This study estimated several life history parameters of the brown-marbled grouper
E. fuscoguttatus population in Saleh Bay, Indonesia using monthly catch data collected during a fisheries landing monitoring program (FLM) from January 2017 to December 2022 and biological samples collected from January 2020 to August 2021. The catch data was used to estimate von Bertalanffy growth parameters, which include the mean asymptotic length (
L∞) and growth coefficient (
k). The biological specimens were used to estimate the length at 50% and 95% maturity (
L50 and
L95), age at maturity, and gonadosomatic index (GSI). This study also used the maximum age of
E. fuscoguttatus on the Great Barrier Reef, Australia calculated by Pears et al. (2006) [
13], as one of the parameters for estimating natural mortality (
M). The life history parameters were employed to estimate key parameters to re-evaluate stock health and the exploitation rate of the
E. fuscoguttatus in Saleh Bay using the length-based spawning potential ratio (LB-SPR) method, providing essential input for its management. This study provides new life history estimation values for
E. fuscoguttatus, offering valuable information for management and scientific authorities in Saleh Bay to evaluate the stock condition and refine management strategies for this species.
4.1. Estimation of Growth and Reproduction
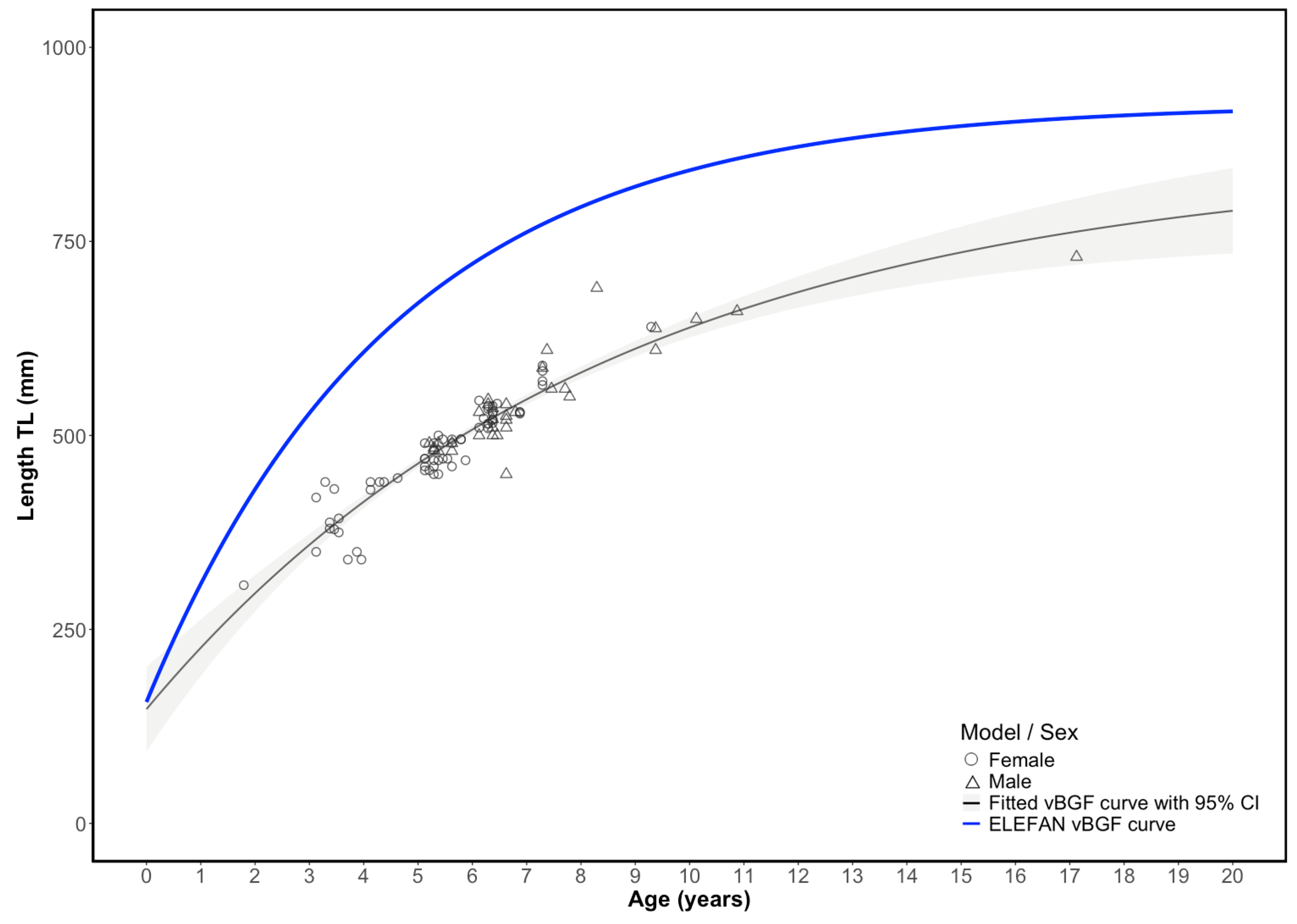
Estimating the von Bertalanffy growth parameters from individual lengths at age yielded lower asymptotic length (
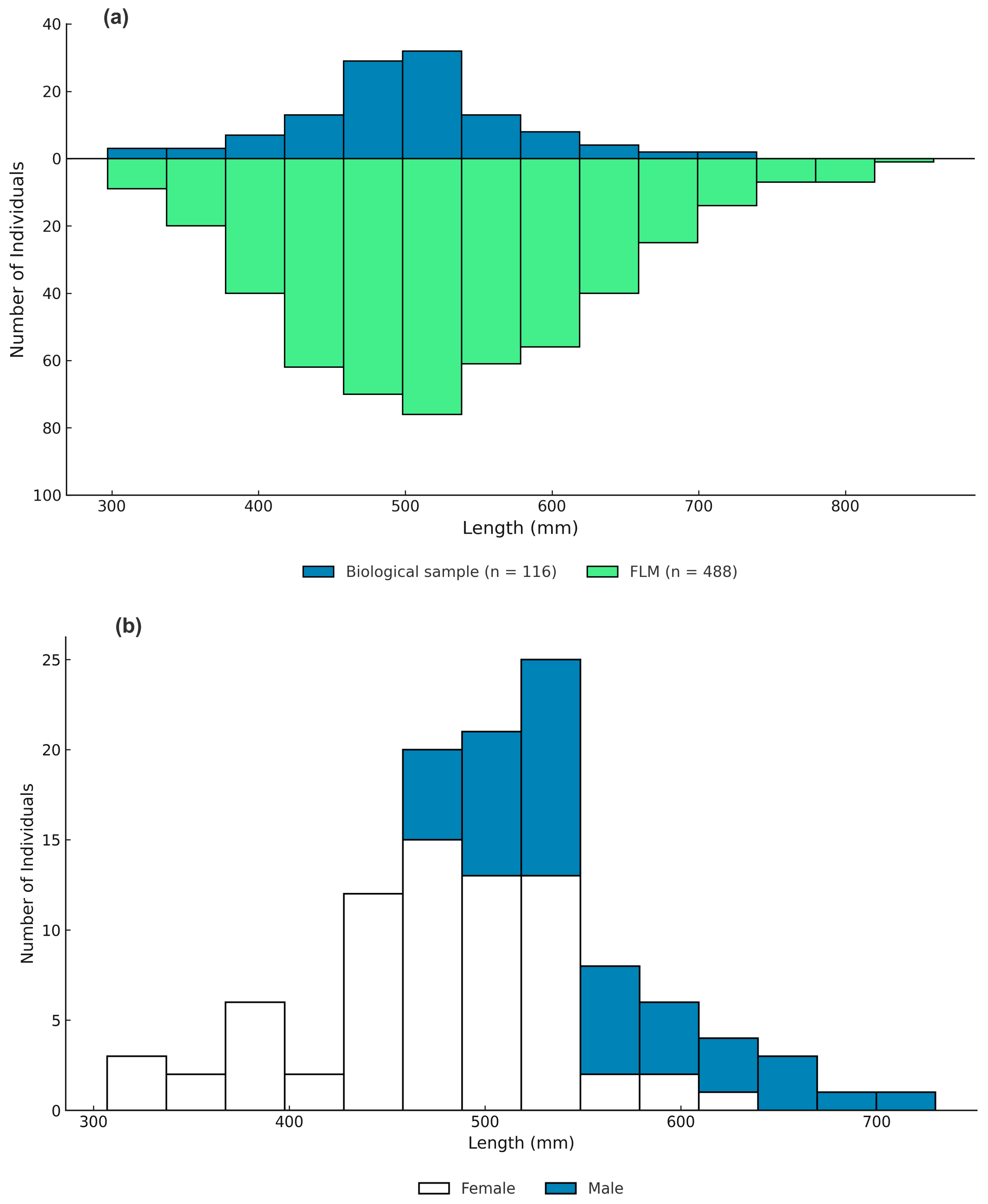
L∞) and growth coefficient (
k) values than those derived from FLM data using ELEFAN optimized method. This discrepancy is expected due to the smaller size range of the biological samples able to be obtained, with the maximum length of fish in the aged sample of only 730 mm TL, compared with 860 mm from the catch data. The estimated
