Impact of Hypoxia on Intestinal Health and Gut Microbiota in Anadara granosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Histological Sectioning
2.3. RNA Extraction and Gene Expression Analysis
2.4. Statistical Analysis
2.5. DNA Extraction, 16S rRNA Amplification and Illumina MiSeq Sequencing
2.6. Bioinformatics Analysis
3. Results
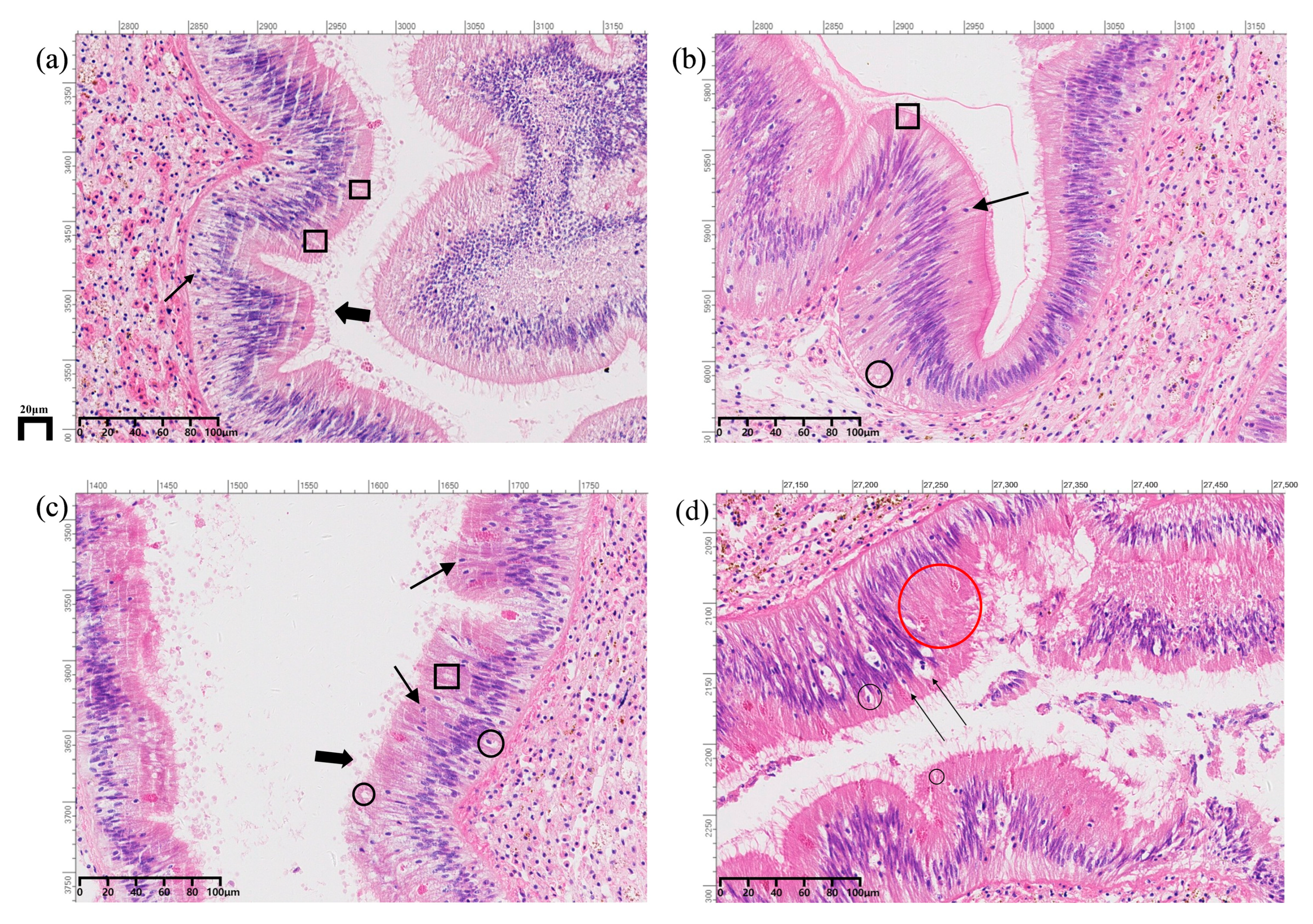
3.1. Histopathological Changes in the Intestine of A. granosa
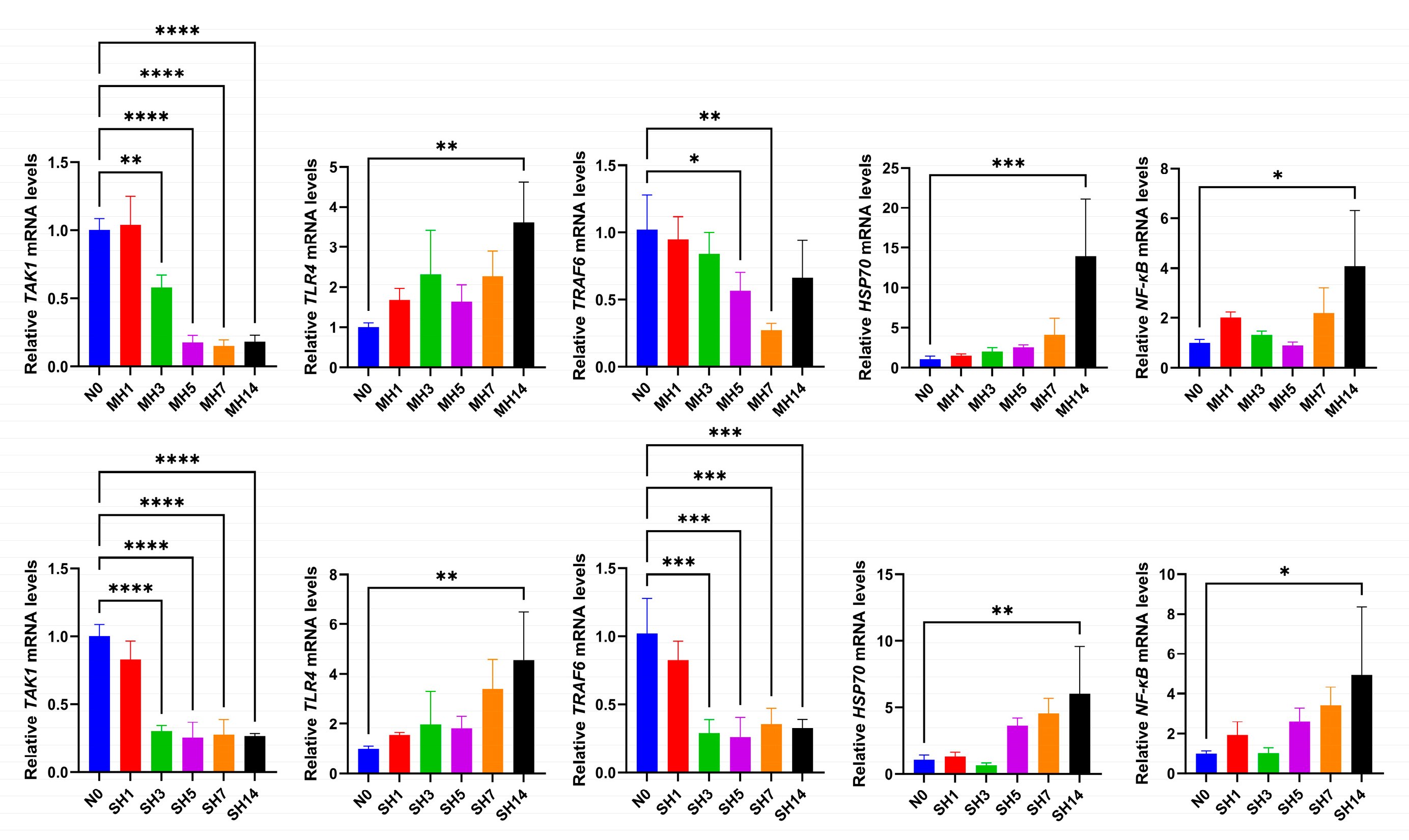
3.2. Effects of Hypoxia on Intestinal Immune-Related Gene Expression
3.3. Response of Intestinal Bacterial Community in A. granosa to Hypoxia Stress
3.4. Alterations in Ecological Mechanisms Regulating Intestinal Microbiota Assembly in A. granosa During Hypoxia Stress
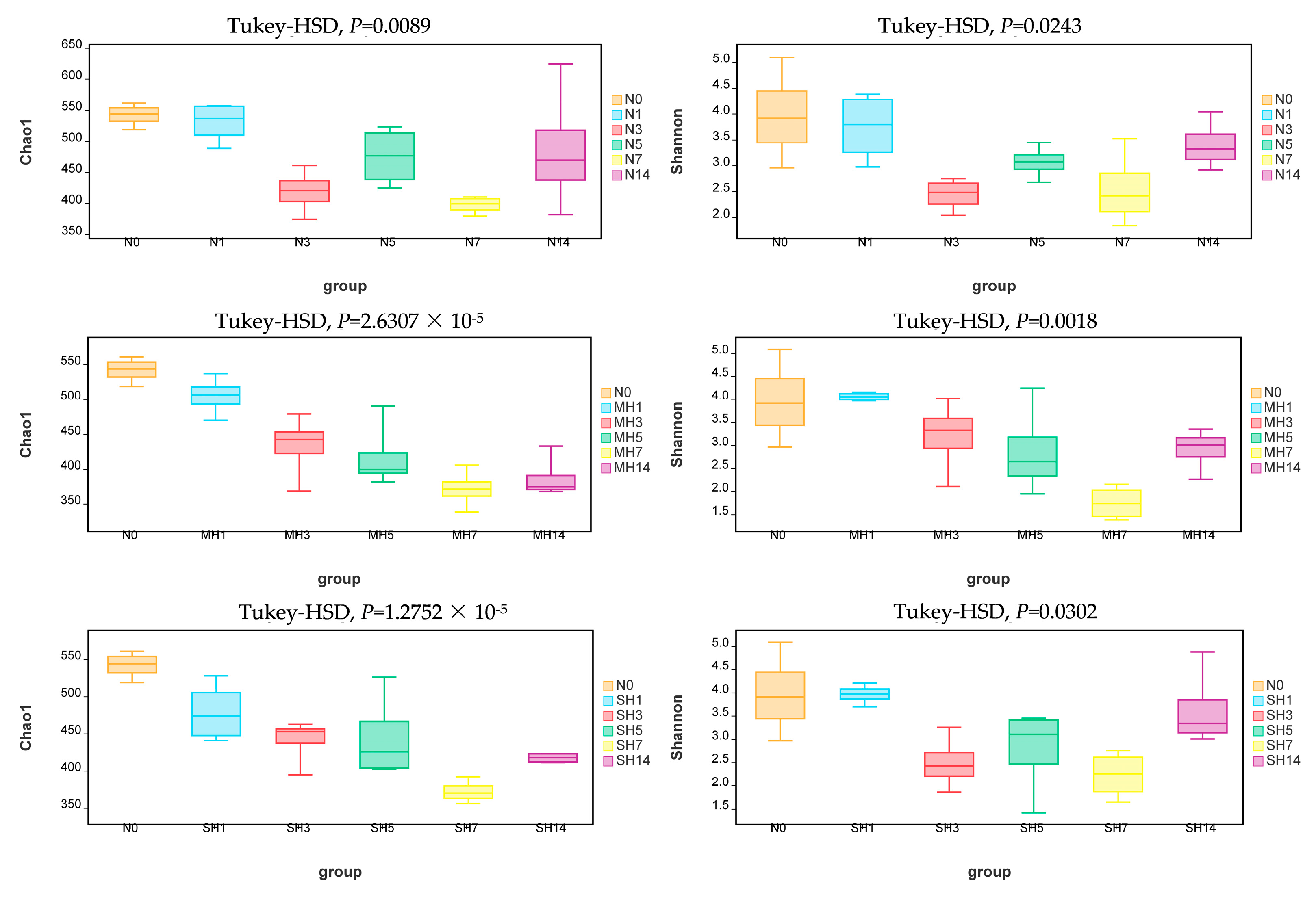
3.5. Diversity Differences in Intestinal Bacterial Community in A. granosa
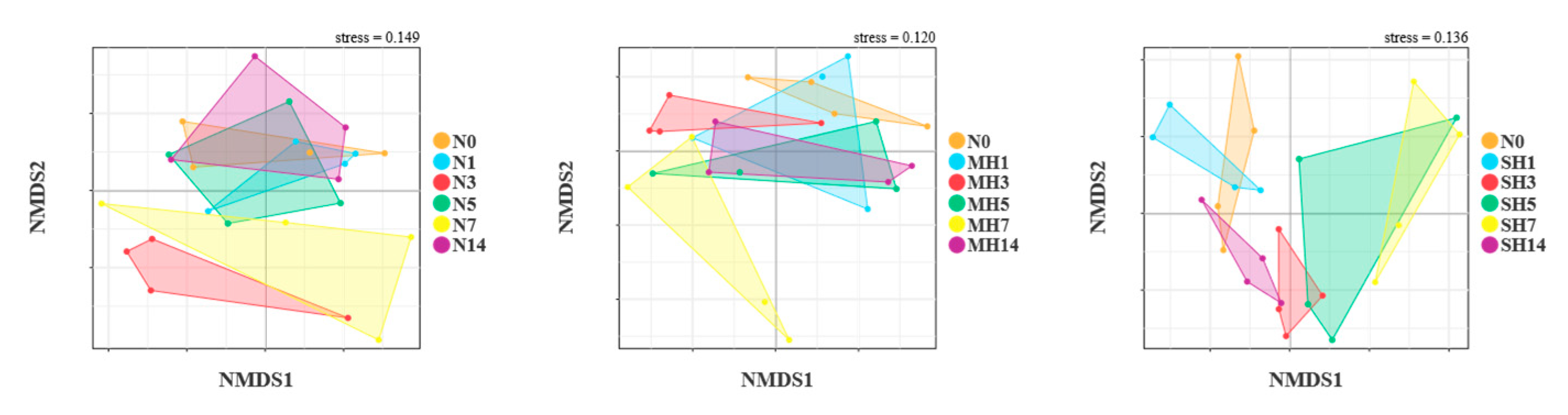
3.6. Structural Differences in Intestinal Bacterial Community in A. granosa
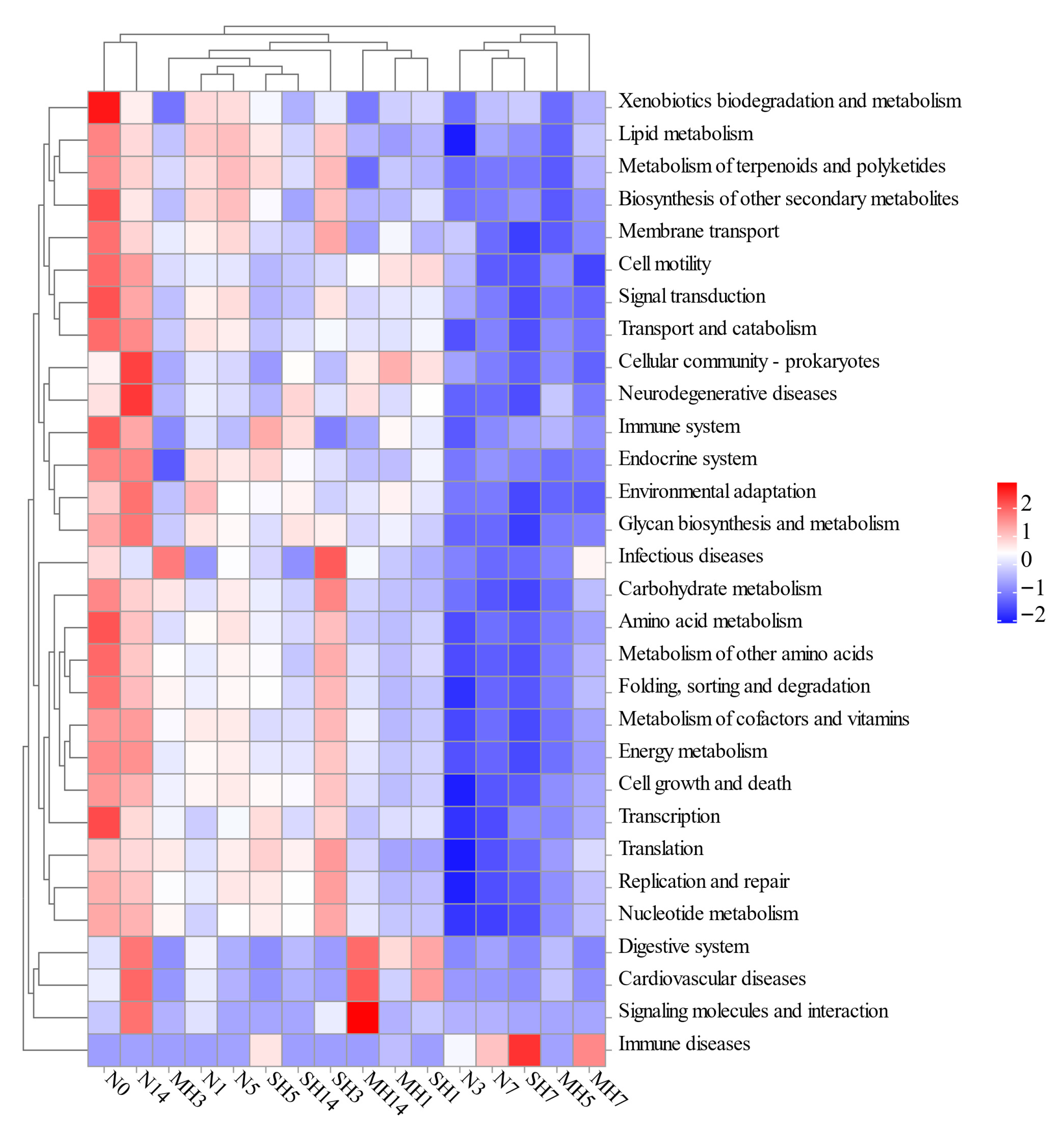
3.7. Functional Differences in Intestinal Bacterial Community in A. granosa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Tawwab, M.; Monier, M.N.; Hoseinifar, S.H.; Faggio, C. Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019, 45, 997–1013. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Guo, Y.; Rong, Z.; Li, B.; Xu, Z.; Li, P.; Li, X. Physical processes causing the formation of hypoxia off the Changjiang estuary after Typhoon Chan-hom, 2015. J. Oceanol. Limnol. 2019, 37, 1–17. [Google Scholar] [CrossRef]
- Lam, P.; Kuypers, M.M.M. Microbial Nitrogen Cycling Processes in Oxygen Minimum Zones. Annu. Rev. Mar. Sci. 2011, 3, 317–345. [Google Scholar] [CrossRef]
- Galic, N.; Hawkins, T.; Forbes, V.E. Adverse impacts of hypoxia on aquatic invertebrates: A meta-analysis. Sci. Total Environ. 2019, 652, 736–743. [Google Scholar] [CrossRef]
