A Scalable Protocol for Ex Vivo Production of CAR-Engineered Human NK Cells
Abstract
1. Introduction
2. Experimental Design
3. Procedure
3.1. Cell Collection
3.1.1. Isolation of Peripheral Blood Mononuclear Cells (PBMCs) from Buffy Coat or Whole Blood (Processing Time: 60–90 min)
- Dilute whole blood with sterile PBS at a 1:1 ratio (e.g., 10 mL blood + 10 mL PBS). For buffy coat, dilute with sterile PBS at a 1:2 or 1:3 ratio (e.g., 10 mL buffy coat + 20 mL or 30 mL PBS) depending on the viscosity observed with buffy coat. Use fresh whole blood or buffy coat (obtained within 24 h), when possible.
- Prepare Ficoll–Paque gradient by gently adding 15 mL of Ficoll-Paque to the bottom of a 50 mL conical tube.
CRITICAL STEP: Gently add the diluted whole blood or buffy coat on the top of the Ficoll–Paque gradient very slowly without disturbing the gradient (tip: tilt the tube and slowly dispense the diluted blood or buffy coat from one side of the tube into the gradient).
- Add up to 25 mL of diluted whole blood or buffy coat per 15 mL of Ficoll–Paque gradient. Add sterile PBS to bring the final volume to 50 mL.
CRITICAL STEP: Centrifuge at 800× g for 20 min at room temperature. Perform the spin with medium acceleration and no brakes for deceleration to avoid disturbing the density gradient.
- After centrifugation, four layers will be visible: Plasma (first); white, a cloudy layer containing PBMCs between the plasma/Ficoll interface (second); Ficoll–Paque medium (third); and a cellular pellet containing the red blood cells (RBCs) and other cells such as granulocytes (fourth) (Figure 1, Step 3).
CRITICAL STEP: Aspirate and discard the fraction containing plasma. Remove as much plasma as possible without disturbing the PBMC “white cloudy” layer. This helps to prevent contamination of PBMCs with platelets.
- After discarding the plasma fraction, carefully aspirate the PBMC layer without disturbing the Ficoll–Paque gradient using a sterile pipette and transfer to a new tube. Discard the tube containing the Ficoll–Paque gradient and RBCs.
- Wash the PBMCs by resuspending in 20 mL of PBS and centrifuge at 300× g for 10 min with full acceleration and braking.
- Discard the supernatant and repeat the wash steps two more times to remove any residual Ficoll gradient and plasma (note: proper washing ensures the removal of platelets and residual Ficoll, which can interfere with downstream applications).
CRITICAL STEP: After completion of the wash steps, the cell pellet should appear white in color. If contamination with RBCs is observed, add 10 times the volume equivalent of the RBC lysis buffer (note: if 10 mL of blood or buffy coat was used as a starting material for PBMC isolation, add 100 mL of RBC lysis buffer). Incubate for 5 min at room temperature to lyse RBCs. Following RBC lysis, wash one time with 20 mL PBS as described above.