L∞ from individual lengths at age (
L∞ = 855.9 mm) is higher than those reported in another study by Pears et al. (2006) [
13] in the Great Barrier Reef, where the estimated
L∞ was 807 mm in fork length (FL). When converted to total length using the commonly applied multiplier (FL × 1.134), the Pears et al. estimate corresponds to approximately 915 mm TL, which is closer to, but still slightly higher, than the otolith-based
L∞ obtained in the present study. Comparisons with other regions also indicate broader size ranges than those represented in our biological samples. For example, in the Selayar Islands (Indonesia), the maximum observed lengths reached approximately 730 mm for females (n = 473) and 970 mm for males (n = 569) [
29].
The comparatively smaller maximum size in our aged subsample (730 mm TL) likely contributed to the lower L∞ estimate derived from otolith data. As a result, the growth parameter estimates derived from the FLM program catch data, which include a fuller representation of large individuals, are considered more representative of the population in Saleh Bay and were used in the estimation of natural mortality, stock condition, and fishing mortality.
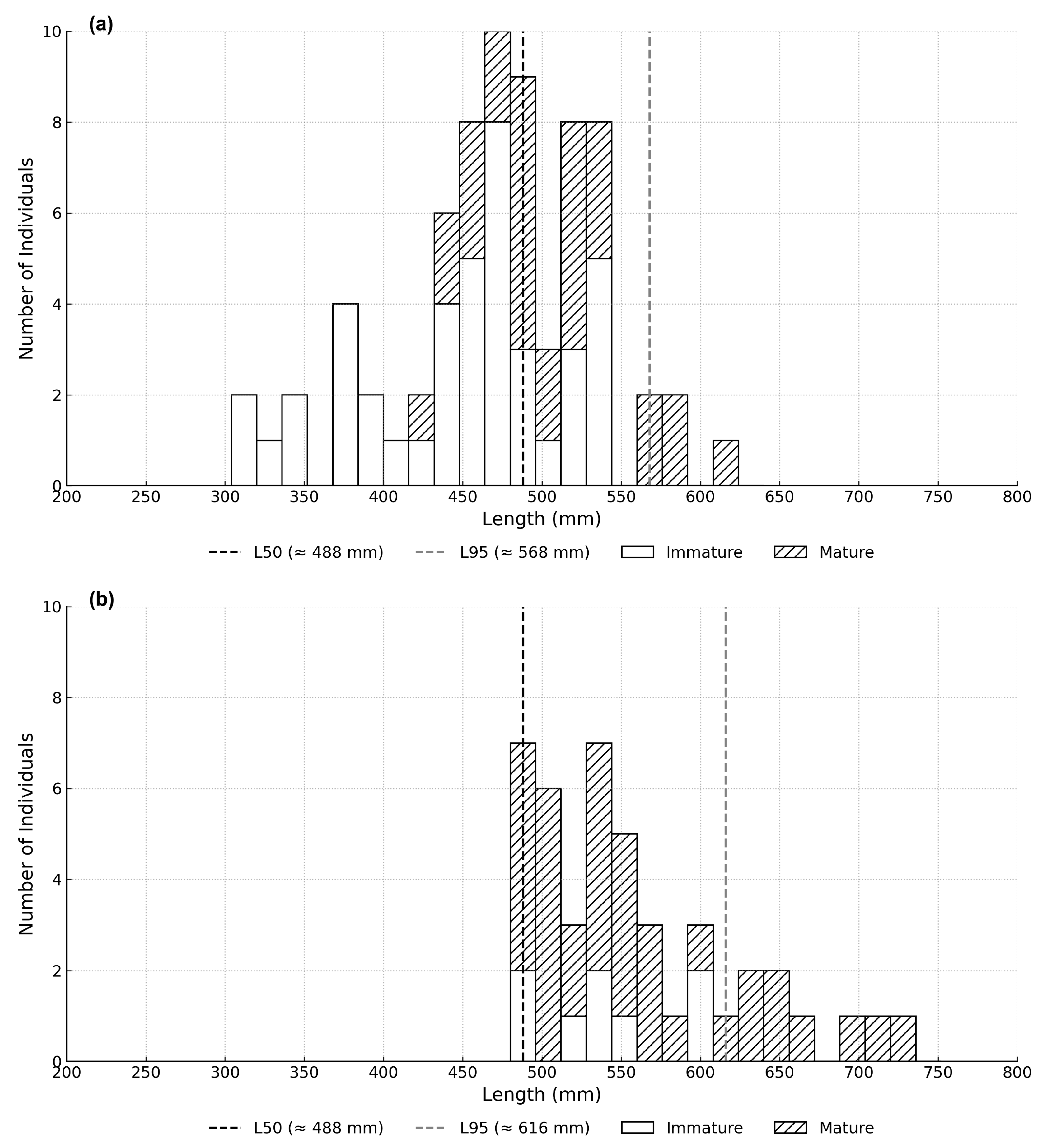
The biological samples under-represented large individuals compared with the FLM data, which introduces uncertainty in the estimation of growth and reproductive parameters, particularly for males after sex change. Because the number of mature individuals was limited and unevenly distributed across size classes, it was not feasible to fit sex-specific logistic maturity ogives. Instead, the L50 was approximated by identifying the first length class in which more than half of females were mature. This approach produced an estimated female L50 of 488 mm TL.
