Extensive Characterization of Arapaima gigas Follicle-Stimulating Hormone (ag-Fsh) Synthesized in HEK293 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Qualitative and Quantitative Analysis of Purified ag-Fsh
2.2. MALDI-TOF-MS
2.3. Immunoenzymatic Assay Based on hFSH ELISA
2.4. In Vitro Bioassay Based on the Release of 11-Ketotestosterone (11-KT) from Immature A. gigas Testes
2.5. Statistical Analysis
3. Results
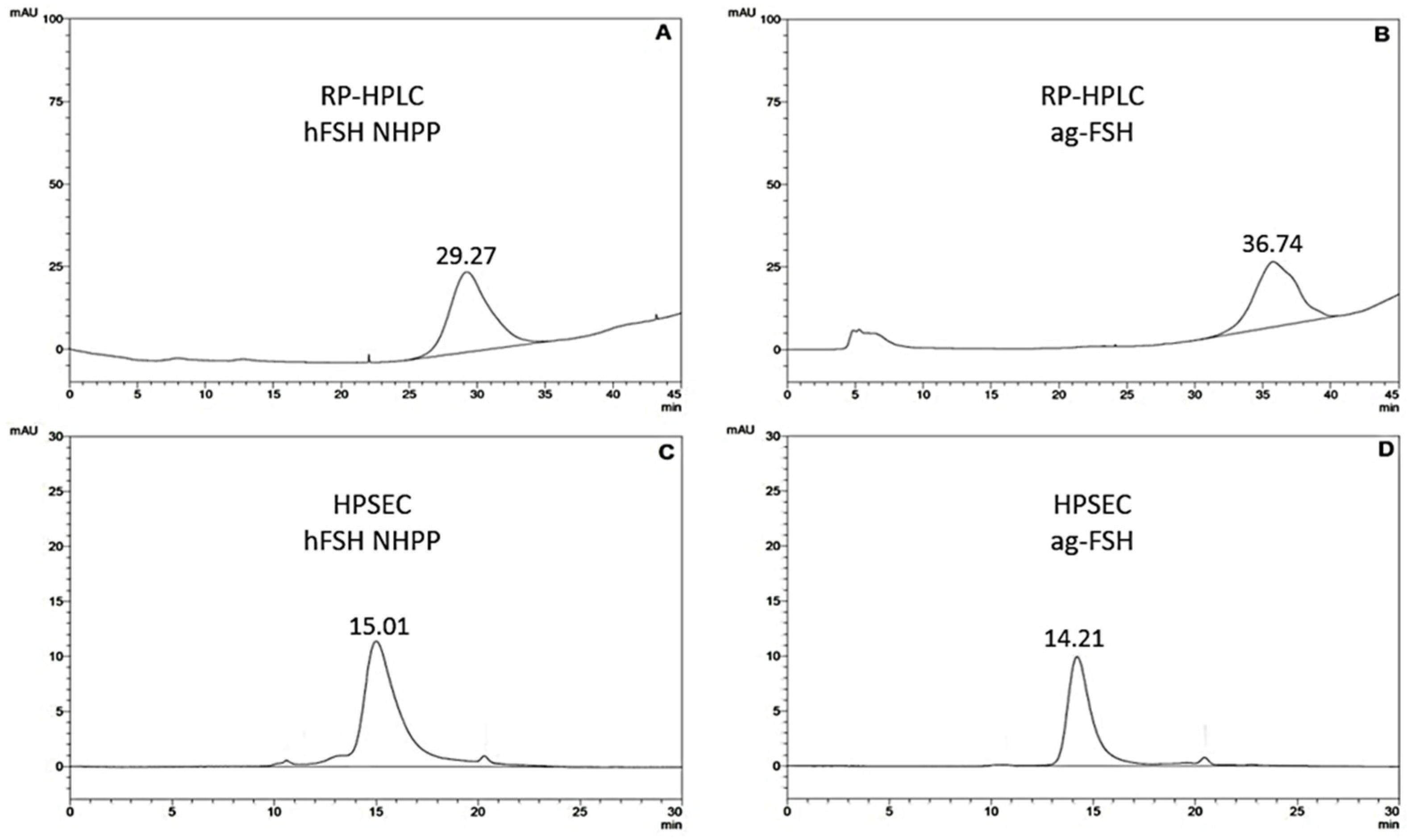
3.1. Qualitative and Quantitative Analysis of ag-Fsh by RP-HPLC and HPSEC
3.2. MALDI-TOF-MS of ag-FSH, in Comparison with the hFSH Standard
3.3. Immunological Activity Determination of ag-Fsh
3.4. In Vitro Bioassay of ag-Fsh, Based on the Stimulated Release of 11-KT from Immature A. gigas Testes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ag-Fsh | Arapaima gigas Follicle-Stimulating Hormone |
| ag-Gh | Arapaima gigas Growth Hormone |
| ag-Lh | Arapaima gigas Luteinizing Hormone |
| hCG | human Chorionic Gonadotropin |
| RP-HPLC | Reversed-Phase High-Performance Liquid Chromatography |
| HPSEC | High-Performance Size-Exclusion Chromatography |
| MALDI-TOF-MS | Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| 11-KT | 11-Ketotestosterone |
| HEK293 | Human Embryonic Kidney 293 cells |
| NHPP | National Hormone and Pituitary Program |
| MM | Molecular Mass |
References
- Huang, H.; Zhang, Y.; Huang, W.R.; Li, S.S.; Zhu, P.; Liu, Y.; Yin, S.W.; Liu, X.C.; Lin, H.R. Molecular characterization of marbled eel (Anguilla marmorata) gonadotropin subunits and their mRNA expression profiles during artificially induced gonadal development. Gen. Comp. Endocrinol. 2009, 162, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Levavi-Sivan, B.; Bogerd, J.; Mañanós, E.; Gómez, A.; Lareyre, J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010, 165, 412–437. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Senthilkumaran, B. Recent advances in meiotic maturation and ovulation: Comparing mammals and pisces. Front. Biosci. 2011, 16, 1898. [Google Scholar] [CrossRef] [PubMed]
- Borella, M.I.; Venturieri, R.; Mancera, J.M. Immunocytochemical identification of adenohypophyseal cells in the pirarucu (Arapaima gigas), an Amazonian basal teleost. Fish Physiol. Biochem. 2009, 35, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef] [PubMed]
- Castello, L.; Stewart, D.J.; Arantes, C.C. Modeling population dynamics and conservation of arapaima in the Amazon. Rev. Fish Biol. Fish. 2011, 21, 623–640. [Google Scholar] [CrossRef]
