First Record of the Arabian Barracuda Sphyraena arabiansis (Sphyraenidae) from Korean Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Data and Measuremensts
2.2. Molecular Analysis
3. Results
3.1. Morphological Characteristics
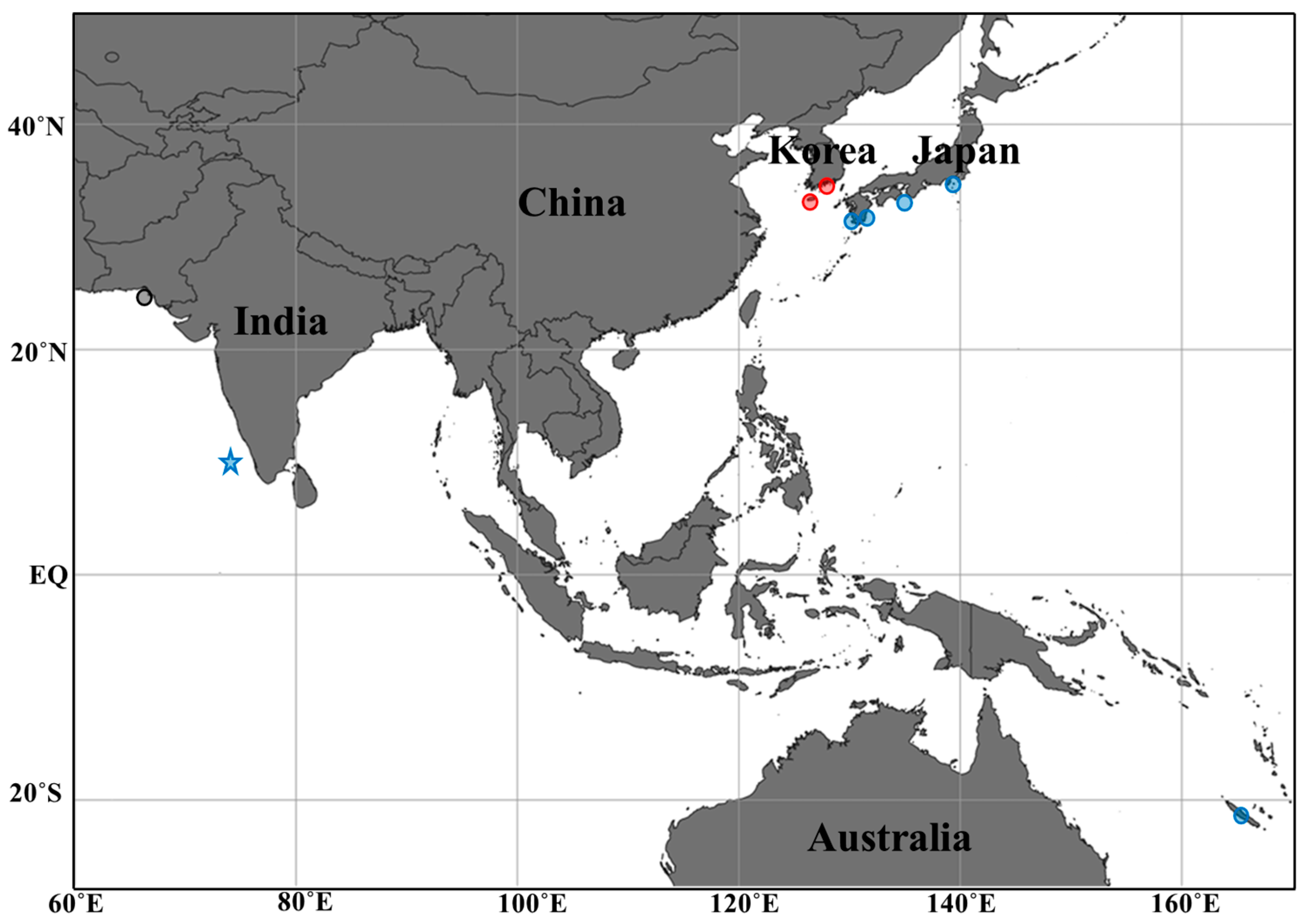
3.2. Distribution
