Abstract
Low-fishmeal feed is increasingly being adopted across the global aquaculture industry. This study evaluated dietary Clostridium butyricum and alanyl-glutamine (Ala-Gln) supplementation in juvenile triploid rainbow trout (Oncorhynchus mykiss) with a low-fishmeal diet. Four diets were tested: basal diet (SBM, 15% fishmeal and 21.6% soybean meal), SBM + 0.5% C. butyricum (CB), SBM + 1.0% Ala-Gln, and SBM + 0.5% C. butyricum + 1.0% Ala-Gln (CB-AG). Fish were fed in 500 L tanks in recirculating aquaculture systems for 8 weeks (62.52 ± 0.47 g). Each group comprised three tanks, with each tank housing 30 fish. Then 10 fish per tank were challenged with Aeromonas salmonicida. CB-AG showed significantly higher weight gain and specific growth rates than the SBM group (p < 0.05). Mortality was significantly lower in CB-AG and AG than in SBM after A. salmonicida challenge. Histomorphology revealed significant differences (p < 0.05) between CB-AG and SBM in muscularis thickness, villus width, and height. SBM sections showed inflammatory infiltration and border damage were attenuated in supplemented groups. Serum malondialdehyde (MDA) and dioxygenase (DAO) were significantly lower in CB-AG than SBM (p < 0.05), while serum and hepatic lysozyme (LZM) and hepatic superoxide dismutase (SOD) were higher. Digestive enzymes indicated significantly higher trypsin and lipase activities in CB-AG (p < 0.05). CB-AG upregulated intestinal tight junction proteins and PepT1 and downregulated pro-inflammatory mediators. Combined 0.5% C. butyricum and 1.0% Ala-Gln inclusion effectively preserved growth performance, antioxidant capacity, gut microbiome homeostasis, and intestinal health in rainbow trout on low-fishmeal diets.
Key Contribution:
The inclusion of 0.5% C. butyricum and 1.0% Ala-Gln in the feed revealed no adverse effects on growth, antioxidant capacity, or gut health in rainbow trout. Furthermore, the combined supplementation exerted more pronounced effects compared to either additive administered alone, demonstrating potential for application in low-fishmeal aquaculture feeds.
1. Introduction
In recent years, driven by intertwined factors—including the depletion of wild fishery resources due to overfishing, volatility in global supply chains, and the enforcement of environmental policies—fishmeal prices have risen steadily, while its supply stability has declined. This trend has not only severely constrained cost control and the sustainable development of the rainbow trout (Oncorhynchus mykiss) farming industry but also driven up feed costs, posing a practical challenge to large-scale aquaculture operations [1]. To address this, the industry has widely adopted alternative protein sources, such as plant proteins (soybean meal, rapeseed meal) and animal by-product proteins (meat and bone meal, blood meal), to reduce fishmeal inclusion in feed formulations [2]. While this strategy mitigates short-term resource pressure, its long-term application reveals that low-fishmeal diets readily induce a series of physiological and metabolic disorders in triploid rainbow trout. Key contributors to these issues include anti-nutritional factors (ANFs) in plant proteins (e.g., trypsin inhibitors and non-starch polysaccharides), amino acid imbalances in animal by-product proteins, and disparities in fatty acid profiles of low-fishmeal diets. These factors collectively inhibit feed efficiency and protein deposition in triploid rainbow trout, ultimately impairing their growth performance [3,4].
More critically, the nutritional imbalance from low-fishmeal diets further compromises intestinal health: it impairs the integrity of the intestinal mucosal barrier and disrupts gut microbiota composition, which in turn elevates the abundance of pathogenic bacteria. This chain of effects triggers intestinal inflammatory responses and impairs disease resistance in triploid rainbow trout. Notably, Aeromonas salmonicida—a prevalent pathogen in rainbow trout aquaculture—can exploit the compromised intestinal mucosa to invade the host, causing diseases such as septicemia and skin ulceration. These diseases lead to significant mortality in farmed populations and result in substantial economic losses for producers [5]. Consequently, mitigating the adverse effects of low-fishmeal diets on the gut health and immune function of triploid rainbow trout has become a critical bottleneck for the sustainable development of this aquaculture sector. This underscores the urgency of exploring targeted nutritional regulation strategies to address the limitations of low-fishmeal feed applications.
Clostridium butyricum is a Gram-positive bacterium possessing endospores which is capable of producing butyric acid and exhibits strong adaptability to elevated temperatures and low pH levels. It flourishes exclusively in anaerobic settings and can multiply within fish digestive systems. This bacterium actively ferments sugars, transforming them and glycerol into biofuel components and biomaterial precursors, including hydrogen, butanol, BA, and 1,3-propanediol [6]. It secretes lipase (LPS), amylase (AMS), and proteases to assist in digesting intestinal chyme and enhancing small molecule uptake [7]. This strain hinders pathogenic bacterial colonization by releasing antimicrobial peptides and adjusting the intestinal acid–base balance, thus modulating dysbiotic gut microbiomes [8]. C. butyricum, a green probiotic, has been extensively utilized in aquaculture in recent years. Its primary modes of action encompass regulating gut microbiota, enhancing immunity, and improving water quality. The short-chain fatty acids (SCFAs) metabolized by C. butyricum, such as butyric acid, are capable of repairing intestinal mucosa, inhibiting pathogenic bacteria like Vibrio, and supplying energy to intestinal epithelial cells. In pisciculture, the addition of 1 × 107–1 × 109 CFU/g of C. butyricum can enhance serum lysozyme activity and elevate the survival rate of fish infected with Aeromonas hydrophila by 30–40% [9].
Glutamine, as the amide derivative of glutamic acid, ranks as the most abundant free amino acid in plasma. Widely distributed in muscle tissue, this amino acid exerts a pivotal regulatory role in protein biosynthesis. Furthermore, it possesses prominent biological activity, as it participates in the biosynthesis of glutathione (GSH)—a process that effectively enhances the antioxidant defense capacity in fish [10]. In addition, glutamine serves as the primary nitrogen donor for the de novo synthesis of nucleotides and non-essential amino acids, while its catabolic metabolism can directly supply energy substrates for cellular metabolic processes [11]. Given its diverse and indispensable physiological functions outlined above, glutamine has been developed into a high-performance additive for the food and feed industries and is widely employed as a nutritional supplement and immune modulator in relevant applications. Ala-Gln dipeptide is commonly used in production as a carrier for Gln application; Ala-Gln is stable and non-toxic at high temperatures and can be rapidly hydrolyzed into Gln in the body to exert its effect, which is more suitable for aquatic application [12].
As a commercially significant cold-water fish species, the structural stability of the gut microbiota of rainbow trout critically influences nutrient absorption efficiency and immune health [9]. The implementation of low-fishmeal diets, which is necessitated by fishmeal resource scarcity, often induces gut microbiota dysbiosis. This study investigated the regulatory mechanisms of specific additives on rainbow trout gut microbiota structure by supplementing low-fishmeal diets with C. butyricum, Ala-Gln, and their combination, while concurrently evaluating growth performance and immune parameters. The primary innovation of this work lies in demonstrating a synergistic effect on the ‘C. butyricum + Ala-Gln’ formulation under low-fishmeal conditions, thereby addressing a research gap where existing studies predominantly focus on ‘probiotic + prebiotic’ combinations or single active substances. This finding provides novel insights for optimizing low-fishmeal feed formulations.
2. Materials and Methods
2.1. Rainbow Trout Management
Triploid rainbow trout were acquired from Egremorin Industrial Co. in Benxi, China. All procedures were approved by the institutional animal care and use committee. During a 14-day adaptation phase using a recirculation aquaculture system, the fish consumed a basal diet twice daily before the experiment commenced. Post-adaptation, 360 fish with an initial weight of 62.52 ± 0.47 g were randomly allocated to 12 experimental tanks, each holding 500 L and containing 30 fish. Throughout the 8-week feeding trial, the fish received test diets until apparent satiety twice daily at 08:00 and 16:00. Body weights were measured every two weeks to track growth and modify meal sizes. Aerated tap water served as the aquatic medium. Water quality was assessed with a YSI 8500 SELECT™ Biochemistry Analyzer, maintaining parameters at a stable temperature of 15 ± 0.5 °C, dissolved oxygen exceeding 6.0 mg/L, nitrite-nitrogen below 0.02 mg/L, pH ranging from 6.8 to 7.1, and ammonia-nitrogen under 0.2 mg/L. Daily replacement of one-third of the water ensured consistent water quality.
2.2. Diets
Four groups in this experiment were identified as the control group (SBM), which was fed the basal diet (crude protein, 37.63%; crude lipid, 15.95%; gross energy, 21.55 MJ/kg), while the other three groups were supplemented with 0.50% C. butyricum (CB), 1.00% Ala-Gln (AG), and 0.50% C. butyricum and 1.00% Ala-Gln (CB-AG), respectively. The choice of treatments (0.50% C. butyricum (CB) and 1.00% Ala-Gln) was based on pilot tests and laboratory observations. Table 1 presents the experimental diet formulations. Ingredients were weighed as per the formulations and then blended. Prior to mixing, they were finely milled to particles under 250 μm. Following the addition of the lipid source, all materials underwent thorough mixing for 25 min. The mixture was subsequently re-homogenized, with distilled water incorporated to attain a caking consistency. Using a pelletizer (GYJ-250B, Dashiqiao Bao’s Feed Machinery Factory, Yingkou, Liaoning, China), pellets measuring 1.50 mm were produced. These pellets were air-dried and preserved under −20 °C.