Comparative estimates from other regions indicate that the maturity values obtained in this study fall within the expected biological range for
E. fuscoguttatus across its distribution. Reported female
L50 values include approximately 453 mm in the Selayar Islands [
29], 482 mm in coastal Kenya [
30] and 408 mm on the Great Barrier Reef [
13]. These comparisons suggest that, despite sample size limitations, the maturity parameters derived for the Saleh Bay population are aligned with values documented in other regions. Although these comparisons indicate that the estimates fall within a biologically reasonable range for the species, larger and more balanced maturity samples are needed in future work to support fitting more robust, sex-specific maturity curves.
Despite the limitations of the estimates, this study suggests that
E. fuscoguttatus in Saleh Bay may mature at a smaller size than previously reported and used for estimating the SPR [
19]. The estimated
L50 for
E. fuscoguttatus in Saleh Bay from the current study was is 488 mm, ~91 mm smaller than the previous estimate of 577.8 mm [
19], which was based on the empirical relationship of length at maturity with length frequency data of historical catch, a model proposed by Froese and Binohlan (2000) [
31]. It is important to note that at 95% maturity, the fish has a higher proportion of male individuals (
Figure 6) confirming the protogynous hermaphroditic characteristics of this species. We suggest that the
L50 and the corresponding SPR estimated in the present study more closely reflect the actual conditions in Saleh Bay than previous estimates.
The interpretation of the maturity estimates also requires caution because E. fuscoguttatus is a protogynous hermaphrodite. Sex-specific L50 values were estimated, and both yielded similar results (L50 = 488 mm for females). However, the number of mature males and females was too limited to support the construction of reliable sex-specific maturity ogives or to apply a sex-specific maturity schedule in the SPR analysis. As a result, a single maturity threshold (i.e., that for females but also the one based on limited numbers of males) was used to represent the size at which approximately half of the population is reproductively mature. This should be considered a practical, population-level indicator rather than a precise maturity benchmark for either sex.
This reproductive biology also has implications for interpreting the SPR results. The LB-SPR model assumes that all mature individuals contribute equally to spawning, but this may not fully apply to protogynous groupers. Individuals undergoing sex transition are temporarily inactive reproductively, and heavy fishing on larger fish can reduce male abundance, causing potential sperm limitation even when female biomass is adequate [
32,
33,
34]. These factors could lead to higher apparent SPR values than the true spawning potential. Although these processes could not be modelled with the available data, acknowledging them provides important context when interpreting SPR estimates for this species.
Differences between the growth parameters obtained from otolith aging and those estimated using the ELEFAN reoutine mainly reflect the broader size range present in the FLM dataset, which includes larger individuals than the aged subsample. Previous evaluations of the LB-SPR method show that SPR estimates are strongly influenced by maturity schedules (
L50/
L∞) and the
M/k ratio, and are less sensitive to moderate variation in
L∞ and
k within plausible biological ranges [
17]. The approximately 90 mm difference in
L50 with the previous estimates for
E. fuscoguttatus in the study area is therefore expected to have a much larger effect on SPR than the differences between the growth parameter sets.
From a growth parameter perspective, the lower k associated with the otolith-derived estimates would reduce M and increase F/M, producing somewhat lower SPR values. However, this effect is likely modest relative to the influence of L50, and the general conclusion that the stock is below the SPR target would remain the same. Additional aging data covering a broader size range would help refine growth estimates and support further evaluation of alternative scenarios in future assessments.
Overall, these findings provide a coherent biological context for interpreting the status of E. fuscoguttatus in Saleh Bay. The combined patterns of lower length at maturity, limited representation of large individuals in the catch, and the reproductive characteristics associated with protogyny suggest that the population may have a reduced capacity to sustain fishing pressure compared with previous assumptions. While some uncertainty persists in the underlying life history parameters, the available growth and maturity information consistently points toward constrained reproductive output. This biological interpretation is central to understanding the subsequent SPR results and indicates the need for cautious evaluation of stock condition.