- Song, J.; Farhadi, A.; Tan, K.; Lim, L.; Tan, K. Impact of anthropogenic global hypoxia on the physiological response of bivalves. Sci. Total Environ. 2024, 926, 172056. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, P.; Xia, F.; Bai, Q.; Zhang, X. Transcriptional and physiological profiles reveal the respiratory, antioxidant and metabolic adaption to intermittent hypoxia in the clam Tegillarca granosa. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 50, 101215. [Google Scholar] [CrossRef]
- Zhan, Y.; Zha, S.; Peng, Z.; Lin, Z.; Bao, Y. Hypoxia-mediated immunotoxicity in the blood clam Tegillarca granosa. Mar. Environ. Res. 2022, 177, 105632. [Google Scholar] [CrossRef]
- Wen, J.; Wu, Y.; Zhang, F.; Wang, Y.; Yang, A.; Lu, W.; Zhao, X.; Tao, H. Neonatal hypoxia leads to impaired intestinal function and changes in the composition and metabolism of its microbiota. Sci. Rep. 2025, 15, 15285. [Google Scholar] [CrossRef]
- Song, Z.; Ye, W.; Tao, Y.; Zheng, T.; Qiang, J.; Li, Y.; Liu, W.; Xu, P. Transcriptome and 16S rRNA Analyses Reveal That Hypoxic Stress Affects the Antioxidant Capacity of Largemouth Bass (Micropterus salmoides), Resulting in Intestinal Tissue Damage and Structural Changes in Microflora. Antioxidants 2022, 12, 1. [Google Scholar] [CrossRef]
- Niklasson, L.; Sundh, H.; Fridell, F.; Taranger, G.L.; Sundell, K. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 2011, 31, 1072–1080. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Qian, X.; Ji, J.; Ning, X.; Zhu, F.; Yin, S.; Zhang, K. Effects of hypoxia and reoxygenation on apoptosis, oxidative stress, immune response and gut microbiota of Chinese mitten crab, Eriocheir sinensis. Aquat. Toxicol. 2023, 260, 106556. [Google Scholar] [CrossRef]
- Nie, H.; Wang, H.; Jiang, K.; Yan, X. Transcriptome analysis reveals differential immune related genes expression in Ruditapes philippinarum under hypoxia stress: Potential HIF and NF-κB crosstalk in immune responses in clam. BMC Genom. 2020, 21, 318. [Google Scholar] [CrossRef]
