Comparison of Muscle Texture Characteristics and Nutritional Composition Between Gene-Edited Intermuscular-Bone-Free Crucian Carp and Other Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
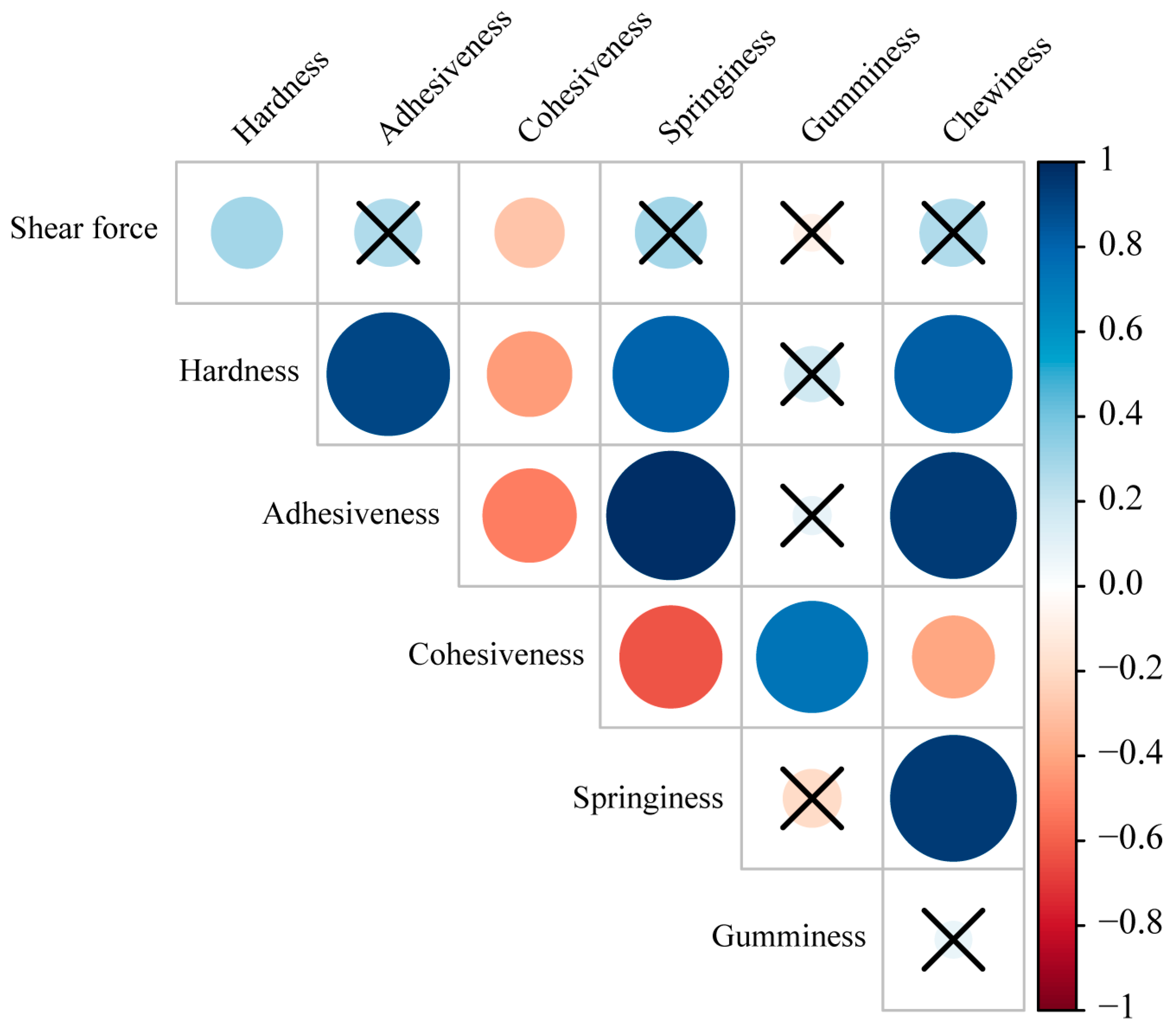
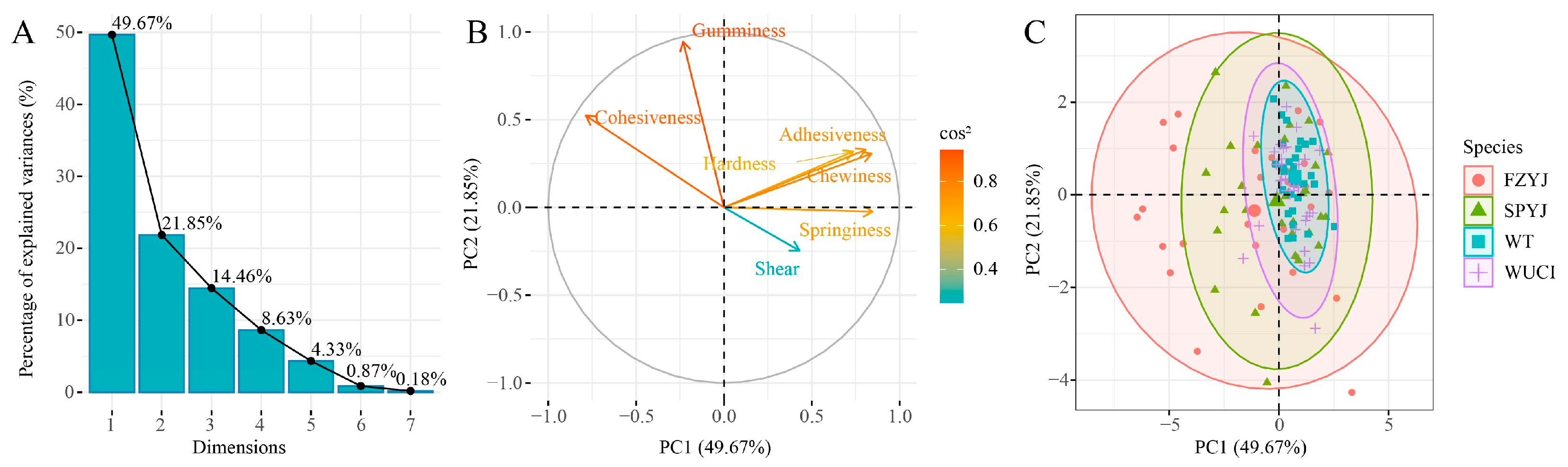
2.2. Texture Measurement
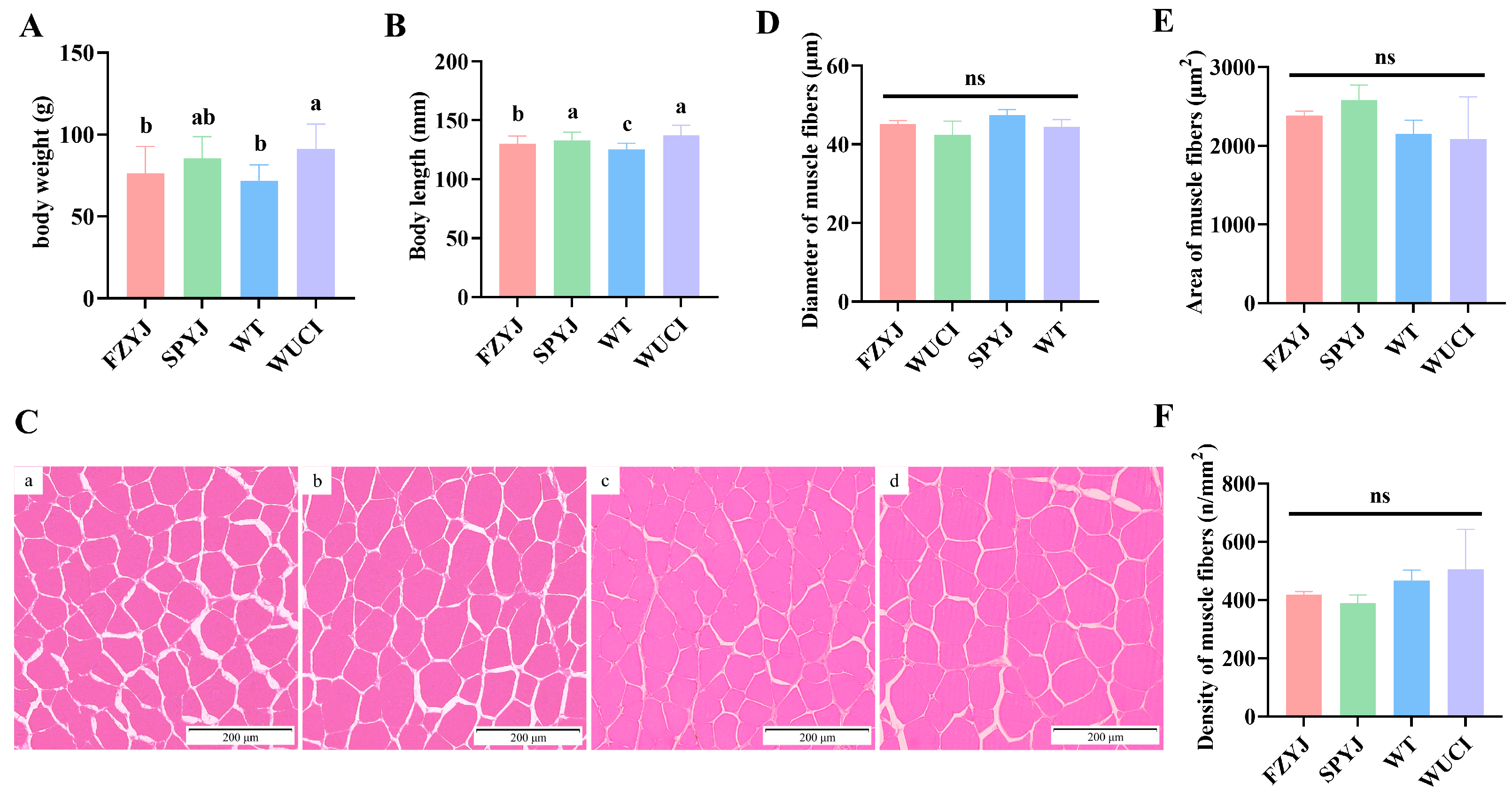
2.3. Muscle Fiber Histological Analysis
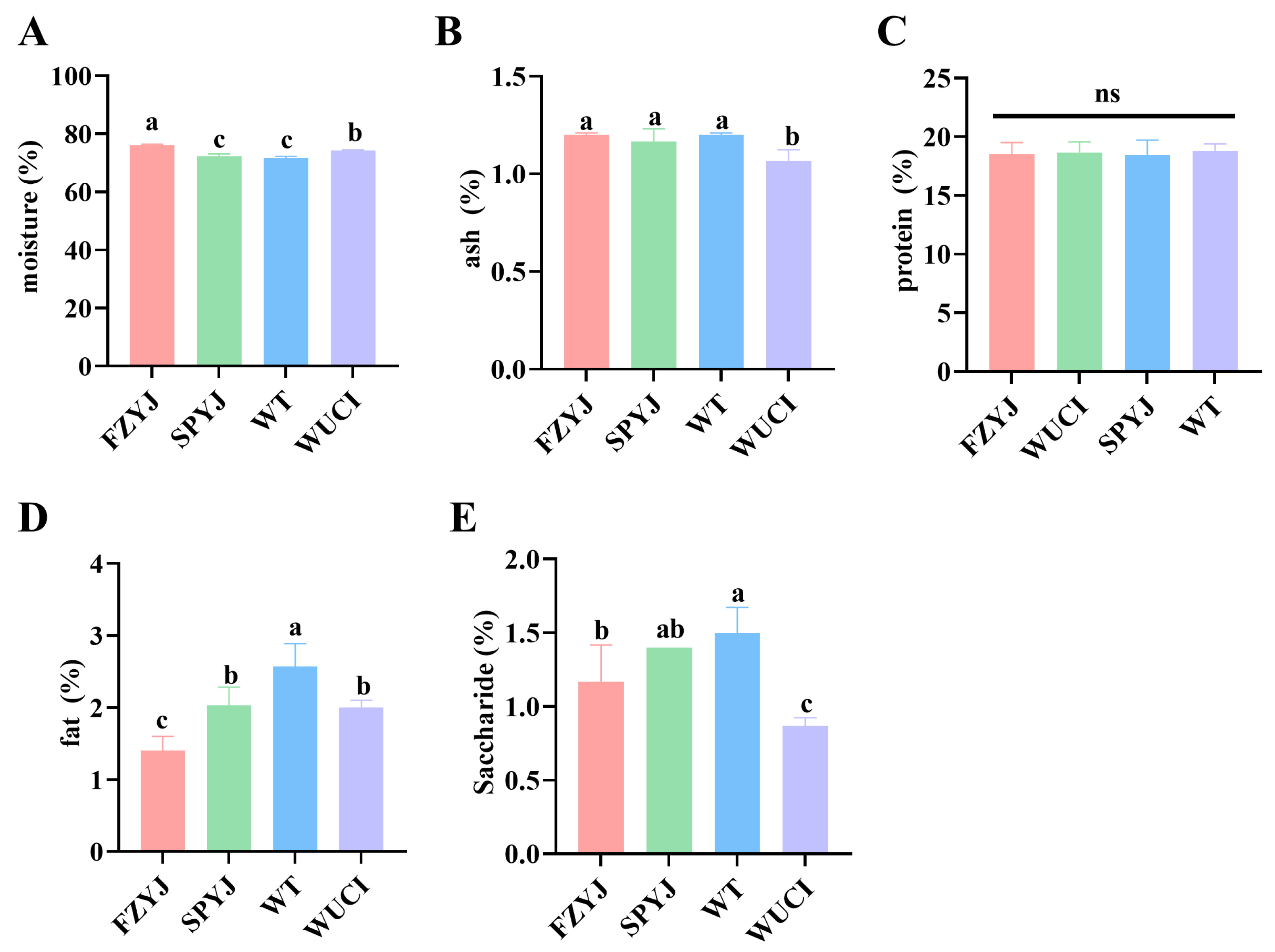
2.4. Muscle Nutritional Composition Determination
2.5. Muscle Nutrition Evaluation
2.6. Statistical Analysis
3. Results
3.1. Comparison of Muscle Fiber Structure
3.2. Comparison of Muscle Texture Characteristics
3.3. Conventional Nutritional Composition of Muscle
3.4. Muscle Amino Acid Composition and Evaluation
3.5. Analysis of Muscle Fatty Acid Composition and Content
4. Discussion
4.1. Analysis of Muscle Texture Characteristics
4.2. Analysis of Conventional Nutritional Components
4.3. Analysis of Amino Acid Composition, Content, and Nutritional Value
4.4. Analysis of Fatty Acid Composition and Content
4.5. Effects of bmp6 Knockout on Muscle Development and Metabolism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| texture profile analysis | (TPA) |
| polyunsaturated fatty acids | (PUFA) |
| essential amino acids | (EAA) |
| hematoxylin-eosin | (HE) |
| amino acid score | (AAS) |
| chemical score | (CS) |
| essential amino acid index | (EAAI) |
| one-way analysis of variance | (ANOVA) |
| European Food Safety Authority | (EFSA) |
| principal component analysis | (PCA) |
| non-essential amino acids | (NEAA) |
| total amino acid content | (TAA) |
| umami amino acid content | (UAA) |
| monounsaturated fatty acids | (MUFA) |
| Transforming Growth Factor-β | (TGF-β) |
| total fatty acid | (TFA) |
| histidine | (His) |
| glycine | (Gly) |
| alanine | (Ala) |
| aspartic | (Asp) |
| glutamic | (Glu) |
| eicosapentaenoic acid | (EPA) |
| docosahexaenoic acid | (DHA) |
References
- Ismail, I.; Huda, N. Current Techniques and Technologies of Meat Quality Evaluation. In Hand Book of Processed Functional Meat Products; Springer: Berlin/Heidelberg, Germany, 2024; pp. 437–512. [Google Scholar]
- Simeanu, D.; Radu-Rusu, R.-M.; Mintas, O.S.; Simeanu, C. Qualitative and Nutritional Evaluation of Paddlefish (Polyodon spathula) Meat Production. Agriculture 2022, 12, 1965. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, F.; Zhang, W.B.; Parisi, G.; Du, Z.Y.; Zhang, M.L. The flesh texture of teleost fish: Characteristics and interventional strategies. Rev. Aquac. 2024, 16, 508–535. [Google Scholar] [CrossRef]
- He, Z.; Wang, J.; Wei, Y.; Yan, X.; Li, Y.; Xie, D.; Nie, G. Optimizing Muscle Quality in Common Carp (Cyprinus carpio L.): Impacts of Body Size on Nutrient Composition, Texture, and Volatile Profile. Foods 2025, 14, 2794. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Sun, W.; Ma, F.; Wang, X.; Yang, Z. New Techniques of Meat Quality Assessment for Detecting Meat Texture. Processes 2025, 13, 640. [Google Scholar] [CrossRef]
- Purlis, E.; Cevoli, C.; Fabbri, A. Modelling volume change and deformation in food products/processes: An overview. Foods 2021, 10, 778. [Google Scholar] [CrossRef]