4.2. Estimation of Life History Parameters for Stock Assessment
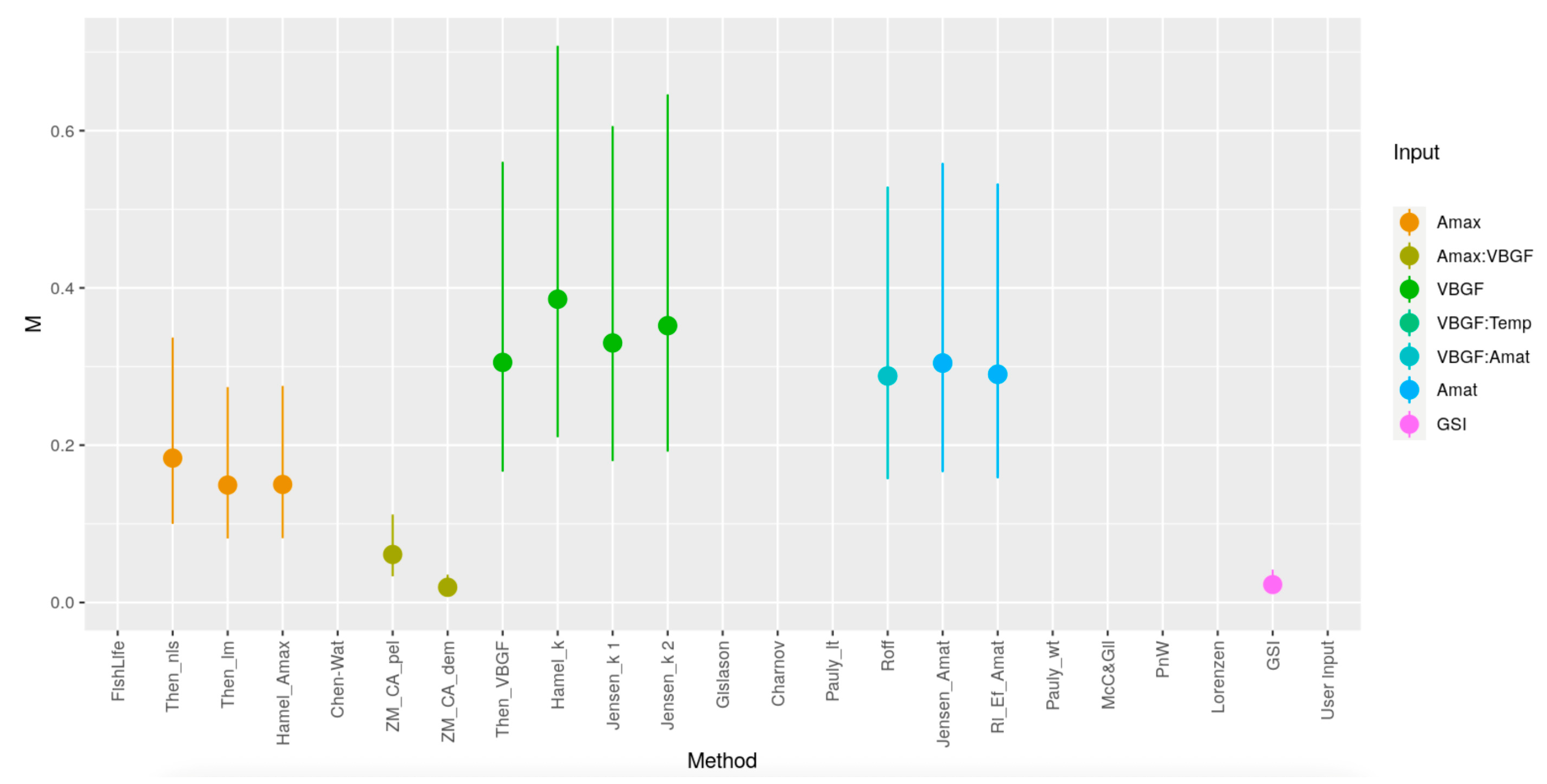
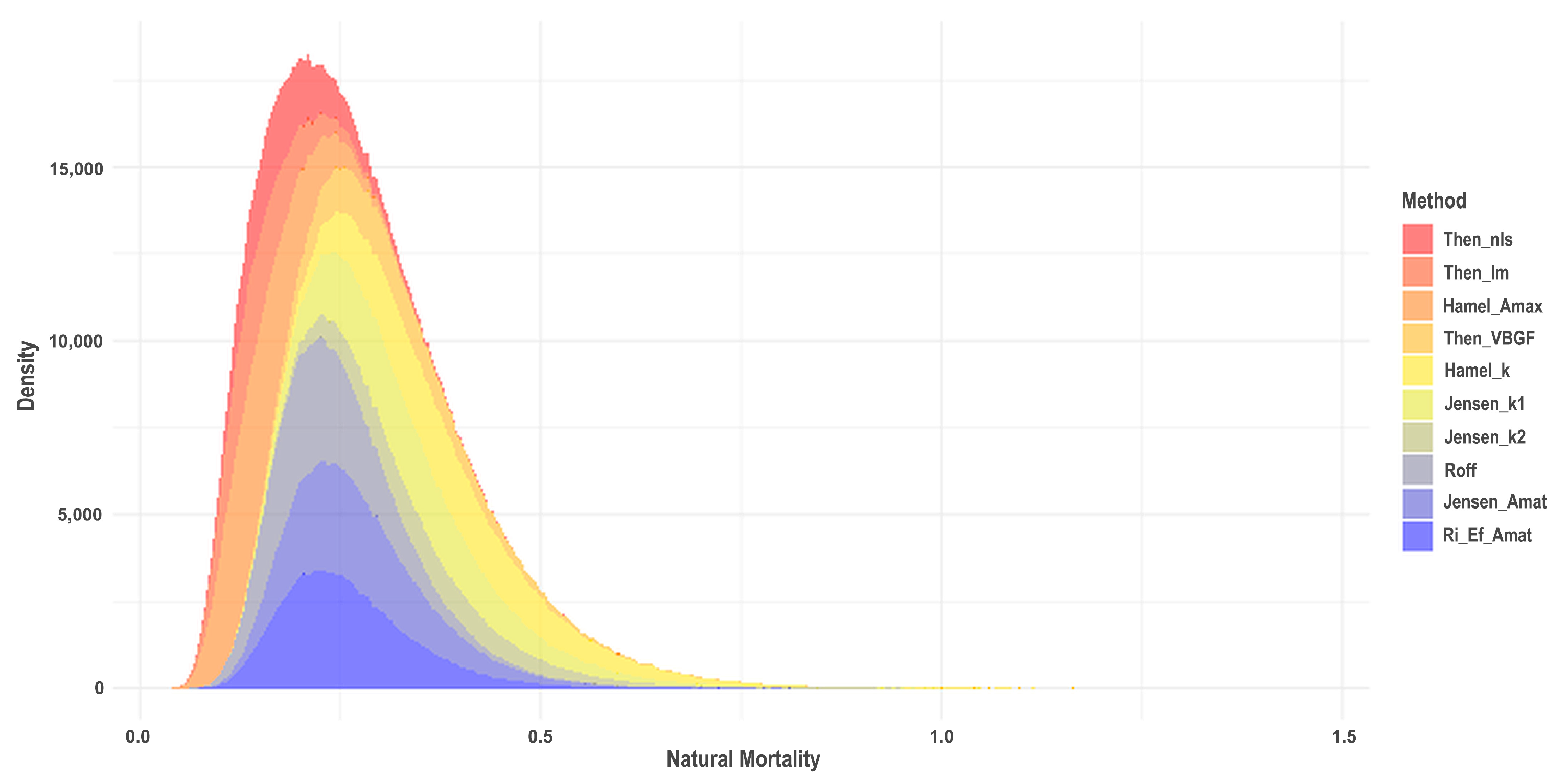
One of the life history parameters employed in assessing stock conditions using the LB-SPR method is the ratio between natural mortality and growth (M/k). A commonly used M/k value for fish follows the Beverton–Holt Life History of M/k = 1.5. However, this study sought to estimate the M/k value derived by independently estimating the M (natural mortality) and k (von Bertalanffy’s growth coefficient) values. Given the challenges and significant uncertainty associated with estimating M, 10 estimation methods were employed to derive M values. These estimates of M were subsequently aggregated to calculate both the average and median values of M.