- Falfushynska, H.; Piontkivska, H.; Sokolova, I.M. Effects of intermittent hypoxia on the cell survival and inflammatory responses in the intertidal marine bivalves Mytilus edulis and Crassostrea gigas. J. Exp. Biol. 2020, 223, jeb217026. [Google Scholar] [CrossRef]
- Montufar-Romero, M.; Valenzuela-Munoz, V.; Valenzuela-Miranda, D.; Gallardo-Escarate, C. Hypoxia in the Blue Mussel Mytilus chilensis Induces a Transcriptome Shift Associated with Endoplasmic Reticulum Stress, Metabolism, and Immune Response. Genes 2024, 15, 658. [Google Scholar] [CrossRef]
- Xie, Z.; Li, Y.; Xiong, K.; Tu, Z.; Waiho, K.; Yang, C.; Deng, Y.; Li, S.; Fang, J.K.H.; Hu, M.; et al. Combined effect of salinity and hypoxia on digestive enzymes and intestinal microbiota in the oyster Crassostrea hongkongensis. Environ. Pollut. 2023, 331, 121921. [Google Scholar] [CrossRef]
- Evariste, L.; Barret, M.; Mottier, A.; Mouchet, F.; Gauthier, L.; Pinelli, E. Gut microbiota of aquatic organisms: A key endpoint for ecotoxicological studies. Environ. Pollut. 2019, 248, 989–999. [Google Scholar] [CrossRef]
- Clerissi, C.; de Lorgeril, J.; Petton, B.; Lucasson, A.; Escoubas, J.M.; Gueguen, Y.; Degremont, L.; Mitta, G.; Toulza, E. Microbiota Composition and Evenness Predict Survival Rate of Oysters Confronted to Pacific Oyster Mortality Syndrome. Front. Microbiol. 2020, 11, 311. [Google Scholar] [CrossRef]
- Dai, W.; Dong, Y.; Ye, J.; Xue, Q.; Lin, Z. Gut microbiome composition likely affects the growth of razor clam Sinonovacula constricta. Aquaculture 2022, 550, 737847. [Google Scholar] [CrossRef]
- Montufar-Romero, M.; Valenzuela-Miranda, D.; Valenzuela-Munoz, V.; Morales-Rivera, M.F.; Gallardo-Escarate, C. Microbiota Dysbiosis in Mytilus chilensis Is Induced by Hypoxia, Leading to Molecular and Functional Consequences. Microorganisms 2025, 13, 825. [Google Scholar] [CrossRef]
