Effects of Astragalus Polysaccharide and Isatis indigotica Extract Synergy on the Antioxidant Status, Inflammation, Autophagy, Apoptosis, and Intestinal Health of Larimichthys crocea Juveniles
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Fish Husbandry
2.2. Sample Collection
2.3. Intestinal Histology
2.4. Biochemical Analysis
2.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
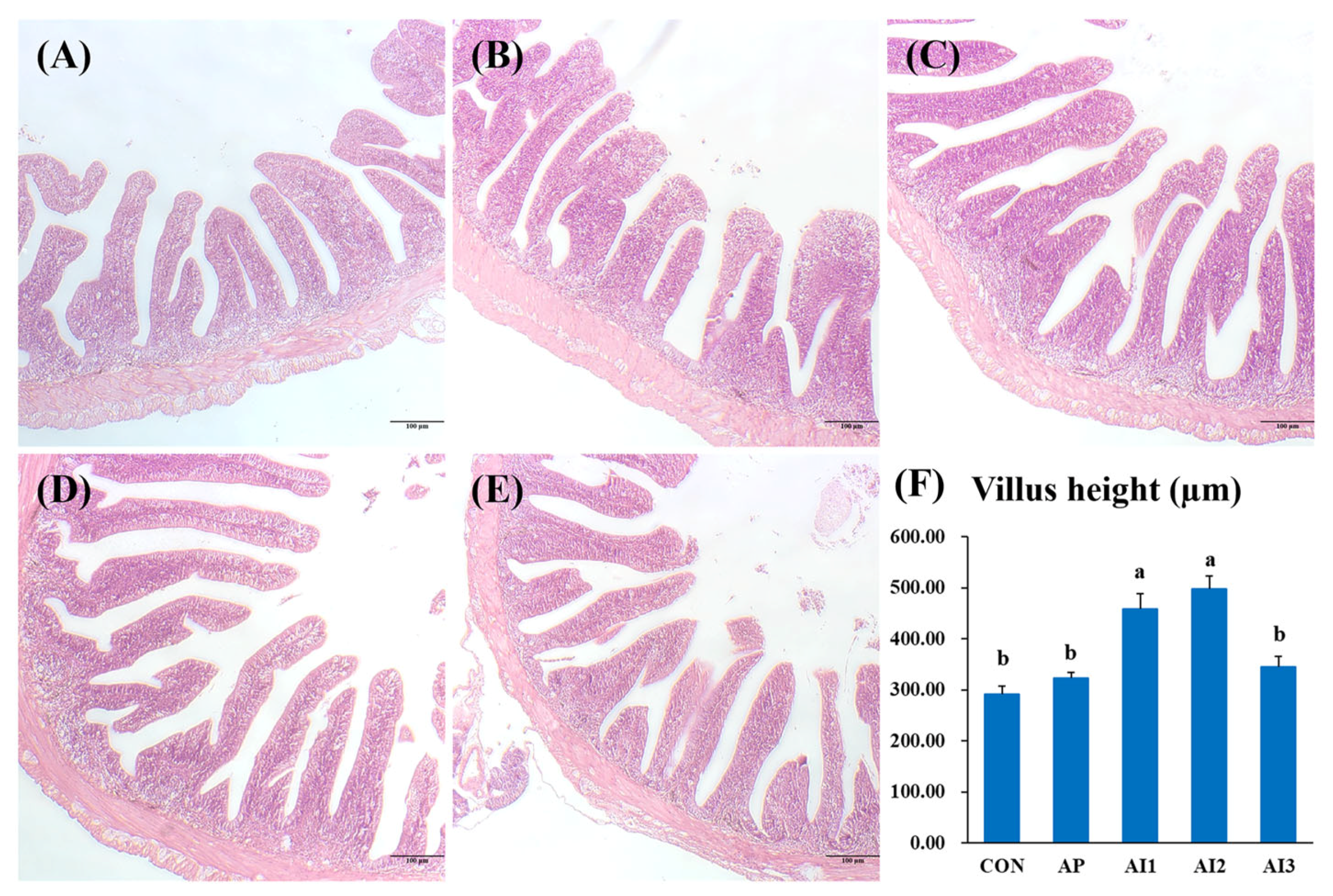
3.1. Intestinal Histology
3.2. Activity of Intestinal Digestive Enzymes
3.3. The Antioxidant Parameters in the Liver of Large Yellow Croaker
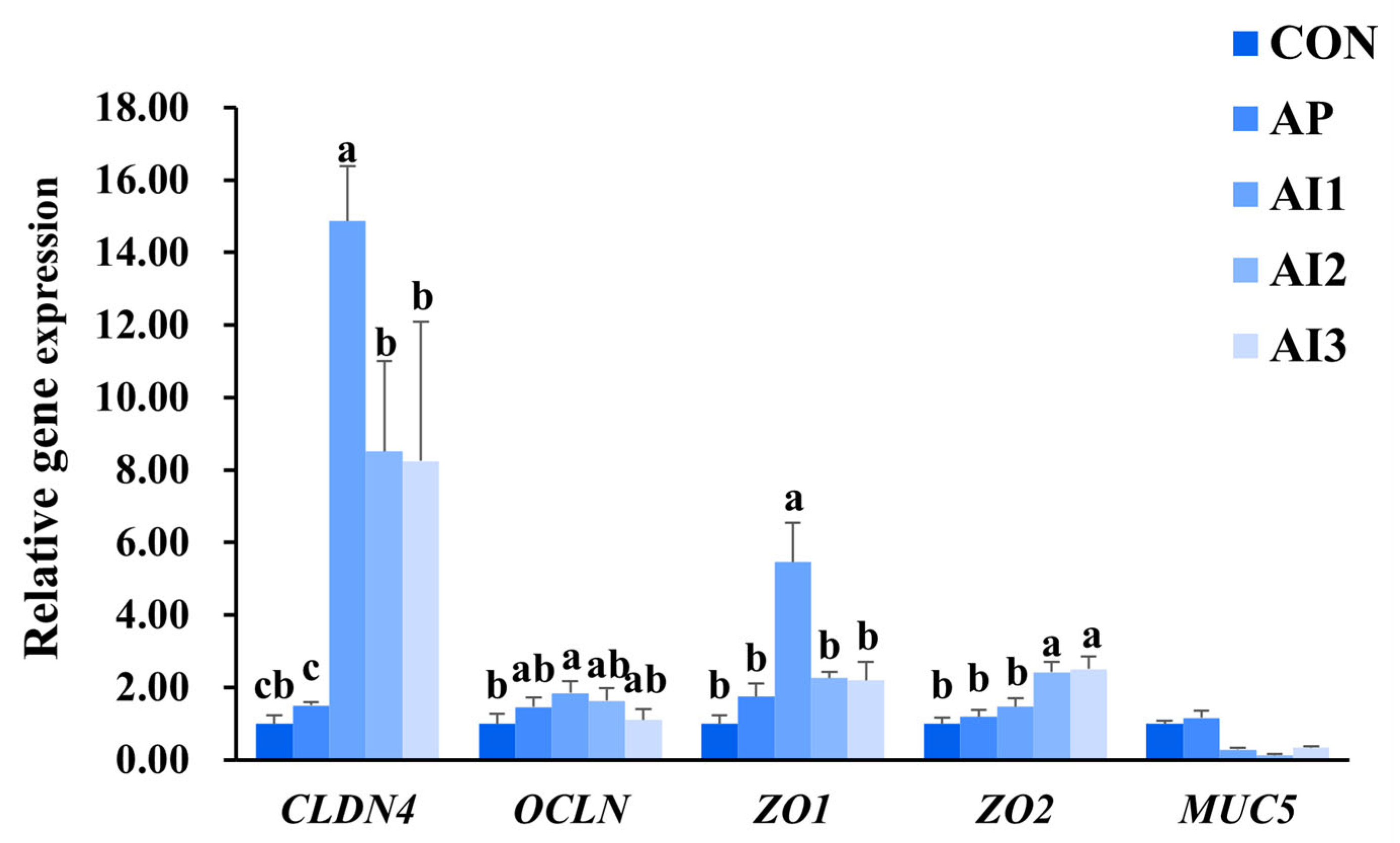
3.4. Relative Gene Expression of Tight Junction and Mucin Proteins in the Intestine of Large Yellow Croaker
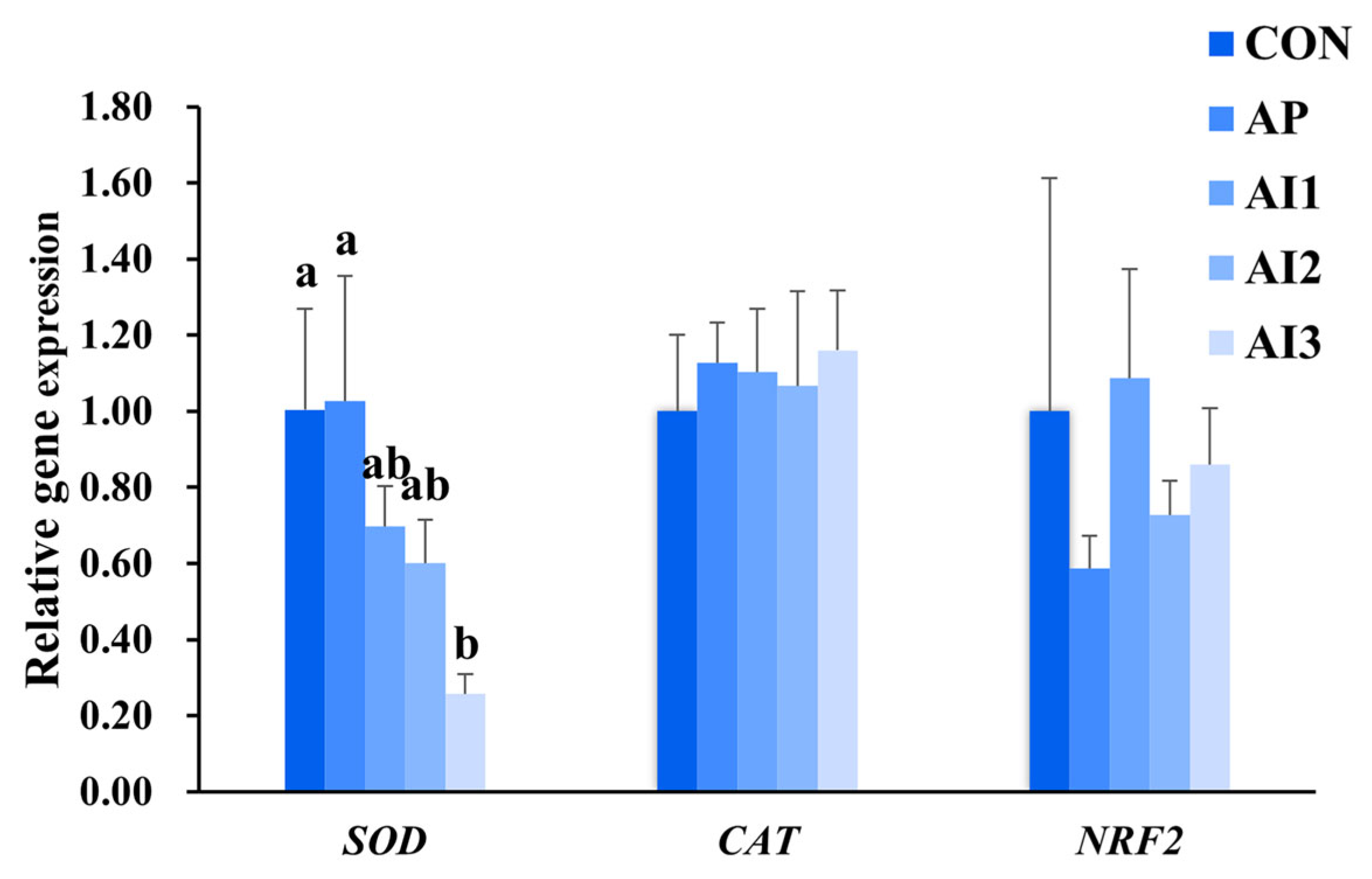
3.5. Antioxidant-Related Gene Expression in the Liver of Large Yellow Croaker
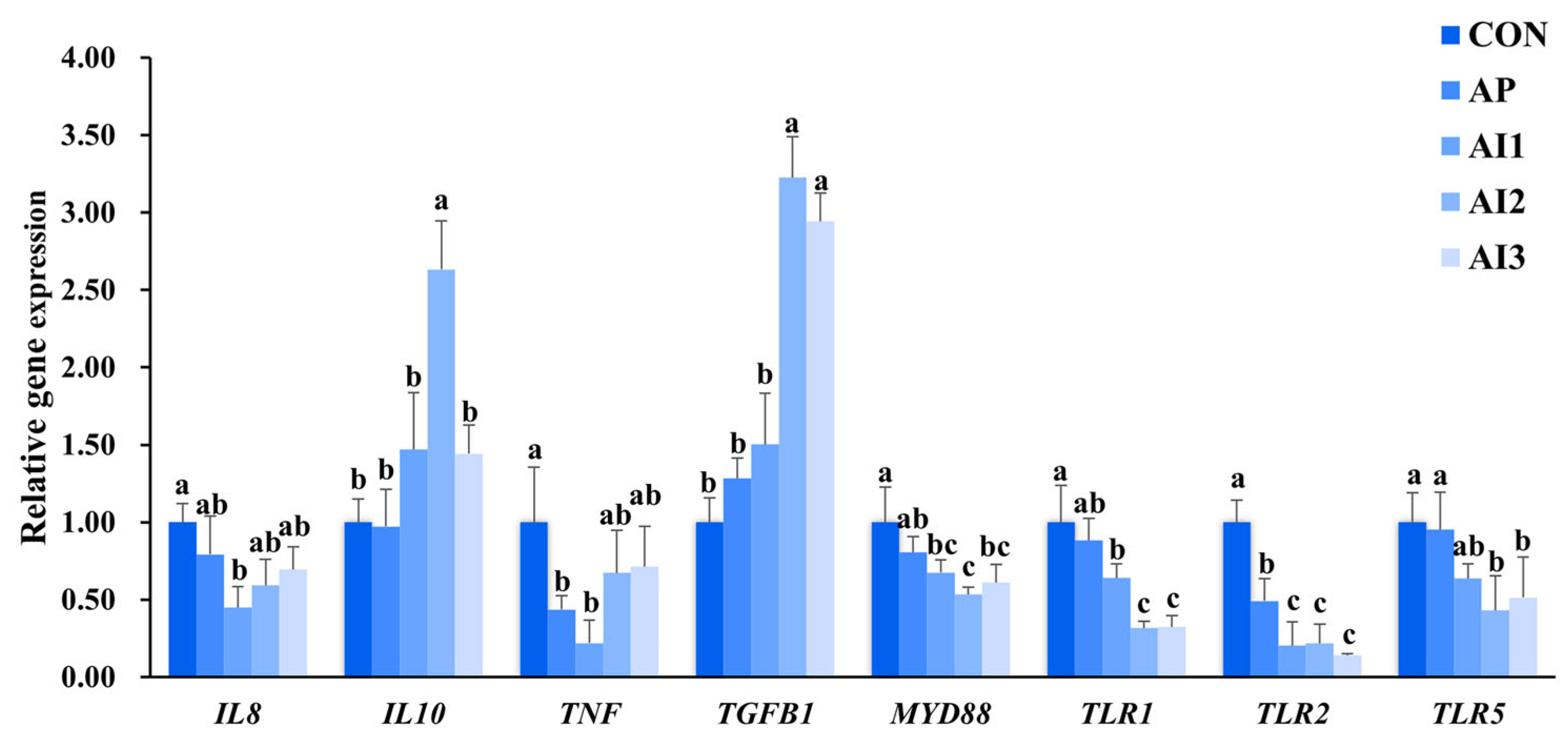
3.6. Inflammation-Related Gene Expression in the Head Kidney of Large Yellow Croaker
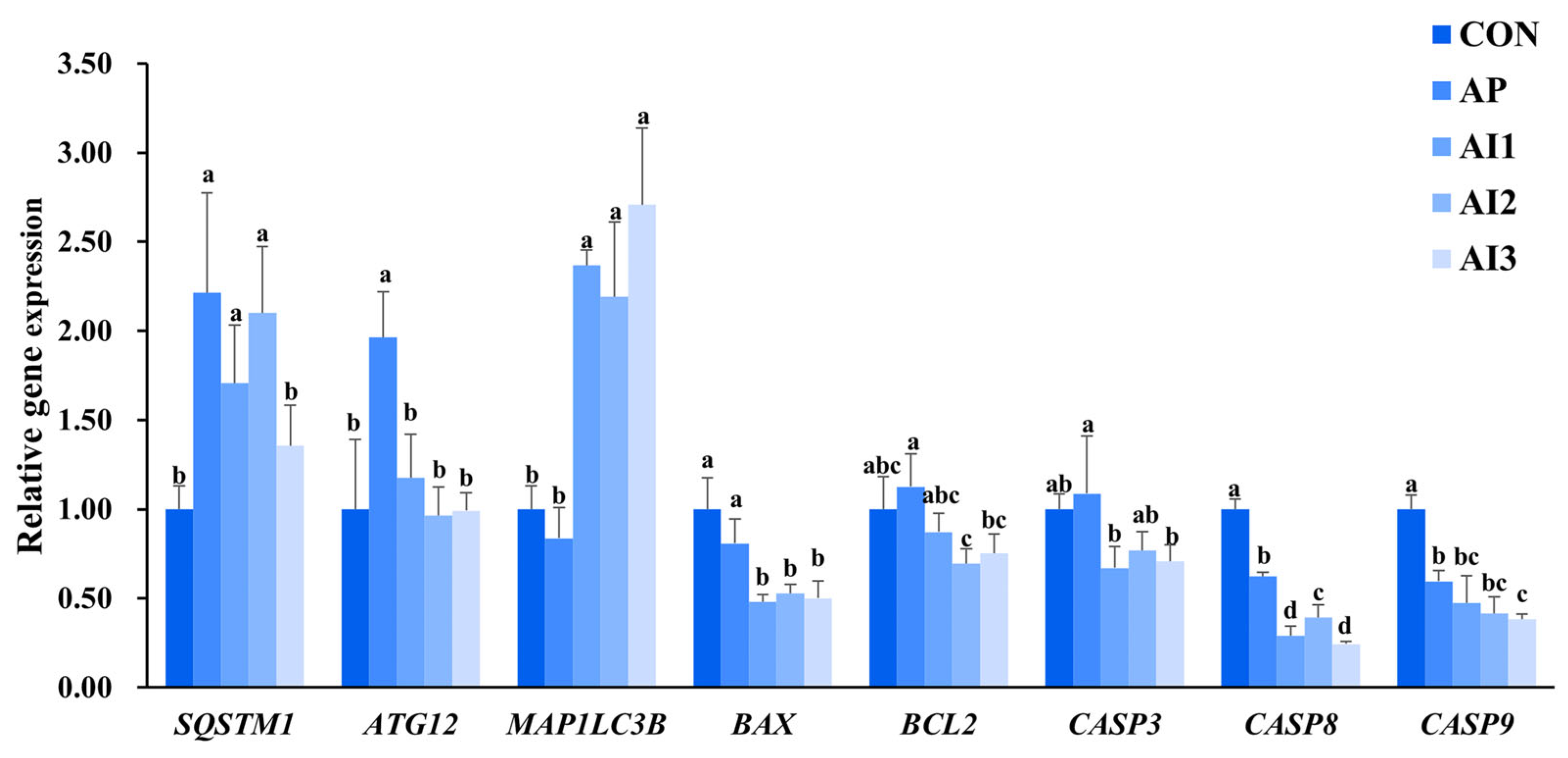
3.7. Autophagy and Apoptosis-Related Gene Expression in the Head Kidney of Large Yellow Croaker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | Actin beta |
| AI | APS and IIE diet |
| AP | APS diet |
| APS | Astragalus polysaccharide |
| ATG12 | Autophagy Related 12 |
| BAX | BCL2-associated x protein |
| BCL2 | BCL2 apoptosis regulator |
| BCL2L1 | BCL2 Like 1 |
| CASP | Caspase |
| CAT | Catalase |
| CLDN | Claudin |
| CON | Control diet |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| GSH | Glutathione |
| IIE | Isatis indigotica extract |
| IL | Interleukin |
| MAP1LC3B | Microtubule-associated protein 1 light chain 3 beta |
| MDA | Malondialdehyde |
| MUC5 | Mucin 5 |
| MyD88 | Myeloid differentiation primary response 88 |
| NF-κB | Nuclear factor kappa B |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| OCLN | Occludin |
| SOD | Superoxide dismutase |
| SQSTM1 | Sequestosome 1 |
| T-AOC | Total antioxidant capacity |
| TGFB1 | transforming growth factor β |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| ZO | Zonula occludens |
References