The instantaneous natural mortality (
M) is very difficult to estimate [
35]. However, this is one of the most important life history parameters in length-based stock assessments [
27]. The estimation of the
M value from 10 different methods yielded two key figures: a mean of 0.27 year
−1 and a median of 0.25 year
−1. The selection of the estimated
M value in this study is important as it is used to determine the
M/k value used in subsequent stock assessments employing the LB-SPR method. Given the considerable variation in estimated
M values resulting from the 10 methods employed (see
Appendix D), the reliability of deciding between utilizing mean and median values remains uncertain. The mean is greatly influenced by extreme values and high variance, whereas the median proves unreliable in cases where the data lacks a uniform and symmetrical distribution [
36]. Nevertheless, the disparity in the
M/k value between the mean and median
M values is relatively small (1.23 vs. 1.13). Consequently, the
M/k value chosen for SPR estimation was established at 1.23. Using the higher estimate of
M/k in this case is also preferable, as the common
M/k value used in the SPR model when the
M/k information is not available is 1.5 [
17,
35].
Variation in M has direct implications for the SPR results because the LB-SPR framework expresses exploitation through the F/M ratio; a lower M increases F/M and reduces SPR, whereas a higher M has the opposite effect. The considerable spread in M estimates across the 10 methods therefore contributes meaningful uncertainty to the absolute SPR values. Much of this variation stems from the fact that many estimators rely on shared theoretical relationships with other life history parameters. Methods such as Then_VBGF and Hamel_k incorporate von Bertalanffy growth parameters, which themselves were derived from historical catch data, adding an additional layer of uncertainty and reducing the independence of the estimators. Although these sources of uncertainty influence the magnitude of SPR estimates, they do not alter the overall conclusion that the stock remains below the reference point. The approach used here clearly documents how M was estimated and acknowledges these limitations, allowing future assessments to refine and replicate the method.
The estimated SPR of
E. fuscoguttatus from the current study (mean = 0.22, range = 0.13 to 0.28) yields higher estimates than those reported previously for the five years between 2017 and 2021 (mean = 0.06, range = 0.05 to 0.07) [
19]. The notable disparity between the two estimates is mainly attributed to the values assigned to
L50 in the SPR estimation. The much smaller
L50 value (488 mm TL) used in the current study than that used previously (577.8 mm TL) resulted in higher estimated SPR values. This pattern is consistent with earlier findings showing that LB-SPR estimates are strongly influenced by the maturity schedule (e.g.,
L50 and
L95) and the
M/k ratio, while being comparatively less sensitive to moderate variation in
L∞ or
k within biologically plausible range [
28].
The consistency of SPR estimates across alternative maturity schedules further supports this interpretation. Sensitivity analyses using sex-combined and male-based maturity parameters produced SPR values that were nearly identical to those obtained under the primary female-based maturity schedule (21–22%). The corresponding F/M estimates were also the same across all scenarios. These results indicate that, within the range of plausible L50 and L95 values for this species, the relative assessment of stock status is stable and not strongly affected by uncertainty in the maturity parameters.
For protogynous species such as
E. fuscoguttatus, it is important to recognize that the LB-SPR metric reflects the reproductive output of mature females and does not explicitly incorporate the availability of functional males. Male groupers tend to be larger and more vulnerable to size selective fishing, which can reduce male abundance and create the potential for sperm limitation, even when female biomass appears adequate [
37,
38]. In addition, the transition from female to male involves a period of reduced reproductive activity while the gonad restructures; experimental studies in epinephelid groupers indicate that this process typically takes approximately 3–7 weeks [
39]. Although the estimated male and female
L50 values in this study were identical (488 mm TL), the LB-SPR model assumes that individuals contribute fully to spawning immediately upon reaching maturity and does not account for this temporary period of inactivity as some individuals transition from females to males. As a result, SPR may be slightly overestimated for
E. fuscoguttatus, and this biological context should be considered when interpreting stock status for protogynous species.