- Maulu, S.; Nawanzi, K.; Abdel-Tawwab, M.; Khalil, H.S. Fish nutritional value as an approach to children’s nutrition. Front. Nutr. 2021, 8, 780844. [Google Scholar] [CrossRef]
- Dong, C.-J.; Li, X.-J.; Sun, X.-W. Research progress of the genetic diversity, origin and evolution of Carassius auratus in China. J. Fish. China 2020, 44, 1046–1062. [Google Scholar]
- Li, B.; Zhang, Y.-W.; Liu, X.; Ma, L.; Yang, J.-X. Molecular mechanisms of intermuscular bone development in fish: A review. Zool. Res. 2021, 42, 362. [Google Scholar] [CrossRef]
- Yang, J.; Tong, G.; Sun, Z.; Zheng, X.; Lv, W.; Cao, D.; Sun, X.; Kuang, Y. Comparative analysis of muscle development in zebrafish with different intermuscular-bones patterns. Pak. J. Zool. 2021, 53, 313. [Google Scholar]
- Tang, G.; Lv, W.; Sun, Z.; Cao, D.; Zheng, X.; Tong, G.; Wang, H.; Zhang, X.; Kuang, Y. Heritability and quantitative trait locus analyses of intermuscular bones in mirror carp (Cyprinus carpio). Aquaculture 2020, 515, 734601. [Google Scholar] [CrossRef]
- Xu, H.; Tong, G.; Yan, T.; Dong, L.; Yang, X.; Dou, D.; Sun, Z.; Liu, T.; Zheng, X.; Yang, J. Transcriptomic analysis provides insights to reveal the bmp6 function related to the development of intermuscular bones in zebrafish. Front. Cell Dev. Biol. 2022, 10, 821471. [Google Scholar] [CrossRef]
- Yang, X.; TONG, G.; YAN, T. The effect of cilp on the development of the intermuscular bones and vertebra in zebrafish. J. Shanghai Ocean Univ. 2023, 32, 460–471. [Google Scholar]
- Yang, J.; Tong, G.-x.; Zheng, X.; Sun, Z.; Lyu, W.; Sun, X.; Kuang, Y. Comparative analysis of embryonic muscle development in wildtype zebrafish and its intermuscular bone deficiency mutant. J. Fish. Sci. China 2019, 26, 296. [Google Scholar] [CrossRef]
- Kuang, Y.; Zheng, X.; Cao, D.; Sun, Z.; Tong, G.; Xu, H.; Yan, T.; Tang, S.; Chen, Z.; Zhang, T. Generate a new crucian carp (Carassius auratus) strain without intermuscular bones by knocking out bmp6. Aquaculture 2023, 569, 739407. [Google Scholar] [CrossRef]
- Ortega, D.L.; Lin, W.; Ward, P.S. Consumer acceptance of gene-edited food products in China. Food Qual. Prefer. 2022, 95, 104374. [Google Scholar] [CrossRef]
- Tang, S.; Li, N.; Sun, Z.; Yan, T.; Zhang, T.; Xu, H.; Chen, Z.; Qin, D.; Kuang, Y. Accumulation Patterns and Health Risk Assessment of Trace Elements in Intermuscular Bone-Free Crucian Carp. Toxics 2025, 13, 595. [Google Scholar] [CrossRef]
- Pematilleke, N.; Kaur, M.; Adhikari, B.; Torley, P.J. Relationship between instrumental and sensory texture profile of beef semitendinosus muscles with different textures. J. Texture Stud. 2022, 53, 232–241. [Google Scholar] [CrossRef]
- Girard, I.; Bruce, H.; Basarab, J.; Larsen, I.; Aalhus, J. Contribution of myofibrillar and connective tissue components to the Warner–Bratzler shear force of cooked beef. Meat Sci. 2012, 92, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Sun, Y.; Chen, Z.; Huang, X.; Li, C.; Gao, L.; Bai, S.; Wang, P.; Hao, Q. Effects of multiple freeze-thaw cycles on protein and lipid oxidation, microstructure and quality characteristics of rainbow trout (Oncorhynchus mykiss). Fishes 2023, 8, 108. [Google Scholar] [CrossRef]