- Ahmad, A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N.I. Aquaculture industry: Supply and demand, best practices, effluent and its current issues and treatment technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N.; Rye, M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture 2012, 350, 117–129. [Google Scholar] [CrossRef]
- Bergqvist, J.; Gunnarsson, S. Finfish aquaculture: Animal welfare, the environment, and ethical implications. J. Agric. Environ. Ethics 2013, 26, 75–99. [Google Scholar] [CrossRef]
- Pu, H.; Li, X.; Du, Q.; Cui, H.; Xu, Y. Research progress in the application of Chinese herbal medicines in aquaculture: A review. Engineering 2017, 3, 731–737. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Cheng, A.; Hao, E.; Huang, X.; Chen, X. Immunomodulatory and antioxidant effects of Astragalus polysaccharide liposome in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2020, 100, 126–136. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Cheng, A.; Liu, R.; Kang, F.; Zhao, J.; Shao, J.; Huang, X.; Chen, X. Protective effects of dietary Astragalus polysaccharides on large yellow croaker (Larimichthys crocea) against Vibrio alginolyticus infection. Aquaculture 2024, 581, 740398. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Chen, H.; Liu, R.; Zhang, W.; Chen, X. Astragalus polysaccharides protect against inactivated Vibrio alginolyticus-induced inflammatory injury in macrophages of large yellow croaker. Fish Shellfish Immunol. 2022, 131, 95–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Li, W.; Xiao, L. Effects of Chinese herbal medicines on growth and nonspecific immunity in yellow catfish Pelteobagrus fulvidraco. Fish. Sci. 2010, 29, 225–228. [Google Scholar]
- Wang, Q.; Zhao, H.; Lv, Z.; Chen, C.; Xing, K. Effects of dietary Angelica sinensis polysaccharideon nonspecific immunity of Epinephelus coioides. J. Anhui Agric. Sci. 2011, 39, 13857–13860. [Google Scholar]
- Meng, Z.; Chen, Y.; Liu, M. Effect of Chinese herbal compounds on nonspecific immunity of Cyprinus carpio var. specularis Juvenile fish. J. Northeast Agric. Univ. 2011, 42, 54–59. [Google Scholar]