- Bao, Y.; Zeng, Q.; Wang, J.; Zhang, Z.; Zhang, Y.; Wang, S.; Wong, N.K.; Yuan, W.; Huang, Y.; Zhang, W.; et al. Genomic Insights into the Origin and Evolution of Molluscan Red-Bloodedness in the Blood Clam Tegillarca granosa. Mol. Biol. Evol. 2021, 38, 2351–2365. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, W.; Liu, H.; Bao, Y. Genome-wide identification and characteristic analysis of ETS gene family in blood clam Tegillarca granosa. BMC Genom. 2023, 24, 700. [Google Scholar] [CrossRef]
- Liu, H.; Zha, S.; Yang, Z.; Zhang, W.; Lin, Z.; Wang, S.; Bao, Y. Acute sulfide exposure induces hemocyte toxicity and microbiota dysbiosis in blood clam Tegillarca granosa. Aquat. Toxicol. 2022, 249, 106224. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Jin, M.; Zhan, Y.; Zhang, X.; Bao, Y.; Liu, M. Hypoxia Activates HIF-1α and Affects Gene Expression and Transcriptional Regulation of PHD in Tegillarca granosa. Fishes 2023, 8, 359. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Bao, Y.; Peng, Z. The Structure Analysis and mRNA Expression of CaV2 Gene Responding to Hypoxia Stress in Anadara granosa. Fishes 2024, 9, 409. [Google Scholar] [CrossRef]
- Shao, Y.; Chai, X.; Xiao, G.; Zhang, J.; Lin, Z.; Liu, G. Population Genetic Structure of the Blood Clam, Tegillarca granosa, Along the Pacific Coast of Asia: Isolation by Distance in the Sea. Malacologia 2016, 59, 303–312. [Google Scholar] [CrossRef]
- Zha, S.; Rong, J.; Guan, X.; Tang, Y.; Han, Y.; Liu, G. Immunotoxicity of four nanoparticles to a marine bivalve species, Tegillarca granosa. J. Hazard. Mater. 2019, 377, 237–248. [Google Scholar] [CrossRef]
- Jing, H.; Liu, Z.; Wu, B.; Tu, K.; Liu, Z.; Sun, X.; Zhou, L. Physiological and molecular responses to hypoxia stress in Manila clam Ruditapes philippinarum. Aquat. Toxicol. 2023, 257, 106428. [Google Scholar] [CrossRef]