- Resuspend the PBMC pellet in complete RPMI media (RPMI 1640 + 1% Pen/Strep + 1% glutamine + 10% heat-inactivated FBS) (tip: use the same volume of complete RPMI to resuspend the PBMC pellet as the initial volume of blood or buffy coat used in Step 1).
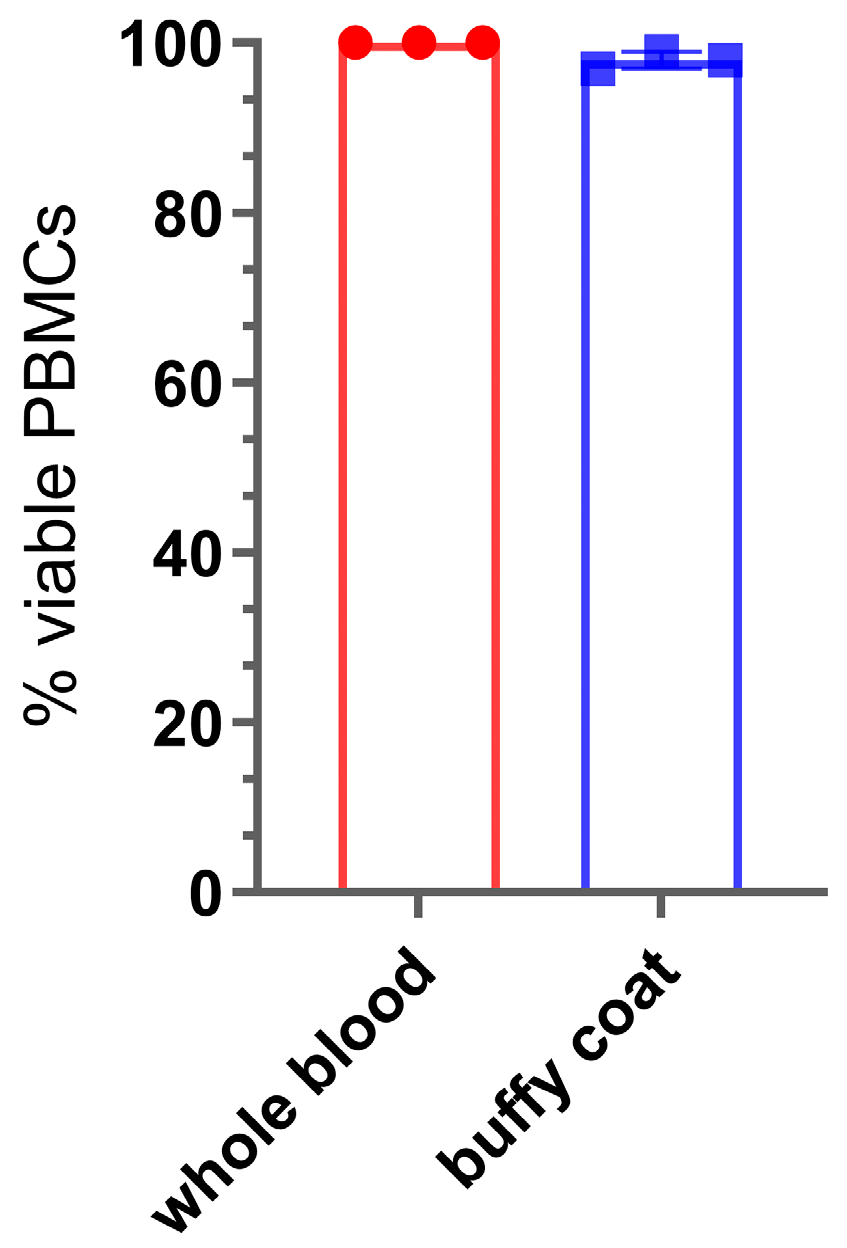
- Count the cells using an appropriate cell counting method (e.g., automated cell counter) to assess viability and cell concentration. Adjust the PBMC concentration to 1 × 107 cells/mL using the complete RPMI media.
- Label the cells as PBMCs.
PAUSE STEP: PBMCs can be either used immediately for CAR-NK cell manufacturing or cryopreserved for future use. For cryopreservation, resuspend PBMCs in freezing media at a concentration of 1 × 107 cells/mL and aliquot in cryovials. Label the cryovials using cryogenic labels and place in a freezing container (e.g., Mr. Frosty) at −80 °C for 24 h. PBMCs may be stored at −80 °C for the short term (e.g., less than 1 week). For long-term storage, move the cryovials from −80 °C to LN2.
3.1.2. Isolation of NK Cells from PBMCs by CD3 Depletion and CD56 Enrichment (Processing Time: 60–90 min)
- For cryopreserved PBMCs, go to Step 2. For fresh PBMCs, go to Step 3.
- Obtain the required number of cryovials containing cryopreserved PBMCs from storage. Thaw the cells using an appropriate method (e.g., 37 °C water bath). After complete thawing, wash the cells once in PBS (10 mL per 107 cells) and resuspend in 100 µL of MACS buffer per 107 cells.
- Add 20 µL of anti-CD3 microbeads per 107 cells to the PBMC suspension (note: any commercial NK isolation kits can be used, following the manufacturer’s instructions).
CRITICAL STEP: Mix the bead and cell suspension gently using pipette and incubate for 10 min on ice or at 4 °C. Ensure slow and uniform mixing of the beads and cells. Strictly follow the manufacturer’s incubation times and keep cells cold to prevent non-specific binding.
- Wash the cells by adding 1 mL of MACS buffer per 107 cells.
- Centrifuge at 300× g for 10 min. Aspirate the supernatant completely.
- Resuspend up to 10 × 107 cells in 500 µL of MACS buffer.
- To isolate cells using the magnetic separator, either use an MS (for up to 2 × 108 cells) or LS column (for up to 2 × 109 cells).
- Rinse the column with MACS buffer (500 µL for the MS column or 3 mL for the LS column).
CRITICAL STEP: Apply the cell suspension to the rinsed column slowly to maintain column integrity and prevent clogging.
- Collect the CD3− flow-through fraction, which contains NK cells and other CD3− cells.
- Wash the column with MACS buffer (500 µL for the MS column or 3 mL for the LS column) collecting all flow-throughs.
- Discard the column containing CD3+ cells unless needed for any other purpose.
- To isolate the NK cells from the CD3− fraction, count the cells and adjust the cell concentration to 1 × 107 cells/100 µL of MACS buffer.