- Chen, Q.; Lan, H.Y.; Peng, W.; Rahman, K.; Liu, Q.C.; Luan, X.; Zhang, H. Isatis indigotica: A review of phytochemistry, pharmacological activities and clinical applications. J. Pharm. Pharmacol. 2021, 73, 1137–1150. [Google Scholar] [CrossRef]
- Song, L.; Guo, Z.; Wang, A. Effects of Isatis root polysaccharide on non-specific immune responses and nutritive indices in obscure pufferfish, Takifugu obscurus. Aquac. Res. 2018, 49, 603–613. [Google Scholar] [CrossRef]
- Pan, T.; Yan, M.; Chen, S.; Wang, X. Effects of ten traditional Chinese herbs on immune response and disease resistance of Sciaenops ocellatus (Actinopterygii: Perciformes: Sciaenidae). Acta Ichthyol. Piscat. 2013, 43, 41–49. [Google Scholar] [CrossRef]
- Wang, E.; Chen, X.; Wang, K.; Wang, J.; Chen, D.; Geng, Y.; Lai, W.; Wei, X. Plant polysaccharides used as immunostimulants enhance innate immune response and disease resistance against Aeromonas hydrophila infection in fish. Fish Shellfish Immunol. 2016, 59, 196–202. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Murthy, A.; Li, Y.; Peng, I.; Reichelt, M.; Katakam, A.K.; Noubade, R.; Roose-Girma, M.; DeVoss, J.; Diehl, L.; Graham, R.R. A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature 2014, 506, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.D.; Kimchi, A. Life in the balance—A mechanistic view of the crosstalk between autophagy and apoptosis. J. Cell Sci. 2012, 125, 5259–5268. [Google Scholar] [CrossRef] [PubMed]
- Wirawan, E.; Vande Walle, L.; Kersse, K.; Cornelis, S.; Claerhout, S.; Vanoverberghe, I.; Roelandt, R.; De Rycke, R.; Verspurten, J.; Declercq, W. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010, 1, e18. [Google Scholar] [CrossRef]
- Luo, S.; Rubinsztein, D. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: An effect rescued by Bcl-xL. Cell Death Differ. 2010, 17, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Criollo, A.; Kroemer, G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 2010, 29, 515–516. [Google Scholar] [CrossRef]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: Impact on the aging process. Ageing Res. Rev. 2013, 12, 520–534. [Google Scholar] [CrossRef]