- Zohar, Y.; Zmora, N.; Trudeau, V.L.; Muñoz-Cueto, J.A.; Golan, M. A half century of fish gonadotropin-releasing hormones: Breaking paradigms. J. Neuroendocr. 2022, 34, e13069. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cavole, L.M.; Arantes, C.C.; Castello, L. How illegal are tropical small-scale fisheries? An estimate for arapaima in the Amazon. Fish. Res. 2015, 168, 1–5. [Google Scholar] [CrossRef]
- Watson, L.C.; Stewart, D.J.; Kretzer, A.M. Genetic Diversity and Population Structure of the Threatened Giant Arapaima in Southwestern Guyana: Implications for Their Conservation. Copeia 2016, 104, 864–872. [Google Scholar] [CrossRef]
- Castello, L.; Stewart, D.J. Assessing CITES non-detriment findings procedures for Arapaima in Brazil. J. Appl. Ichthyol. 2010, 26, 49–56. [Google Scholar] [CrossRef]
- Chu-Koo, F.; Dugué, R.; Aguilar, M.A.; Daza, A.C.; Bocanegra, F.A.; Veintemilla, C.C.; Duponchelle, F.; Renno, J.-F.; Tello, S.; Nuñez, J. Gender determination in the Paiche or Pirarucu (Arapaima gigas) using plasma vitellogenin, 17β-estradiol, and 11-ketotestosterone levels. Fish Physiol. Biochem. 2009, 35, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Tello, S.; Vargas, G.; Duponchelle, F. Patterns of commercial fish landings in the Loreto region (Peruvian Amazon) between 1984 and 2006. Fish Physiol. Biochem. 2009, 35, 53–67. [Google Scholar] [CrossRef]
- Torati, L.S.; Lima, A.F.; Kirschnik, L.N.G.; Migaud, H. Endoscopy and Cannulation as Non-Invasive Tools to Identify Sex and Monitor Reproductive Development in Arapaima gigas. Ichthyol. Herpetol. 2019, 107, 287–296. [Google Scholar] [CrossRef]
- Torati, L.S.; Taylor, J.F.; Mesquita, P.E.; Migaud, H. GnRHa implants and size pairing effects on plasma and cephalic secretion sex steroids in Arapaima gigas. Gen. Comp. Endocrinol. 2020, 299, 113614. [Google Scholar] [CrossRef]
- Freire, R.P.; Hernandez-Gonzalez, J.E.; Lima, E.R.; Suzuki, M.F.; de Oliveira, J.E.; Torati, L.S.; Bartolini, P.; Soares, C.R.J. Molecular Cloning and AlphaFold Modeling of Thyrotropin (ag-TSH) From the Amazonian Fish Pirarucu (Arapaima gigas). Bioinform. Biol. Insights 2023, 17, 11779322231154148. [Google Scholar] [CrossRef]
- Gen, K.; Soyano, K.; Okuzawa, K.; Kagawa, H. Production of biologically active red seabream gonadotropins in a baculovirus system. Cybium 2008, 32, 218–219. [Google Scholar]
- Kobayashi, M.; Hayakawa, Y.; Park, W.; Banba, A.; Yoshizaki, G.; Kumamaru, K.; Kagawa, H.; Kaki, H.; Nagaya, H.; Sohn, Y.C. Production of recombinant Japanese eel gonadotropins by baculovirus in silkworm larvae. Gen. Comp. Endocrinol. 2010, 167, 379–386. [Google Scholar] [CrossRef]