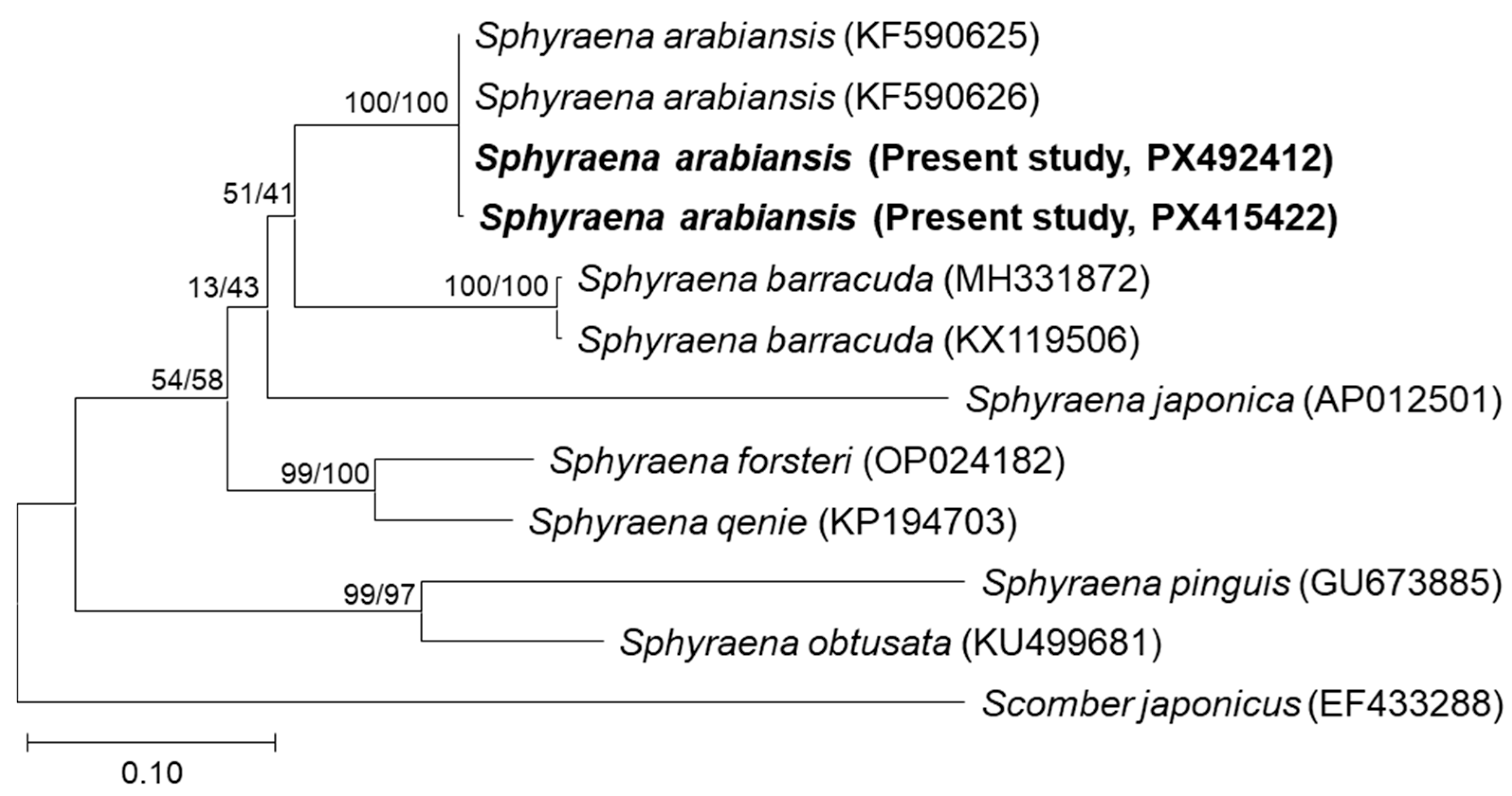
3.3. Molecular Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Froese, R.; Pauly, D. (Eds.) FishBase; World Wide Web Electronic Publication: Lexington, MA, USA, 2025; Available online: www.fishbase.org (accessed on 1 April 2025).