- Park, H.-J.; Park, S.-H. Induction of apoptosis by Ethyl Acetate Fraction of Astragalus membranaceus in human non-small cell lung cancer cells:-apoptosis induction by Astragalus membranaceus. J. Pharmacopunct. 2018, 21, 268. [Google Scholar] [CrossRef]
- Shan, H.; Zheng, X.; Li, M. The effects of Astragalus membranaceus active extracts on autophagy-related diseases. Int. J. Mol. Sci. 2019, 20, 1904. [Google Scholar] [CrossRef]
- Chang, C.-C.; Su, H.J.; You, H.L.; Kao, C.W.; Hung, I.L.; Huang, S.T. Isatis indigotica Inhibits Influenza A Virus (H1N1) Virulent Protein Production and Autophagosome Accumulation. J. Herb. Med. 2024, 43, 100827. [Google Scholar] [CrossRef]
- Chung, Y.C.; Tang, F.Y.; Liao, J.W.; Chung, C.H.; Jong, T.T.; Chen, S.S.; Tsai, C.H.; Chiang, E.P. Isatis indigotica induces hepatocellular cancer cell death via caspase-independent apoptosis-inducing factor translocation apoptotic pathway in vitro and in vivo. Integr. Cancer Ther. 2011, 10, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, C.; Wu, X.; Zhang, J.; Wang, L.; Chen, X.; Shao, J. Synergistic effects of dietary Forsythia suspensa extract and Astragalus membranaceus polysaccharides on growth enhancement, antioxidant boost, and inflammation alleviation in juvenile Larimichthys crocea. Aquaculture 2025, 598, 742029. [Google Scholar] [CrossRef]
- Ao, J.; Mu, Y.; Xiang, L.X.; Fan, D.; Feng, M.; Zhang, S.; Shi, Q.; Zhu, L.Y.; Li, T.; Ding, Y. Genome sequencing of the perciform fish Larimichthys crocea provides insights into molecular and genetic mechanisms of stress adaptation. PLoS Genet. 2015, 11, e1005118. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Qin, P.; Chen, X.; Lou, B.; Wu, T.; Yang, J.; Wang, W.; Huang, G.; Chen, X. Outbreak of trypanosomiasis in cage-cultured large yellow croaker in China. J. Fish Dis. 2024, 48, e13952. [Google Scholar] [CrossRef]