- Kobayashi, M.; Morita, T.; Ikeguchi, K.; Yoshizaki, G.; Suzuki, T.; Watabe, S. In vivo biological activity of recombinant goldfish gonadotropins produced by baculovirus in silkworm larvae. Aquaculture 2006, 256, 433–442. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Morita, T.; Kitamura, W.; Kanda, S.; Banba, A.; Nagaya, H.; Hotta, K.; Sohn, Y.C.; Yoshizaki, G.; Kobayashi, M. Biological activities of single-chain goldfish follicle-stimulating hormone and luteinizing hormone. Aquaculture 2008, 274, 408–415. [Google Scholar] [CrossRef]
- Aizen, J.; Kasuto, H.; Golan, M.; Zakay, H.; Levavi-Sivan, B. Tilapia Follicle-Stimulating Hormone (FSH): Immunochemistry, Stimulation by Gonadotropin-Releasing Hormone, and Effect of Biologically Active Recombinant FSH on Steroid Secretion1. Biol. Reprod. 2007, 76, 692–700. [Google Scholar] [CrossRef]
- Kasuto, H.; Levavi-Sivan, B. Production of biologically active tethered tilapia LHβα by the methylotrophic yeast Pichia pastoris. Gen. Comp. Endocrinol. 2005, 140, 222–232. [Google Scholar] [CrossRef]
- Yu, X.; Lin, S.-W.; Kobayashi, M.; Ge, W. Expression of recombinant zebrafish follicle-stimulating hormone (FSH) in methylotropic yeast Pichia pastoris. Fish Physiol. Biochem. 2010, 36, 273–281. [Google Scholar] [CrossRef]
- Sanchís-Benlloch, P.J.; Nocillado, J.; Ladisa, C.; Aizen, J.; Miller, A.; Shpilman, M.; Levavi-Sivan, B.; Ventura, T.; Elizur, A. In-vitro and in-vivo biological activity of recombinant yellowtail kingfish (Seriola lalandi) follicle stimulating hormone. Gen. Comp. Endocrinol. 2017, 241, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Aizen, J.; Hollander-Cohen, L.; Shpilman, M.; Levavi-Sivan, B. Biologically active recombinant carp LH as a spawning-inducing agent for carp. J. Endocrinol. 2017, 232, 391–402. [Google Scholar] [CrossRef]
- Aizen, J.; Sharma, S.; Elizur, A.; Joy, K.; Chaube, R. Regulation of steroid production and key genes in catfish Heteropneustes fossilis using recombinant gonadotropins. Fish Physiol. Biochem. 2023, 49, 911–923. [Google Scholar] [CrossRef]
- Choi, E.; Ko, H.; Shin, J.; Kim, M.-A.; Sohn, Y.C. Expression of gonadotropin genes in Manchurian trout Brachymystax lenok and production of recombinant gonadotropins. Fish. Sci. 2005, 71, 1193–1200. [Google Scholar] [CrossRef]
- Kazeto, Y.; Ito, R.; Tanaka, T.; Suzuki, H.; Ozaki, Y.; Okuzawa, K.; Gen, K. Establishment of cell-lines stably expressing recombinant Japanese eel follicle-stimulating hormone and luteinizing hormone using CHO-DG44 cells: Fully induced ovarian development at different modes. Front. Endocrinol. 2023, 14, 1201250. [Google Scholar] [CrossRef] [PubMed]