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; 705p. [Google Scholar] [CrossRef]
- Morishita, S.; Motomura, H. Sphyraena stellata, a new barracuda from the Indo-Pacific, with redescriptions of S. helleri Jenkins, 1901 and S. novaehollandiae Günther, 1860 (Perciformes: Sphyraenidae). Zootaxa 2020, 4772, 545–566. [Google Scholar] [CrossRef] [PubMed]
- MABIK (National Marine Biodiversity Institute of Korea). National List of Marine Species. I. Marine Vertebrate; National Marine Biodiversity Institute of Korea: Seocheon, Republic of Korea, 2023; 147p. [Google Scholar]
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006; 601p. [Google Scholar]
- Betancu-R, R.; Wiley, E.O.; Arratia, G.; Acero, A.; Bailly, N.; Miya, M.; Lecointre, G.; Ortí, G. Arratia Phylogenetic classification of bony fishes. BMC Ecol. Evol. 2017, 17, 162. [Google Scholar] [CrossRef]
- Near, T.J.; Thacker, C.E. Phylogenetic Classification of Living and Fossil Ray-Finned Fishes (Actinopterygii). Bull. Peabody Mus. Nat. Hist. 2024, 65, 3–302. [Google Scholar] [CrossRef]
- Abdussamad, E.M.; Retheesh, T.B.; Thangaraja, R.; Bineesh, K.K.; Prakasan, D. First record of Arabian barracuda, Sphyraena arabiansis from India. Indian J. Fish. 2015, 62, 1–6. [Google Scholar]
- Manzoor, H.; Osmany, H.B.; Zohra, K. First record of Arabian barracuda, Sphyraena arabiansis from Pakistan. Int. J. Biol. Biotechnol. 2020, 17, 463–468. [Google Scholar]
- Morishita, S.; Miki, R.; Senou, H.; Motomura, H. First record of Sphyraena arabiansis (Perciformes: Sphyraenidae), with a revised species diagnosis and morphological comparisons with S. barracuda. Japan. J. Ichthyol. 2020, 67, 73–83. [Google Scholar] [CrossRef]
- Doiuchi, R.; Nakabo, T. The Sphyraena obtusata group (Perciformes: Sphyraenidae) with a description of a new species from southern Japan. Ichthyol. Res. 2005, 52, 132–151. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D.N. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular evolutionary genetic analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; pp. 1–333. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Han, I.S.; Lee, J.S.; Jung, H.K. Long-term pattern changes of sea surface temperature during summer and winter due to climate change in the Korean Waters. Fish. Aquat. Sci. 2023, 26, 639–648. [Google Scholar] [CrossRef]


| Meristic Characters | Present Study | Abdussamad et al. [8] (Holotype) | Manzoor et al. [9] | Morishita et al. [10] |
|---|---|---|---|---|
| No. of specimens | 2 | 1 | 1 | 5 |
| Counts | ||||
| Dorsal fin rays | V-I, 9 | V-I, 10 | V-I, 10 | V-I, 9 |
| Pectoral fin rays | ii, 12 | 14 | 14 | ii, 12 |
| Pelvic fin rays | I, 5 | I, 5 | I, 5 | I, 5 |
| Anal fin rays | II, 8 | I, 9 | I, 9 | II, 8 |
| Lateral line scales | 118, 121 | 120 | 120 | 118–126 |
| Scales above lateral line | 11 | 11 | 11 | 10–11 |
| Scales below lateral line | 14 | 13 | 13 | 13–14 |
| Gill rakers | 0 | 0 | 0 | 0 |
| Branchiostegal rays | 7 | 7 | 7 | - |
