The Tasmanian Estuary Perch Population—A Threatened Species?
Abstract
1. Introduction
2. Materials and Methods
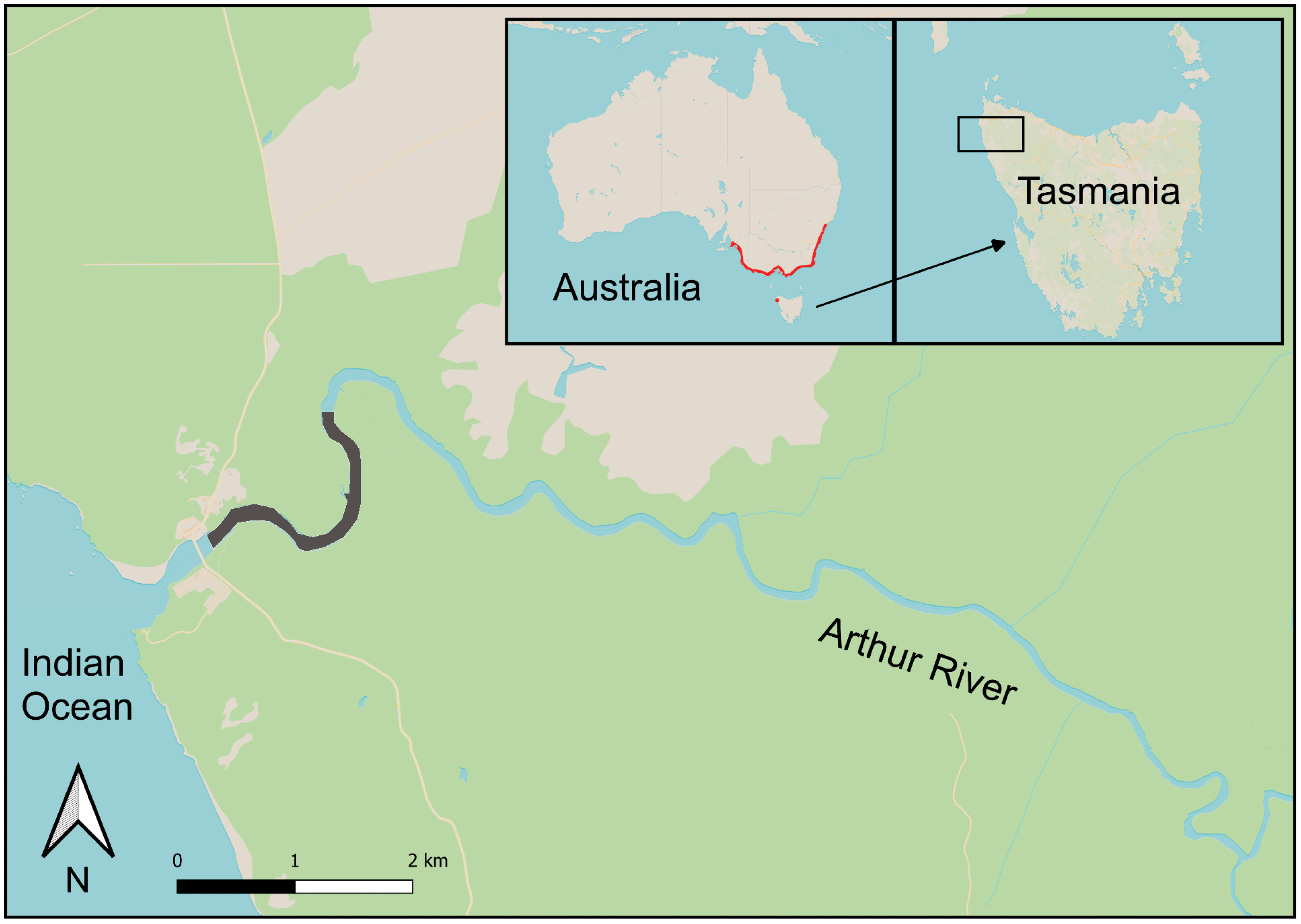
2.1. Capture Techniques and Effort
2.2. Catch and Release Process
2.3. Retained Fish Process
2.4. Data Analysis
3. Results
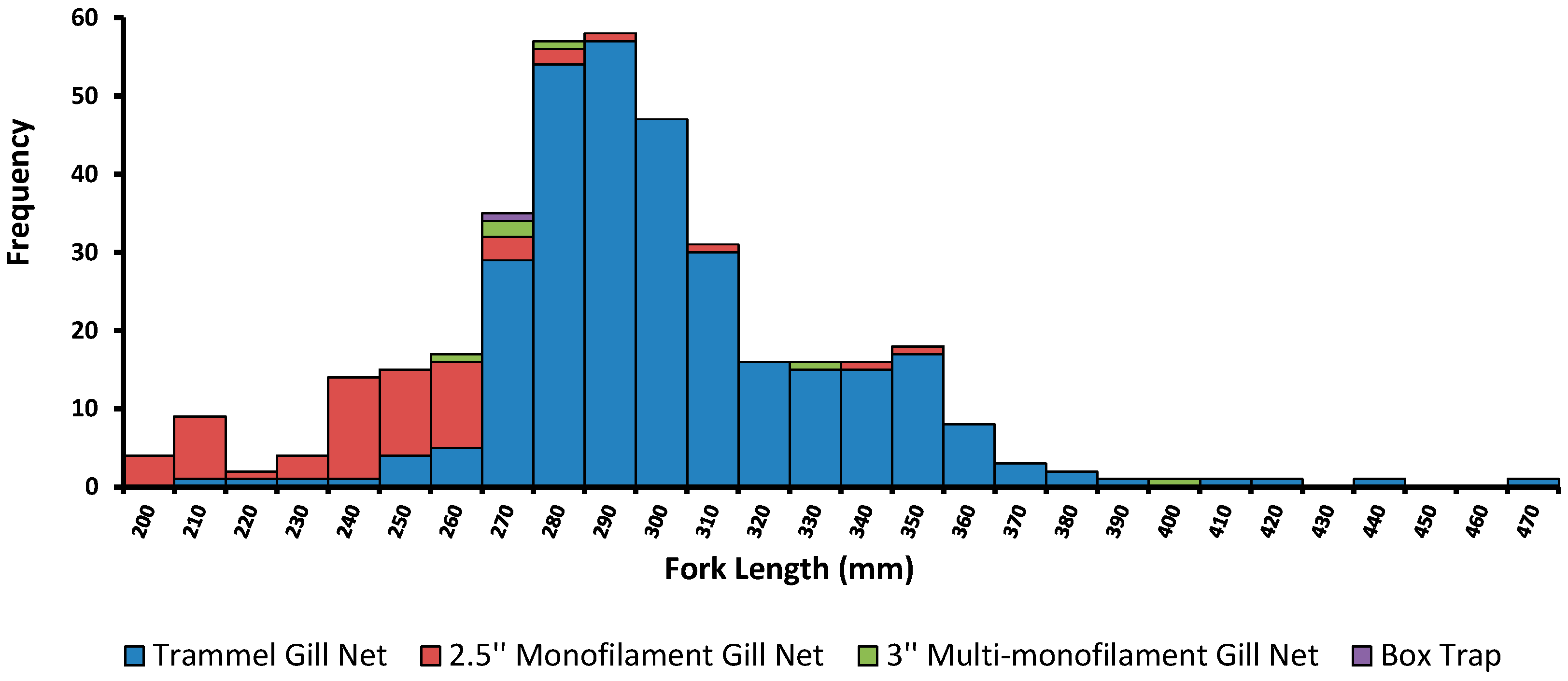
3.1. Catch Effort
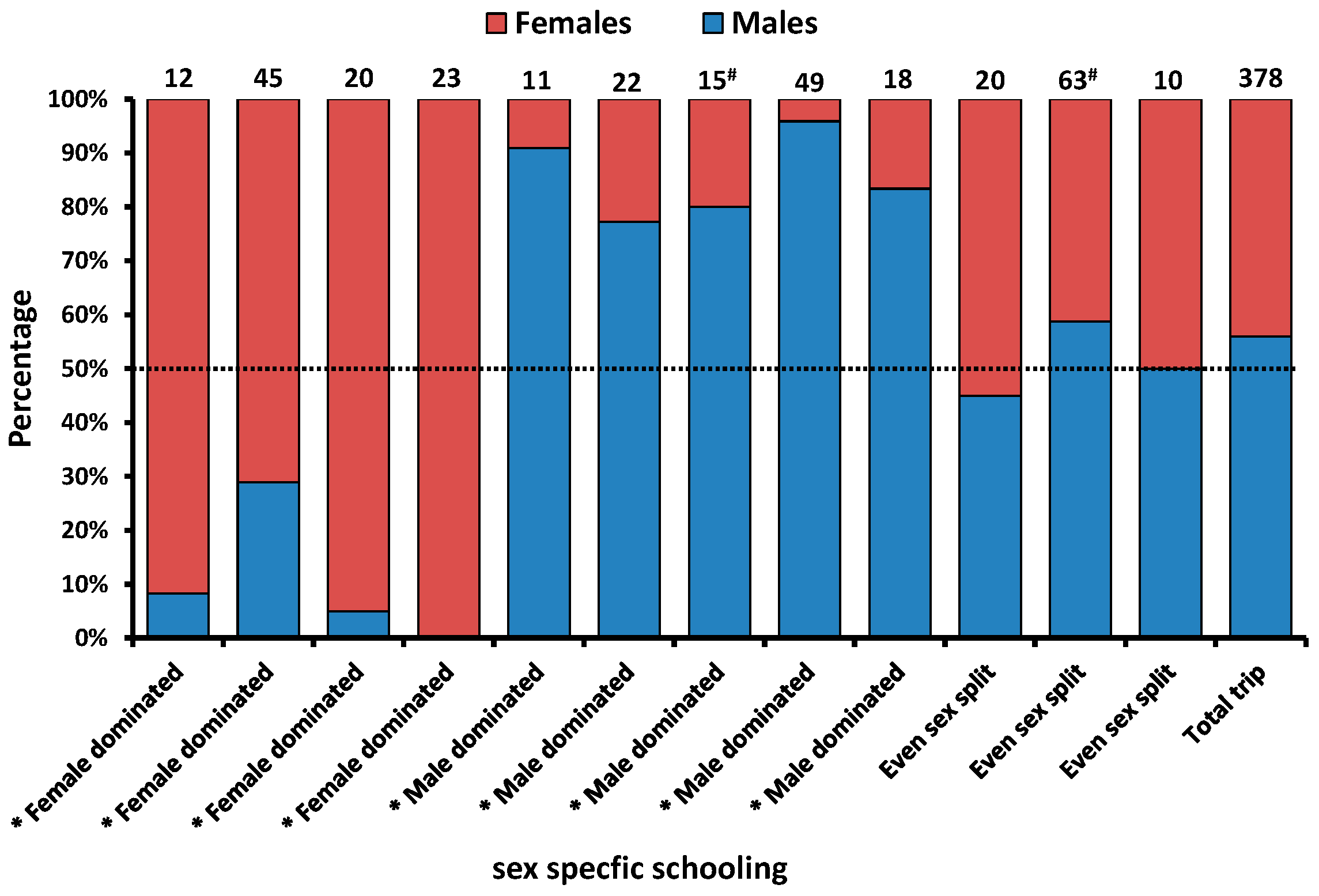
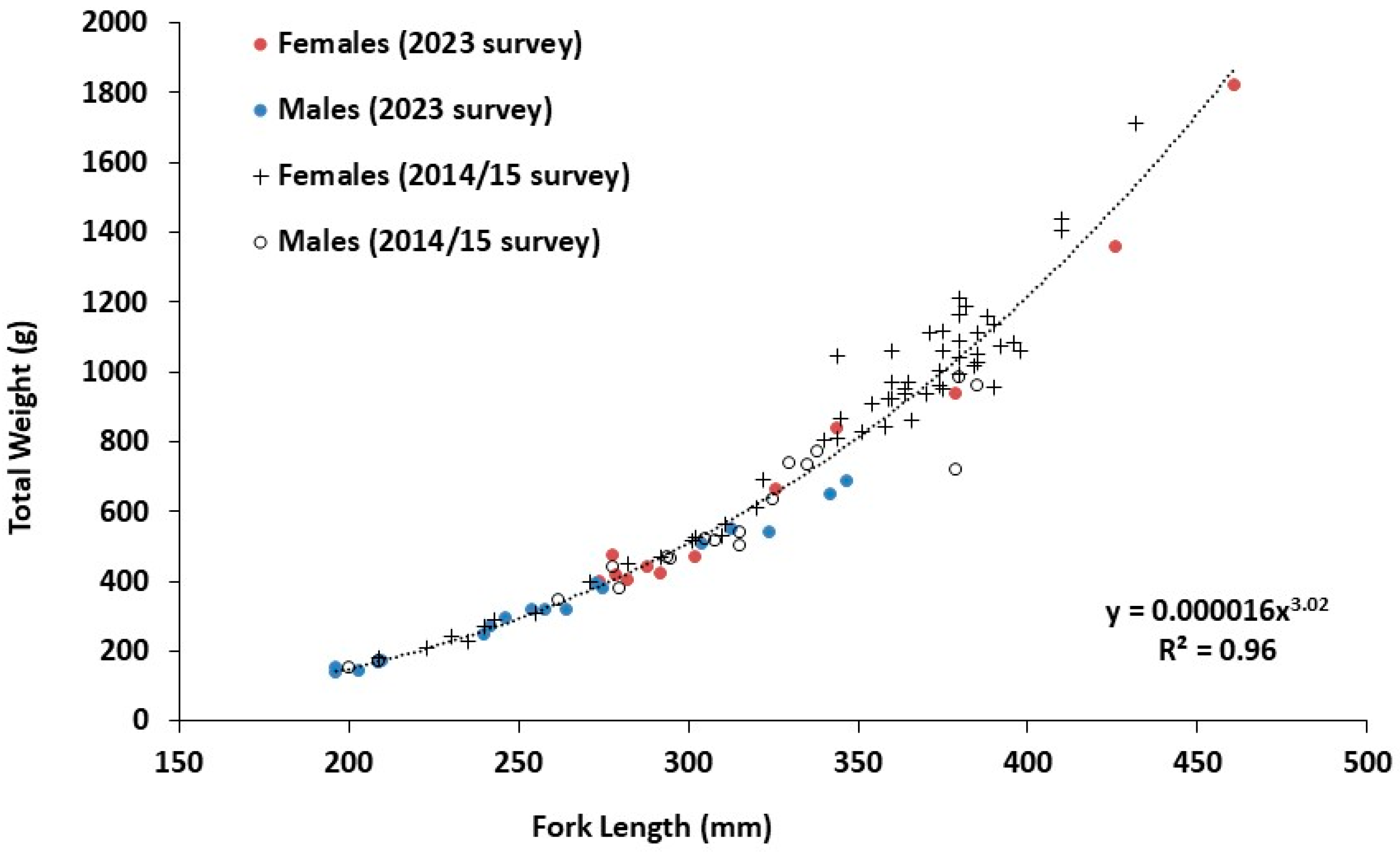
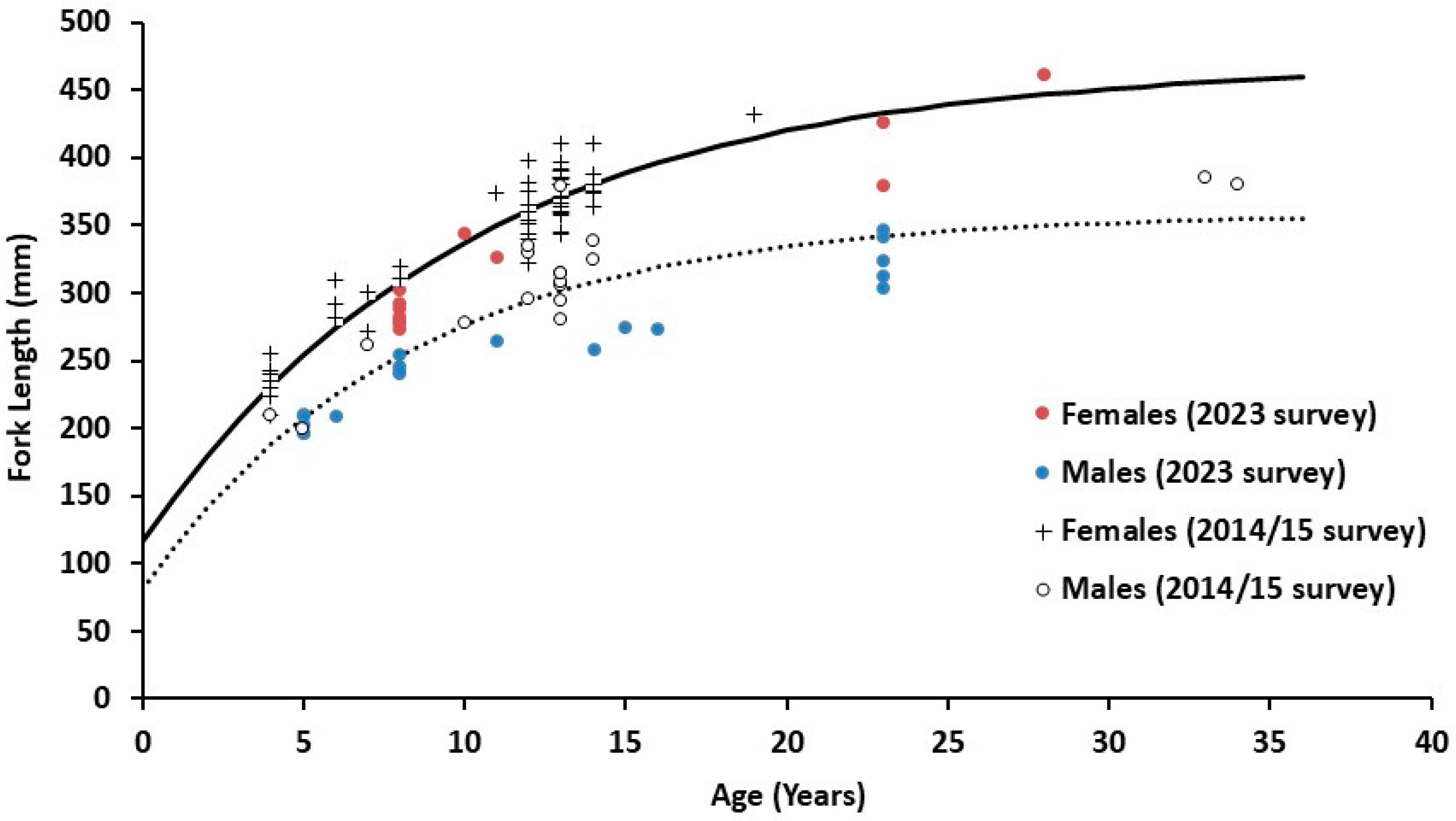
3.2. Biological Information