- Vischer, H.; Granneman, J.; Linskens, M.; Schulz, R.; Bogerd, J. Both recombinant African catfish LH and FSH are able to activate the African catfish FSH receptor. J. Mol. Endocrinol. 2003, 31, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mazón, M.J.; Molés, G.; Rocha, A.; Crespo, B.; Lan-Chow-Wing, O.; Espigares, F.; Muñoz, I.; Felip, A.; Carrillo, M.; Zanuy, S.; et al. Gonadotropins in European sea bass: Endocrine roles and biotechnological applications. Gen. Comp. Endocrinol. 2015, 221, 31–41. [Google Scholar] [CrossRef]
- Molés, G.; Gómez, A.; Rocha, A.; Carrillo, M.; Zanuy, S. Purification and characterization of follicle-stimulating hormone from pituitary glands of sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol. 2008, 158, 68–76. [Google Scholar] [CrossRef]
- Molés, G.; Gómez, A.; Carrillo, M.; Zanuy, S. Development of a homologous enzyme-linked immunosorbent assay for European sea bass FSH. Reproductive cycle plasma levels in both sexes and in yearling precocious and non-precocious males. Gen. Comp. Endocrinol. 2012, 176, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Molés, G.; Hausken, K.; Carrillo, M.; Zanuy, S.; Levavi-Sivan, B.; Gómez, A. Generation and use of recombinant gonadotropins in fish. Gen. Comp. Endocrinol. 2020, 299, 113555. [Google Scholar] [CrossRef] [PubMed]
- Mazón, M.J.; Zanuy, S.; Muñoz, I.; Carrillo, M.; Gómez, A. Luteinizing Hormone Plasmid Therapy Results in Long-Lasting High Circulating Lh and Increased Sperm Production in European Sea Bass (Dicentrarchus labrax). Biol. Reprod. 2013, 88, 32. [Google Scholar] [CrossRef]
- Rambabu, K.M.; Rao, S.H.N.; Rao, N.M. Efficient expression of transgenes in adult zebrafish by electroporation. BMC Biotechnol. 2005, 5, 29. [Google Scholar] [CrossRef]
- Lv, W.; Jiang, P.; Wang, W.; Wang, X.; Wang, K.; Chang, L.; Fang, Y.; Chen, J. Electrotransfer of single-chain LH gene into skeletal muscle induces early ovarian development of orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2018, 259, 12–19. [Google Scholar] [CrossRef]
- Iwaizumi, M.; Yokoi, H.; Suzuki, T. Plasmid delivery by electroporation into fish skeletal muscle for recombinant protein secretion and uptake by oocytes. Fish Physiol. Biochem. 2020, 46, 1121–1130. [Google Scholar] [CrossRef]
- Faria, M.T.; Carvalho, R.F.; Sevilhano, T.C.A.; Oliveira, N.A.J.; Silva, C.F.P.; Oliveira, J.E.; Soares, C.R.J.; Garcez, R.; Santo, P.R.E.; Bartolini, P. Isolation of the pituitary gonadotrophic α-subunit hormone of the giant amazonian fish: Pirarucu (Arapaima gigas). Fish Physiol. Biochem. 2013, 39, 683–693. [Google Scholar] [CrossRef]