Table 1.
Ingredients and their contents in the test diets (air-dry basis, g/kg).
2.3. Sample Collection
After a 56-day feeding period, rainbow trout were fasted for 24 h prior to sampling. Following MS-222 anesthesia (75 mg/L, CAS 886-86-2, Sigma, St. Louis, MO, USA), fish were weighed to compute growth indices. Draw blood from the venous sinuses on both sides of the caudal peduncle using a 5 mL syringe. Tail vein blood samples were centrifuged at 3000× g for 10 min at 4 °C to isolate serum and stored at −20 °C for biochemical analyses. Mid intestines from six fish were collected and preserved at −80 °C for biochemical testing. Concurrently, proximal intestine samples from three fish were frozen in liquid nitrogen and stored at −80 °C for gene expression studies. For microbiota examination, two fish were sterilized with 75% alcohol (CAS 64-17-5, Runjie Technology Development Co., Ltd., Shanghai, China), and distal intestinal mucosa was excised under sterile conditions. Finally, mid intestines from six fish per group were flushed and fixed in Bouin’s solution (Xinyu Biochemical Co., Ltd. Shanghai, China) for histological assessment. The growth performance parameters were calculated as follows:
where W0 represents the initial body weight (g), W56 represents the final body weight (g), Wf represents the feed intake (g), and L56 represents the final body length (cm).
Weight gain rate (WGR; %) = 100 × (W56 − W0)/W0
Specific growth rate (SGR; %/d) = 100 × (lnW56 − lnW0)/56
Protein efficiency ratio (PER) = (W56 − W0)/(Wf × feed protein content)
Feed conversion ratio (FCR) = Wf/(W56 − W0)
Hepatosomatic index (HSI; %) = 100 × (liver weight (g)/body weight (g))
Condition factor (CF; %) = 100 × W56/L563
Viscerosomatic index (VSI; %) = 100 × (viscera (g)/body weight (g))
Survival rate (SR, %) = 100 × W56/W0
Specific growth rate (SGR; %/d) = 100 × (lnW56 − lnW0)/56
Protein efficiency ratio (PER) = (W56 − W0)/(Wf × feed protein content)
Feed conversion ratio (FCR) = Wf/(W56 − W0)
Hepatosomatic index (HSI; %) = 100 × (liver weight (g)/body weight (g))
Condition factor (CF; %) = 100 × W56/L563
Viscerosomatic index (VSI; %) = 100 × (viscera (g)/body weight (g))
Survival rate (SR, %) = 100 × W56/W0
2.4. Nutrient Content
The dietary and whole-body composition was assessed following AOAC (2012) methodologies [14]. The moisture content of samples was determined by oven-drying at 105 °C for three hours. Crude protein was evaluated using the Kjeldahl method (2001.11), determining nitrogen content and applying a 6.25 conversion factor (2300, FOSS, Sweden). Ash concentration was quantified via combustion at 600 °C for two hours (method 942.05). Crude lipid content was assessed via Soxhlet extraction (method 920.39).
2.5. Digestive Enzyme
The activities of intestinal lipase (LPS), amylase (AMS), trypsin (TPS), and total protein were evaluated using commercial assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Samples were homogenized in 9 volumes (w/v) of ice-cold 5.3% saline and centrifuged at 861× g for 10 min at 4 °C, and the supernatant was stored at −80 °C until analysis. All absorbance data were acquired with a microplate reader (Synergy 2, BoiTek, Winooski, VT, USA).
2.6. Oxidative Stress and Antioxidant Enzyme Analysis
Each liver sample was mixed with 9 volumes (w/v) of pre-chilled 0.53% saline solution. A high-speed homogeniser (DP-200S, Yaoudepeng Technology Co., Ltd., Beijing, China) was used to prepare a 10% liver tissue homogenate. This was centrifuged at 861× g for 10 min at 4 °C. The supernatant was stored at −80 °C for subsequent assays.
The activity of superoxide dismutase (SOD)in serum and liver tissue was measured using the WST-1 assay, malondialdehyde (MDA) content was determined by the TBA assay, the activity of diamine oxidase (DAO) was assessed via ultraviolet colorimetry, and lysozyme (LZM) content was determined by the turbidimetric method (Nanjing Jianjian Bioengineering Institute, Nabjing, China). The specific calculation formulas are as follows:
SOD activity (U/mgprot) = [(Acontrol − Acontrol blank) − (Atreatment − Atreatment blank)]/(Acontrol − Acontrol blank)/2 × 12/TP concentration;
MDA content (nmol/mgprot) = (ODtreatment − ODcontrol)/(ODstandard − ODblank) × standard concentration/TP concentration;
TP concentration (mg/mL) = (ODtreatment − ODblank)/(ODstandard − ODblank) × standard concentration × dilution ratio;
DAO activity (U/L) = (A20″ − A10′20″) × 103/(6.3 × 0.5 × 10) × 880 μL/80 μL;
LZM content (μg/mL) = ΔTtreatment/ΔTstandard × Cstandard × dilution ratio.
MDA content (nmol/mgprot) = (ODtreatment − ODcontrol)/(ODstandard − ODblank) × standard concentration/TP concentration;
TP concentration (mg/mL) = (ODtreatment − ODblank)/(ODstandard − ODblank) × standard concentration × dilution ratio;
DAO activity (U/L) = (A20″ − A10′20″) × 103/(6.3 × 0.5 × 10) × 880 μL/80 μL;
LZM content (μg/mL) = ΔTtreatment/ΔTstandard × Cstandard × dilution ratio.
2.7. Histological Examination
For each biological replicate, four rainbow trout intestinal segments free of alimentary canal contents were randomly selected and fixed in Bouin’s fixative (Xinyu Biochemical Co., Ltd., Shanghai, China). They were subsequently rinsed repeatedly with distilled water to remove residual fixative and then transferred to 70% ethanol (CAS 64-17-5, Runjie Technology Development Co., Ltd., Shanghai, China), for gradient dehydration until the ethanol remained colorless. The dehydrated midgut segments were embedded via conventional paraffin infiltration. Paraffin-embedded samples were sectioned into 4 μm thick slices using a microtome (Model MD400B, Leica, Wetzlar, German). After deparaffinization and rehydration, the sections were stained with hematoxylin-eosin (HE, Solarbio Science & Technology Co., Ltd., Beijing, China) and mounted with neutral balsam. In each experimental group, 30 midgut intestine sections were observed under a light microscope (Echo Revolve, Chicago, IL, USA). Villi height (VH), villus width (VW), and muscle layer thickness (MT) in the midgut of rainbow trout were measured by ImageJ (Java8, National Institutes of Health, Bethesda, MD, USA) [13].
2.8. RT-PCR Analysis
The ground rainbow trout gut samples were frozen in liquid nitrogen at −80 °C. RNA was derived with TRIzol and reverse transcribed to cDNA. Quality was evaluated via NanoDrop spectrophotometry (A260/A280: 1.8–2.0, Thermo Fisher Scientific, Waltham, MA, USA). Primer sequences are in Table 2. β-actin normalized cDNA loading. cDNA served as a template for RT-PCR (Table 3). Gene expression was analyzed using the 2−ΔΔCT method [15].

Table 2.
Real-time PCR primer sequences.

Table 3.
Real-time PCR amplification procedure.
2.9. Intestinal Microbiota Analysis
Following extraction of DNA from rainbow trout intestinal contents, the V3-V4 region of the 16S rRNA gene was amplified, and the amplified data were screened [13]. Sequencing was conducted on an Illumina MiSeq platform (300 bp paired-end reads) at Shanghai Meiji Biopharmaceutical Technology Co., Ltd., Shanghai, China. OTU clustering and species taxonomy were analyzed, followed by alpha diversity (Mothur) and beta diversity (Qiime) assessments. UCHIME identified chimeric sequences, and raw dates were uploaded to NCBI SRA (PRJNA1255308). Community structure was examined by taxonomic level, including phylum and genus. PCA evaluated beta diversity, LEfSe identified differential taxa, and wTO (weighted topological) analyzed interactions with padj < 0.05. PICRUSt2 (https://github.com/picrust/picrust2, accessed on 12 May 2024) predicted KEGG pathways, BugBase (http://bugbase.cs.umn.edu, accessed on 12 May 2024) predicted phenotypes, and co-occurrence networks were built with Spearman correlations >0.6 or <−0.6 and p < 0.01.
2.10. Aeromonas Salmonicida Challenge
After sampling, the experimental fish were fed the test diets for 7 days. Thirty fish in each group were randomly assigned to 3 tanks (10 fish per tank) to detect A. salmonicida infection. The bacteria were cultured in TSB at 28 °C for 14 h, then diluted with PBS (Solarbio Science & Technology Co., Ltd., Beijing, China) to a bacterial suspension of 1.65 × 108 CFU/mL (LD50). A 100 μL aliquot of this suspension was injected into the base of the pectoral fin of each fish. For 7 days after infection (during which the fish were starved), the number of dead fish was recorded daily.