- Cheng, H.; Peng, Z.; Zhao, C.; Jin, H.; Bao, Y.; Liu, M. The transcriptomic and biochemical responses of blood clams (Tegillarca granosa) to prolonged intermittent hypoxia. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2024, 270, 110923. [Google Scholar] [CrossRef]
- Zha, S.; Zhang, W.; Liu, H.; Huang, S.; Sun, C.; Bao, Y. Two common nanoparticles exert immunostimulatory and protective effects in Tegillarca granosa against Vibrio parahaemolyticus. Fish Shellfish Immunol. 2023, 137, 108774. [Google Scholar] [CrossRef]
- Su, W.; Zha, S.; Wang, Y.; Shi, W.; Xiao, G.; Chai, X.; Wu, H.; Liu, G. Benzo[a]pyrene exposure under future ocean acidification scenarios weakens the immune responses of blood clam, Tegillarca granosa. Fish Shellfish Immunol. 2017, 63, 465–470. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Todgham, A.E.; Stillman, J.H. Physiological responses to shifts in multiple environmental stressors: Relevance in a changing world. Integr. Comp. Biol. 2013, 53, 539–544. [Google Scholar] [CrossRef]
- Clavel, T.; Gomes-Neto, J.C.; Lagkouvardos, I.; Ramer-Tait, A.E. Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunol. Rev. 2017, 279, 8–22. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Ma, R.; Wang, J.; Liu, Z.; Liu, Y.; Xu, X.; Chu, P.; Zhu, L. Dysregulation of gut barrier and microbiota in Asiatic hard clams (Meretrix petechialis) exposed to environmentally relevant bisphenol A. Environ. Res. 2025, 279, 121913. [Google Scholar] [CrossRef]
- Quigley, E.M.; Stanton, C.; Murphy, E.F. The gut microbiota and the liver. Pathophysiological and clinical implications. J. Hepatol. 2013, 58, 1020–1027. [Google Scholar] [CrossRef]
- Wang, W.-z.; Huang, J.-s.; Zhang, J.-d.; Wang, Z.-l.; Li, H.-j.; Amenyogbe, E.; Chen, G. Effects of hypoxia stress on the intestinal microflora of juvenile of cobia (Rachycentron canadum). Aquaculture 2021, 536, 736419. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, Z.; Dong, Y.; He, L.; Xue, Q.; Lin, Z. Acute Salinity Stress Disrupts Gut Microbiota Homeostasis and Reduces Network Connectivity and Cooperation in Razor Clam Sinonovacula constricta. Mar. Biotechnol. 2023, 25, 1147–1157. [Google Scholar] [CrossRef]