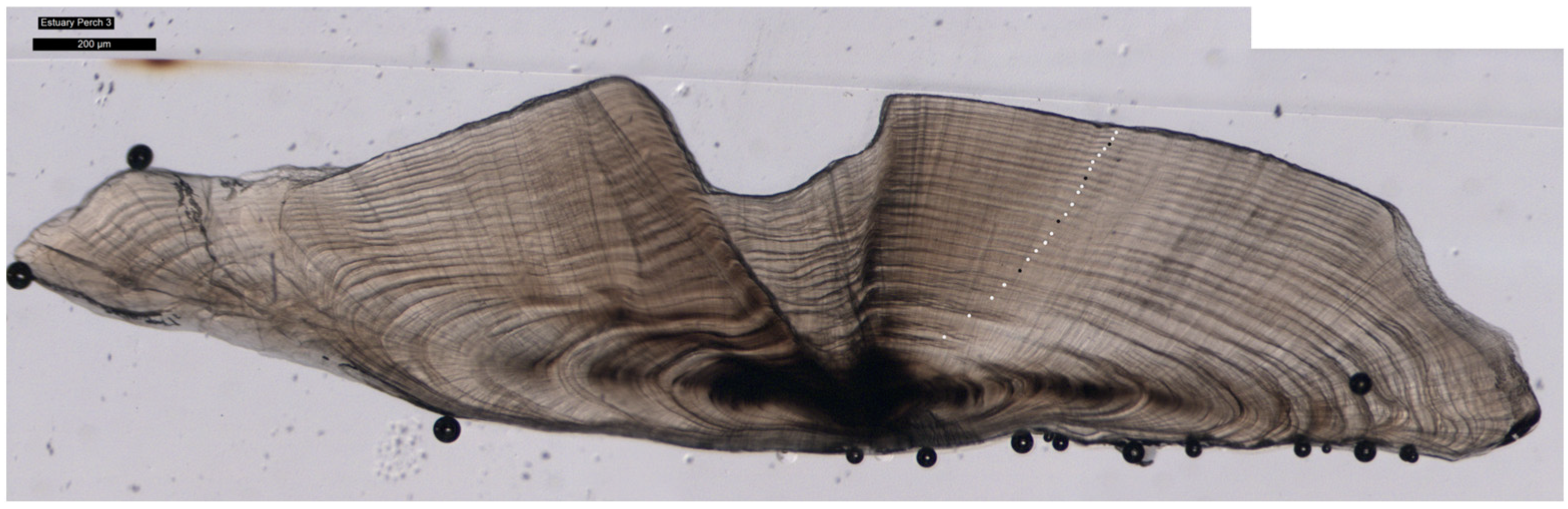
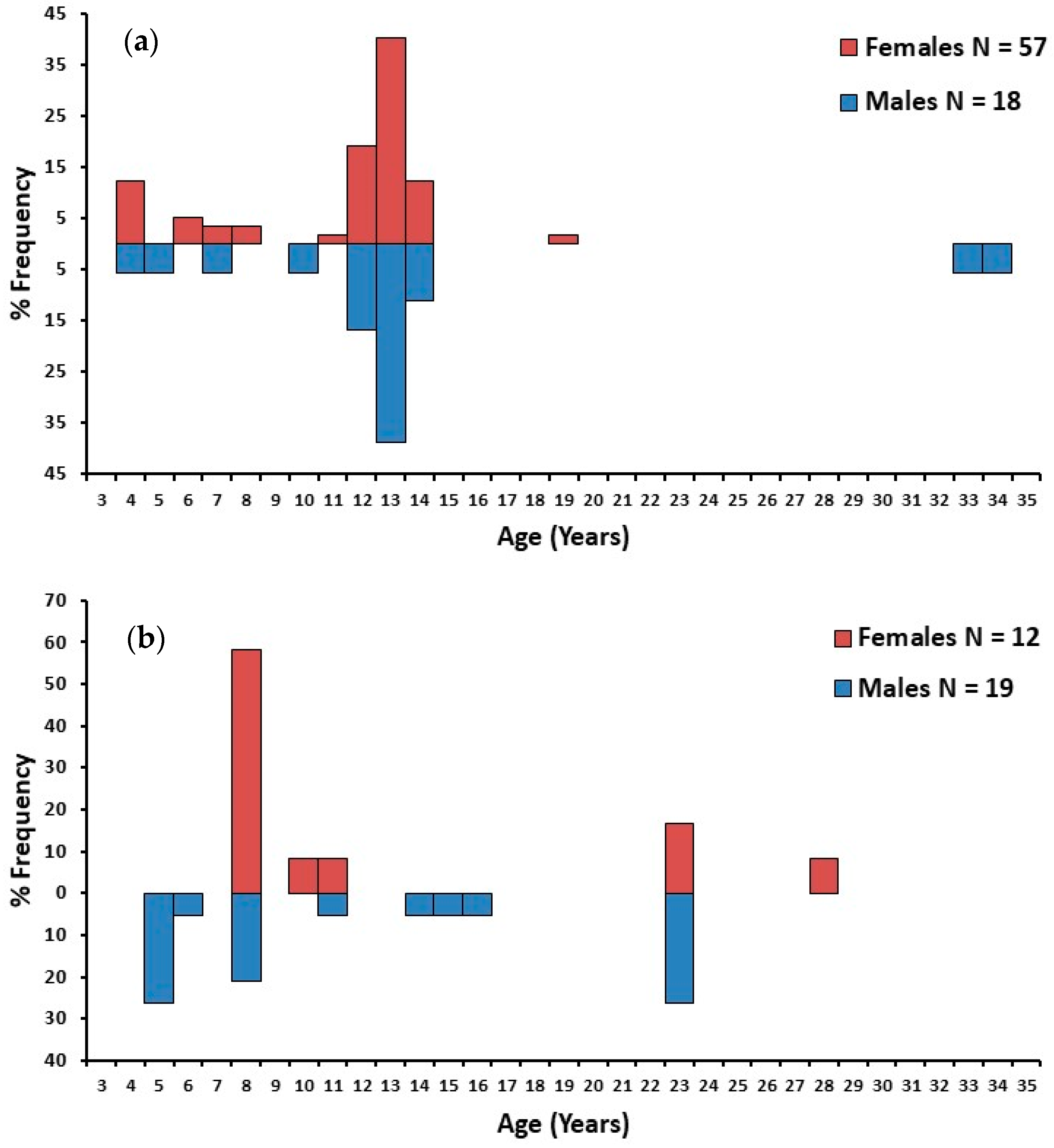
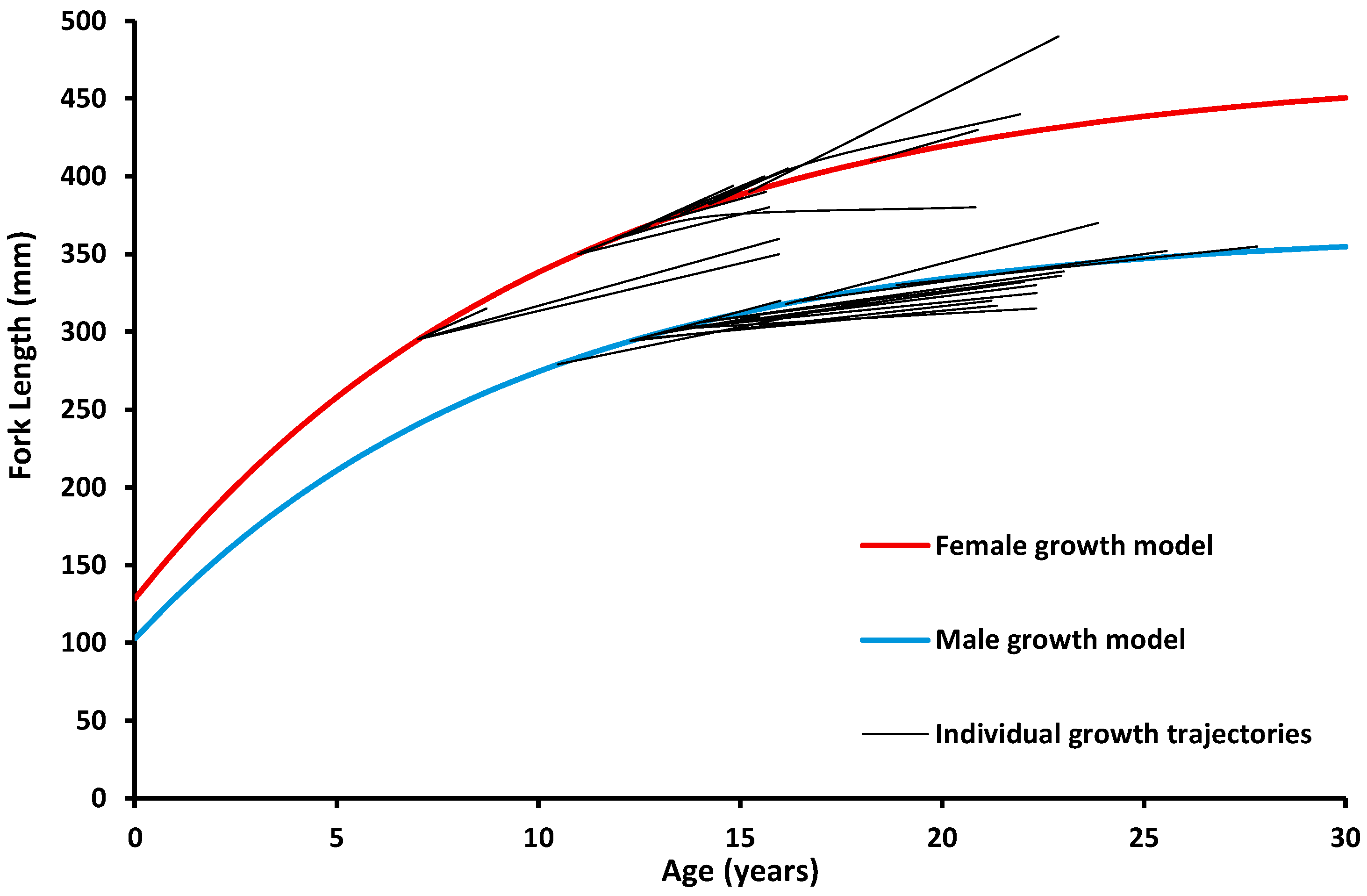
3.3. Ageing and Cohorts
3.4. Recaptured Tagged Fish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- McDowall, R.M. Freshwater Fishes of South-Eastern Australia; Reed Books: Terrey Hills, NSW, Australia, 1996. [Google Scholar]
- Walsh, C.T.; Gray, C.A.; West, R.J.; Williams, L.F.G. Reproductive Biology and Spawning Strategy of the Catadromous