- Add 20 µL of anti-CD56 microbeads per 107 cells.
CRITICAL STEP: Mix the bead and cell suspension gently using a pipette and incubate for 10 min on ice or at 4 °C. Ensure slow and uniform mixing of the beads and cells. Strictly follow the manufacturer’s incubation times and keep cells cold to prevent non-specific binding.
- Wash the cells by adding 1 mL of MACS buffer per 107 cells.
- Centrifuge at 300× g for 10 min.
- Resuspend the cells in MACS buffer. Use 1 mL MACS buffer per 1 × 107 cells.
- Select an appropriate MACS column as in Step 8. Rinse the column with MACS buffer (500 µL for an MS column or 3 mL for an LS column).
- Apply the cell suspension to the MACS column and collect the flow-through as the CD3−CD56− fraction. Discard the flow-through (CD3−CD56− fraction) unless needed for any other purpose.
- Wash the column with MACS buffer (500 µL for the MS column or 3 mL for the LS column).
- To collect CD56+ NK cells from the column, remove the column from the magnet and add 1 mL of MACS buffer per 107 cells into the column. Using a plunger, flush the cells out of the column into a clean 15 mL conical tube. Label the tube as CD3−CD56+ NK cells.
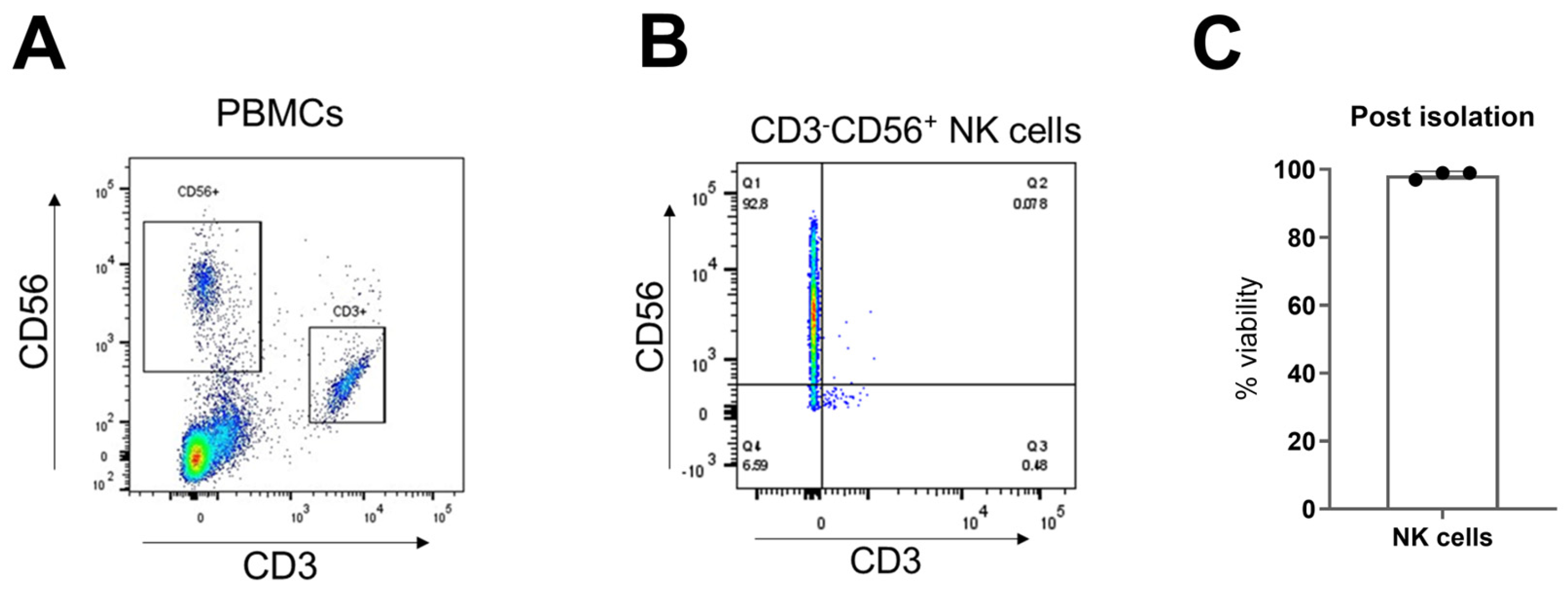
CRITICAL STEP: Count the cells using an appropriate cell counting method (e.g., automated cell counter) to assess viability and cell concentration.
- Assess isolated NK cell purity by flow cytometry after staining with anti-CD3 and anti-CD56 antibodies. Note: We recommend obtaining ≥ 90% CD3−CD56+ NK cells to minimize T cell contamination in CAR-NK cell product.