- Steffen, J.B.M.; Falfushynska, H.I.; Piontkivska, H.; Sokolova, I.M. Molecular Biomarkers of the Mitochondrial Quality Control Are Differently Affected by Hypoxia-Reoxygenation Stress in Marine Bivalves Crassostrea gigas and Mytilus edulis. Front. Mar. Sci. 2020, 7, 604411. [Google Scholar] [CrossRef]
- Andreyeva, A.Y.; Kukhareva, T.A.; Gostyukhina, O.L.; Vialova, O.Y.; Tkachuk, A.A.; Chelebieva, E.S.; Podolskaya, M.S.; Borovkov, A.B.; Bogacheva, E.A.; Lavrichenko, D.S.; et al. Impacts of ocean acidification and hypoxia on cellular immunity, oxygen consumption and antioxidant status in Mediterranean mussel. Fish Shellfish Immunol. 2024, 154, 109932. [Google Scholar] [CrossRef]
- Liu, T.; Lu, Y.; Sun, M.; Shen, H.; Niu, D. Effects of acute hypoxia and reoxygenation on histological structure, antioxidant response, and apoptosis in razor clam Sinonovacula constricta. Fish Shellfish Immunol. 2024, 145, 109310. [Google Scholar] [CrossRef]
- He, H.; Huang, S.; Geng, N.; Weng, S.; He, J.; Li, C. Acute hypoxia stress mediates HIF-1alpha-Yki-Cactus axis to facilitate the infection of Vibrio parahaemolyticus in Litopenaeus vannamei. Front. Immunol. 2024, 15, 1476309. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Liu, T.; Chen, M.; Li, W.; Zhang, X. The transcriptomic responses of the ark shell, Anadara broughtonii, to sulfide and hypoxia exposure. Mol. Biol. Rep. 2019, 46, 4245–4257. [Google Scholar] [CrossRef]
- Rivera-Ingraham, G.A.; Rocchetta, I.; Meyer, S.; Abele, D. Oxygen radical formation in anoxic transgression and anoxia-reoxygenation: Foe or phantom? Experiments with a hypoxia tolerant bivalve. Mar. Environ. Res. 2013, 92, 110–119. [Google Scholar] [CrossRef]