2.11. Calculations and Statistical Analysis
The growth performance parameters were calculated based on Liu et al. [13]. Statistical analyses were conducted utilizing SPSS 26.0 for Windows. Data conforming to a normal distribution were then analyzed using one-way ANOVA, followed by Tukey’s multiple range test for post hoc comparisons. Statistical significance was established at p < 0.05, while a p of less than 0.01 was considered highly significant. Dates are presented as mean ± standard error (S.E.). For the graphical representation of gene expression data, GraphPad Prism 8.0 for Windows was employed.
3. Results
3.1. Growth Performance
Final body weight (FBW), WGR, and SGR were significantly higher in CB-AG compared to SBM (p < 0.05). The value of FCR was significantly lower in CB-AG than SBM (p < 0.05). There were no significant differences in SR, HSI, VSI, and CF among all groups (p > 0.05) (Table 4).

Table 4.
The growth performance of triploid rainbow trout fed diets containing different C. butyricum levels.
3.2. Body Composition
Table 5 showed that dietary C. butyricum and Ala-Gln did not affect the moisture, crude lipid, crude protein, and ash content of rainbow trout (p > 0.05).

Table 5.
Body composition (wet weight, %).
3.3. Antioxidant Indices
The antioxidant indices in the liver and serum were assayed and shown in Table 6. The SOD activity in serum was not significantly different among the four groups (p > 0.05). The MDA concentration in both the serum and liver was significantly lower in CB-AG than in SBM (p < 0.05). The maximum value of LZM activity in serum and SOD and LZM activity in liver were found in CB-AG (p < 0.05). The DAO activity in serum in both AG and CB-AG was significantly lower than that of SBM (p < 0.05), but the lowest value existed in CB-AG.

Table 6.
Antioxidant enzyme activities in serum and livers of triploid rainbow trout in each experimental group.
3.4. Digestive Capacity
As illustrated in Table 7, TPS and LPS activities in CB-AG were significantly higher than in the other three groups (p < 0.05). The minimum activity of AMS was held by CB-AG (p < 0.05). Villi height (VH), villus width (VW), and muscle layer thickness (MT) in the midgut of rainbow trout were significantly elevated by the addition of C. butyricum and Ala-Gln (CB-AG) to fish on a low-fishmeal diet and were significantly higher than in SBM (p < 0.05) (Figure 1). VH was significantly higher in the treatment groups than in SBM and was significantly higher in CB-AG than CB and AG, with an increase of 30% compared to SBM (p < 0.05).

Table 7.
Digestive enzymes and morphology of intestines.
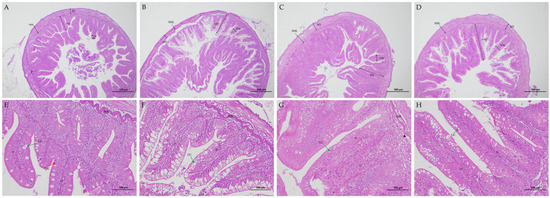
Figure 1.
Effects of dietary C. butyricum and Ala-Gln on gut morphology of triploid rainbow trout. Note: Distal intestine section from rainbow trout in SBM (A,E), CB (B,F), AG (C,G), and CB-AG (D,H) groups. VH: villi height; VW: villus width; MT: muscle layer thickness; SML: submucous layer; LP: lamina propria; GC: goblet cell (green arrows); MV: microvilli (arrowheads); N: nucleus; EGC: eosinophilic granular cell (red arrow).
Figure 1A depicts an incomplete midgut structure in SBM, with poorly developed microvilli and indistinct morphological contours of the striated margin. This group exhibited clear inflammatory signs, including epithelial cell atrophy, nuclear displacement, and observable inflammatory cell infiltration. Upon dietary supplementation with C. butyricum and Ala-Gln, inflammatory responses in the rainbow trout midgut significantly diminished. The intestinal tissue morphology of CB-AG was optimal for gut health, marked by a substantial increase in goblet cell numbers, well-formed microvilli free from fusion or detachment, and orderly arranged columnar epithelial cells (Figure 1).
3.5. Relative Gene Expression in the Intestine
As illustrated in Figure 2, rainbow trout receiving diets supplemented with CB-AG showed reduced expression of genes linked to inflammation, such as IL-1β, IL-8, IKK, NF-κB, IκB-α, and TNF-α, compared to those on different diets (p < 0.05). The expression of MLCK and MLC-3 in the intestine was considerably down-regulated in rainbow trout given diets containing CB-AG (p < 0.05). The expression of TGF-β1, cldn-1, ZO-1, Ocldn, and PepT1 were markedly elevated with dietary CB-AG (p < 0.05).
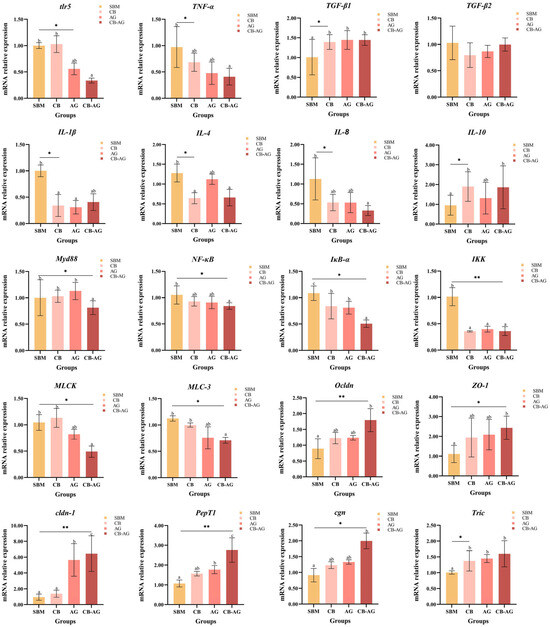
Figure 2.
Dietary C. butyricum and alanine-glutamine on mRNA expression in the guts of triploid rainbow trout. ** represents extremely significant (p < 0.01), * represents significant (p < 0.05), and blank represents not significant (p > 0.05). Values within the same column bearing distinct superscript letters differ significantly (p < 0.05).
3.6. Intestinal Microbiota
From 24 sequenced samples, the Illumina NextSeq2000 platform generated an average of 52,474 sequences per sample, yielding 526,952,601 high-quality sequences. Sequence lengths ranged from 205 to 540 bp, with a mean length of 418 bp. Venn diagram analysis was employed to assess the similarities and overlaps of OTUs across the different groups. The four groups shared 330 core OTUs, while the number of unique OTUs per group was 653 (SBM), 776 (CB), 804 (AG), and 408 (CB-AG), respectively (Figure 3A).
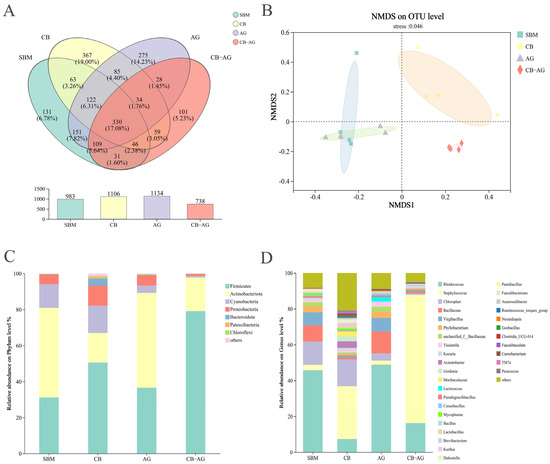
Figure 3.
Venn diagram showing the number of shared and unique OTUs in SBM, CB, AG and CB-AG groups (A). PCA based on OUT level (B). Comparative abundance of gut microorganisms in rainbow trout at the phylum (C) and genus (D) levels.
As illustrated in Figure 3B, the gut microbial compositions of rainbow trout across various treatment groups demonstrated significant representativeness, with a stress value of 0.046. There were found to be 7 distinct bacterial phyla and 32 genera. The relative abundances of Firmicutes and Staphylococcus were significantly higher in the CB and CB-AG than in SBM and AG, while Actinobacteriota and Rhodococcus had lower relative abundances (Figure 3C,D)
We conducted LEfSe analysis (Figure 4A) on the four groups and identified Phyllobacterium, Paenibacillus, Thermobispora, Paenisporosarcina, Thermobifida, and Stackebrandtia as the genus-level biomarkers in the non-supplemented diet (SBM). Proteobacteria and Bacteroidota were the phylum-level biomarkers in a 0.10% C. butyricum-supplemented diet (CB). Acinetobacter was the genus-level biomarker in CB. Rhodococcus, norank-f-Bacillaceae, and Virgibacillus were the genus-level biomarkers in a 0.10% Ala Gln-supplemented diet (AG). Actinobacteriota was the phylum-level biomarker in AG. Staphylococcales was the genus-level biomarker in CB-AG. Firmicutes was the phylum-level biomarker in CB-AG (Figure 4B).