- He, X.; Shu, H.; Xu, T.; Huang, Y.; Mo, J.; Ai, C. Effects of broad bean diet on the growth performance, muscle characteristics, antioxidant capacity, and intestinal health of Nile tilapia (Oreochromis niloticus). Animals 2023, 13, 3705. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.L.; Mu, Y.C.; Xu, J.H.; Yang, Z.; Lu, X.R.; Zhang, X.F.; Sun, H.W.; Qiao, W. Nutrient composition analysis and quality evaluation system construction of Litopenaeus vannamei from different aquaculture water environments. Food Res. 2024, 5, 100661. [Google Scholar] [CrossRef]
- Pellett, P.L.; Young, V.R.; UN University. Nutritional Evaluation of Protein Foods; The United National University Publishing Company: Tokyo, Japan, 1980; pp. 26–29. [Google Scholar]
- Surányi, J.; Zaukuu, J.-L.Z.; Friedrich, L.; Kovacs, Z.; Horváth, F.; Németh, C.; Kókai, Z. Electronic tongue as a correlative technique for modeling cattle meat quality and classification of breeds. Foods 2021, 10, 2283. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, S.; Tao, Y.; Hua, J.; Zhuge, Y.; Chen, W.; Qiang, J. Characteristic Muscle Quality Parameters of Male Largemouth Bass (Micropterus salmoides) Distinguished from Female and Physiological Variations Revealed by Transcriptome Profiling. Biology 2024, 13, 1029. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Xie, Q.; Wang, Y.; Yu, J.; Ma, X. Physicochemical and structural changes of myofibrillar proteins in muscle foods during thawing: Occurrence, consequences, evidence, and implications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3444–3477. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-M.; Fan, Y.-C.; Xu, S.-J.; Liu, Y.-X.; Wu, Z.-X.; Zhou, D.-Y.; Zhu, B.-W. Effects of antioxidants on the texture and protein quality of ready-to-eat abalone muscles during storage. J. Food Compos. Anal. 2022, 108, 104456. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, Y.; Wang, G.; Zheng, G.; Zou, S. Study on flesh quality and physiological response of grass carp cultivated at higher density in-pond raceway system. J. World Aquac. Soc. 2023, 54, 686–700. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, M.; Zhang, X.; Qin, N.; Zhu, W.; Wang, L.; Fu, J.; Dong, Z. Evaluation of muscle quality and movement-related traits in two common carp strains under a rice–fish farming system. Aquac. Int. 2025, 33, 309. [Google Scholar] [CrossRef]
- Johnston, I.A.; Li, X.; Vieira, V.L.; Nickell, D.; Dingwall, A.; Alderson, R.; Campbell, P.; Bickerdike, R. Muscle and flesh quality traits in wild and farmed Atlantic salmon. Aquaculture 2006, 256, 323–336. [Google Scholar] [CrossRef]
- Wang, M.; Feng, L.; Wu, P.; Liu, Y.; Ren, H.; Jin, X.; Zhou, X.; Jiang, W. Regulation of muscle springiness and hardness: The role of myo-inositol in enhancing fish flesh texture. Food Chem. X 2025, 29, 102788. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.; Miller, R.; Ha, M.; Wheeler, T.L.; Dunshea, F.; Li, X.; Vaskoska, R.; Purslow, P.; Wheeler, T. Meat tenderness: Underlying mechanisms, instrumental measurement, and sensory assessment. Meat Muscle Biol. 2021, 4, 1–25. [Google Scholar]
