Metabolic Responses and Oxidative Stress Adaptation Mechanisms of the Pituitary Gland in the Tiger Puffer Under Low-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Management and Cultivation of Experimental Fish
2.2. Experimental Design and Sample Collection
2.3. Metabolite Extraction
2.4. Metabolomics Data Analysis
2.5. Detection of Apoptosis by the TUNEL Assay
2.6. Assay of Oxidative Stress-Related Enzyme Activity
2.7. Muscle Histology Observations
2.8. Real-Time Quantitative PCR (RT-qPCR) Validation
2.9. Statistical Analysis
3. Results
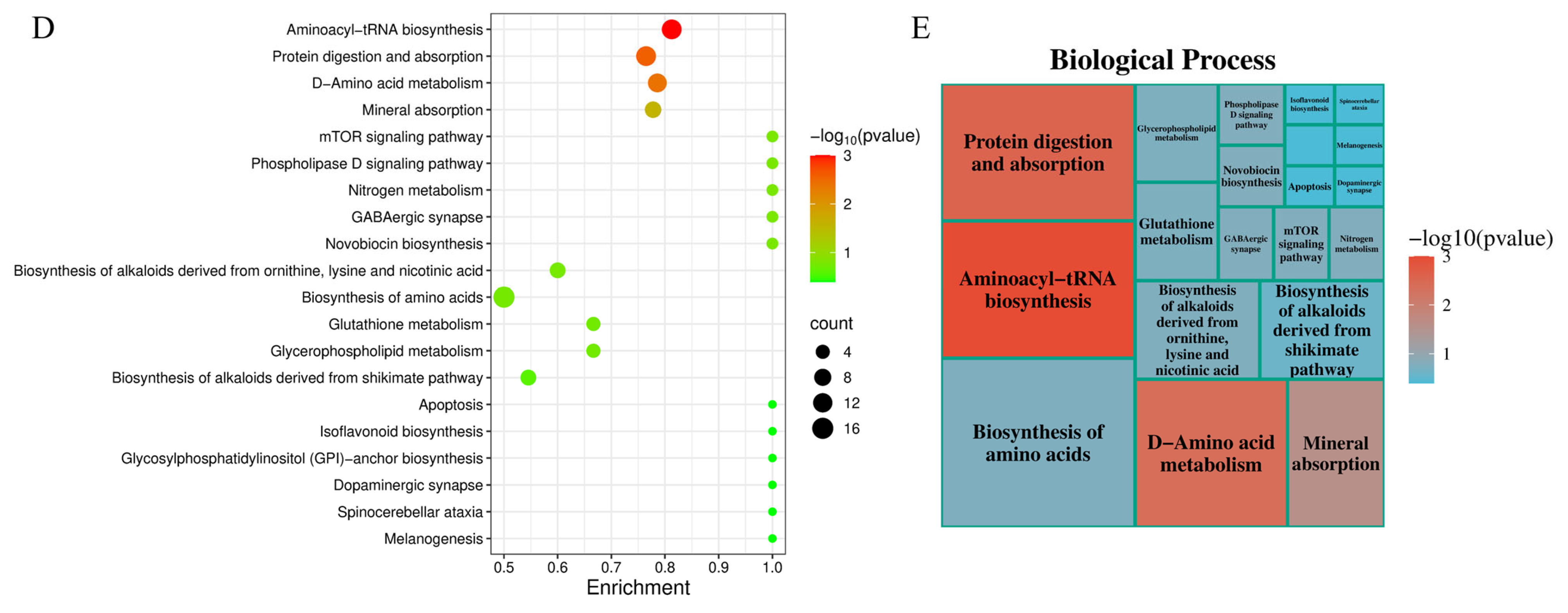
3.1. Metabolomic Analysis of the Effects of Low-Temperature Stress on the Pituitary Gland of the Tiger Puffers
3.2. Low-Temperature Stress Induces Oxidative Stress Damage to the Pituitary Gland
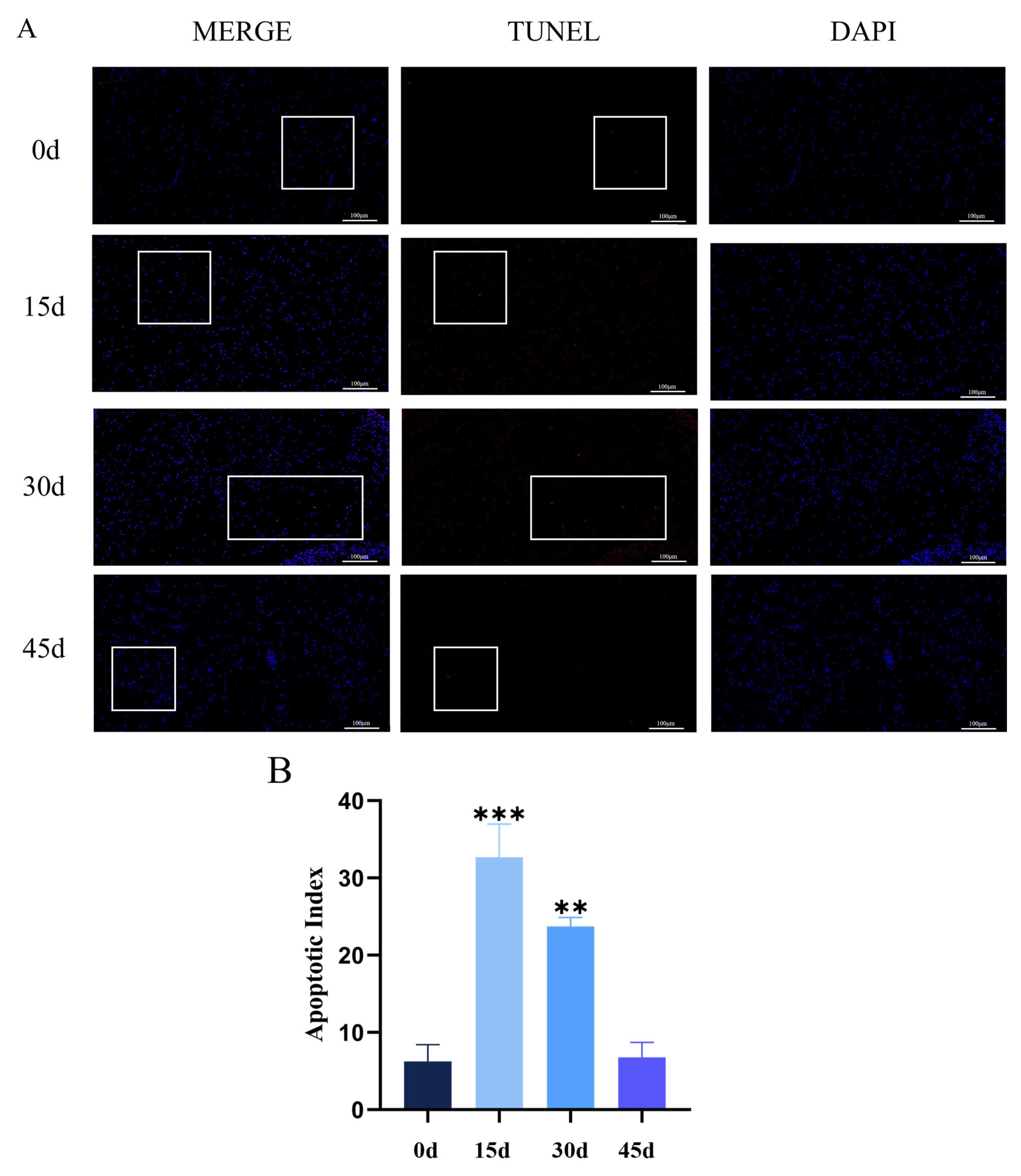
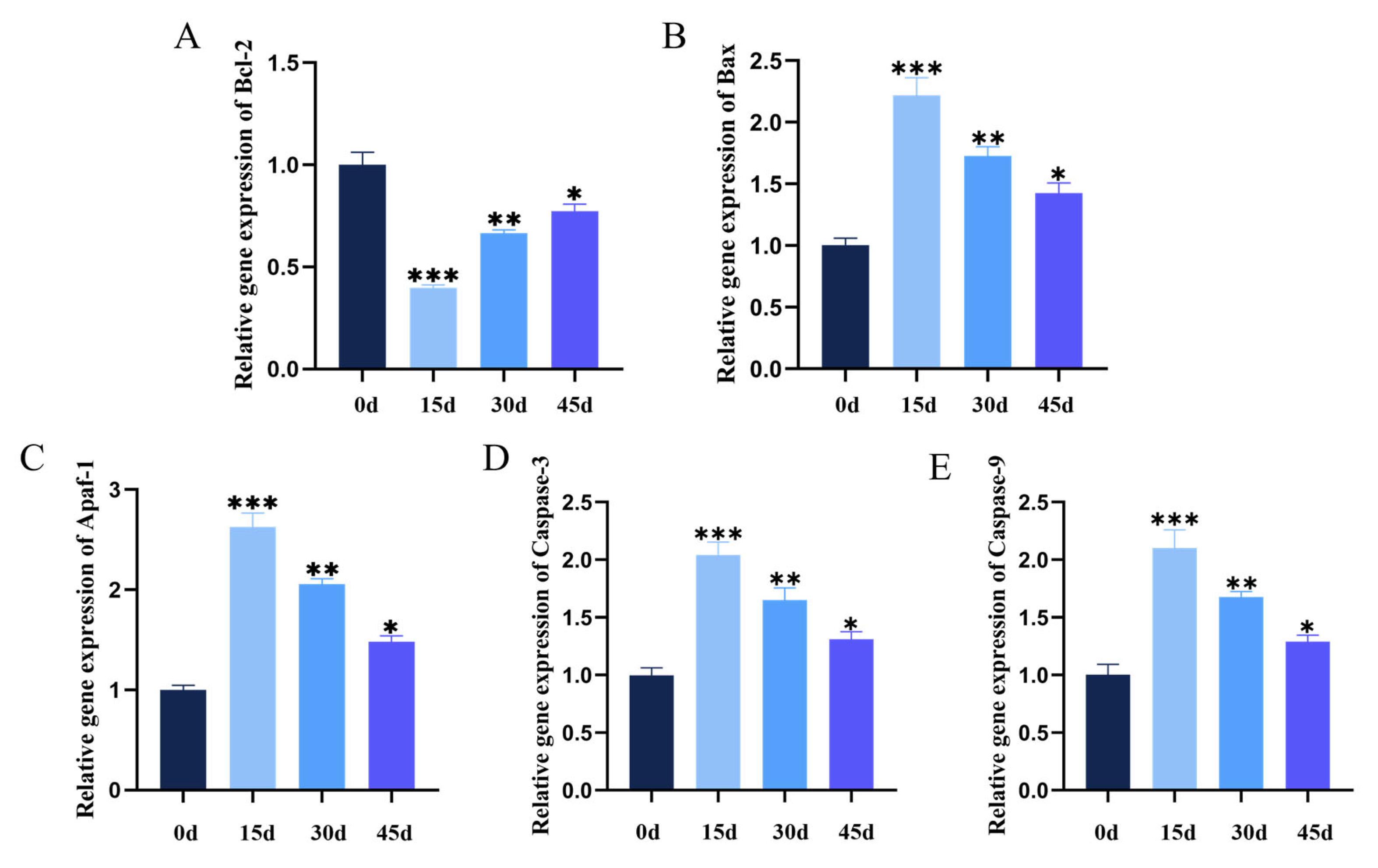
3.3. Chronic Hypothermic Stress Induces Apoptosis in Pituitary Cells
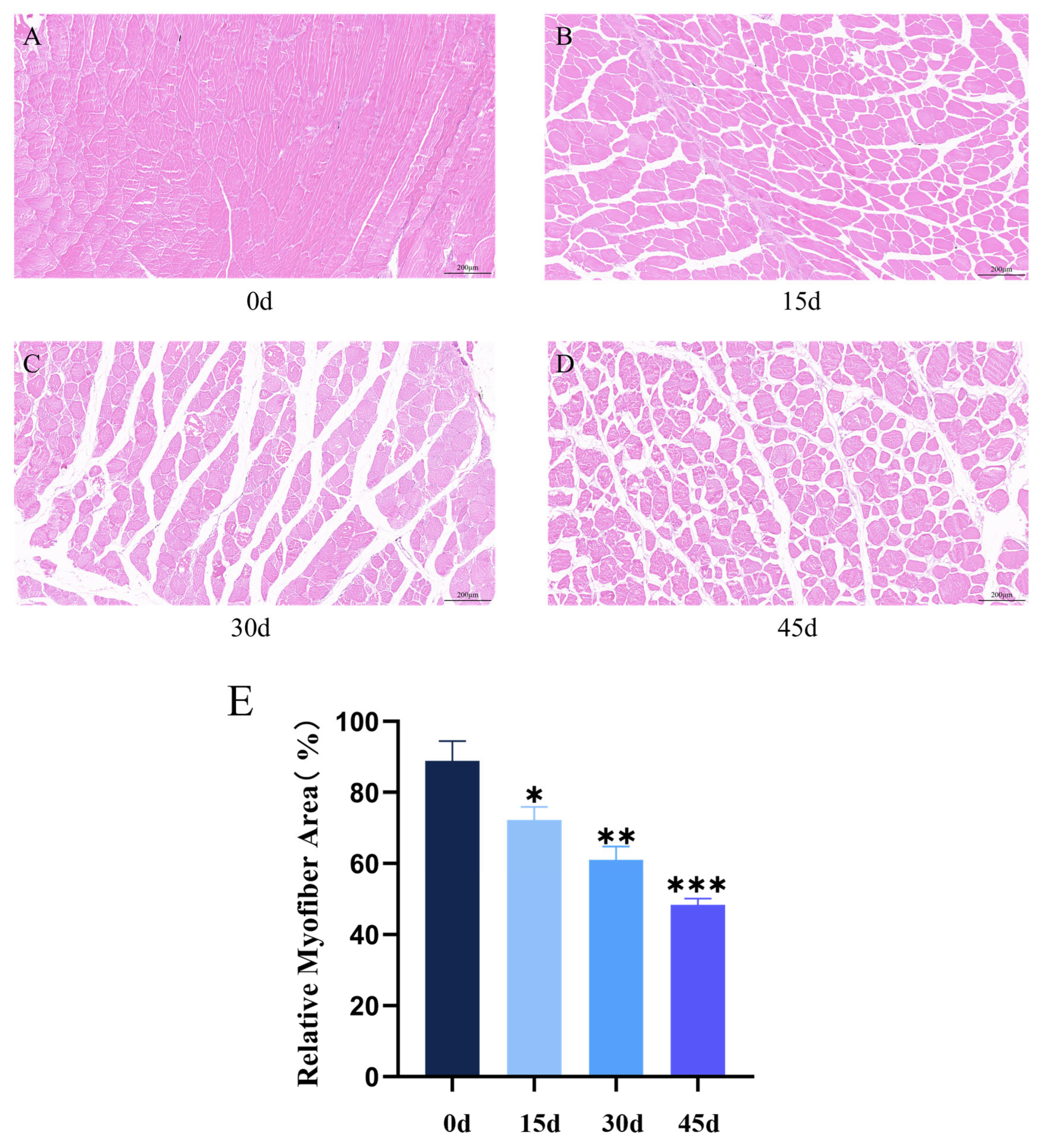
3.4. Low Temperatures Significantly Inhibit the Growth and Development of Muscle Tissue
4. Discussion
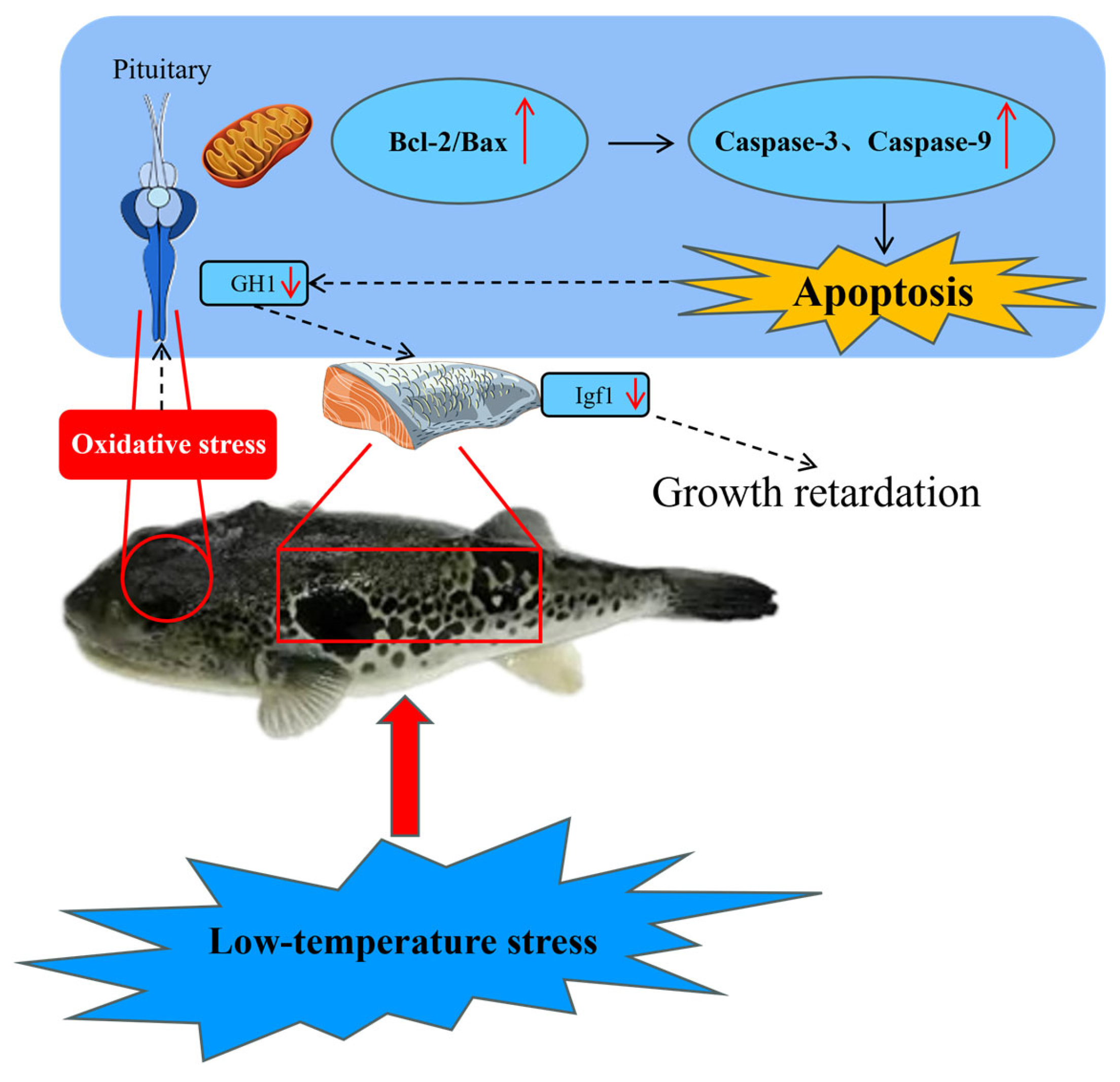
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Campana, S.E.; Stefánsdóttir, R.B.; Jakobsdóttir, K.; Sólmundsson, J. Shifting fish distributions in warming sub-Arctic oceans. Sci. Rep. 2020, 10, 16448. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Johansen, R.B.; Hansen, Ø.J.; Puvanendran, V. Temperature preference of juvenile lumpfish (Cyclopterus lumpus) originating from the southern and northern parts of Norway. J. Therm. Biol. 2020, 89, 102562. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Abdul Kadir, S.R. Diversity, distribution and different habitat use among the tropical freshwater eels of genus Anguilla. Sci. Rep. 2017, 7, 7593. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Villegas-Ríos, D.; Moland, E.; Olsen, E.M. Sea temperature effects on depth use and habitat selection in a marine fish community. J. Anim. Ecol. 2021, 90, 1787–1800. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Niu, M.; Hu, L.; Chen, L. Cold Acclimation for Enhancing the Cold Tolerance of Zebrafish Cells. Front. Physiol. 2021, 12, 813451. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, C.; Ding, H.; Li, P.; Gao, Y.; Zhong, K.; Bao, Z.; Qu, Z.; Wang, B.; Hu, J. Effects of cold stress on the blood-brain barrier in Plectropomus leopardus. BMC Genom. 2024, 25, 1031. [Google Scholar] [CrossRef]