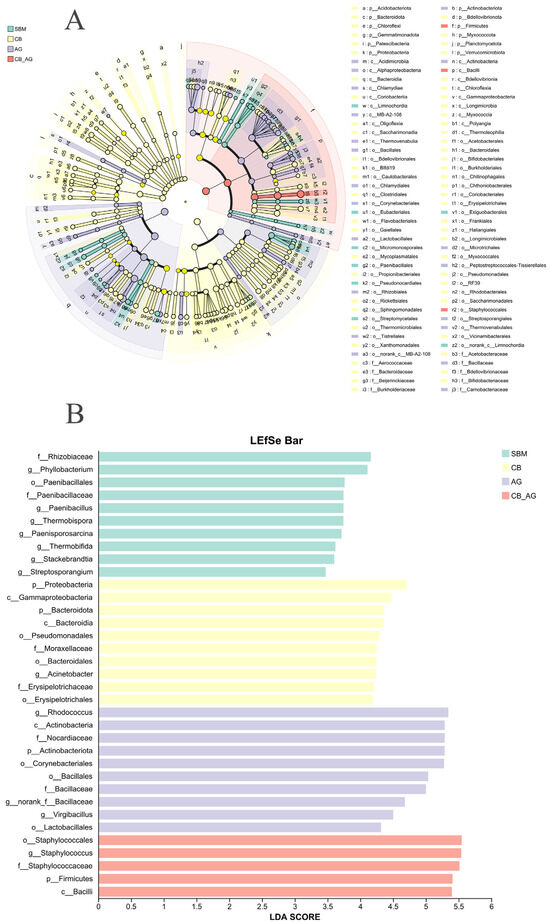
Figure 4.
The figure shows a clustering tree, inter-group variation in the relative abundance of intestinal microbial communities (A). Sample clustering tree and histogram combination analysis plot, the graph shows the LDA scores obtained by LDA analysis (linear regression analysis) of the microbial taxa that had a significant role among the four statistical groups (B).
As shown in Figure 5A, the microbiota in the guts of rainbow trout is dominated by interactions between the phylum Actinobacteria and the phylum Firmicutes.
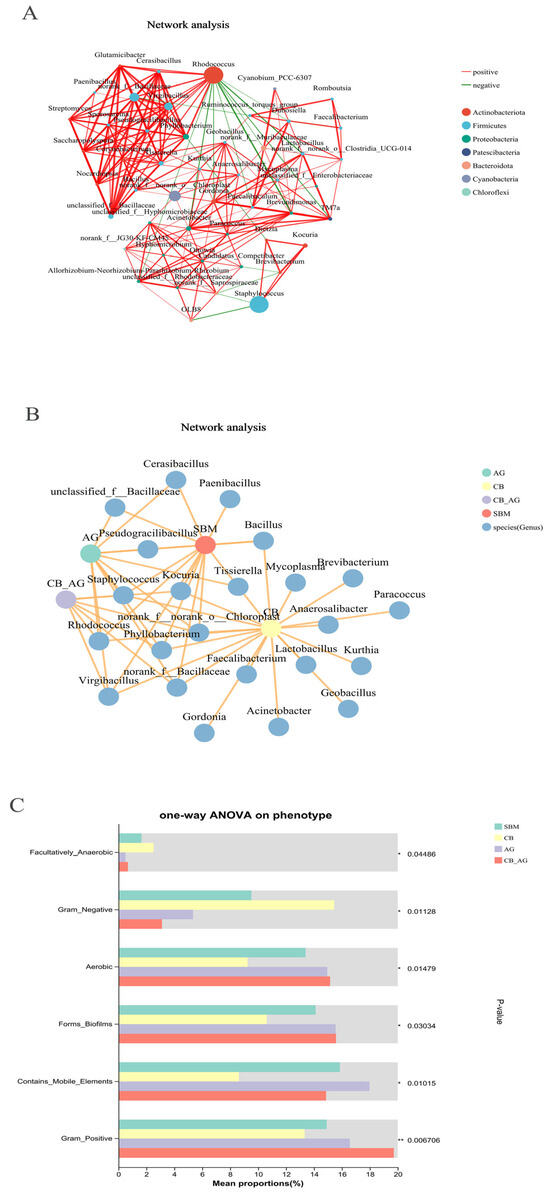
Figure 5.
Network interaction graph for hindgut microbial communities at the genus level, using wTO network analysis (A,B). BugBase phenotype prediction of the gut microbiota in rainbow trout (C). Line color indicates correlation type: red for positive, green for negative. Thickness shows correlation strength, with thicker lines meaning stronger correlations. More lines signify closer species connections. ** represents extremely significant (p < 0.01), * represents significant (p < 0.05), and blank represents not significant (p > 0.05).
The abundance of pathways such as carbohydrate metabolism, amino acid metabolism, and energy metabolism was relatively higher in AG. The abundance of pathways including methane metabolism, sulfur metabolism, and TCA cycle was relatively higher in CB-AG (Figure 6).
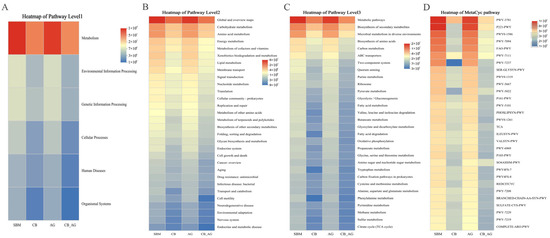
Figure 6.
Predictive metagenomic analysis of rainbow trout fecal microbiota functional profiling. Bacterial gene functions were predicted from the 16S rRNA gene-based microbial compositions using the PICRUSt2 algorithm and inferences from KEGG databases. Heatmap of pathway level1 (A), pathway level2 (B), pathway level3 (C) and MetaCyc pathway (D) in the gut microbiota of rainbow trout.
3.7. Challenge with A. salmonicida
As illustrated in Figure 7, the deaths of the rainbow trout started 24 h after A. salmonicida infection and lasted until the fifth day after infection. Seven days after A. salmonicida infection, the mean mortality rates of rainbow trout in AG and CB-AG (30.00%) were significantly lower than those in SBM (73.33%) and CB (53.33%).
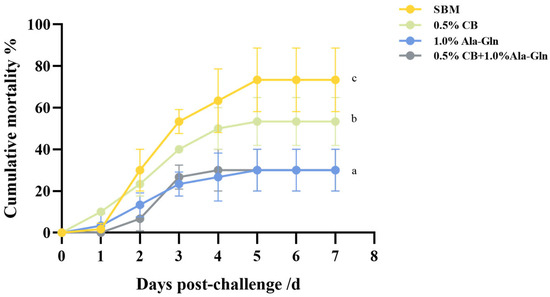
Figure 7.
Effect of C. butyricum and Ala-Gln supplementation on survival against bacterial infection. Values within the same column bearing distinct superscript letters differ significantly (p < 0.05).
As shown in Figure 8, AG displayed significantly higher expression of pro-inflammatory genes like TNF-α, NF-κB, and MyD88 compared to SBM (p < 0.05). In contrast, CB-AG exhibited markedly lower expression of pro-inflammatory factors, including IL-6, IL-8, NF-κB, TLR4, and MyD88, relative to SBM, while concurrently showing significantly increased expression of TGF-β (p < 0.05).
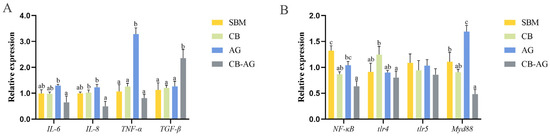
Figure 8.
Gene expression of intestine in rainbow trout after infection with A. salmonicida ((A): IL-6, IL-8, TNF-α and TGF-β; (B): NF-κB, tlr4, tlr5 and myd88). Values within the same column bearing distinct superscript letters differ significantly (p < 0.05).
4. Discussion
As carnivorous species, rainbow trout rely heavily on fishmeal. Adding a high proportion of soybean meal in their feed diminishes their utilization of food-borne proteins, hindering growth and development while reducing overall growth performance. Building on prior investigations, this study explored the effects of supplementing a basal diet—containing 40.00% soybean meal as a fishmeal substitute—with 0.50% CB and 1.00% Ala-Gln. It revealed that these additions, both alone and combined, boosted WGR and SGR and lowered FCR of rainbow trout. Clostridium butyricum has demonstrated its capacity to enhance growth performance, feed utilization, and protein deposition in yellow catfish (Pelteobagrus fulvidraco, 4.5 × 107, 5.1 × 108, 3.6 × 109 CFU/kg), aucha perch (Siniperca chuatsi, 1.0 × 108 CFU/g), and large yellow croakers (Larimichthys crocea, 5 × 108 CFU/g) [16,17,18]. Typically, C. butyricum promotes the transformation of macromolecular nutrients into smaller, more readily absorbed molecules through fermentation reactions and the release of digestive enzymes [9]. This process facilitates the deposition of substances essential for organismal growth, thereby accelerating development.
Xu et al. reported that the addition of 1.00% Ala-Gln to feed significantly improved the growth performance and intestinal function of young hybrid sturgeon (Acipenser schrenckii ♀ × Huso dauricus ♂) [19]. In studies on grass carp (Ctenopharyngodon Idella), carp (Cyprinus carpio), and yellow catfish, the addition of 0.50–0.75% Ala-Gln to the feed promoted growth performance and feed efficiency [20,21]. Gln can act as a nitrogen source, provide substrates for protein synthesis, promote the synthesis of muscle proteins and reduce their consumption and catabolism, increase the volume of myocytes, and promote myocyte proliferation [22]. At the same time, it promotes the secretion of growth hormone so that an organism is in a growing state to improve its own growth capacity [11]. Therefore, based on the information mentioned above, the purpose of adding Gln to the feed was to stimulate the secretion and release of growth hormone, promote protein synthesis and prevent its decomposition, and improve intestinal digestion and absorption capacity, thus improving the growth capacity of aquatic animals.