- Shao, J.; Wu, L.; Wu, X.; Zhang, J.; Wang, L.; Chen, X.; Zhao, W. Synergistic benefits of Eleutherococcus senticosus extract and Astragalus membranaceus polysaccharides in juvenile Larimichthys crocea: Enhanced growth, amplified antioxidant activity, and reduced inflammation. Aquaculture 2024, 598, 742028. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Ma, Y.; Li, J.; Chen, X. The effective components of herbal medicines used for prevention and control of fish diseases. Fish Shellfish Immunol. 2022, 126, 73–83. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, S.; Ou, W.; Ai, Q.; Zhang, W.; Mai, K.; Zhang, Y. Host-associated Bacillus velezensis T20 improved disease resistance and intestinal health of juvenile turbot (Scophthalmus maximus). Aquac. Rep. 2024, 35, 101927. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Sadiq, I.Z. Free radicals and oxidative stress: Signaling mechanisms, redox basis for human diseases, and cell cycle regulation. Curr. Mol. Med. 2023, 23, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Ali, M.F.; Soliman, A.A.; Gewaily, M.S.; Abdel-Kader, T.Y.; Amer, A.A.; Zaineldin, A.I.; Al-Asgah, N.A.; Younis, E.M.; Abdel-Warith, A.W.A.; Sewilam, H. Isatis phytogenic relieved atrazine induced growth retardation, hepato-renal dysfunction, and oxidative stress in Nile tilapia. Saudi J. Biol. Sci. 2022, 29, 190–196. [Google Scholar] [CrossRef]
- Chen, Z.; Ceballos-Francisco, D.; Guardiola, F.A.; Huang, D.; Esteban, M.Á. The alleviation of skin wound-induced intestinal barrier dysfunction via modulation of TLR signalling using arginine in gilthead seabream (Sparus aurata L). Fish Shellfish Immunol. 2020, 107, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, D.; Yongyut, P.; Li, G.; Esteban, M.Á.; Jintasataporn, O.; Deng, J.; Zhang, W.; Ai, Q.; Mai, K. Vitamin D3 deficiency induced intestinal inflammatory response of turbot through nuclear factor-κB/inflammasome pathway, accompanied by the mutually exclusive apoptosis and autophagy. Front. Immunol. 2022, 13, 986593. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Z.; Zheng, J.; Dai, J.; Ou, W.; Xu, W.; Ai, Q.; Zhang, W.; Niu, J.; Mai, K. Citric acid mitigates soybean meal induced inflammatory response and tight junction disruption by altering TLR signal transduction in the intestine of turbot, Scophthalmus maximus L. Fish Shellfish Immunol. 