- Li, C.; Liu, D.; Zhou, G.; Xu, X.; Qi, J.; Shi, P.; Xia, T. Meat quality and cooking attributes of thawed pork with different low field NMR T21. Meat Sci. 2012, 92, 79–83. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Liu, C.; Feng, D.; Huang, J.; Jin, Z.; Ma, F.; Xu, J.; Xu, Y.; Zhang, M. Effects of water flow treatment on muscle quality, nutrient composition and volatile compounds in common carp (Cyprinus carpio). Food Chem. X 2025, 26, 102257. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hegazy, A.M.; Zhang, X. Swimming exercise as potential measure to improve flesh quality of cultivable fish: A review. Aquac. Res. 2021, 52, 5978–5989. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Cabrera-Álvarez, M.J.; Maia, C.M.; Saraiva, J.L. Environmental enrichment in fish aquaculture: A review of fundamental and practical aspects. Rev. Aquac. 2022, 14, 704–728. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Abdollahi, M.; Wu, H.; Undeland, I. Impact of processing technology on macro-and micronutrient profile of protein-enriched products from fish backbones. Foods 2021, 10, 950. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, J.; Xu, C.; Qin, C.; Zhang, Y.; Yang, L.; Zhi, S.; Feng, J.; Nie, G. Comparison of muscle nutritional composition, texture quality, carotenoid metabolites and transcriptome to underling muscle quality difference between wild-caught and pond-cultured Yellow River carp (Cyprinus carpio haematopterus). Aquaculture 2024, 581, 740392. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Fan, S.; Zhou, Z.; Zhou, R.; Wu, C.; Gong, D.; Wen, M.; Wang, Y.; Tao, M. Comparative analysis of muscle nutrient in two types of hybrid bream and native bream. Reprod. Breed. 2022, 2, 71–77. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Zheng, X.; Wang, Z.; Wang, Z.; Cheng, X. Nutritional value and bioaccumulation of heavy metals in nine commercial fish species from Dachen Fishing Ground, East China Sea. Sci. Rep. 2022, 12, 6927. [Google Scholar] [CrossRef]
- Peng, J.; Lu, X.; Fan, R.; Ren, Y.; Sun, H.; Yu, Y.; Cheng, B. Analysis and evaluation of muscle quality in different parts of the bighead carp (Aristichthys nobilis). Foods 2023, 12, 4430. [Google Scholar] [CrossRef]
- Wang, L.; Jia, S.-p.; Zhang, L.; Ma, F.-r.; Zhang, M.; Yu, M.; Jiang, H.-x.; Qiao, Z.-g.; Li, X.-j. Comparative study on nutritional quality and volatile flavor compounds of muscle in Cyprinus carpio haematopterus under wild, traditional pond and in-pond raceway system culture. Aquac. Rep. 2022, 25, 101194. [Google Scholar] [CrossRef]
- Ackman, R. Seafood lipids and fatty acids. Food Rev. Int. 1990, 6, 617–646. [Google Scholar] [CrossRef]
- Ryu, B.; Shin, K.-H.; Kim, S.-K. Muscle protein hydrolysates and amino acid composition in fish. Mar. Drugs 2021, 19, 377. [Google Scholar] [CrossRef]
- Cai, L.; Tong, F.; Tang, T.; Ao, Z.; Wei, Z.; Yang, F.; Shu, Y.; Liu, S.; Mai, K. Comparative evaluation of nutritional value and flavor quality of muscle in triploid and diploid common carp: Application of genetic improvement in fish quality. Aquaculture 2021, 541, 736780. [Google Scholar] [CrossRef]