- Parisi, M.A.; Franklin, C.E.; Cramp, R.L. Can slowing the rate of water temperature decline be utilized to reduce the impacts of cold water pollution from dam releases on fish physiology and performance? J. Fish Biol. 2022, 100, 979–987. [Google Scholar] [CrossRef]
- Ridgway, M.R.; Scott, G.R. Constant temperature and fluctuating temperature have distinct effects on hypoxia tolerance in killifish (Fundulus heteroclitus). J. Exp. Biol. 2023, 226, jeb245425. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, K.; Liang, X.F.; Li, J.; Tang, S.; Zhang, Y.; Cai, W.; Xiao, Q.; Zhang, Q. Effects of early low temperature exposure on the growth, glycolipid metabolism and growth hormone (gh) gene methylation in the late stage of Chinese perch (Siniperca chuatsi). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 259, 110705. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, S.; Liu, Y.; Chu, P.; Han, B.; Ning, X.; Wang, T.; Yin, S. Acute cold stress leads to zebrafish ovarian dysfunction by regulating miRNA and mRNA. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 48, 101139. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, N.; Liu, X.; Deng, W.; Dong, R.; Jiang, Q. Mechanisms of Low Temperature-induced GH Resistance via TRPA1 Channel Activation in Male Nile Tilapia. Endocrinology 2025, 166, bqaf013. [Google Scholar] [CrossRef]
- Ge, G.; Long, Y.; Song, G.; Li, Q.; Cui, Z.; Yan, H. Transcriptomic Profiling Revealed Signaling Pathways Associated with the Spawning of Female Zebrafish under Cold Stress. Int. J. Mol. Sci. 2022, 23, 7494. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental toxicity, oxidative stress and apoptosis: Ménage à Trois. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Marcondes, M.-C.G.; Bajova, H.; Dugan, L.L.; Conti, B. Metabolic Depression and Increased Reactive Oxygen Species Production by Isolated Mitochondria at Moderately Lower Temperatures. J. Biol. Chem. 2010, 285, 32522–32528. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Nuño, S.; Sanahuja, I.; Fernández-Alacid, L.; Ordóñez-Grande, B.; Carbonell, T.; Ibarz, A. Oxidative attack during temperature fluctuation challenge compromises liver protein homeostasis of a temperate fish model. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 236, 110311. [Google Scholar] [CrossRef]
- Fadhlaoui, M.; Couture, P. Combined effects of temperature and metal exposure on the fatty acid composition of cell membranes, antioxidant enzyme activities and lipid peroxidation in yellow perch (Perca flavescens). Aquat. Toxicol. 2016, 180, 45–55. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Z.; Yang, Y.; Wang, J.; Yang, T.; Chen, X.; Liang, L.; Mu, W. Histology, physiology, and glucose and lipid metabolism of Lateolabrax maculatus under low temperature stress. J. Therm. Biol. 2022, 104, 103161. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, R.; Wang, X.; Zhu, H.; Tian, Z. Transcriptome analysis reveals molecular mechanisms responsive to acute cold stress in the tropical stenothermal fish tiger barb (Puntius tetrazona). BMC Genom. 2020, 21, 737. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Yang, Y.; Li, J.; Yang, X.; Li, L.; Zheng, Z.; Yang, B.; Zhang, P.; Liu, H. Effects of chronic cold stress and thermal stress on growth performance, hepatic apoptosis, oxidative stress, immune response and gut microbiota of juvenile hybrid sturgeon (Acipenser baerii ♀ × A. schrenkii ♂). Fish Shellfish Immunol. 2025, 157, 110078. [Google Scholar] [CrossRef]
- Zhou, T.; Gui, L.; Liu, M.; Li, W.; Hu, P.; Duarte, D.F.C.; Niu, H.; Chen, L. Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2019, 84, 1145–1156. [Google Scholar] [CrossRef]
- Liu, R.; Liu, R.; Song, G.; Li, Q.; Cui, Z.; Long, Y. Mitochondria Dysfunction and Cell Apoptosis Limit Resistance of Nile Tilapia (Oreochromis niloticus) to Lethal Cold Stress. Animals 2022, 12, 2382. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M.; Liu, J.H.; Shu, L.H.; Chen, C.H. Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Canosa, L.F.; Chang, J.P.; Peter, R.E. Neuroendocrine control of growth hormone in fish. Gen. Comp. Endocrinol. 