. Fishes 2024, 9, 413. https://doi.org/10.3390/fishes9100413
Zhao C, Ren L, Xu S, Wu Y, Han H, Li B, Zheng Y, Chen Y, Wang X, Fan N, et al. Analysis of the Polyculture Model of the Bivalves Anadara broughtonii and Chlamys farreri in Suspension Cages in Shallow Seas. Fishes. 2024; 9(10):413. https://doi.org/10.3390/fishes9100413
Chicago/Turabian StyleZhao, Chunnuan, Liqun Ren, Shuai Xu, Yuping Wu, Haiying Han, Bo Li, Yanxin Zheng, Yang Chen, Xiwen Wang, Nini Fan, and et al. 2024. "Analysis of the Polyculture Model of the Bivalves Anadara broughtonii and Chlamys farreri in Suspension Cages in Shallow Seas" Fishes 9, no. 10: 413. https://doi.org/10.3390/fishes9100413
APA StyleZhao, C., Ren, L., Xu, S., Wu, Y., Han, H., Li, B., Zheng, Y., Chen, Y., Wang, X., Fan, N., Li, J., Xie, C., Cai, S., & Yu, T. (2024). Analysis of the Polyculture Model of the Bivalves Anadara broughtonii and Chlamys farreri in Suspension Cages in Shallow Seas. Fishes, 9(10), 413. https://doi.org/10.3390/fishes9100413