- PAUSE STEP: Isolated NK cells can be either used immediately for CAR-NK cell manufacturing or cryopreserved for future use. For cryopreservation, resuspend the NK cells in freezing media at a concentration of 1 × 107 cells/mL and aliquot in cryovials. Label the cryovials using cryogenic labels and place in a freezing container (e.g., Mr. Frosty) at −80 °C for 24 h. PBMCs may be stored at −80 °C for the short term (e.g., less than 1 week). For long-term storage, move the cryovials from −80 °C to LN2.
3.2. NK Cell Activation and Transduction by a Lentiviral Vector for CAR Expression (Processing Time: 24–48 h)
- Wash the isolated fresh NK cells with PBS and resuspend in complete RPMI media in the presence of IL-2 (200–500 IU/mL). If cryopreserved NK cells are used, obtain the required number of cryovials containing cryopreserved NK cells from storage. Thaw the cells using an appropriate method (e.g., 37 °C water bath). After complete thawing, resuspend and wash the cells in 10 mL PBS and resuspend in complete RPMI media.
- Count the cells using an appropriate cell counting method (e.g., automated cell counter) to assess viability and cell concentration. Adjust the NK cell concentration to 5 × 106 cells per mL.
- To activate NK cells using feeder cells, co-culture NK cells (5 × 106 cells) with irradiated, replication-incompetent K562 cells at a 1:1 ratio for 24 h.
- Alternatively, to activate NK cells independent of feeder cells, add PMA (50 ng/mL) and Ionomycin (1 µg/mL) to NK cells (5 × 106 cells) for 24 h.
CRITICAL STEP: Coat the non-tissue culture-treated plate (6 or 12 well) with retronectin (10 µg/mL in PBS). Add retronectin (0.5 mL) into each well of a 24-well plate or 2 mL into each well of a 6-well plate (note: retronectin coating improves the lentiviral vector transduction efficiency of NK cells).
- Incubate for 2 h at room temperature or overnight at 4 °C.
- Wash the retronectin-coated plate with 1 mL PBS and add 1 mL of Superblock solution or 2% BSA in PBS for 30 min at room temperature.
- PAUSE STEP: The retronectin-coated plate can be sealed with parafilm and kept at 4 °C for up to 2 weeks (note: preparing the retronectin-coated plate in advance ensures the plate’s availability for the transduction step).
- 8.
- Wash the retronectin-coated plate two times with 1 mL PBS before use.
- 9.
- Add the lentiviral vector and activated NK cells in the retronectin-coated wells at a multiplicity of infection (MOI) of 20 (note: we recommend optimizing the MOI using primary NK cells or a NK cell line to achieve the desired level of CAR gene expression before executing experiments).
- 10.
- Centrifuge the retronectin-coated plate containing the lentiviral vector and NK cells at 1800× g for 60 min at room temperature.
- 11.
- After centrifugation, incubate the plate at 37 °C and 5% CO2.
- 12.
- After 12–24 h, wash the cells once using PBS and count the cells using an appropriate cell counting method (e.g., automated cell counter) to assess viability and cell concentration.
- 13.
- Resuspend transduced cells in complete RPMI media supplemented with IL-2 (200–500 IU/mL), IL-15 (5 ng/mL), and IL-21 (25 ng/mL) at 0.5–1.0 × 106 cells per mL. Culture transduced cells in a tissue culture plate (e.g., 12-well or 6-well).
- 14.
- Incubate the cells at 37 °C and 5% CO2 for 48 h.
- 15.