2007, 151, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Durán-Prado, M.; Luque, R.M.; Martínez-Fuentes, A.J.; Quintero, A.; Gutiérrez-Pascual, E.; Córdoba-Chacón, J.; Malagón, M.M.; Gracia-Navarro, F.; Castaño, J.P. Understanding the multifactorial control of growth hormone release by somatotropes: Lessons from comparative endocrinology. Ann. N. Y. Acad. Sci. 2009, 1163, 137–153. [Google Scholar] [CrossRef]
- Vélez, E.J.; Unniappan, S. A Comparative Update on the Neuroendocrine Regulation of Growth Hormone in Vertebrates. Front. Endocrinol. 2020, 11, 614981. [Google Scholar] [CrossRef]
- Bergan-Roller, H.E.; Sheridan, M.A. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen. Comp. Endocrinol. 2018, 258, 119–133. [Google Scholar] [CrossRef]
- Becerra, S.; Arriagada-Solimano, M.; Escobar-Aguirre, S.; Palomino, J.; Aedo, J.; Estrada, J.M.; Barra-Valdebenito, V.; Zuloaga, R.; Valdes, J.A.; Dettleff, P. High temperature induces oxidative damage, immune modulation, and atrophy in the gills and skeletal muscle of the teleost fish black cusk-eel (Genypterus maculatus). Dev. Comp. Immunol. 2025, 164, 105332. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, M.; Yan, K.; Zhang, Y.; Li, Y.; Zhu, J.; Wang, G.; Wang, X.; Li, Y.; Huang, X.; et al. Cold Stress Induces Apoptosis in Silver Pomfret via DUSP-JNK Pathway. Mar. Biotechnol. 2023, 25, 846–857. [Google Scholar] [CrossRef]
- Peng, H.; Yang, B.; Li, B.; Cai, Z.; Cui, Q.; Chen, M.; Liu, X.; Yang, X.; Jiang, C. Comparative transcriptomic analysis reveals the gene expression profiles in the liver and spleen of Japanese pufferfish (Takifugu rubripes) in response to Vibrio harveyi infection. Fish Shellfish Immunol. 2019, 90, 308–316. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, H.; Shen, X.; Jiang, J.; Yuan, Z.; Liu, Q.; Wang, Z.; Bai, L.; Zhang, L.; Song, C.; et al. Effects of different light conditions on growth, muscle nutrients content, and clock gene circadian expression of Takifugu rubripes. Aquac. Rep. 2022, 26, 101294. [Google Scholar] [CrossRef]