2019, 92, 181–187. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.; Wang, T. The innate and adaptive immune system of fish. In Infectious Disease in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–68. [Google Scholar]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A review of recent research progress on the Astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, H.; Chen, J.; Chen, X.; Wen, Y.; Xu, L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med. 2018, 18, 83. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
| Ingredients | CON | AP | AI1 | AI2 | AI3 |
|---|---|---|---|---|---|
| Fish meal 1 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 |
| Soybean meal 1 | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
| Wheat flour 1 | 21.90 | 21.80 | 21.75 | 21.70 | 21.65 |
| Fish oil | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 |
| Soybean lecithin | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Brewer’s yeast | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Vitamin premix 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral premix 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Isatis indigotica extract | 0.00 | 0.00 | 0.05 | 0.10 | 0.15 |
| Astragalus polysaccharides | 0.00 | 0.10 | 0.10 | 0.10 | 0.10 |
| Proximate analysis | |||||
| Moisture | 9.41 | 9.58 | 9.42 | 9.55 | 9.43 |
| Crude protein | 43.27 | 43.19 | 43.46 | 43.36 | 43.58 |
| Crude lipid | 9.42 | 9.28 | 9.35 | 9.32 | 9.35 |
| Ash | 10.26 | 10.19 | 10.30 | 10.16 | 10.14 |
| Target Genes | Primer Sequence (5′-3′) | Primer Amplification Efficiencies | Accession Number |
|---|---|---|---|
| SOD | F-ATGGTGATCCACGAGAAGGC | 2.01 | NM_001303360.1 |
| R-CTACCAGCGTTGCCAGTCTT | |||
| CAT | F-GACGCTCTACTGTTCCCGTC | 2.09 | XM_010735178.3 |
| R-GCAAACCTCGATCGCTGAAC | |||
| NRF2 | F-GATGGAAATGGAGGTGATGC | 2.02 | XM_010737768.3 |
| R-CATGTTCTTTCTGTCGGTGG | |||
| CLDN4 | F-CGTCCAGGATTTCTACAACCC | 2.08 | XM_010734071.3 |
| R-CCCAGCTCTCTTTTGTGCAT | |||
| ZO1 | F-TGTCAAGTCCCGCAAAAATG | 1.97 | XM_019260744.2 |
| R-CAACTTGCCCTTTGACCTCT | |||
| ZO2 | F-ACCCGACCTGTTTGTTATTG | 1.98 | XM_019272858.2 |