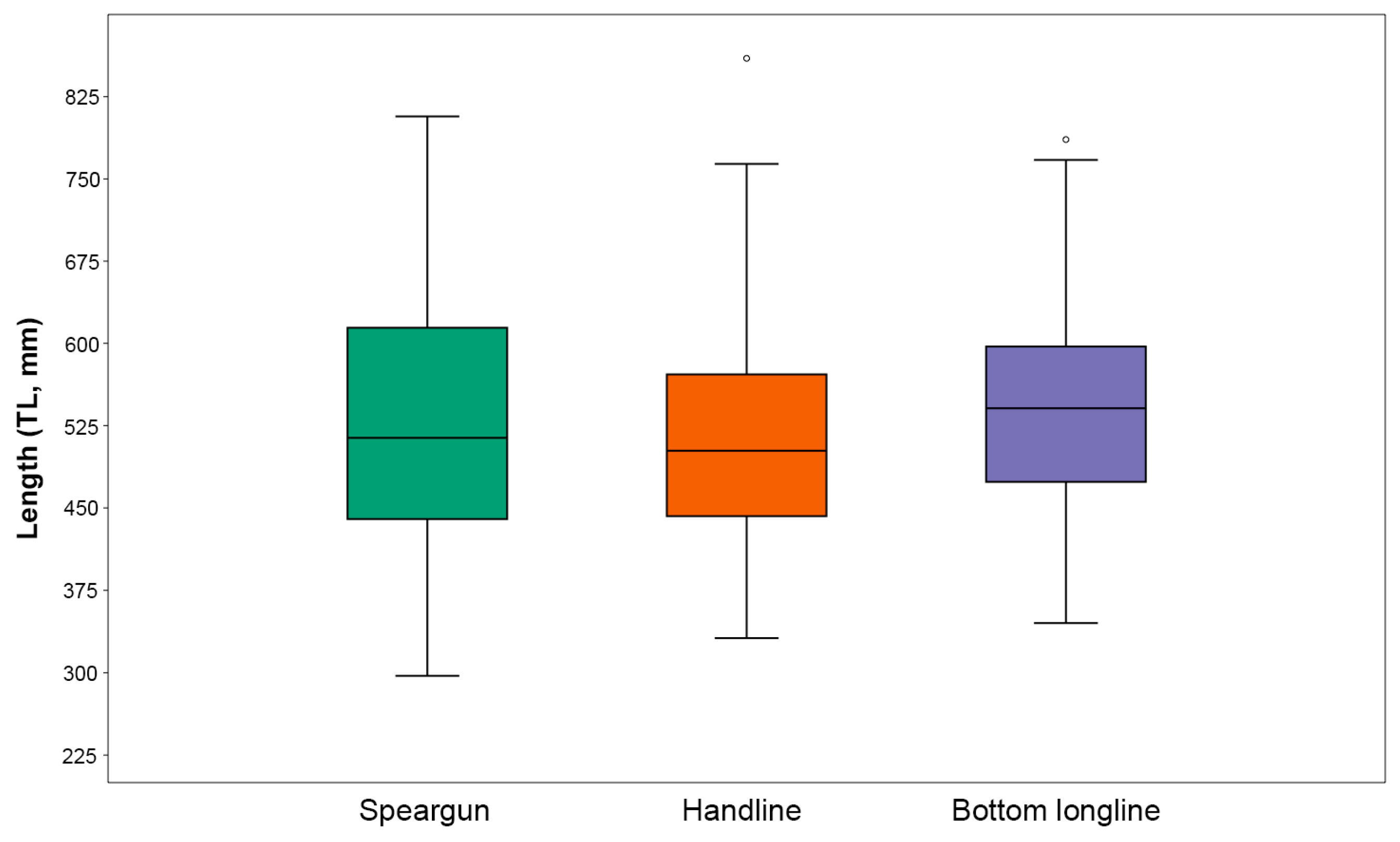
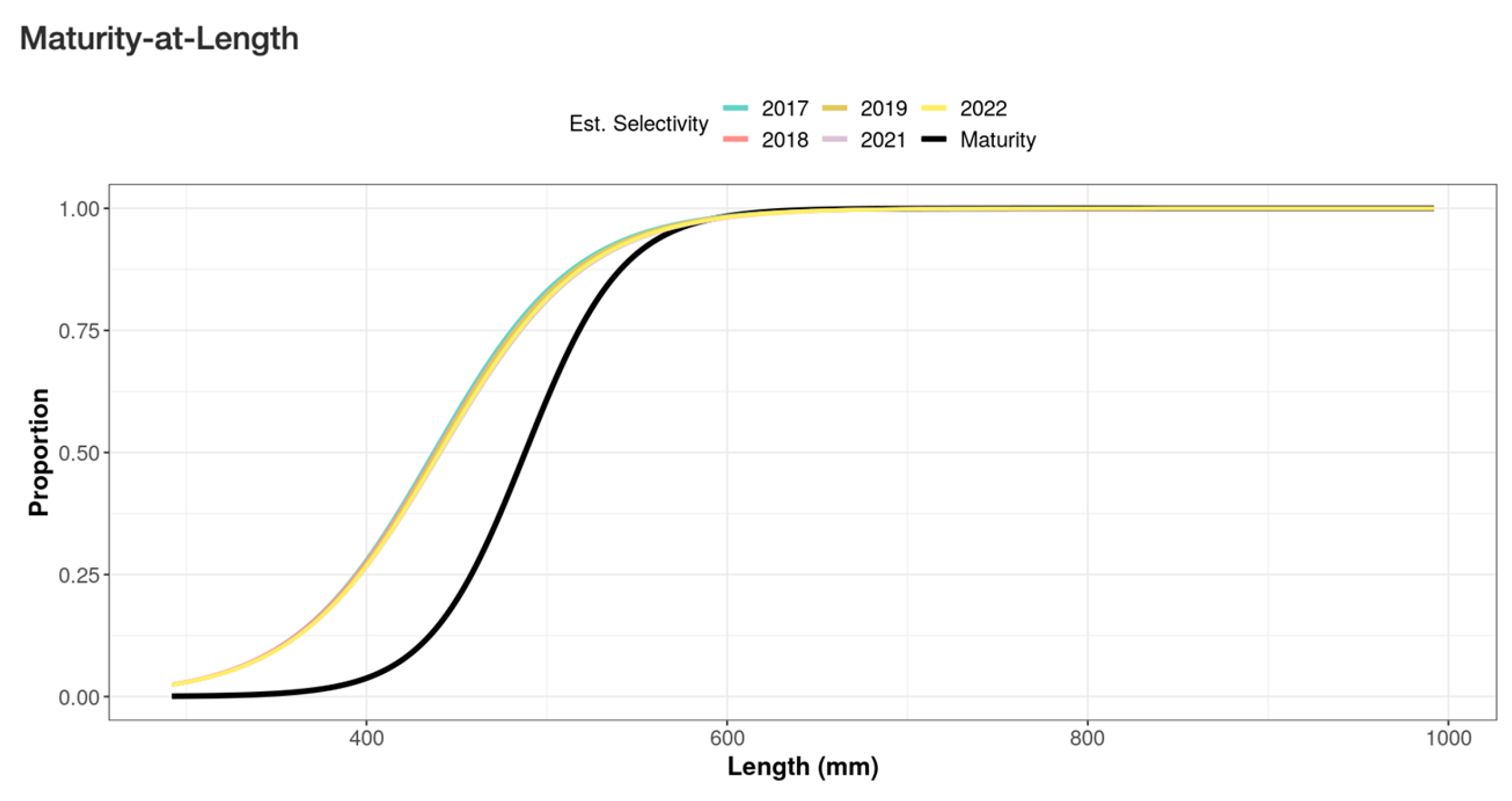
Another factor that may have contributed to the very low previous SPR estimates is some of the model assumptions. The LB-SPR model assumes asymptotic selectivity at length (see [
17]). However, it is noteworthy that preliminary data exploration revealed that 96.1% of the catches originated from three fishing methods, i.e., spearguns, handlines, and bottom longlines, that are each likely to have dome-shaped selectivity. Estimating the SPR from catch data derived from dome-shaped selectivity fishing gears is expected to underestimate the SPR value because the asymptotic selectivity assumption posits that the absence of larger fish in catch data is attributed to fishing mortality [
28]. However, in reality, larger fish are likely not captured by dome-shaped selectivity fishing gears, e.g., by smaller hook size or inaccessible deeper water by spearguns where larger fish are likely to occur ([
17,
40].