It is noteworthy that the WGR and SGR of CB-AG with 0.50% CB and 1.00% Ala-Gln co-addition were elevated compared to the other three groups, which suggests that the effect of the co-addition of C. butyricum and Ala-Gln is better than that of the individual additions, and that the two do not antagonistically interact with each other in fish. This is similar to results in carp, where the combination of 3.60 g/kg Ala-Gln and 54.00 mg/kg γ-aminobutyric acid had the best effect on growth, feed conversion, and protein synthesis [23]. The combination of 5 g/kg Ala-Gln and 50 IU/kg vitamin E also promoted the growth of C. butyricum in black king fish (Rachycentron canadum) growth promotion [24]. He et al. reported that in, American black bass (Micropterus salmoides) fed diets containing 0.20% CB and 0.60% Ala-Gln with 40.00% fermented soybean meal replacing fishmeal, these supplements exhibited no significant growth-promoting effect. The authors attributed this result to the absence of a synergistic effect between the two feed additives and insufficient Ala-Gln supplementation [25]. Ala-Gln and C. butyricum have been reported to exhibit limited efficacy when applied alone, but it is reasonable to hypothesize that the acidic metabolites produced by C. butyricum may not induce denaturation of Gln—thereby potentially avoiding impairment of Gln’s intestinal absorption and utilization. Additionally, the transporters for butyric acid and Gln in intestinal epithelial cells might be distinct, suggesting a low likelihood of transport antagonism between them; this could enable their coordinated action in intestinal tissue repair, reducing tissue damage and alleviating enteritis. Furthermore, the active enzymes secreted by C. butyricum could potentially facilitate Gln absorption and its subsequent conversion into proteins in the intestine [9]. Collectively, these observations support the proposition that the enhanced growth performance of O. mykiss may be attributed to the synergistic effects of C. butyricum and Gln.
SOD activity and MDA content are important parameters reflecting the antioxidant capacity of fish; SOD can catalyze the generation of H2O2 from O2− in various organs and tissues to scavenge free radicals [26]. MDA can reflect the rate and intensity of lipid peroxidation in tissues and respond to the degree of tissue peroxidative damage [27]. The MDA content in the livers and serum of all three test groups in this experiment was lower than that in SBM. The SOD activity in the livers of the 0.50% CB and 1.00% Ala-Gln addition group was higher than that of the other three groups. In studies on aquatic organisms such as nile tilapia, yellow catfish, and largemouth bass, it was proven that C. butyricum can activate the antioxidant systems of aquatic organisms. Gln has a similar promoting effect on the antioxidant capacity of aquatic organisms. For example, 0.75% Ala-Gln significantly increased the total antioxidant capacity and SOD and decreased MDA in the serum and brains of yellow catfish [28].
Beyond antioxidant indices, the activity of immune-related enzymes also reflects fish health status. LZM mediates fish immune defense by decomposing polysaccharides in bacterial cell walls and forming complexes with viral proteins, thereby exerting bacteriolytic and viral-inactivating effects [29]. Experimental results showed that serum and hepatic LZM levels in rainbow trout from the three experimental groups were higher than those in the control group; notably, CB and CB-AG exhibited significantly higher LZM levels than the other two groups. This suggests that C. butyricum is more effective than Gln in enhancing fish resistance to bacterial and viral infections, and the combined use of C. butyricum and Gln yields a more potent effect. DAO is an intracellular enzyme with high activity in the upper villi of the small intestinal mucosa. It protects the intestinal mucosa by maintaining intracellular ion homeostasis, regulating signaling pathways, and promoting cellular repair [30]. Intestinal damage triggers rapid release of DAO from the intestinal mucosa into the blood and lymph [31]. In this study, serum DAO levels in rainbow trout from the three experimental groups were significantly lower than those in the control group; specifically, CB-AG showed the lowest serum DAO levels among all groups. This indicates that C. butyricum and Gln effectively repair intestinal damage and alleviate enteritis in rainbow trout, with their combination exerting a faster and more pronounced effect. DAO research in aquatic organisms remains limited, as it is primarily investigated in the context of intestinal mucosal mechanical barriers in humans and mammals. Previous studies have shown that sodium butyrate (NaB) reduces serum DAO levels while increasing jejunal mucosal DAO levels in rainbow trout, which is consistent with the findings of this experiment [13]. This consistency may be attributed to the fact that butyric acid and Gln both serve as energy sources for intestinal cells; exogenous supplementation accelerates their absorption and utilization by rainbow trout, thereby promoting intestinal mucosal repair and inhibiting the occurrence and progression of enteritis.
Soya bean meal significantly reduced the height of intestinal mucosa, blurred the boundaries of epithelial cells, and even caused separation, and its effect on intestinal digestion and absorption limits the efficiency of protein utilization in fish and even triggers enteritis, which eventually results in diminished growth performance [32,33]. The high or low activity of digestive enzymes also represents the decomposition of nutrients in fish, which determines the intestinal health of fish. Therefore, protecting the gut health of fish is essential to improve fish production. Research has indicated that incorporating C. butyricum into the diets of aquatic organisms such as nile tilapia (Oreochromis niloticus) and blue discus (Symphysodon aequifasciata) increased both TPS and AMS viability, and the addition of Ala-Gln increased the levels of TPS and LPS of cultured species [34]. The findings from this study were similar to previous findings, as the TPS and LPS levels of the 0.50% CB and 1.00% Ala-Gln addition group were markedly elevated compared to those of the single-addition groups. However, addition of C. butyricum, Ala-Gln, or a combination of both did not affect the activities of digestive enzymes in a study on largemouth bass (Anguilla japonica) [25]. This may have been due to the differences between the species and amounts added.
Compared with omnivorous fish, the rainbow trout has a shorter digestive tract, lower digestive enzyme content, and shorter feed residence time in the colon—thus its intestinal absorptive surface area is closely linked to its digestive absorptive capacity. Intestinal villus length and width are the most intuitive indicators for calculating this surface area [35]. Research shows dietary supplementation of C. butyricum in aquatic organisms (e.g., largemouth bass) significantly improves intestinal morphology and increases mucosal fold height and thickness [9,36]. Meanwhile, dietary Ala-Gln increases VH and mucosal fold depth in yellow catfish but has no significant effect on hybrid sturgeon [37]. The present study showed similar trends: all three experimental groups had significantly higher VH than the controls. However, CB-AG showed no significant differences in VW or MT vs. the control group—notably, these two groups had significantly higher VH, VW, and MT than the other three groups. He et al. found C. butyricum did not significantly improve largemouth bass intestinal morphology, but Ala-Gln enhanced villus structure [25]. Thus, C. butyricum and Ala-Gln exhibited a significant synergistic effect here, possibly because Ala-Gln is more effective than CB at intestinal mucosa restoration and more efficiently utilized by intestinal cells. Both CB’s metabolite (butyric acid) and Ala-Gln provide energy/raw materials for intestinal cell development, stimulate intracellular mRNA and protein synthesis, help form a tight intestinal physical barrier against rainbow trout bacterial infection, and alleviate inflammation (leukocyte infiltration)—thereby regulating intestinal health and promoting growth.
CB and Ala-Gln also regulate intestinal inflammation in rainbow trout at the molecular pathway level. In a study on yellow catfish, the addition of CB to feed significantly reduced the concentration of pro-inflammatory factors and the levels of pro-inflammatory factor expression, and Li et al. concluded that the optimal level of CB being added to feed suppressed the inflammatory response of the intestinal tract [16]. Yin et al. illustrated that incorporating 0.10–0.20% CB into the diet could diminish the expression of IFN-γ, IL-1β, and IL-8, thereby safeguarding large yellow croakers against pathogenic infections [18]. The NF-κB pathway constitutes a central regulatory axis governing intestinal inflammatory responses in rainbow trout, with its activation status directly correlating with the risk of stress-induced enteritis in aquaculture environments. Administration of C. butyricum alone exerts inhibitory effects via its metabolic products, specifically SCFAs (notably butyrate), which suppress IKK phosphorylation within rainbow trout intestinal epithelial cells. This inhibition reduces IκBα protein degradation, thereby blocking nuclear translocation of the p65 subunit—a mechanism consistent with previously reported NF-κB regulatory pathways in freshwater teleosts [38]. Concurrently, glutamine dipeptide, serving as the preferential energy substrate for intestinal epithelium, maintains intracellular glutathione levels to attenuate ROS-mediated NF-κB activation. Excessive ROS accumulation promotes IκBα ubiquitination and degradation through oxidative modification, a process effectively counteracted by glutamine dipeptide supplementation [39].