- Yan, H.; Shen, X.; Jiang, J.; Zhang, L.; Yuan, Z.; Wu, Y.; Liu, Q.; Liu, Y. Gene Expression of Takifugu rubripes Gonads During AI- or MT-induced Masculinization and E2-induced Feminization. Endocrinology 2021, 162, bqab068. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, Y.; Li, X.; Zhou, Z.; Ma, K.; Guo, W.; Liang, Y.; Xie, X.; Zhang, J.; Wang, Q.; et al. A Transcriptomic Analysis of Gonads from the Low-Temperature-Induced Masculinization of Takifugu rubripes. Animals 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yan, H.; Hu, M.; Zhou, H.; Zhang, Q.; Gao, R.; Liu, Q.; Sun, Q. A detailed transcriptome study uncovers the epigenetic characteristics associated with Aromatase inhibitor-induced masculinization in Takifugu rubripes larvae gonads. BMC Genom. 2025, 26, 380. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Wang, H.C.; Chang, C.J.; Lee, S.Y. Mitochondrial Glutathione in Cellular Redox Homeostasis and Disease Manifestation. Int. J. Mol. Sci. 2024, 25, 1314. [Google Scholar] [CrossRef]
- Guillen, A.C.; Borges, M.E.; Herrerias, T.; Kandalski, P.K.; de Souza, M.; Donatti, L. Gradual increase of temperature trigger metabolic and oxidative responses in plasma and body tissues in the Antarctic fish Notothenia rossii. Fish Physiol. Biochem. 2022, 48, 337–354. [Google Scholar] [CrossRef]
- Zhai, C.; Li, Y.; Wang, R.; Han, H.; Zhang, Y.; Ma, B. Combined Impacts of Acute Heat Stress on the Histology, Antioxidant Activity, Immunity, and Intestinal Microbiota of Wild Female Burbot (Lota Lota) in Winter: New Insights into Heat Sensitivity in Extremely Hardy Fish. Antioxidants 2025, 14, 947. [Google Scholar] [CrossRef]
- Gould, R.L.; Pazdro, R. Impact of Supplementary Amino Acids, Micronutrients, and Overall Diet on Glutathione Homeostasis. Nutrients 2019, 11, 1056. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. Biomed. Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Liu, A.; Duan, G.; Yang, L.; Hu, Y.; Zhou, H.; Wang, H. Low-Temperature Stress-Induced Hepatic Injury in Darkbarbel Catfish (Pelteobagrus vachelli): Mediated by Gut-Liver Axis Dysregulation. Antioxidants 2025, 14, 762. [Google Scholar] [CrossRef]