| R-ATGCCGTGCTTGCTGTC | |||
| OCLN | F-AGGCTACGGCAACAGTTATG | 1.92 | XM_010734512.3 |
| R-GTGGGTCCACAAAGCAGTAA | |||
| MUC5 | ATAAATAAGCTCCAGTCAGCAC | 1.95 | XM_027285416.1 |
| ACCACAGACTCTTTAGCCTT | |||
| IL8 | F-CCCTGCTTGTCGTCGTAACT | 2.01 | XM_010754327.3 |
| R-CTCACCAGGCCAGCTATCAC | |||
| IL10 | F-AGTCGGTTACTTTCTGTGGTG | 2.07 | XM_010738826.3 |
| R-TGTATGACGCAATATGGTCTG | |||
| TNF | F-ACACCTCTCAGCCACAGGAT | 1.98 | XM_010745990 |
| R-CCGTGTCCCACTCCATAGTT | |||
| TGFB1 | F-AGCAACCACCGTACATCCTG | 1.99 | XM_027280465.1 |
| R-AGGTATCCCGTTGGCTTGTG | |||
| MYD88 | F-TCGGCCTTTGATTGATGAGGA | 2.03 | XM_010753272.3 |
| R-GTGAGACCAACCCTGTCTCG | |||
| TLR5 | F-GGCACAGTGAGGAAAGGT | 2.06 | XM_019267725.2 |
| R-TAGCAAGCGTCCACATAC | |||
| TLR2 | F-GTCCGACAACCTGCTGACTGA | 2.10 | KKF22682.1 |
| R-CAGGTGGGTGAGTTTGGAGAG | |||
| TLR1 | F-CTTTGTCAAGAGCGAGTGGT | 1.98 | KF318376.1 |
| R-GGTTCATCATGGCCTTCAGC | |||
| SQSTM1 | F-GTGGAGGTCTGGCTTTTTTATG | 1.99 | XM_019265187.2 |
| R-AGTGATCGTTGTGGCTATTG | |||
| ATG12 | F-CCTCACCGGATCAAGACGTA | 2.03 | XM_027286876.1 |
| R-AAGAGAGCAGTTTAACCCCAG | |||
| MAP1LC3B | F-TGGGTCAGAACCACCACAGAACT | 2.05 | XM_027285483.1 |
| R-GCTACTACGTGGGCCTGCAATG | |||
| BAX | F-GGTGGCTGGGAGGGTATTCGTT | 1.99 | XM_019258722.2 |
| R-TCCCGTCCACATTTCCCTTCGT | |||
| BCL2 | F-TGCTTGCGGACACGGACTCA | 1.92 | XM_010729997.2 |
| R-CACAGCACAGGCGTATCCACAA | |||
| CASP3 | F-GATGGCACTACAAAGATCCCTGTG | 1.95 | EU878546.1 |
| R-GCTACTCTGTGGCTGAAGGTTAC | |||
| CASP8 | F-TCAGCGAAGACCACAACC | 2.06 | XM_010754584.3 |
| R-CACCACAGTGAAGCCAAG | |||
| CASP9 | F-GCGCAACACAGACAAATCTCG | 1.95 | XM_010755472.3 |
| R-TGATCCCGGCGCTCTTTATC | |||
| ACTB | F-GACCTGACAGACTACCTCATG | 1.99 | GU584189 |
| R-AGTTGAAGGTGGTCTCGTGGA | |||
| GAPDH | F-CGGAGTCAACGGATTTGGTC | 2.10 | XM_010743420.3 |
| R-AGCCTTCTCCATGGTCGTGA |
| CON | AP | AI1 | AI2 | AI3 | |
|---|---|---|---|---|---|
| Amylase (U/mg protein) | 1.06 b ± 0.04 | 0.91 a ± 0.03 | 1.06 b ± 0.01 | 1.36 c ± 0.02 | 1.56 d ± 0.02 |
| Lipase (U/g protein) | 2.62 ab ± 0.11 | 2.847 ab ± 0.22 | 2.02 a ± 0.45 | 3.48 b ± 0.76 | 3.36 b ± 0.25 |