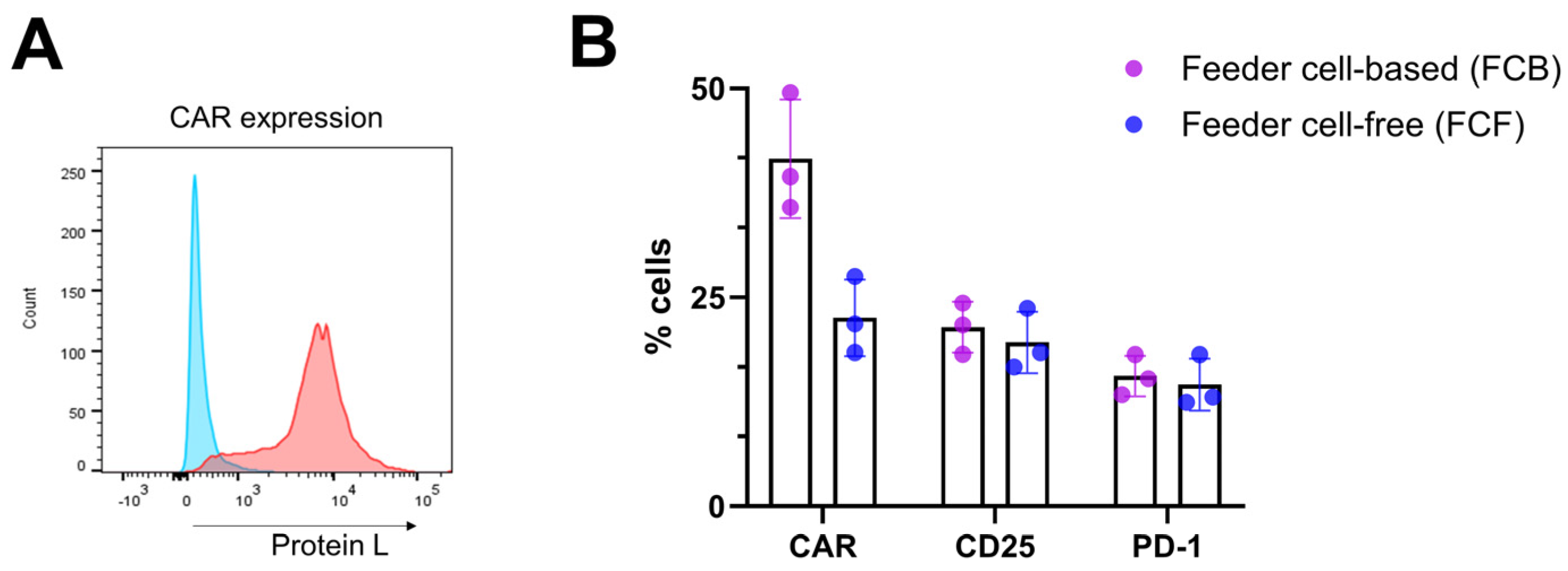
CRITICAL STEP: Assess CAR protein expression using an appropriate reagent (e.g., protein L, anti-Fab etc.) using flow cytometry.
- 16.
- If CAR expression is acceptable, proceed with CAR-NK expansion.
3.3. Expansion of Transduced CAR-NK Cells (Processing Time: 10–14 Days)
- Wash the transduced CAR-NK cells using 10 mL PBS and resuspend in 10 mL NK cell expansion media containing all three cytokines (IL-2, IL-15, and IL-21).
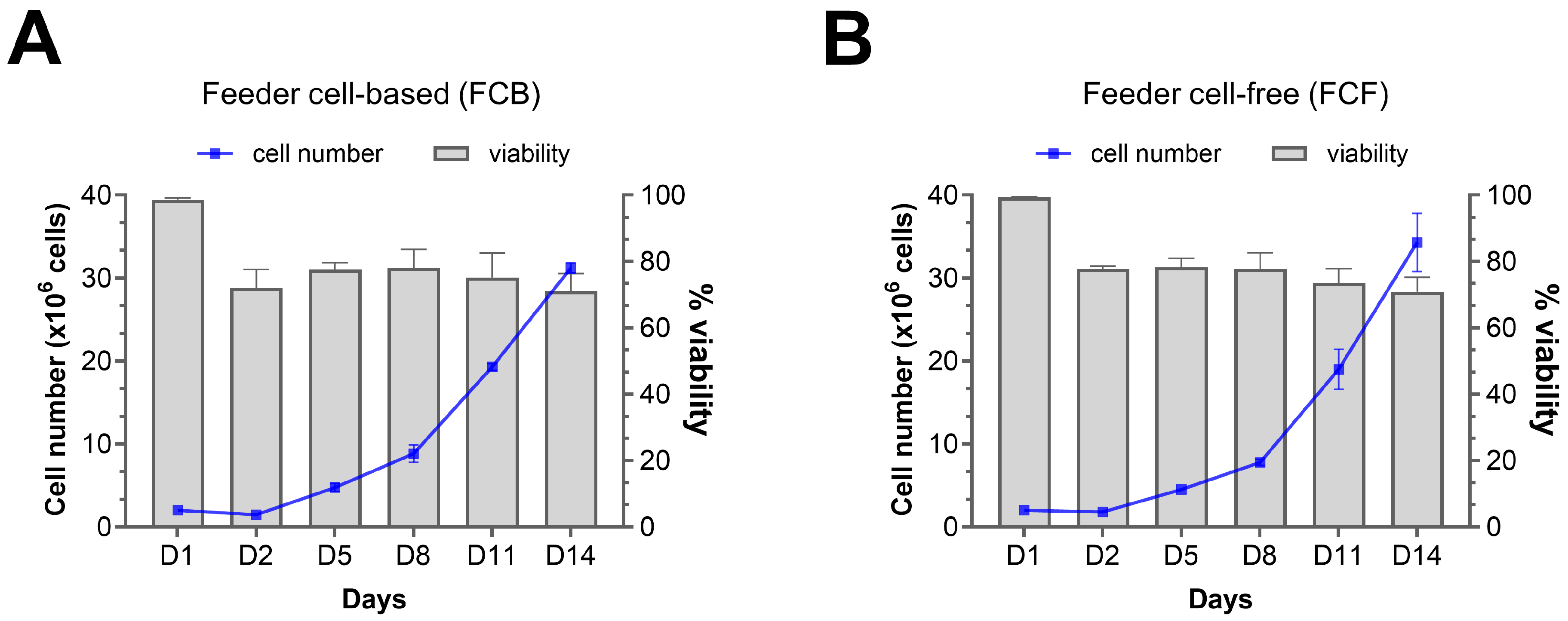
- Transfer the cells into an appropriate system for cellular expansion. We recommend using the G-Rex system for CAR-NK cell expansion using NK cell expansion media (note: resuspend 5 × 106 CAR-NK cells in 10 mL expansion media per well of a G-Rex 6-well plate). Maintain CAR-NK cell density between 0.5 and 1.0 × 106 cells per mL and incubate at 37 °C and 5% CO2.