- Huang, J.; Fu, Z.; Bai, J.; Ma, Z. Cold stress disrupts gill homeostasis in juvenile yellowfin tuna (Thunnus albacares) by altering oxidative, metabolic, and immune responses. Mar. Environ. Res. 2025, 210, 107300. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, L.; Conde de la Rosa, L.; Torres, S.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial cholesterol: Metabolism and impact on redox biology and disease. Redox Biol. 2023, 61, 102643. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Tajeddine, N. How do reactive oxygen species and calcium trigger mitochondrial membrane permeabilisation? Biochim. Biophys. Acta 2016, 1860, 1079–1088. [Google Scholar] [CrossRef]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssière, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Liu, S.; Huang, B.; Cao, J.; Wang, Y.; Xiao, H.; Zhu, Y.; Zhang, H. ROS fine-tunes the function and fate of immune cells. Int. Immunopharmacol. 2023, 119, 110069. [Google Scholar] [CrossRef]
- Cheng, C.H.; Ye, C.X.; Guo, Z.X.; Wang, A.L. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress. Fish Shellfish Immunol. 2017, 64, 137–145. [Google Scholar] [CrossRef]
- Liu, E.; Zhao, X.; Li, C.; Wang, Y.; Li, L.; Zhu, H.; Ling, Q. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, L.; Ma, R.; Wang, J.; Du, L. FoxO signaling and mitochondria-related apoptosis pathways mediate tsinling lenok trout (Brachymystax lenok tsinlingensis) liver injury under high temperature stress. Int. J. Biol. Macromol. 2023, 251, 126404. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Yang, F.F.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L.; Tan, J.W.; Chen, X.Y. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; Roose, H.; Vennekens, A.; Vankelecom, H. Pituitary stem cell regulation: Who is pulling the strings? J. Endocrinol. 2017, 234, R135–R158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russell, J.P.; Lodge, E.J.; Andoniadou, C.L. Basic Research Advances on Pituitary Stem Cell Function and Regulation. Neuroendocrinology 2018, 107, 196–203. [Google Scholar] [CrossRef]
- Blanco, A.M. Hypothalamic- and pituitary-derived growth and reproductive hormones and the control of energy balance in fish. Gen. Comp. Endocrinol. 2020, 287, 113322. [Google Scholar] [CrossRef]
- Fontaine, R.; Rahmad Royan, M.; Henkel, C.; Hodne, K.; Ager-Wick, E.; Weltzien, F.A. Pituitary multi-hormone cells in mammals and fish: History, origin, and roles. Front. Neuroendocrinol. 2022, 67, 101018. [Google Scholar] [CrossRef]