- Xie, X.-D.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Ren, H.-M.; Jin, X.-W.; Zhang, R.-N.; Zhou, X.-Q. From antioxidant to muscle enhancer: Resveratrol’s role in grass carp (Ctenopharyngodon idella) nutrition. Aquac. Rep. 2024, 39, 102499. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Tian, J.; Li, C.; Ge, Y.; Hu, X.; Cheng, L.; Shi, X.; Shi, L.; Jia, Z. Nutritional components, biochemical characteristics, enzyme activities, and growth differences of five freshwater fish species? Fishes 2022, 7, 285. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, J.; Li, C.; Wu, S.; Huang, L.; Qin, D.; Hao, Q.; Gao, L. Texture, Nutrition, and Flavor of Different Freshwater Fish Muscles: Comparative Study and Molecular Docking. Foods 2025, 14, 2258. [Google Scholar] [CrossRef]
- Xue, R.; Chen, L.; Sun, C.; Muhammad, A.; Shao, Y. Mulberry Leaf Protein: Extraction Technologies, Functional Attributes and Food Applications. Foods 2025, 14, 2602. [Google Scholar] [CrossRef]
- Tomičić, Z.; Spasevski, N.; Lazarević, J.; Čabarkapa, I.; Tomičić, R. Diversity of amino acids composition in cereals. Food Feed Res. 2022, 49, 11–22. [Google Scholar] [CrossRef]
- Niu, M.; Chen, X.; Zhou, W.; Guo, Y.; Yuan, X.; Cui, J.; Shen, Z.; Su, N. Multi-omics analysis provides insights into lysine accumulation in quinoa (Chenopodium quinoa Willd.) Sprouts. Food Res. Int. 2023, 171, 113026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wen, S.; Wei, L.; Gan, B.; Lu, Y.; Mo, F. Nutrient composition and quality evaluation of Cyprinus multitaeniata. Anim. Breed. Feed 2024, 23, 1–6. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, X.; Tang, D.; Zhu, Y.; Wang, J.; Zhu, J. Analysis and evaluation of nutritional components in muscle of cultured Opsariichthys bidens. J. Ningbo Univ. (Nat. Sci. Eng. Ed.) 2019, 32, 15–19. [Google Scholar]
- Li, Z.; Hong, T.; Shen, G.; Gu, Y.; Guo, Y.; Han, J. Amino acid profiles and nutritional evaluation of fresh sweet–waxy corn from three different regions of China. Nutrients 2022, 14, 3887. [Google Scholar] [CrossRef]
- Nandi, S.K.; Siddik, M.A.; Hridoy, M.A.A.M.; Bapary, M.A.J.; Hasan, S.; Kabir, M.A.; Islam, M.J. Effects of Replacing Commercial Fish Meal With Invasive Suckermouth Catfish on Growth, Health, and Economic Performance of Climbing Perch Anabas testudineus. Aquac. Res. 2025, 2025, 3610730. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Xie, C.; Li, D.; Xu, J.; Zhang, M.; Zhang, M. Lipid contents, fatty acid profiles and nutritional quality of nine wild caught freshwater fish species of the Yangtze Basin, China. J. Food Nutr. Res. 2014, 2, 388–394. [Google Scholar] [CrossRef]
- Hong, H.; Zhou, Y.; Wu, H.; Luo, Y.; Shen, H. Lipid content and fatty acid profile of muscle, brain and eyes of seven freshwater fish: A comparative study. J. Am. Oil Chem. Soc. 2014, 91, 795–804. [Google Scholar] [CrossRef]
- Kaneniwa, M.; Miao, S.; Yuan, C.; Lida, H.; Fukuda, Y. Lipid components and enzymatic hydrolysis of lipids in muscle of Chinese freshwater fish. J. Am. Oil Chem. Soc. 2000, 77, 825. [Google Scholar] [CrossRef]
- Jerab, D.; Blangero, F.; da Costa, P.C.T.; de Brito Alves, J.L.; Kefi, R.; Jamoussi, H.; Morio, B.; Eljaafari, A. Beneficial Effects of Omega-3 Fatty Acids on Obesity and Related Metabolic and Chronic Inflammatory Diseases. Nutrients 2025, 17, 1253. [Google Scholar] [CrossRef] [PubMed]