Combined administration elicits a synergistic “dual inhibition” effect: Firstly, C. butyricum diminishes pro-inflammatory signal sources, such as LPS, through microbiota modulation, thereby indirectly attenuating upstream stimulation of the NF-κB pathway. Secondly, glutamine dipeptide enhances the intestinal physical barrier integrity by upregulating the tight junction proteins occludin and ZO-1, reducing LPS binding to tlr4 on intestinal epithelial cells and further inhibiting tlr4-mediated NF-κB signaling activation [40]. At the molecular level, the combined treatment group exhibited lower mRNA expression of p65 and IKKβ in their intestines compared to the single-treatment groups, while mRNA and protein expression of IκBα were significantly elevated. This indicates that the combined intervention not only transcriptionally suppresses NF-κB components but also modulates signaling dynamics [41]. Crucially, the stabilization of IκBα protein enhances regulatory efficacy, a key mechanism for alleviating chronic inflammation in intensive rainbow trout aquaculture C. butyricum exhibits “indirect regulation” of the MLCK pathway: by modulating butyrate produced through gut microbiota metabolism, it inhibits HDAC activity and promotes the expression of MLCP. MLCP reverses the phosphorylation state of MLC, thereby antagonizing the action of MLCK. When combined, they form a synergistic mechanism of “downregulation + functional antagonism”: the combined group showed a reduction in MLCK mRNA expression compared to the single-addition groups, while MLCP mRNA expression increased. Additionally, the tight junction proteins occludin and ZO-1 were concentrated at intercellular junctions. These findings indicate that combined supplementation not only directly inhibits MLCK transcription via glutamine dipeptide but also enhances MLCP functional antagonism through C. butyricum, providing dual safeguards for rainbow trout intestinal barrier integrity. This holds significant practical value for reducing nutrient loss and pathogenic bacterial invasion in aquaculture.
A substantial rise in the comparative levels of disease-causing Vibrio was detected. This correlates with the intestinal inflammatory response induced by the low-fishmeal diet [42]. Following addition of C. butyricum, the proportion of Firmicutes within the intestine microbiota increased, concurrent with a rise in the abundance of the lactic acid-producing genus Lactococcus and a decrease in the proportion of Vibrio. This indicates that C. butyricum can competitively inhibit harmful bacteria for nutrients and space, thereby promoting the proliferation of beneficial bacteria. This finding aligns closely with results from a study on rainbow trout probiotics. CB exhibited a substantial rise in Firmicutes levels, a marked decrease in Proteobacteria, and heightened abundance of beneficial phyla such as Fusobacteria, confirming that C. butyricum has a species-independent regulatory effect on salmonid gut microbiota [43].
AG indicated a rise in the abundance of the Bacteroidetes phylum. The polysaccharide-degrading function of Bacteroidetes may function synergistically with the nutrient absorption-enhancing properties of Ala-Gln. This phenomenon finds analogous support in large yellow croaker studies: in glutamine-supplemented groups, the relative abundance of Bacteroidetes significantly increased compared to controls, concurrent with an elevated abundance of Bacteroides, which is associated with intestinal nutrient absorption [44]. This suggests that the promotional effect of glutamine-like substances on Bacteroidetes may represent a cross-species common mechanism. The combined bacteriopeptide group exhibited a dual-dominant microbial structure of ‘Firmicutes + Bacteroidetes,’ alongside the lowest abundance of potential pathogens. This demonstrates a synergistic effect between microbial regulation by C. butyricum and the intestinal barrier repair function of Aln-Gln, consistent with the established paradigm observed in combined probiotic applications [45]. In the aforementioned rainbow trout trial, the T3 group (C. butyricum + Bacillus coagulans) similarly formed a dual-dominant community structure of Firmicutes and Bacteroidetes, while the proportion of Proteobacteria decreased to the lowest level among all groups [46]. This demonstrates that the combined strategy of ‘direct probiotic regulation + active substance barrier repair’ can establish a more stable intestinal microecological system.
Analysis of predicted microbial metabolic functions revealed that all supplementation groups enhanced the expression of functional genes associated with carbohydrate and amino acid metabolism within the gut microbiota. Among these, the combined group exhibited the highest enrichment in pathways related to vitamin synthesis and SCFA production. This finding correlates with shifts in microbial composition. The increased Firmicutes abundance promoted SCFAs synthesis, while enriched Bacteroidetes facilitated nutrient degradation [47]. Their combined action further optimized metabolic networks, providing enhanced nutritional support for rainbow trout growth. Additionally, the combined group exhibited significantly downregulated expression of inflammation-related pathways. This suggests it mitigates intestinal inflammatory responses by improving microbial composition—consistent with the “gut microbiota-immune axis” regulation theory. This mechanism was further substantiated in probiotic trials with Nile tilapia. Bacillus tequilensis Bt-CO significantly suppressed the pro-inflammatory factors IL-1β and TNF-α while upregulating the anti-inflammatory factor TGF-β through gut microbiota optimization [48]. This provides cross-species validation for the inflammatory pathway regulation observed in the combined group of this study. This suggests that the regulatory chain of ‘microbiota optimization—inflammation alleviation’ holds universal applicability across both freshwater and marine fish species. However, further studies are required to validate the efficacy of dietary C. butyricum and alanyl-glutamine supplementation in fish.
Studies indicate that the pathogenicity of A. salmonicida is linked to several virulence factors, and gcaT is its main virulence factor due to its cytotoxicity, haemolytic activity, and thermal stability [49]. A. salmonicida infection triggers inflammation in the skin and intestines of rainbow trout, compromising physical barriers and heightening intestinal cell permeability. The results of this experiment showed that the addition of both CB and Ala-Gln can help rainbow trout improve their resistance to infection and that a synergistic effect is more favorable. Regarding the capacity of CB to resist pathogenic bacteria, Li demonstrated in a study conducted on Oreochromis niloticus that dietary supplementation with both 1 × 106, 1 × 107 CFU/g of CB significantly reduced the cumulative mortality rate following challenge with Streptococcus agalactiae. CB-treated rainbow trout showed enhanced resistance to Vibrio, and the numbers of Vibrio organisms in the kidneys, livers, and blood of these trout were lower than those in the untreated group after a Vibrio vulnificus infection of 2.7 × 105 CFU/mL, and they also showed an increased number of renal phagocytes [50]. Ala-Gln has also been reported to enhance intestinal resistance. Injection of Gln can alleviate hemorrhagic necrosis of the liver and spleen, atrophy of the midgut mucosa, and breakdown of the villi caused by Aeromonas hydrophila infection in softshell turtles (Trionyx Sinensis) and reduce the mortality rate [51]. A trinitrobenzene sulfonic acid ethanol mixture solution was used to perfuse the anuses of grass carp to establish an intestinal injury model, and the carp were fed feed containing 1.2% Gln for 7 consecutive days, which effectively improved the VH, VW, and MT in their intestines. A study on the effect of 1 mL of Bifidobacterium bifidum solution (1 × 107 CFU/mL) with 0.04 g/L Gln mixture delivered by gavage demonstrated that it could alleviate symptoms of intestinal dysfunction and diarrhea caused by stress in mice [52]. In the present experiment, the combination of C. butyricum and Ala-Gln also significantly reduced the mortality of trout after infection with A. salmonicida. C. butyricum protects the intestinal barrier and reduces infections by hindering colonization of trout by A. salmonicida and releasing BA to stimulate the development of intestinal cells. Gln has a direct function in intestinal cells in resisting bacterial infections and accelerates the repair of intestinal mucosa. Both BA and Gln can also act as small molecule signaling factors to regulate the NF-κB and MLCK-MLC signaling pathways and stimulate the rapid development of the immune response and the secretion of TJ proteins in rainbow trout.
5. Conclusions
Research findings demonstrate that combined supplementation with 0.5% C. butyricum and 1.0% Ala-Gln significantly enhances the growth performance of rainbow trout juveniles. This enhancement is mediated through the elevation of hepatic and serum antioxidant markers, facilitation of intestinal mucosal repair, upregulation of TJ protein expression, and downregulation of NF-κB and MLCK-MLC signaling pathways, thereby strengthening resistance to A. salmonicida. Furthermore, incorporating 0.5% C. butyricum and 1.0% Ala-Gln into low-fishmeal diets resulted in gut microbiota compositions more favorable for the growth and development of triploid rainbow trout.
Author Contributions
Conceptualization, C.W.; methodology, C.W.; resources, H.J. and H.L.; data curation, S.L. (Siyuan Liu); writing—original draft preparation, S.L. (Siyuan Liu); writing—review and editing, S.L. (Siyuan Liu), L.C. and C.W.; visualization, S.L. (Siyuan Liu), S.Z. and Y.W.; supervision, C.W., S.L. (Shaoxia Lu) and H.L.; project administration, C.W., S.H. and H.L.; funding acquisition, C.W. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2023YFD2400405, the China Agriculture Research System of MOF and MARA, grant number CARS-46, the Identification and Assessment of Germplasm Resources for Special Aquatic Species and Development of Enhanced Varieties in Heilongjiang Province, the Central Public-interest Scientific Institution Basal Research Fund, grant number CAFS2025XT0102, 2023TD60 and 2023TD96, the Key Research and Development Program of Heilongjiang Province, grant number 2024ZX10B06, Natural Science Fund of Heilongjiang Province, grant number LH2023C057, the Fund of Key Laboratory of Efficient Utilization and Processing of Marine Fishery Resources of Hainan Province, grant number KLEU-2024-3, the National Postdoctoral Fund, grant number 2022MD713817.