Percichthyid, Macquaria Colonorum (Günther, 1863). Environ. Biol. Fishes 2011, 91, 471–486. [Google Scholar] [CrossRef]
- Hunt, T.L.; Ingram, B.A. Investigating the Historical Abundance of Estuary Perch in Lake Tyers; Fisheries Victoria: Melbourne, Australia, 2014. [Google Scholar]
- Van Wyk, B. Reproduction, Growth and Population Dynamics of Estuary Perch (Percalates Colonorum) in the Arthur River, Tasmania. Honour’s Thesis, University of Tasmania, Hobart, Australia, 2015. [Google Scholar]
- Walsh, C.T.; Gray, C.A.; West, R.J.; van der Meulen, D.E.; Williams, L.F.G. Growth, Episodic Recruitment and Age Truncation in Populations of a Catadromous Percichthyid, Macquaria Colonorum. Mar. Freshw. Res. 2010, 61, 397–407. [Google Scholar] [CrossRef]
- Beard, J.; Crawford, C.; Hirst, A. Developing a Monitoring Program for Six Key Estuaries in North-West Tasmania; University of Tasmania: Hobart, Australia, 2008. [Google Scholar]
- Stoessel, D.J.; van Rooyen, A.R.; Beheregaray, L.B.; Raymond, S.M.C.; van Wyk, B.; Haddy, J.; Lieschke, J.; Weeks, A.R. Population Genetic Structure of Estuary Perch (Percalates Colonorum Gunther) in South-Eastern Australia. Mar. Freshw. Res. 2020, 72, 263–274. [Google Scholar] [CrossRef]
- Karakulak, F.S.; Erk, H. Gill Net and Trammel Net Selectivity in the Northern Aegean Sea, Turkey. Sci. Mar. 2008, 72, 527–540. [Google Scholar] [CrossRef]
- Haddon, M. Modelling and Quantitative Methods in Fisheries, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2011; ISBN 978-0-429-10949-2. [Google Scholar]
- King, M. Fisheries Biology, Assessment and Management; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-68804-5. [Google Scholar]
- Biophysical Character of Rivers in the Arthur River Catchment. In Water Assessment Aquatic Ecology Report Series; Report No. WA 14/02; Water and Marine Resources Division, Department of Primary, Industries, Parks, Water and Environment: Hobart, Australia, 2014.