- Monitor cell growth every other day and change the media as needed to maintain cell density (note: to change the media, gently remove the top 80% of the media without disturbing the cells settled at the bottom and add the required amount of fresh NK cell expansion media).
- After 10 days of expansion, count cells, and if the desired cell number is achieved, prepare the cells for harvest. If additional cell expansion is needed, maintain the cell culture for additional days up to Day 14.
- Before harvesting CAR-NK cells, assess their total cell number, viability, and CAR protein expression. To harvest cells, gently collect CAR-NK cells from G-Rex to a sterile 50 mL conical tube. Centrifuge at 300× g for 10 min. Wash the cells twice with sterile PBS.
- PAUSE STEP: CAR-NK cells can be used immediately for experiments or cryopreserved for future use. For cryopreservation, resuspend CAR-NK cells in freezing media at a concentration of 1 × 107 cells/mL and aliquot in cryovials. Label the cryovials using cryogenic labels and place in freezing container (e.g., Mr. Frosty) at −80 °C for 24 h. PBMCs may be stored at −80 °C for the short term (e.g., less than 1 week). For long-term storage, move the cryovials from −80 °C to LN2.
- 6.
- Resuspend the CAR-NK cells in an appropriate NK cell media for experiments. Before using CAR-NK cells for experiments, perform additional testing or characterization as needed (e.g., assess activation and exhaustion markers, the presence of non-NK cellular impurities, sterility, and endotoxin testing, etc.).
4. Expected Results
4.1. PBMC Isolation
4.2. NK Cell Isolation
4.3. NK Cell Activation and Transduction
4.4. CAR-NK Cell Expansion
4.5. Final CAR-NK Cell Product
5. Recommendations and Operational Notes
5.1. Functional Assays
5.1.1. Cytotoxicity Measurement
5.1.2. Degranulation Analysis (CD107a)
5.1.3. Cytokine Secretion Profiling
5.2. Donor Variability
- Starting NK cell purity: Samples with higher CD56+CD3− purity (>90%) demonstrated higher and more consistent CAR-NK product attributes.
- Age: NK cells from donors (age < 40 years) typically showed higher transduction and expansion compared with donors (age > 40), consistent with age-related changes in NK cell activation state and cytotoxicity profiles.
- Metabolic state of the NK cell population: Donors with higher baseline NK cell activity showed improved transduction.
5.2.1. Vector Dose Optimization
- Low responders (<20% transduction at MOI 10): Increase the MOI to more than 20 while monitoring cytotoxicity.
- High responders (>80% transduction at MOI 5): Consider reducing to MOI 5 or below to minimize vector-induced toxicity.
5.2.2. Cytokine Supplementation
- IL-15: Increase from the standard 10 ng/mL to 15–20 ng/mL for donors demonstrating poor expansion.
- IL-2: Add fresh IL-2 200 IU/mL on Days 3–5 to improve expansion.
- IL-21: Add 25 ng/mL of IL-21 during G-Rex expansion to improve cellular expansion.