- Sevilhano, T.; de Carvalho, R.F.; Oliveira, N.A.d.J.; Oliveira, J.E.; Maltarollo, V.G.; Trossini, G.; Garcez, R.; Bartolini, P.; Vaudry, H. Molecular cloning and characterization of pirarucu (Arapaima gigas) follicle-stimulating hormone and luteinizing hormone β-subunit cDNAs. PLoS ONE 2017, 12, e0183545. [Google Scholar] [CrossRef]
- Kamei, H.; Ohira, T.; Yoshiura, Y.; Uchida, N.; Nagasawa, H.; Aida, K. Expression of a biologically active recombinant follicle stimulating hormone of Japanese Eel Anguilla japonica using methylotropic yeast, Pichia pastoris. Gen. Comp. Endocrinol. 2003, 134, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.R.; Freire, R.P.; Suzuki, M.F.; Oliveira, J.E.; Yosidaki, V.L.; Peroni, C.N.; Sevilhano, T.; Zorzeto, M.; Torati, L.S.; Soares, C.R.J.; et al. Isolation and Characterization of the Arapaima gigas Growth Hormone (ag-GH) cDNA and Three-Dimensional Modeling of This Hormone in Comparison with the Human Hormone (hGH). Biomolecules 2023, 13, 158. [Google Scholar] [CrossRef]
- Migaud, H.; Davie, A.; Taylor, J.F. Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J. Fish Biol. 2010, 76, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, R.F.; de Oliveira, J.E.; Torjesen, P.A.; Bartolini, P.; Ribela, M.T.C. Analysis of intact human follicle-stimulating hormone preparations by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2006, 1136, 10–18. [Google Scholar] [CrossRef]
- Almeida, B.; Oliveira, J.; Damiani, R.; Dalmora, S.; Bartolini, P.; Ribela, M. A pilot study on potency determination of human follicle-stimulating hormone: A comparison between reversed-phase high-performance liquid chromatography method and the in vivo bioassay. J. Pharm. Biomed. Anal. 2011, 54, 681–686. [Google Scholar] [CrossRef]
- Núñez, J.; Duponchelle, F. Towards a universal scale to assess sexual maturation and related life history traits in oviparous teleost fishes. Fish Physiol. Biochem. 2009, 35, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Kazeto, Y.; Suzuki, H.; Ozaki, Y.; Gen, K. C-terminal peptide (hCTP) of human chorionic gonadotropin enhances in vivo biological activity of recombinant Japanese eel follicle-stimulating hormone and luteinizing hormone produced in FreeStyle 293-F cell lines. Gen. Comp. Endocrinol. 2021, 306, 113731. [Google Scholar] [CrossRef]
- Miura, C.; Miura, T.; Yamashita, M.; Yamauchi, K.; Nagahama, Y. Hormonal induction of all stages of spermatogenesis in germ-somatic cell coculture from immature Japanese eel testis. Dev. Growth Differ. 1996, 38, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kamei, H.; Kawazoe, I.; Kaneko, T.; Aida, K. Purification of follicle-stimulating hormone from immature Japanese eel, Anguilla japonica, and its biochemical properties and steroidogenic activities. Gen. Comp. Endocrinol. 2005, 143, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kamei, H.; Kaneko, T.; Aida, K. In vivo gonadotropic effects of recombinant Japanese eel follicle-stimulating hormone. Aquaculture 2006, 261, 771–775. [Google Scholar] [CrossRef]