- Morison, A.K.; Coutin, P.C.; Robertson, S.G. Age Determination of Black Bream, Acanthopagrus Butcheri (Sparidae), from the Gippsland Lakes of South-Eastern Australia Indicates Slow Growth and Episodic Recruitment. Mar. Freshw. Res. 1998, 49, 491–498. [Google Scholar] [CrossRef]
- Staunton-Smith, J.; Robins, J.B.; Mayer, D.G.; Sellin, M.J.; Halliday, I.A. Does the Quantity and Timing of Fresh Water Flowing into a Dry Tropical Estuary Affect Year-Class Strength of Barramundi (Lates Calcarifer)? Mar. Freshw. Res. 2004, 55, 787–797. [Google Scholar] [CrossRef]
- Morrongiello, J.R.; Walsh, C.T.; Gray, C.A.; Stocks, J.R.; Crook, D.A. Environmental Change Drives Long-Term Recruitment and Growth Variation in an Estuarine Fish. Glob. Change Biol. 2014, 20, 1844–1860. [Google Scholar] [CrossRef] [PubMed]
- Stoessel, D.J.; Morrongiello, J.R.; Raadik, T.A.; Lyon, J.P.; Nicol, M.D. Determinants of Year Class Strength and Growth of Estuary Perch Macquaria Colonorum in a Highly Regulated System. Mar. Freshw. Res. 2018, 69, 1663–1673. [Google Scholar] [CrossRef]
- Feyrer, F.; Nobriga, M.L.; Sommer, T.R. Multidecadal Trends for Three Declining Fish Species: Habitat Patterns and Mechanisms in the San Francisco Estuary, California, USA. Can. J. Fish. Aquat. Sci. 2007, 64, 723–734. [Google Scholar] [CrossRef]
- Walsh, C.T.; Reinfelds, I.V.; Ives, M.C.; Gray, C.A.; West, R.J.; van der Meulen, D.E. Environmental Influences on the Spatial Ecology and Spawning Behaviour of an Estuarine-Resident Fish, Macquaria Colonorum. Estuar. Coast. Shelf Sci. 2013, 118, 60–71. [Google Scholar] [CrossRef]
- McCarraher, D.B.; McKenzie, J.A. Observations on the Distribution, Growth, Spawning and Diet of Estuary Perch (Macquaria Colonorum) in Victorian Waters; Technical Report Series; Arthur Rylah Institute for Environmental Research: Victoria, Australia, 1986. [Google Scholar]
- Trnski, T.; Hay, A.C.; Fielder, D.S. Larval Development of Estuary Perch (Macquaria Colonorum) and Australian Bass (M. Novemaculeata) (Perciformes: Percichthyidae), and Comments on Their Life History. Fish. Bull. 2005, 103, 183–194. [Google Scholar]
- Bureau of Meteorology Website. Available online: Australia’s Official Weather Forecasts & Weather Radar—Bureau of Meteorology (accessed on 22 July 2024).
- Díaz, N.; Ribas, L.; Piferrer, F. The Relationship between Growth and Sex Differentiation in the European Sea Bass (Dicentrarchus Labrax). Aquaculture 2013, 408–409, 191–202. [Google Scholar] [CrossRef]
- Douglas, J. Estuary Perch Movement and Habitat Use in the Snowy River Recreational Fishing Grant Program—Research Report; Department of Primary Industries: Fisheries Victoria, Australia, 2010. [Google Scholar]
- Wearmouth, V.J.; Sims, D.W. Chapter 2 Sexual Segregation in Marine Fish, Reptiles, Birds and Mammals: Behaviour Patterns, Mechanisms and Conservation Implications. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 2008; Volume 54, pp. 107–170. [Google Scholar]




| Males | |
| Stages | Macroscopic Characteristics |
| 1. Indeterminate | Small, moderately translucent to pink, determination of sex is difficult. |
| 2. Developing | Much larger than stage 1, colourless to white, transverse sections triangular. |
| 3. Mature | Testes larger in diameter, white in colour, slightly vascularized. |
| 4. Ripe | Swollen, soft and white testes. Milt flows from the urogenital pore. |
| 5. Spent | Rubbery, reduced in size, bloodshot and grey in appearance. |
| Females | |
| Stages | Macroscopic Characteristics |
| 1. Indeterminate | Small, moderately translucent to pink, determination of sex is difficult. |