5.3. Cell Viability and CAR Expression
- Day 0 (post-isolation): ≥95% viable cells;
- Day 1 (post-transduction): ≥90% viable cells;
- Day 3–7 (early expansion): ≥80% viable cells;
- Day 8–14 (late expansion): ≥70% viable cells;
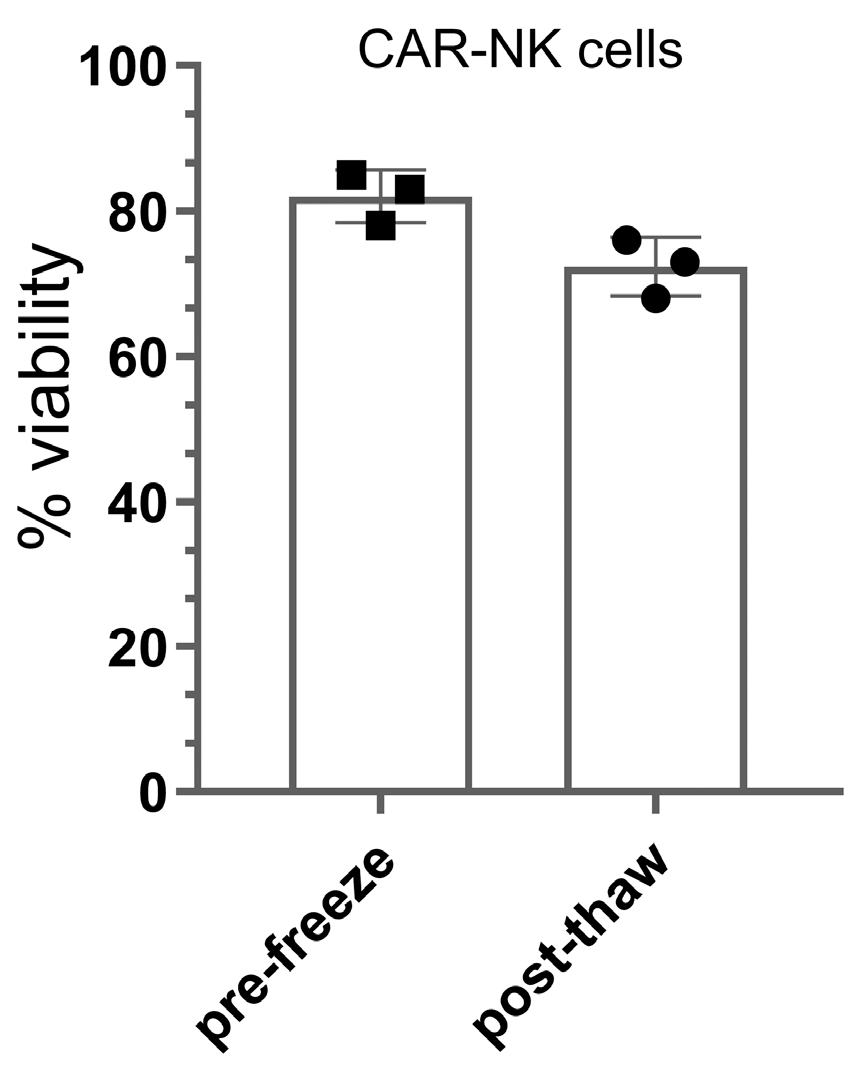
- Post-cryopreservation: ≥60% viable cells (post-thaw).
5.3.1. Lower Viability During Expansion
- Early stage (Days 3–5, <90%): Check contamination, use fresh media, and maintain cell density (around 1 × 106 cells/mL).
- Mid-stage (Days 7–10, <85%): Assess exhaustion markers and supplement metabolites (2 mM glutamine and 10 mM glucose).
- Late stage (Days 11–14, <80%): Continue fresh cytokine supplementation, maintain cell density, or consider early harvest or cryopreservation.
5.3.2. Poor Cell Expansion (<10-Fold by Day 14)
- Cytokine boost: Increase cytokine concentrations, namely IL-15 to 20 ng/mL and/or IL-21 (15–25 ng/mL) post-transduction.
- Feeder cell co-culture: Optimize the effector–target ratios using irradiated K562-mbIL21 cells if expansion is <5-fold by Day 7.
- Metabolic enhancement: Supplement with 2 mM glutamine and 10 mM glucose.
5.3.3. Low CAR Expression (<20% CAR+ Cells)
- MOI optimization: Increase MOI gradually from 10 to 15–20, monitoring for any vector-related toxicity.
- Transduction enhancement: Use 8 μg/mL polybrene or retronectin coating.
- Timing adjustment: Perform transduction 18–24 h post-activation.