- Klase, G.; Lee, S.; Liang, S.; Kim, J.; Zo, Y.G.; Lee, J. The microbiome and antibiotic resistance in integrated fishfarm water: Implications of environmental public health. Sci. Total Environ. 2019, 649, 1491–1501. [Google Scholar] [CrossRef]
- Sun, S.; Yang, M.; Fu, H.; Ge, X.; Zou, J. Altered intestinal microbiota induced by chronic hypoxia drives the effects on lipid metabolism and the immune response of oriental river prawn Macrobrachium nipponense. Aquaculture 2020, 526, 735431. [Google Scholar] [CrossRef]
- Carrillo-Barragan, P.; Cassola, G.E.; Burkhardt-Holm, P. Microbial colonisation of polyethylene in offshore marine environments: Insights from the Southern and South Atlantic Oceans. Sci. Total Environ. 2025, 996, 180189. [Google Scholar] [CrossRef]
- Chang, Y.; Ko, H.; Wu, P.; Kumar, R.; Wang, H.; Lu, H. Gut microbiota of Pacific white shrimp (Litopenaeus vannamei) exhibits distinct responses to pathogenic and non-pathogenic Vibrio parahaemolyticus. Microbiol. Spectr. 2023, 11, e0118023. [Google Scholar] [CrossRef]
- Yamada, Y.; Saito, Y.; Shimanuki, K.; Izumi, S.; Gojobori, T.; Akiyama, N. Monitoring the gut microbiota of rainbow trout during seawater acclimation. Fish Sci. 2025, 91, 943–959. [Google Scholar] [CrossRef]
- Akter, S.; Wos-Oxley, M.L.; Catalano, S.R.; Hassan, M.M.; Li, X.; Qin, J.G.; Oxley, A.P. Host Species and Environment Shape the Gut Microbiota of Cohabiting Marine Bivalves. Microb. Ecol. 2023, 86, 1755–1772. [Google Scholar] [CrossRef]
- Pierce, M.L.; Ward, J.E. Gut Microbiomes of the Eastern Oyster (Crassostrea virginica) and the Blue Mussel (Mytilus edulis): Temporal Variation and the Influence of Marine Aggregate-Associated Microbial Communities. mSphere 2019, 4, e00730-19. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, M.; Jiang, H.; Wang, R.; Qian, Y.; Li, M. Ammonia stress disrupts intestinal microbial community and amino acid metabolism of juvenile yellow catfish (Pelteobagrus fulvidraco). Ecotoxicol. Environ. Saf. 2021, 227, 112932. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]