To address the selectivity incompatibility issue, Hommik et al. (2020) [
41] introduced a modification to the LB-SPR model to accommodate dome-shaped selectivity. This modification was applied to catch data for brown trout (
Salmo trutta) from gillnet fisheries in Ireland. However, the LB-SPR model tested for dome-shaped selectivity in hook and lines and spearguns is currently unavailable. In addition, further work is needed to develop a dome-shaped selectivity model for the three primary fishing methods in Saleh Bay. For instance, the construction of this model requires information on catch at length associated with various hook sizes employed by handlines and bottom longlines in Saleh Bay, which, unfortunately, is currently unavailable. In addition, further investigation is also required as there is no compelling evidence to confirm the true dome-shaped selectivity of these three fishing gears. The absence of large-sized fish caught may not be attributable to the selectivity of the fishing gear. Still, it could instead be influenced by pattern of fishing effort, such as avoiding catching larger fish due to price considerations or limitation of the fishing methods in accessing deeper waters where larger fish typically reside. Therefore, this study can only estimate the SPR value using the standard LB-SPR model, which assumes asymptotic selectivity.
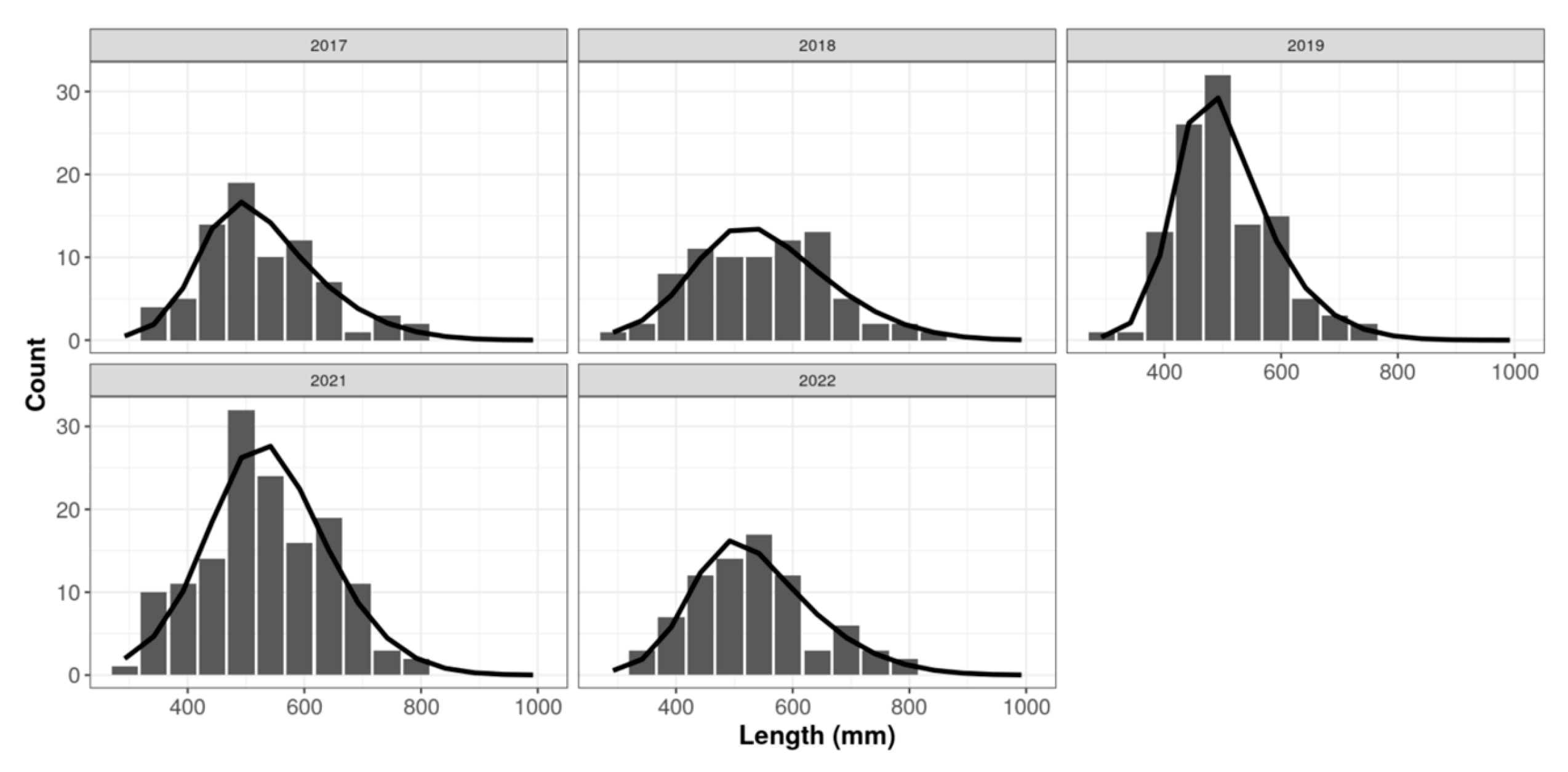
The lowest SPR estimated during the current study (0.11) was in 2019, the year in which
E. fuscoguttatus had the smallest size range, mean, and median total lengths. Hence, the disparities in sizes at selectivity (
SL50 and
SL95) compared to
L∞,
L50, and
L95 are greater in 2019 than in the other years, resulting in the lowest SPR estimate. The comparatively smaller size of
E. fuscoguttatus caught in 2019 may be attributed to at least two factors: (i) a decline in stock conditions due to fishing mortality, and/or (ii) limited sampling during the FLM program. Examining the decreasing trend in SPR from 2018 to 2019 suggests that fishing pressure is also a likely cause of the reduced SPR. This is substantiated by the higher SPR in 2021 and 2022 compared to 2019. Notably, the COVID-19 pandemic in 2020–2021 led to a significant decline in fishing activities in Saleh Bay when there was a 10% decrease in the market value of fish [
42]. The pandemic also caused delayed product distribution, reduced fishing trips and catches, and lower demand for fisheries products, which led to a decline in fishers’ income [
42]. The drastic reduction in fishing pressure during the pandemic likely provides an opportunity for stocks to recover in the short-term, and this impact might be reflected in the higher SPR values of 2021 and 2022. The significant decline in fishing pressures due to the COVID-19 pandemic has also been documented in other areas in Indonesia, for example, gillnet fisheries in Indramayu on the Northern coast of Java, small-scale fisheries in Southeast Sulawesi, and coastal fisheries in North Bali [
43,
44,
45], as well as in other countries, including the northwestern coast of India and northwestern Mediterranean Sea, where a wide range of fisheries were affected [
46,
47]. This reduction is a consequence of implementing travel restrictions, social distancing measures, decreased demand from domestic and export markets due to trade route closures, and the shutdown of fishing industries (see [
44,
48,
49]). In addition,
E. fuscoguttatus is among the grouper species that are highly traded in the export market [
10], making the COVID-19 pandemic likely to significantly reduce fishing and trade activities for this species in Saleh Bay.