| 2. Developing | Much larger than stage 1, colourless to cream with fine granular texture. Slightly vascularized |
| 3. Mature | Ovaries larger in diameter, yellow in colour with extensive vascularization. Oocytes visible, mature |
| 4. Ripe | Hydrated translucent oocytes visible through ovary wall, yellow to amber in colour, gonads take up three quarters of body cavity |
| 5. Ovulation | Oocytes extruded from the genital papilla with gentle gravitational pressure |
| 6. Spent | Ovaries reduced in length, leathery, bloodshot towards posterior end. |
| Fish Numbers | Number of Sets | Total Soak Time (Hrs) | Total Length of Net Soaked (m) | Standardised 100 M/hr | Mean CPUE * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day No. | Day | Evening | Day | Evening | Day | Evening | Day | Evening | Day | Evening | Day | Evening |
| 1 | - | 57 | 2 | 1.5 | 70 | 0.5 | 99.5 | |||||
| 2 | 9 | 38 | 6 | 5 | 5.8 | 3.5 | 297 | 175 | 3.2 | 1.2 | 2.6 | 24.0 |
| 3 | 14 | 30 | 5 | 3 | 8.1 | 2.4 | 426 | 90 | 6.7 | 0.7 | 1.7 | 36.9 |
| 4 | 16 | 70 | 6 | 4 | 13.8 | 2.6 | 484 | 170 | 11.1 | 1.1 | 1.2 | 52.6 |
| 5 | 70 | 76 | 3 | 8 | 6.0 | 6.9 | 276 | 280 | 5.5 | 2.4 | 10.6 | 29.4 |
| Trip Totals | 109 | 271 | 20 | 22 | 33.7 | 16.8 | 1483 | 785 | 26.5 | 6.0 | 3.1 | 39.7 |
| Recapture Year | Initial Length (mm) | Recapture Length (mm) | Time at Liberty (Yrs) | Total Growth (mm) | Growth per Year (mm) | Sex (F/M/I) |
|---|---|---|---|---|---|---|
| 2017 | 298 | 310 | 2.7 | 12 | 4.5 | M |
| 2018 | 294 | 320 | 3.7 | 26 | 7.0 | M |
| 2020 | 385 | 420 | 5.1 | 35 | 6.9 | M |
| 2022 | 279 | 320 | 7.7 | 41 | 5.3 | M |
| 2022 | 318 | 370 | 7.7 | 52 | 6.7 | M |
| 2023 * | 302 | 315 | 8.9 | 13 | 1.5 | M |
| 2023 * | 300 | 332 | 8.9 | 32 | 3.6 | M |
| 2023 * | 302 | 330 | 8.9 | 28 | 3.1 | M |
| 2023 * | 330 | 355 | 8.9 | 25 | 2.8 | M |
| 2023 * | 295 | 317 | 8.9 | 22 | 2.5 | M |
| 2023 * | 294 | 320 | 8.9 | 26 | 2.9 | M |
| 2023 * | 306 | 336 | 8.9 | 30 | 3.4 | M |
| 2023 * | 302 | 325 | 8.9 | 23 | 2.6 | M |
| 2023 * | 320 | 352 | 9.0 | 32 | 3.5 | M |
| 2024 | 306 | 339 | 9.0 | 33 | 3.7 | M |
| 2017 | 306 | 345 | 2.7 | 39 | 14.7 | I |
| 2016 | 295 | 315 | 1.7 | 20 | 11.8 | F |
| 2016 | 351 | 368 | 1.7 | 17 | 10.0 | F |
| 2017 | 356 | 380 | 2.6 | 24 | 9.1 | F |
| 2017 | 386 | 420 | 2.6 | 34 | 12.8 | F |
| 2017 | 382 | 410 | 2.6 | 28 | 10.6 | F |
| 2017 | 363 | 394 | 2.7 | 31 | 11.7 | F |
| 2017 | 371 | 390 | 2.7 | 19 | 7.2 | F |
| 2017 | 376 | 405 | 2.7 | 29 | 10.9 | F |
| 2017 | 360 | 375 | 2.7 | 15 | 5.7 | F |
| 2017 | 410 | 430 | 2.7 | 20 | 7.5 | F |
| 2018 | 360 | 400 | 3.7 | 40 | 10.8 | F |
| 2019 | 350 | 380 | 4.7 | 30 | 6.3 | F |
| 2021 | 355 | 420 | 6.7 | 65 | 9.7 | F |
| 2022 | 390 | 490 | 7.7 | 100 | 13.0 | F |
| 2022 | 382 | 440 | 7.7 | 58 | 7.5 | F |
| 2023 * | 360 | 380 | 8.9 | 20 | 2.2 | F |
| 2023 * | 295 | 350 | 8.9 | 55 | 6.1 | F |
| 2023 * | 295 | 360 | 8.9 | 65 | 7.3 | F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yick, J.L.; Van Wyk, B.; Haddy, J. The Tasmanian Estuary Perch Population—A Threatened Species? Fishes 2025, 10, 31. https://doi.org/10.3390/fishes10010031
Yick JL, Van Wyk B, Haddy J. The Tasmanian Estuary Perch Population—A Threatened Species? Fishes. 2025; 10(1):31. https://doi.org/10.3390/fishes10010031
Chicago/Turabian StyleYick, Jonah L., Bryan Van Wyk, and James Haddy. 2025. "The Tasmanian Estuary Perch Population—A Threatened Species?" Fishes 10, no. 1: 31. https://doi.org/10.3390/fishes10010031
APA StyleYick, J. L., Van Wyk, B., & Haddy, J. (2025). The Tasmanian Estuary Perch Population—A Threatened Species? Fishes, 10(1), 31. https://doi.org/10.3390/fishes10010031