| Morphometric Characters | Present Study | Abdussamad et al. [8] (Holotype) | Manzoor et al. [9] | Morishita et al. [10] |
|---|---|---|---|---|
| No. of specimens | 2 | 1 | 1 | 5 |
| Total length (mm) | 1164–1187 | - | - | - |
| Fork length (mm) | 1120–1150 | 950 | 950 | - |
| Standard length (mm) | 1053–1086 | 850 | 850 | 648.5–1162.9 |
| In % standard length | ||||
| Head length | 25.9 | 27.53 | 25.5 | 26.1–29.1 |
| Body depth at pelvic fin insertion | 12.5–14.9 | 13.05 | 13.5 | 12.7–15.2 |
| Body depth at 2nd dorsal fin origin | 12.6–13.3 | 12.66 | 11.0 | 12.4–14.0 |
| 1st dorsal fin base length | 7.2–7.3 | 6.34 | 7.8 | 6.0–6.8 |
| 2nd dorsal fin base length | 8.4–8.5 | 8.99 | 9.3 | 8.5–9.7 |
| Anal fin base length | 7.2–7.5 | 8.13 | 7.3 | 7.4–8.5 |
| Pre-dorsal fin length | 37.4–38.5 | 41.18 | 37.0 | 38.7–40.9 |
| Pre-2nd dorsal fin length | 66.8–67.9 | 69.41 | 62.0 | 68.8–71.2 |
| Prepectoral fin length | 26.0–27.1 | 28.24 | 25.0 | 27.3–29.3 |
| Pre-pelvic fin length | 32.6–35.1 | 35.41 | 31.5 | 34.7–36.8 |
| Pre-anal fin length | 69.0–70.2 | 70.59 | 63.2 | 71.2–72.3 |
| 1st dorsal fin height | 6.7–7.2 | 7.75 | 8.0 | 7.1–8.0 |
| 2nd dorsal fin height | 8.8–8.9 | 10.59 | 9.2 | 7.8–10.1 |
| Pectoral fin length | 10.2–10.4 | 10.94 | 9.5 | 10.4–11.5 |
| Pelvic fin length | 7.0 | 8.29 | 7.5 | 6.7–8.8 |
| Distance from origin of first dorsal to origin of second dorsal fin | 29.4–29.9 | - | - | 28.3–31.1 |
| Distance from pelvic fin to anal fin | 34.9–36.0 | - | - | 36.3–37.6 |
| Snout length | 11.0–11.5 | 13.29 | 11.91 | 12.1–12.9 |
| Orbital diameter | 2.7 | 3.06 | 2.9 | 2.8–3.3 |
| Orbital depth | 2.4–2.6 | - | - | 2.6–3.3 |
| Interorbital length | 6.0–6.6 | 5.36 | 7.2 | 6.4–7.0 |
| Upper jaw length | 11.4–12.6 | 12.00 | 10.5 | 12.2–12.8 |
| Postorbital length | 12.4–12.6 | 11.53 | 11.5 | 10.7–13.2 |
| Caudal peduncle length | 17.3–17.8 | 19.53 | 19.0 | 20.1–20.8 |
| Caudal peduncle depth | 6.0 | 6.06 | 6.0 | 6.0–6.8 |
| Caudal peduncle width | 3.7–4.2 | 3.61 | 3.5 | - |
| Snout to anus length | 64.3–70.2 | 67.06 | 60.0 | - |
| Upper caudal fin length | 18.0–18.3 | - | - | 18.6–20.7 |
| Lower caudal fin length | 16.2–17.0 | - | - | 16.5–19.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Chae, Y.M.; Seong, G.C.; Song, C.B. First Record of the Arabian Barracuda Sphyraena arabiansis (Sphyraenidae) from Korean Waters. Fishes 2025, 10, 608. https://doi.org/10.3390/fishes10120608
Kim MJ, Chae YM, Seong GC, Song CB. First Record of the Arabian Barracuda Sphyraena arabiansis (Sphyraenidae) from Korean Waters. Fishes. 2025; 10(12):608. https://doi.org/10.3390/fishes10120608
Chicago/Turabian StyleKim, Maeng Jin, Yu Min Chae, Gi Chang Seong, and Choon Bok Song. 2025. "First Record of the Arabian Barracuda Sphyraena arabiansis (Sphyraenidae) from Korean Waters" Fishes 10, no. 12: 608. https://doi.org/10.3390/fishes10120608
APA StyleKim, M. J., Chae, Y. M., Seong, G. C., & Song, C. B. (2025). First Record of the Arabian Barracuda Sphyraena arabiansis (Sphyraenidae) from Korean Waters. Fishes, 10(12), 608. https://doi.org/10.3390/fishes10120608