- Nave, C.B.d.; Pereira, P.; Silva, M.L. The Effect of Polyunsaturated Fatty Acid (PUFA) Supplementation on Clinical Manifestations and Inflammatory Parameters in Individuals with Sjögren’s Syndrome: A Literature Review of Randomized Controlled Clinical Trials. Nutrients 2024, 16, 3786. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, X.; He, X.; Sun, X.; Yu, X.; Cheng, Y.; Yu, R.-Q.; Wu, Y. Fatty acid composition analyses of commercially important fish species from the Pearl River Estuary, China. PLoS ONE 2020, 15, e0228276. [Google Scholar] [CrossRef]
- Simopoulos, A. Human requirement for N-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Ochi, E.; Tsuchiya, Y. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in muscle damage and function. Nutrients 2018, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Martin, L.; Wagner, A.; Jahreis, G. n− 3 LC-PUFA-enriched dairy products are able to reduce cardiovascular risk factors: A double-blind, cross-over study. Clin. Nutr. 2010, 29, 592–599. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Larson, D.E.; Fisher-Wellman, K.H.; Galpin, A.J.; Schilling, B.K. Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: A randomized, placebo controlled, cross-over study. Lipids Health Dis. 2009, 8, 36. [Google Scholar] [CrossRef]
- Wang, L.; Shan, T. Factors inducing transdifferentiation of myoblasts into adipocytes. J. Cell. Physiol. 2021, 236, 2276–2289. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.-P. The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, L.; Wu, P.; Feng, L.; Jiang, W.; Liu, Y.; Kuang, S.; Li, S.; Mi, H.; Tang, L. Dietary protein levels changed the hardness of muscle by acting on muscle fiber growth and the metabolism of collagen in sub-adult grass carp (Ctenopharyngodon idella). J. Anim. Sci. Biotechnol. 2022, 13, 109. [Google Scholar] [CrossRef]
- Chen, M.-M.; Zhao, Y.-P.; Zhao, Y.; Deng, S.-L.; Yu, K. Regulation of myostatin on the growth and development of skeletal muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef]
- Sharma, A.; Huard, C.; Vernochet, C.; Ziemek, D.; Knowlton, K.M.; Tyminski, E.; Paradis, T.; Zhang, Y.; Jones, J.E.; von Schack, D. Brown fat determination and development from muscle precursor cells by novel action of bone morphogenetic protein 6. PLoS ONE 2014, 9, e92608. [Google Scholar] [CrossRef]
- Salanga, C.M.; Salanga, M.C. Genotype to phenotype: CRISPR gene editing reveals genetic compensation as a mechanism for phenotypic disjunction of morphants and mutants. Int. J. Mol. Sci. 2021, 22, 3472. [Google Scholar] [CrossRef] [PubMed]
- Diofano, F.; Weinmann, K.; Schneider, I.; Thiessen, K.D.; Rottbauer, W.; Just, S. Genetic compensation prevents myopathy and heart failure in an in vivo model of Bag3 deficiency. PLoS Genet. 2020, 16, e1009088. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.A.; Østbye, T.K.K.; Kettunen, A.H.; Coates, A.; Barrett, L.T.; Robledo, D.; Dempster, T. A guide to assess the use of gene editing in aquaculture. Rev. Aquac. 2024, 16, 775–784. [Google Scholar] [CrossRef]
- Van Eenennaam, A.L. New Genomic Techniques (NGT) in animals and their agri/food/feed products. EFSA Support. Publ. 2023, 20, 8311E. [Google Scholar] [CrossRef]

| Amino Acid (g/100 g) | FZYJ | SPYJ | WT | WUCI |
|---|---|---|---|---|