| DO (mg/L) | Time (d) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 7 | 14 | |
| 7.0 (N) | N0 | N1 | N3 | N5 | N7 | N14 |
| 2.0 (MH) | MH1 | MH3 | MH5 | MH7 | MH14 | |
| 0.5 (SH) | SH1 | SH3 | SH5 | SH7 | SH14 | |
| Primer | Sequence (5′-3′) |
|---|---|
| TAK1-F | CGACTCTGTTGATTACTCTC |
| TAK1-R | CATTGTAAGTTGGCTCAAGA |
| TLR4-F | CAATGAGCATCTTCGTATCG |
| TLR4-R | TTCTGTCTTCATCTGCGTAT |
| TRAF6-F | CATGAAGGCACCTCCTGGAA |
| TRAF6-R | CAATCACACCAACAGAGTCAGT |
| HSP70-F | AACCAGGTGTCACAATACA |
| HSP70-R | TAACGTCGATCTGAGGTAT |
| NF-κB-F | AATCAAGCAGGTGTAGTAGAC |
| NF-κB-R | CAGACAGGACAGCCAGAT |
| 18S-F | CTTTCAAATGTCTGCCCTATCAACT |
| 18S-R | TCCCGTATTGTTATTTTTCGTCACT |
| Df | Sum of Sqs | Mean Sqs | Fvalue | R2 | p Value | |
|---|---|---|---|---|---|---|
| N | 5 | 2.5539 | 0.5108 | 1.6277 | 0.3114 | 0.012 |
| MH | 5 | 2.4829 | 0.4966 | 1.5978 | 0.3074 | 0.014 |
| SH | 5 | 3.1586 | 0.6317 | 2.3269 | 0.3926 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cheng, G.; Jin, J.; Ji, Y.; Zhang, X.; Bao, Y.; Peng, Z. Impact of Hypoxia on Intestinal Health and Gut Microbiota in Anadara granosa. Fishes 2025, 10, 584. https://doi.org/10.3390/fishes10110584
Li Y, Cheng G, Jin J, Ji Y, Zhang X, Bao Y, Peng Z. Impact of Hypoxia on Intestinal Health and Gut Microbiota in Anadara granosa. Fishes. 2025; 10(11):584. https://doi.org/10.3390/fishes10110584
Chicago/Turabian StyleLi, Yueyue, Guangzhi Cheng, Jiaqi Jin, Yangguang Ji, Xiaolin Zhang, Yongbo Bao, and Zhilan Peng. 2025. "Impact of Hypoxia on Intestinal Health and Gut Microbiota in Anadara granosa" Fishes 10, no. 11: 584. https://doi.org/10.3390/fishes10110584
APA StyleLi, Y., Cheng, G., Jin, J., Ji, Y., Zhang, X., Bao, Y., & Peng, Z. (2025). Impact of Hypoxia on Intestinal Health and Gut Microbiota in Anadara granosa. Fishes, 10(11), 584. https://doi.org/10.3390/fishes10110584