- Campbell, G.S. Growth-hormone signal transduction. J. Pediatr. 1997, 131, S42–S44. [Google Scholar] [CrossRef]
- Carter-Su, C.; Rui, L.; Herrington, J. Role of the tyrosine kinase JAK2 in signal transduction by growth hormone. Pediatr. Nephrol. 2000, 14, 550–557. [Google Scholar] [CrossRef]
- Xu, B.C.; Wang, X.; Darus, C.J.; Kopchick, J.J. Growth hormone promotes the association of transcription factor STAT5 with the growth hormone receptor. J. Biol. Chem. 1996, 271, 19768–19773. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Duan, C.; Yang, L.; Zhou, D.; Zhang, Z.; Chen, H.; Li, G.; Zhu, C.; Tian, C. Effects of chronic high-temperature stress on muscle tissue integrity and metabolism-related genes in Clarias fuscus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 55, 101497. [Google Scholar] [CrossRef]
- Renaud, C.; de Lamballerie, M.; Guyon, C.; Astruc, T.; Venien, A.; Pottier, L. Effects of high-pressure treatment on the muscle structure of salmon (Salmo salar). Food Chem. 2022, 367, 130721. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Einarsdottir, I.E.; Valdes, J.A.; Alvarez, M.; Molina, A.; Björnsson, B.T. Inherent growth hormone resistance in the skeletal muscle of the fine flounder is modulated by nutritional status and is characterized by high contents of truncated GHR, impairment in the JAK2/STAT5 signaling pathway, and low IGF-I expression. Endocrinology 2012, 153, 283–294. [Google Scholar] [CrossRef]



| Commercial Reagent Kit | Item Number | Manufacturer |
|---|---|---|
| Reactive Oxygen Species (ROS)Assay Kit | E004-1-1 | Nanjing Jian Cheng Bioengineering Institute |
| Total protein (TP) quantitative Assay Kit | A045-2-2 | Nanjing Jian Cheng Bioengineering Institute |
| Reduced glutathione (GSH) Assay Kit | A006-2-1 | Nanjing Jian Cheng Bioengineering Institute |
| Glutathione Peroxidase (GSH-PX) Assay Kit | A005-1-2 | Nanjing Jian Cheng Bioengineering Institute |
| Total antioxidant capacity(T-AOC) Assay Kit | A015-1-2 | Nanjing Jian Cheng Bioengineering Institute |
| Catalase (CAT) Assay Kit | A007-1-1 | Nanjing Jian Cheng Bioengineering Institute |
| Superoxide Dismutase (SOD) typed Assay Kit | A001-2-1 | Nanjing Jian Cheng Bioengineering Institute |