Institutional Review Board Statement
The animal study protocol was approved by the Committee for the Welfare and Ethics of the Laboratory Animals of Heilongjiang River Fisheries Research Institute, approval code: CAFS 20200615 and approval date: 15 June 2020.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank the participants who gave their time to the trial.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Ala-Gln | Alanyl-glutamine |
| CB | Clostridium butyricum |
| MDA | Malondialdehyde |
| DAO | Diamine oxidase |
| LZM | Lysozyme |
| SOD | Superoxide dismutase |
| ANF | Anti-nutritional factors |
| BA | Butyric acid |
| SCFAs | Short-chain fatty acids |
| LPS | Lipase |
| AMS | Amines |
| TPS | Trypsin |
| RT-PCR | Real-time polymerase chain reaction |
| PCA | Principal component analysis |
| TCA | Tricarboxylic acid |
| wTO | Weighted topological |
| KEGG | Kyoto encyclopedia of genes and genomes |
| OTU | Operational taxonomic units |
| TSB | Trypticase soy broth |
| PBS | Phosphate-buffered saline |
| S.E. | Standard error |
| FBW | Final body weight |
| WGR | Weight gain rate |
| SR | Survival rate |
| FCR | Feed conversion ratio |
| SGR | Specific growth rate |
| CF | Condition factor |
| HSI | Hepatosomatic index |
| VSI | Viscerosomatic index |
| VH | Villus length |
| VW | Villus width |
| MT | Muscular layer thickness |
| MV | Microvilli |
| N | Nucleus |
| EGC | Eosinophilic granular cell |
| SML | Submucous layer |
| LP | Lamina propria |
| GC | Goblet cell |
| IL | Interleukin |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
| IκB-α | Inhibitor of κB α |
| IKK | IκB kinase |
| NF-κB | Nuclear factor-kappa B |
| MLCK | Myosin light chain kinase |
| MLC | Myosin light chainmyosin |
| Zo-1 | Zonula occluden-1 |
| tric | Tricellulin |
| Ocln | Occluding |
| Cgn | Cingulin |
| cldn-3 | Claudin-3 |
| tlr | Toll-like receptor |
| HDAC | Histone deacetylase |
| MLCP | Myosin light chain phosphatase |
| gcaT | Glycine C-acetyltransferase |
| TJ | Tight junction |
References
- Nasopoulou, C.; Zabetakis, I. Benefits of Fish Oil Replacement by Plant Originated Oils in Compounded Fish Feeds. A Review. LWT 2012, 47, 217–224. [Google Scholar] [CrossRef]
- Opstvedt, J.; Aksnes, A.; Hope, B.; Pike, I.H. Efficiency of Feed Utilization in Atlantic Salmon (Salmo salar L.) Fed Diets with Increasing Substitution of Fish Meal with Vegetable Proteins. Aquaculture 2003, 221, 365–379. [Google Scholar] [CrossRef]
- Lin, S.; Luo, L. Effects of Different Levels of Soybean Meal Inclusion in Replacement for Fish Meal on Growth, Digestive Enzymes and Transaminase Activities in Practical Diets for Juvenile Tilapia, Oreochromis niloticus × O. aureus. Anim. Feed. Sci. Technol. 2011, 168, 80–87. [Google Scholar] [CrossRef]
- Buttle, L.G.; Burrells, A.C.; Good, J.E.; Williams, P.D.; Southgate, P.J.; Burrells, C. The Binding of Soybean Agglutinin (SBA) to the Intestinal Epithelium of Atlantic Salmon, Salmo Salar and Rainbow Trout, Oncorhynchus mykiss, Fed High Levels of Soybean Meal. Vet. Immunol. Immunopathol. 2001, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Soleto, I.; Morel, E.; Muñoz-Atienza, E.; Díaz-Rosales, P.; Tafalla, C. Aeromonas salmonicida Activates Rainbow Trout IgM+ B Cells Signalling through Toll like Receptors. Sci. Rep. 2020, 10, 16810. [Google Scholar] [CrossRef] [PubMed]
- Calusinska, M.; Hamilton, C.; Monsieurs, P.; Mathy, G.; Leys, N.; Franck, F.; Joris, B.; Thonart, P.; Hiligsmann, S.; Wilmotte, A. Genome-Wide Transcriptional Analysis Suggests Hydrogenase- and Nitrogenase-Mediated Hydrogen Production in Clostridium Butyricum CWBI 1009. Biotechnol. Biofuels 2015, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Wangari, M.R.; Gao, Q.; Sun, C.; Liu, B.; Song, C.; Tadese, D.A.; Zhou, Q.; Zhang, H.; Liu, B. Effect of Dietary Clostridium butyricum and Different Feeding Patterns on Growth Performance, Antioxidant and Immune Capacity in Freshwater Prawn (Macrobrachium rosenbergii). Aquac. Res. 2021, 52, 12–22. [Google Scholar] [CrossRef]
- Hagihara, M.; Yamashita, R.; Matsumoto, A.; Mori, T.; Inagaki, T.; Nonogaki, T.; Kuroki, Y.; Higashi, S.; Oka, K.; Takahashi, M.; et al. The Impact of Probiotic Clostridium butyricum MIYAIRI 588 on Murine Gut Metabolic Alterations. J. Infect. Chemother. 2019, 25, 571–577. [Google Scholar] [CrossRef]
- Cai, K.; Chen, J.; Zhang, Z.; Ye, Y.; Sang, S.; Luo, X.; Wang, Y.; Shan, K.; Ou, C.; Jia, L. Recent Progress of Clostridium butyricum in Fish Culture: Maintenance of Intestinal Homeostasis, Improvement of Disease Resistance, Activation of Immune Signaling Pathways, and Positive Effects on Fish. Aquaculture 2025, 596, 741723. [Google Scholar] [CrossRef]
- Newsholme, P.; Lima, M.M.R.; Procopio, J.; Pithon-Curi, T.C.; Doi, S.Q.; Bazotte, R.B.; Curi, R. Glutamine and Glutamate as Vital Metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef]
- Ardawi, M.S.; Newsholme, E.A. Glutamine Metabolism in Lymphocytes of the Rat. Biochem J 1983, 212, 835–842. [Google Scholar] [CrossRef]
- Macedo Rogero, M.; Tirapegui, J.; Pedrosa, R.G.; Santana de Oliveira Pires, I.; Alves de Castro, I. Plasma and Tissue Glutamine Response to Acute and Chronic Supplementation with L-Glutamine and L-Alanyl-L-Glutamine in Rats. Nutr. Res. 2004, 24, 261–270. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Wang, Y.; Lu, S.; Han, S.; Liu, Y.; Jiang, H.; Wang, C.; Liu, H. Dietary Sodium Butyrate Improves Intestinal Health of Triploid Oncorhynchus mykiss Fed a Low Fish Meal Diet. Biology 2023, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Al-Mentafji, H.N. A.O.A.C 2005. 2016. Available online: https://www.researchgate.net/publication/292783651_AOAC_2005 (accessed on 12 May 2024).