- Ko, H.; Park, W.; Kim, D.-J.; Kobayashi, M.; Sohn, Y.C. Biological activities of recombinant Manchurian trout FSH and LH: Their receptor specificity, steroidogenic and vitellogenic potencies. J. Mol. Endocrinol. 2007, 38, 99–111. [Google Scholar] [CrossRef]
- Kazeto, Y.; Kohara, M.; Miura, T.; Miura, C.; Yamaguchi, S.; Trant, J.M.; Adachi, S.; Yamauchi, K. Japanese Eel Follicle-Stimulating Hormone (Fsh) and Luteinizing Hormone (Lh): Production of Biologically Active Recombinant Fsh and Lh by Drosophila S2 Cells and Their Differential Actions on the Reproductive Biology1. Biol. Reprod. 2008, 79, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Nagaya, H.; Kaki, H.; Hotta, K.; Kobayashi, M. Induction of spermatogenesis in Japanese eel by recombinant goldfish gonadotropins. Fish. Sci. 2009, 75, 137–144. [Google Scholar] [CrossRef]
- Almeida, B.; Oliveira, J.; Carvalho, C.; Dalmora, S.; Bartolini, P.; Ribela, M. Analysis of human luteinizing hormone and human chorionic gonadotropin preparations of different origins by reversed-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2010, 53, 90–97. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, E.R.; Sevilhano, T.C.A.; Feitosa, T.C.; Oliveira, J.E.; Suzuki, M.F.; Torati, L.S.; Bartolini, P.; Peroni, C.N. Extensive Characterization of Arapaima gigas Follicle-Stimulating Hormone (ag-Fsh) Synthesized in HEK293 Cells. Fishes 2025, 10, 607. https://doi.org/10.3390/fishes10120607
Lima ER, Sevilhano TCA, Feitosa TC, Oliveira JE, Suzuki MF, Torati LS, Bartolini P, Peroni CN. Extensive Characterization of Arapaima gigas Follicle-Stimulating Hormone (ag-Fsh) Synthesized in HEK293 Cells. Fishes. 2025; 10(12):607. https://doi.org/10.3390/fishes10120607
Chicago/Turabian StyleLima, Eliana R., Thais C. A. Sevilhano, Thais C. Feitosa, João E. Oliveira, Miriam F. Suzuki, Lucas S. Torati, Paolo Bartolini, and Cibele N. Peroni. 2025. "Extensive Characterization of Arapaima gigas Follicle-Stimulating Hormone (ag-Fsh) Synthesized in HEK293 Cells" Fishes 10, no. 12: 607. https://doi.org/10.3390/fishes10120607
APA StyleLima, E. R., Sevilhano, T. C. A., Feitosa, T. C., Oliveira, J. E., Suzuki, M. F., Torati, L. S., Bartolini, P., & Peroni, C. N. (2025). Extensive Characterization of Arapaima gigas Follicle-Stimulating Hormone (ag-Fsh) Synthesized in HEK293 Cells. Fishes, 10(12), 607. https://doi.org/10.3390/fishes10120607