| Malondialdehyde (MDA) Assay Kit | A003-1-2 | Nanjing Jian Cheng Bioengineering Institute |
| Graph 5. | Primer Sequence (5′-3′) |
|---|---|
| Caspase9-F | ATCCTTCAAGCCTCTACGATGGG |
| Caspase9-R | TCTGATTAACTGCCTAGCCTGGTC |
| Caspase3-F | CGACCAGACAGTGAAGCAGATG |
| Caspase3-R | ATGACTCAGCAGAACGCACAC |
| Bcl-2-F | CCTCCTCCATCTCGTGCTTCTC |
| Bcl-2-R | GGGCTTTGAAGACATCCAGAACTG |
| Bax-F | CGATGATGTCACCGCCACTTG |
| Bax-R | TGACCGCCGACGCCTATAT |
| Apaf-1-F | CCTCTTCGCCACCACCTCTG |
| Apaf-1-R | TCCGCACGCACTCCTGATG |
| gh1-F | GAAGCAGAGCAACAATGGACAAAG |
| gh1-R | TGAGCAAGCAGGTGGAGGTG |
| igf1-F | GCATCGGTCATCTATTCGGAGTC |
| igf1-R | GCTGTTCCTTCTAATCGGCTCTG |
| stat5a-F | GGCAGAACACCTCAGACAACAAC |
| stat5a-R | GAACTGTGGCGGCGAACTTG |
| jak2a-F | GCGGAGGAGGTGGAGGTGAG |
| jak2a-R | TTGTCCTGCTTGGTGATGGTAACG |
| ghra-F | TCATTGTGCTCGTTGCTGTATCTC |
| ghra-R | CGGCTCGTCTCGGTAGAACTC |
| β-actin-F | CCAGAAAGACAGCTACGTTGG |
| β-actin-R | GCAACTCTCAGCTCGTTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, T.; Yao, M.; Li, C.; Jiang, Z.; Pan, H.; Wang, W.; Li, Y.; Zhou, H. Metabolic Responses and Oxidative Stress Adaptation Mechanisms of the Pituitary Gland in the Tiger Puffer Under Low-Temperature Stress. Fishes 2025, 10, 572. https://doi.org/10.3390/fishes10110572
Li Y, Li T, Yao M, Li C, Jiang Z, Pan H, Wang W, Li Y, Zhou H. Metabolic Responses and Oxidative Stress Adaptation Mechanisms of the Pituitary Gland in the Tiger Puffer Under Low-Temperature Stress. Fishes. 2025; 10(11):572. https://doi.org/10.3390/fishes10110572
Chicago/Turabian StyleLi, Yifan, Taicheng Li, Meihui Yao, Chuan Li, Zibin Jiang, Hongyu Pan, Wei Wang, Yajuan Li, and He Zhou. 2025. "Metabolic Responses and Oxidative Stress Adaptation Mechanisms of the Pituitary Gland in the Tiger Puffer Under Low-Temperature Stress" Fishes 10, no. 11: 572. https://doi.org/10.3390/fishes10110572
APA StyleLi, Y., Li, T., Yao, M., Li, C., Jiang, Z., Pan, H., Wang, W., Li, Y., & Zhou, H. (2025). Metabolic Responses and Oxidative Stress Adaptation Mechanisms of the Pituitary Gland in the Tiger Puffer Under Low-Temperature Stress. Fishes, 10(11), 572. https://doi.org/10.3390/fishes10110572