| Trypsin (U/mg protein) | 1183.39 b ± 234.33 | 1672.12 ab ± 303.71 | 1434.12 ab ± 57.50 | 1926.27 a ± 317.78 | 1476.98 ab ± 396.79 |
| CON | AP | AI1 | AI2 | AI3 | |
|---|---|---|---|---|---|
| SOD (U/mg protein) | 11.72 a ± 0.99 | 12.94 a ± 1.07 | 12.06 ab ± 2.87 | 5.57 c ± 0.50 | 9.06 b ± 0.12 |
| T-AOC (mmol/g protein) | 0.155 ± 0.014 | 0.152 ± 0.01 | 0.123 ± 0.026 | 0.146 ± 0.01 | 0.156 ± 0.019 |
| MDA (nmol/mg protein) | 5.63 a ± 1.05 | 5.08 ab ± 0.65 | 4.84 ab ± 0.34 | 4.61 ab ± 0.36 | 4.14 b ± 0.37 |
| CAT (nmol/mg protein) | 7.85 b ± 1.62 | 8.04 b ± 1.20 | 13.59 a ± 1.26 | 12.57 a ± 1.23 | 9.71 b ± 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zeng, C.; Wang, A.; Wang, H.; Zhi, X.; Chen, Z.; Lv, H.; Qi, Q.; Wang, P.; Shao, J.; et al. Effects of Astragalus Polysaccharide and Isatis indigotica Extract Synergy on the Antioxidant Status, Inflammation, Autophagy, Apoptosis, and Intestinal Health of Larimichthys crocea Juveniles. Fishes 2025, 10, 593. https://doi.org/10.3390/fishes10110593
Chen Z, Zeng C, Wang A, Wang H, Zhi X, Chen Z, Lv H, Qi Q, Wang P, Shao J, et al. Effects of Astragalus Polysaccharide and Isatis indigotica Extract Synergy on the Antioxidant Status, Inflammation, Autophagy, Apoptosis, and Intestinal Health of Larimichthys crocea Juveniles. Fishes. 2025; 10(11):593. https://doi.org/10.3390/fishes10110593
Chicago/Turabian StyleChen, Zhichu, Chao Zeng, Ai Wang, Huiyu Wang, Xin Zhi, Zhengbang Chen, Huiyuan Lv, Qiong Qi, Pan Wang, Jianchun Shao, and et al. 2025. "Effects of Astragalus Polysaccharide and Isatis indigotica Extract Synergy on the Antioxidant Status, Inflammation, Autophagy, Apoptosis, and Intestinal Health of Larimichthys crocea Juveniles" Fishes 10, no. 11: 593. https://doi.org/10.3390/fishes10110593
APA StyleChen, Z., Zeng, C., Wang, A., Wang, H., Zhi, X., Chen, Z., Lv, H., Qi, Q., Wang, P., Shao, J., & Chen, X. (2025). Effects of Astragalus Polysaccharide and Isatis indigotica Extract Synergy on the Antioxidant Status, Inflammation, Autophagy, Apoptosis, and Intestinal Health of Larimichthys crocea Juveniles. Fishes, 10(11), 593. https://doi.org/10.3390/fishes10110593