- Guo, Y.; Huang, D.; Chen, F.; Ma, S.; Zhou, W.; Zhang, W.; Mai, K. Lipid Deposition in Abalone Haliotis discus hannai Affected by Dietary Lipid Levels through AMPKα2/PPARα and JNK/mTOR/SREBP-1c Pathway. Aquaculture 2021, 532, 736040. [Google Scholar] [CrossRef]
- Li, P.; Hou, D.; Zhao, H.; Wang, H.; Peng, K.; Cao, J. Dietary Clostridium butyricum Improves Growth Performance and Resistance to Ammonia Stress in Yellow Catfish (Pelteobagrus fulvidraco). Aquac. Nutr. 2022, 2022, 6965174. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.-F.; He, S.; Feng, H.; Li, L. Dietary Supplementation of Exogenous Probiotics Affects Growth Performance and Gut Health by Regulating Gut Microbiota in Chinese Perch (Siniperca chuatsi). Aquaculture 2022, 547, 737405. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Q.; Liu, Y.; Gao, S.; He, Y.; Yao, C.; Huang, W.; Gong, Y.; Mai, K.; Ai, Q. Early Life Intervention Using Probiotic Clostridium butyricum Improves Intestinal Development, Immune Response, and Gut Microbiota in Large Yellow Croaker (Larimichthys crocea) Larvae. Front. Immunol. 2021, 12, 640767. [Google Scholar] [CrossRef]
- Qiyou, X.; Qing, Z.; Hong, X.; Chang’an, W.; Dajiang, S. Dietary Glutamine Supplementation Improves Growth Performance and Intestinal Digestion/Absorption Ability in Young Hybrid Sturgeon (Acipenser schrenckii ♀ × Huso dauricus ♂). J. Appl. Ichthyol. 2011, 27, 721–726. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, Q.-Z. Dietary Glutamine Supplementation Improves Structure and Function of Intestine of Juvenile Jian Carp (Cyprinus carpio Var. Jian). Aquaculture 2006, 256, 389–394. [Google Scholar] [CrossRef]
- Wang, C.A.; Xu, Q.Y.; Xu, H.; Zhu, Q.; Yang, J.L.; Sun, D.J. Dietary L-Alanyl-l-Glutamine Supplementation Improves Growth Performance and Physiological Function of Hybrid Sturgeon Acipenser schrenckii ♀ × A. baerii ♂. J. Appl. Ichthyol. 2011, 27, 727–732. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine Reliance in Cell Metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, W.; Chen, X.M.; Wang, G.Q.; Zhang, D.M.; Cong, L.M. Effects of Propylene Ammonia Acyl-glutamine and γ-Aminobutyric Acid on Growth, Feed Utilization and Body Composition of Jian carp, Cyprinus carpio var.jian. J. South China Agric. Univ. 2016, 37, 7–13. [Google Scholar] [CrossRef]
- Ding, Z.; Li, W.; Huang, J.; Yi, B.; Xu, Y. Dietary Alanyl-Glutamine and Vitamin E Supplements Could Considerably Promote the Expression of GPx and PPARα Genes, Antioxidation, Feed Utilization, Growth, and Improve Composition of Juvenile Cobia. Aquaculture 2017, 470, 95–102. [Google Scholar] [CrossRef]
- Ming, H. Study on The Effects of Fermented Soybean Meal in Fish Meal Replacement and The Improvement of Glutamine and Clostridium butyricum on Its Effectiveness in The Diet of Largemouth Bass (Micropterus salmoides). Shanghai Ocean Univ. 2021. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.Z.; Li, M.; Zhang, Q.; Wang, R.X.; Jiang, H.B. Effects of Alanyl-Glutamine Dipeptide Supplementation on Growth Performance, Antioxidant Status, Immune Response and Stress Resistance of Juvenile Yellow Catfish (Pelteobagrus fulvidraco). Chin. J. Anim. Nutr. 2019, 31, 3197–3206. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune System and Immune Responses in Fish and Their Role in Comparative Immunity Study: A Model for Higher Organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Biegański, T. Biochemical, Physiological and Pathophysiological Aspects of Intestinal Diamine Oxidase. Acta Physiol. Pol. 1983, 34, 139–154. [Google Scholar]
- Raithel, M.; Riedel, A.; Kuefner, M.; Donhauser, N.; Hahn, E. Evaluation of Gut Mucosal Diamine Oxidase Activity (DAO) in Patients with Food Allergy and Ulcerative Colitis, Idiopathic Ulcerative Colitis and Crohn’s Disease. Z. Für Gastroenterol. 2005, 43, 54. [Google Scholar] [CrossRef]
- Rimoldi, S.; Finzi, G.; Ceccotti, C.; Girardello, R.; Grimaldi, A.; Ascione, C.; Terova, G. Butyrate and Taurine Exert a Mitigating Effect on the Inflamed Distal Intestine of European Sea Bass Fed with a High Percentage of Soybean Meal. Fish. Aquat. Sci. 2016, 19, 40. [Google Scholar] [CrossRef]
- Maruyama, N.; Katsube, T.; Wada, Y.; Oh, M.H.; Barba De La Rosa, A.P.; Okuda, E.; Nakagawa, S.; Utsumi, S. The Roles of the N-Linked Glycans and Extension Regions of Soybean Beta-Conglycinin in Folding, Assembly and Structural Features. Eur. J. Biochem. 1998, 258, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, B.; Lai, X.; Chen, Z.; Hou, L.; Shu, R.; Huang, Y.; Shu, H. Effects of Clostridium butyricum on Growth, Digestive Enzyme Activity, Antioxidant Capacity and Gut Microbiota in Farmed Tilapia (Oreochromis niloticus). Aquac. Res. 2021, 52, 1573–1584. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Elbialy, Z.I.; Abdelhamid, A.I. Dietary Sodium Butyrate Ameliorated the Blood Stress Biomarkers, Heat Shock Proteins, and Immune Response of Nile Tilapia (Oreochromis niloticus) Exposed to Heat Stress. J. Therm. Biol. 2020, 88, 102500. [Google Scholar] [CrossRef]
- Meng, X.; Cai, H.; Li, H.; You, F.; Jiang, A.; Hu, W.; Li, K.; Zhang, X.; Zhang, Y.; Chang, X.; et al. Clostridium butyricum-Fermented Chinese Herbal Medicine Enhances the Immunity by Modulating the Intestinal Microflora of Largemouth Bass (Micropterus salmoides). Aquaculture 2023, 562, 738768. [Google Scholar] [CrossRef]
- Shazhou, Y.; Jie, Z.; Haimin, C.; Qicun, Z.; Fangzhou, Z. Effects of Glutamine on Growth Performance, Intestinal Morphology and Non-Specific Immune Related Gene Expression of Juvenile Yellow Catfish (Pelteobagrus fulvidraco). Chin. J. Anim. Nutr. 2016, 28, 468–476. [Google Scholar] [CrossRef]
- Ye, D.; Ma, T.Y. Cellular and Molecular Mechanisms That Mediate Basal and Tumour Necrosis Factor-Alpha-Induced Regulation of Myosin Light Chain Kinase Gene Activity. J. Cell. Mol. Med. 2008, 12, 1331–1346. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Zhu, H.; Yao, X.M.; Qian, J.P.; Yang, J.; Pan, X.D.; Chen, X.D. Metformin Regulates Tight Junction of Intestinal Epithelial Cells via MLCK-MLC Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5239–5246. [Google Scholar] [CrossRef]
- Gu, M.; Pan, S.; Deng, W.; Li, Q.; Qi, Z.; Chen, C.; Bai, N. Effects of Glutamine on the IKK/IκB/NF-κB System in the Enterocytes of Turbot Scophthalmus maximus L. Stimulated with Soya-Saponins. Fish Shellfish Immunol. 2021, 119, 373–378. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, B.; Lin, M.; Zhou, P.; Li, J.; Zhang, L.; Gao, F.; Zhou, G. Effects of Alanyl-Glutamine Supplementation on the Small Intestinal Mucosa Barrier in Weaned Piglets. Asian-Australas. J. Anim. Sci. 2017, 30, 236–245. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.F.; Hu, D.G.; Wang, H. Effect of Clostridium butyricum on Intestinal Microbiota and Resistance to Vibrio Alginolyticus of Penaeus Vannamei. Fish Shellfish Immunol. 2023, 138, 108790. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, F.; Wang, D.; Lu, S.; Han, S.; Gu, W.; Jiang, H.; Li, Z.; Liu, H. Enhancing Growth and Intestinal Health in Triploid Rainbow Trout Fed a Low-Fish-Meal Diet through Supplementation with Clostridium butyricum. Fishes 2024, 9, 178. [Google Scholar] [CrossRef]
- Xie, X.; Sun, K.; Liu, A.; Miao, R.; Yin, F. Analysis of Gill and Skin Microbiota in Larimichthys crocea Reveals Bacteria Associated with Cryptocaryoniasis Resistance Potential. Fish Shellfish Immunol. 2025, 161, 110228. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Ramos, M.A.; Gonçalves, J.F.M.; Batista, S.; Costas, B.; Pires, M.A.; Rema, P.; Ozório, R.O.A. Growth, Immune Responses and Intestinal Morphology of Rainbow Trout (Oncorhynchus mykiss) Supplemented with Commercial Probiotics. Fish Shellfish Immunol. 2015, 45, 19–26. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The Interaction among Gut Microbes, the Intestinal Barrier and Short Chain Fatty Acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Ge, X.; Pan, J.; Mo, K.; Chen, H.; Sun, J.; Huang, H.; Zhang, Y.; Mai, K.; Gu, H.; Hu, Y. The Versatility of Bacillus tequilensis Bt-CO as an Additive: Antagonizing Pathogens, Enhancing Immunity, Promoting Intestinal Health, and Optimizing Gut Microbiota of Tilapia. Anim. Nutr. 2025, in press. [CrossRef]
- Lee, K.K.; Ellis, A.E. Glycerophospholipid: Cholesterol Acyltransferase Complexed with Lipopolysaccharide (LPS) Is a Major Lethal Exotoxin and Cytolysin of Aeromonas salmonicida: LPS Stabilizes and Enhances Toxicity of the Enzyme. J. Bacteriol. 1990, 172, 5382–5393. [Google Scholar] [CrossRef]
- Sakai, M.; Yoshida, T.; Atsuta, S.; Kobayashi, M. Enhancement of Resistance to Vibriosis in Rainbow Trout, Oncorhynchus Mykiss (Walbaum), by Oral Administration of Clostridium butyricum Bacterin. J. Fish Dis. 1995, 18, 187–190. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Feng, H.; Guo, Q.; Dai, H. Acute Phase Response in Chinese Soft-Shelled Turtle (Trionyx sinensis) with Aeromonas Hydrophila Infection. Dev. Comp. Immunol. 2011, 35, 441–451. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, S.Y.; Yoon, Y.; Park, C.; Sohn, J.; Jeong, J.J.; Jeon, B.-N.; Jang, M.; An, C.; Lee, S.; et al. Bifidobacterium Bifidum Strains Synergize with Immune Checkpoint Inhibitors to Reduce Tumour Burden in Mice. Nat. Microbiol. 2021, 6, 277–288. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).