| Thr * | 0.66 ± 0.05 a | 0.64 ± 0.02 ab | 0.59 ± 0.01 c | 0.62 ± 0.02 ab |
| Val * | 0.73 ± 0.05 | 0.73 ± 0.02 | 0.68 ± 0.02 | 0.70 ± 0.03 |
| Met * | 0.30 ± 0.02 | 0.27 ± 0.08 | 0.30 ± 0.06 | 0.29 ± 0.06 |
| Leu * | 1.20 ± 0.09 a | 1.16 ± 0.03 ab | 1.08 ± 0.03 b | 1.12 ± 0.04 ab |
| Ile * | 0.64 ± 0.05 | 0.63 ± 0.02 | 0.61 ± 0.01 | 0.61 ± 0.02 |
| Lys * | 1.46 ± 0.09 a | 1.37 ± 0.03 ab | 1.30 ± 0.03 c | 1.41 ± 0.06 a |
| Phe * | 0.67 ± 0.05 | 0.65 ± 0.02 | 0.62 ± 0.02 | 0.63 ± 0.01 |
| His | 0.51 ± 0.04 | 0.49 ± 0.02 | 0.47 ± 0.02 | 0.48 ± 0.01 |
| Arg | 0.82 ± 0.06 | 0.80 ± 0.06 | 0.74 ± 0.02 | 0.77 ± 0.04 |
| Gly # | 0.78 ± 0.04 | 0.82 ± 0.12 | 0.71 ± 0.05 | 0.72 ± 0.03 |
| Ala # | 0.91 ± 0.06 a | 0.87 ± 0.05 ab | 0.80 ± 0.02 b | 0.84 ± 0.03 ab |
| Pro | 0.47 ± 0.03 | 0.49 ± 0.07 | 0.43 ± 0.01 | 0.45 ± 0.01 |
| Tyr | 0.45 ± 0.04 | 0.42 ± 0.02 | 0.41 ± 0.02 | 0.43 ± 0.02 |
| Ser | 0.58 ± 0.05 a | 0.53 ± 0.03 ab | 0.50 ± 0.02 b | 0.52 ± 0.01 ab |
| Asp # | 1.52 ± 0.11 a | 1.45 ± 0.03 ab | 1.36 ± 0.03 b | 1.41 ± 0.04 ab |
| Glu # | 2.04 ± 0.17 a | 1.91 ± 0.09 ab | 1.78 ± 0.06 b | 1.87 ± 0.08 ab |
| Cys | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.03 |
| EAA | 6.19 ± 0.40 a | 5.94 ± 0.13 ab | 5.65 ± 0.09 b | 5.86 ± 0.22 ab |
| NEAA | 8.17 ± 0.58 a | 7.87 ± 0.47 ab | 7.31 ± 0.20 b | 7.60 ± 0.27 ab |
| TAA | 13.85 ± 0.95 a | 13.32 ± 0.58 ab | 12.49 ± 0.27 b | 12.98 ± 0.48 ab |
| UAA | 5.25 ± 0.37 a | 5.05 ± 0.29 ab | 4.65 ± 0.16 b | 4.84 ± 0.17 ab |
| EAA/TAA (%) | 41.01 ± 0.11 | 40.94 ± 0.98 | 41.48 ± 0.35 | 41.45 ± 0.36 |
| EAA/NEAA (%) | 69.52 ± 0.32 | 69.34 ± 2.77 | 70.89 ± 1.01 | 70.80 ± 1.06 |
| Essential Amino Acid | FAO/WHO Standard | Egg Protein | FZYJ | SPYJ | ||||
|---|---|---|---|---|---|---|---|---|
| Content (mg/g N) | AAS | CS | Content (mg/g N) | AAS | CS | |||
| Ile | 250 | 331 | 217.34 | 0.87 | 0.66 | 214.74 | 0.86 | 0.65 |
| Leu | 440 | 534 | 406.53 | 0.92 | 0.76 | 393.31 | 0.89 | 0.74 |
| Thr | 250 | 292 | 224.1 | 0.90 | 0.77 | 215.87 | 0.86 | 0.74 |
| Val | 310 | 441 | 247.75 | 0.8 | 0.56 | 246.38 | 0.79 | 0.56 |
| Met + Cys | 220 | 386 | 135.81 | 0.62 | 0.35 | 125.11 | 0.57 | 0.32 |
| Phe + Tyr | 380 | 565 | 379.5 | 1.00 | 0.67 | 363.92 | 0.96 | 0.64 |
| Lys | 340 | 441 | 494.37 | 1.45 | 1.12 | 464.51 | 1.37 | 1.05 |
| EAAI | 66.43 | 63.88 | ||||||
| Essential Amino Acid | FAO/WHO Standard | Egg Protein | WT | WUCI | ||||
| Content (mg/g N) | AAS | CS | Content (mg/g N) | AAS | CS | |||
| Ile | 250 | 331 | 202.79 | 0.81 | 0.61 | 204.24 | 0.82 | 0.62 |
| Leu | 440 | 534 | 359.04 | 0.82 | 0.67 | 375.00 | 0.85 | 0.7 |
| Thr | 250 | 292 | 197.25 | 0.79 | 0.68 | 207.59 | 0.83 | 0.71 |
| Val | 310 | 441 | 227.17 | 0.73 | 0.51 | 235.49 | 0.76 | 0.53 |
| Met + Cys | 220 | 386 | 130.54 | 0.59 | 0.33 | 133.37 | 0.61 | 0.35 |
| Phe + Tyr | 380 | 565 | 342.42 | 0.9 | 0.61 | 354.91 | 0.93 | 0.63 |
| Lys | 340 | 441 | 432.18 | 1.27 | 0.98 | 473.21 | 1.39 | 1.07 |
| EAAI | 60.24 | 62.75 | ||||||
| Fatty Acid (g/100 g) | FZYJ | SPYJ | WT | WUCI |
|---|---|---|---|---|
| SFA | 0.15 ± 0.02 c | 0.35 ± 0.00 b | 0.42 ± 0.03 a | 0.43 ± 0.05 a |
| C14:0 | 0.00 ± 0.00 b | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| C15:0 | 0.004 ± 0.000 b | - | 0.005 ± 0.000 a | 0.004 ± 0.000 b |