4.3. Management Implications and Further Studies
Apart from the findings of this study, which demonstrate that the SPR value of
E. fuscoguttatus in Saleh Bay is greater than previous estimates (
Table 4), it is noteworthy that SPR of 0.11 was recorded for this species in 2019, which is considerably lower than the SPR limit reference point of 0.2. Furthermore, the SPR value of 0.21 in 2022 remains below the target reference point, in Saleh Bay of >0.3 [
7]. Fishing pressure on this species is expected to increase as the global fishing industry resumes normal operations following the end of COVID-19 pandemic. Furthermore, this species is one of the main targets of speargun fishers who generally catch smaller fish compared to other fishing methods in Saleh Bay [
7]. Although the mean and median sizes of fish caught by the three main fishing methods are greater than the approximation for
L50, they are still smaller than the approximation for
L95 (see
Figure 3). Thus, the overall proportions of immature fish caught are still relatively high.
The maturity data indicates that most individuals below approximately 560 mm TL are female and that males occur predominantly in larger size classes. Given that the fishery removes many individuals below this threshold, the catch is effectively sex-selective by size; based on the observed length distribution, roughly three quarters of individuals caught prior to the size at sex change are likely to be female. The high proportion of smaller fish caught, particularly those smaller than their length at maturity, is likely to reduce the natural reproductive capacity of this species, leading to a substantial decline in its stock condition in the future.
Despite implementing minimum size limit regulations by regulating hook and mesh sizes since 2018 [
7], the current study revealed that catches generally have substantial proportions of fish smaller than the length at maturity. This phenomenon is also identified in another study in Saleh Bay by Efendi et al. (2021) [
16] and other regions, such as grouper and snapper fisheries in Eastern Indonesia (e.g., refs. [
4,
28]). This issue was also identified in previous studies in Saleh Bay [
15]. Therefore, finding mechanisms to ensure effective compliance is essential. This may be achieved by prioritizing the enforcement of regulations, implementing effective monitoring, or other mechanisms to ensure compliance with minimum size limits in Saleh Bay. In addition to enhancing the enforcement of regulations and monitoring their effectiveness, management authorities should also consider exploring the possibility of implementing an incentive system for fishers and fish collectors who comply with the existing regulations [
15].
Taken together, these findings highlight important implications for the management of E. fuscoguttatus in Saleh Bay. The combination of low SPR values (0.13–0.28), SL50 values that are consistently smaller than the estimated L50 for maturity, and the predominance of fish below 500 mm TL in the catch indicate that the stock is experiencing both growth overfishing and recruitment overfishing. Growth overfishing is reflected in the harvest of fish before they reach sizes that maximize biomass contribution, while recruitment overfishing is suggested by SPR values remaining below the 0.30 threshold. Together, these indicators imply that current fishing pressure is reducing both the size structure and reproductive capacity of the population.
Strengthening compliance with minimum size limits and reducing the harvest of immature fish are immediate priorities for improving stock condition. Effective implementation may include stronger enforcement, consideration of slot limits that protect both small individuals and larger males, and spatial or temporal closures during peak reproductive periods to support rebuilding of the spawning stock. In the medium term, adjustments to gear selectivity, particularly for spearguns and handlines, that reduce the vulnerability of fish below 500 mm TL would help shift fishing pressure toward sizes that contribute more to spawning output. Protecting larger males is especially important for this protogynous species, and area-based measures in locations where males aggregate may further enhance recovery. Continued biological sampling and refinement of maturity, selectivity, and male abundance information will improve the reliability of future assessments and guide more effective management interventions.
The analysis of reproductive patterns from the biological samples revealed that the approximation for L50 value of E. fuscoguttatus was lower than the value previously employed in estimating SPR. However, the relatively small sample size and the narrower length distribution of the samples relative to the catch data, contribute to the high uncertainty of the estimate. Additionally, the method used for estimating life history parameters and the SPR also entails model limitations, further contributing to uncertainty. Because SPR outcomes are influenced by maturity schedules, natural mortality, and gear selectivity, these uncertainties should be considered when interpreting the stock status results. To reduce the uncertainty of estimating the life history parameters and stock assessment, there are at least two priority areas for future studies: (i) continuing research on the growth and reproduction pattern of this species with better length distribution of samples, particularly for the larger and older E. fuscogutattus, and (ii) developing an LB-SPR model that incorporates dome-shaped selectivity for the main fishing methods in Saleh Bay. In addition, future research should refine the calculation of L50 and evaluate potential interannual variability, which could not be assessed in this study due to limited maturity samples.