| C16:0 | 0.11 ± 0.01 c | 0.24 ± 0.00 b | 0.31 ± 0.03 a | 0.32 ± 0.03 a |
| C18:0 | 0.04 ± 0.00 b | 0.09 ± 0.00 a | 0.09 ± 0.00 a | 0.09 ± 0.01 a |
| MUFA | 0.21 ± 0.03 b | 0.59 ± 0.03 a | 0.68 ± 0.10 a | 0.71 ± 0.06 a |
| C16:1 | 0.02 ± 0.00 b | 0.05 ± 0.00 a | 0.06 ± 0.01 a | 0.06 ± 0.00 a |
| C18:1n9c | 0.17 ± 0.02 b | 0.50 ± 0.03 a | 0.58 ± 0.09 a | 0.61 ± 0.05 a |
| C20:1 | 0.01 ± 0.00 b | 0.04 ± 0.00 a | 0.03 ± 0.00 a | 0.04 ± 0.00 a |
| C22:1n9 | 0.005 ± 0.001 | 0.004 ± 0.000 | 0.006 ± 0.002 | 0.005 ± 0.001 |
| C24:1 | 0.003 ± 0.002 | - | 0.001 ± 0.002 | - |
| PUFA | 0.14 ± 0.01 c | 0.30 ± 0.02 b | 0.43 ± 0.10 a | 0.37 ± 0.03 ab |
| C18:2n6c | 0.08 ± 0.00 d | 0.15 ± 0.00 c | 0.28 ± 0.06 a | 0.21 ± 0.02 b |
| C18:3n3 | 0.01 ± 0.00 b | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.03 ± 0.00 a |
| C18:3n6 | - | - | 0.002 ± 0.004 | - |
| C20:2 | 0.00 ± 0.00 b | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| C20:3n6 | 0.00 ± 0.00 c | 0.01 ± 0.00 b | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| C20:4n6 | 0.01 ± 0.00 b | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.03 ± 0.00 a |
| C20:5n3 (EPA) | 0.00 ± 0.00 b | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.01 ± 0.00 a |
| C22:6n3 (DHA) | 0.02 ± 0.00 b | 0.04 ± 0.01 a | 0.05 ± 0.02 a | 0.05 ± 0.00 a |
| n3 | 0.04 ± 0.00 b | 0.09 ± 0.01 a | 0.09 ± 0.03 a | 0.09 ± 0.01 a |
| n6 | 0.10 ± 0.01 c | 0.20 ± 0.01 b | 0.33 ± 0.07 a | 0.27 ± 0.02 ab |
| n3/n6 | 0.35 | 0.44 | 0.28 | 0.35 |
| TFA | 0.50 ± 0.06 c | 1.24 ± 0.04 b | 1.53 ± 0.23 a | 1.51 ± 0.13 a |
| SFA/TFA(%) | 30.28 ± 1.29 | 28.09 ± 1.02 | 27.61 ± 2.56 | 28.68 ± 1.28 |
| MUFA/TFA(%) | 41.36 ± 1.17 c | 47.89 ± 1.31 a | 44.65 ± 0.45 b | 46.98 ± 0.13 a |
| PUFA/TFA(%) | 28.36 ± 1.59 a | 24.02 ± 1.43 b | 27.74 ± 2.91 ab | 24.34 ± 1.41 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Sun, Z.; Zhang, T.; Zhang, T.; Yan, T.; Xu, H.; Wei, M.; Yin, Y.; Li, N.; Kuang, Y.; et al. Comparison of Muscle Texture Characteristics and Nutritional Composition Between Gene-Edited Intermuscular-Bone-Free Crucian Carp and Other Varieties. Fishes 2025, 10, 606. https://doi.org/10.3390/fishes10120606
Zhou H, Sun Z, Zhang T, Zhang T, Yan T, Xu H, Wei M, Yin Y, Li N, Kuang Y, et al. Comparison of Muscle Texture Characteristics and Nutritional Composition Between Gene-Edited Intermuscular-Bone-Free Crucian Carp and Other Varieties. Fishes. 2025; 10(12):606. https://doi.org/10.3390/fishes10120606
Chicago/Turabian StyleZhou, Huijie, Zhipeng Sun, Tan Zhang, Tingting Zhang, Ting Yan, Huan Xu, Mingliang Wei, Yashan Yin, Na Li, Youyi Kuang, and et al. 2025. "Comparison of Muscle Texture Characteristics and Nutritional Composition Between Gene-Edited Intermuscular-Bone-Free Crucian Carp and Other Varieties" Fishes 10, no. 12: 606. https://doi.org/10.3390/fishes10120606
APA StyleZhou, H., Sun, Z., Zhang, T., Zhang, T., Yan, T., Xu, H., Wei, M., Yin, Y., Li, N., Kuang, Y., & Tong, G. (2025). Comparison of Muscle Texture Characteristics and Nutritional Composition Between Gene-Edited Intermuscular-Bone-Free Crucian Carp and Other Varieties. Fishes, 10(12), 606. https://doi.org/10.3390/fishes10120606