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.H. The application of natural killer cell immunotherapy for the treatment of cancer. Front. Immunol. 2015, 6, 169989. [Google Scholar] [CrossRef]
- Wang, X.; Rivière, I. Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Mol. Ther.-Oncolytics 2016, 3, 16015. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. eBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Vivier, E.; Rebuffet, L.; Narni-Mancinelli, E.; Cornen, S.; Igarashi, R.Y.; Fantin, V.R. Natural killer cell therapies. Nature 2024, 626, 727–736. [Google Scholar] [CrossRef]
- Wang, X.; Byrne, M.E.; Liu, C.; Ma, M.T.; Liu, D. Scalable process development of NK and CAR-NK expansion in a closed bioreactor. Front. Immunol. 2024, 15, 1412378. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, J. Emerging roles of CAR-NK cell therapies in tumor immunotherapy: Current status and future directions. Cell Death Discov. 2024, 10, 318. [Google Scholar] [CrossRef]
- Khanal, S.; Baer, A.; Hossain, M.K.; Colon-Moran, W.; Panthi, S.; Bhattarai, N. Soluble factors released by peripheral blood-derived CAR-NK cells cause bystander myeloid cell activation. Front. Immunol. 2024, 15, 1519415. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, M.; Romee, R.; Shapiro, R.M. Innovative strategies to improve the clinical application of NK cell-based immunotherapy. Front. Immunol. 2022, 13, 859177. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Li, Y.; Basar, R.; Rafei, H.; Daher, M.; Dou, J.; Mohanty, V.; Dede, M.; Nieto, Y.; Uprety, N. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: A phase 1/2 trial. Nat. Med. 2024, 30, 772–784. [Google Scholar] [CrossRef]
- Hassan, S.H.; Alshahrani, M.Y.; Saleh, R.O.; Mohammed, B.A.; Kumar, A.; Almalki, S.G.; Alkhafaji, A.T.; Ghildiyal, P.; Al-Tameemi, A.R.; Elawady, A. A new vision of the efficacy of both CAR-NK and CAR-T cells in treating cancers and autoimmune diseases. Med. Oncol. 2024, 41, 127. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, Y.; Gu, Z.; Shi, M.; La Cava, A.; Liu, A. CAR immunotherapy in autoimmune diseases: Promises and challenges. Front. Immunol. 2024, 15, 1461102. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Pyrzynska, B. CAR-NK as a rapidly developed and efficient immunotherapeutic strategy against cancer. Cancers 2022, 15, 117. [Google Scholar] [CrossRef]
- Khanal, S.; Baer, A.; Bhattarai, N. Feeder cell-free production of CAR-NK cells via activation of PKC and calcium signaling pathways. Cytotherapy 2025, 27, 1013–1022. [Google Scholar] [CrossRef]
- Albinger, N.; Müller, S.; Kostyra, J.; Kuska, J.; Mertlitz, S.; Penack, O.; Zhang, C.; Möker, N.; Ullrich, E. Manufacturing of primary CAR-NK cells in an automated system for the treatment of acute myeloid leukemia. Bone Marrow Transplant. 2024, 59, 489–495. [Google Scholar] [CrossRef]
- Nakazawa, T.; Maeoka, R.; Morimoto, T.; Matsuda, R.; Nakamura, M.; Nishimura, F.; Yamada, S.; Nakagawa, I.; Park, Y.-S.; Ito, T. An efficient feeder-free and chemically-defined expansion strategy for highly purified natural killer cells derived from human cord blood. Regen. Ther. 2023, 24, 32–42. [Google Scholar] [CrossRef]
- Reina-Ortiz, C.; Constantinides, M.; Fayd-Herbe-de-Maudave, A.; Présumey, J.; Hernandez, J.; Cartron, G.; Giraldos, D.; Díez, R.; Izquierdo, I.; Azaceta, G. Expanded NK cells from umbilical cord blood and adult peripheral blood combined with daratumumab are effective against tumor cells from multiple myeloma patients. Oncoimmunology 2021, 10, 1853314. [Google Scholar] [CrossRef]
- Abou-el-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Till, B.G.; Bauer, G.; Savoldo, B. Scalable manufacturing of CAR T cells for cancer immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Zale, N.E.; Frary, E.D.; Lomakin, J.A. Feeder-cell-free and serum-free expansion of natural killer cells using Cloudz microspheres, G-Rex6M, and human platelet lysate. Front. Immunol. 2022, 13, 803380. [Google Scholar] [CrossRef] [PubMed]
- Ojo, E.O.; Sharma, A.A.; Liu, R.; Moreton, S.; Checkley-Luttge, M.A.; Gupta, K.; Lee, G.; Lee, D.A.; Otegbeye, F.; Sekaly, R.P.; et al. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci. Rep. 2019, 9, 14916. [Google Scholar] [CrossRef]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef]
- Heinze, A.; Grebe, B.; Bremm, M.; Huenecke, S.; Munir, T.A.; Graafen, L.; Frueh, J.T.; Merker, M.; Rettinger, E.; Soerensen, J.; et al. The Synergistic Use of IL-15 and IL-21 for the Generation of NK Cells From CD3/CD19-Depleted Grafts Improves Their ex vivo Expansion and Cytotoxic Potential Against Neuroblastoma: Perspective for Optimized Immunotherapy Post Haploidentical Stem Cell Transplantation. Front. Immunol. 2019, 10, 2816. [Google Scholar] [CrossRef]
- Felices, M.; Lenvik, A.J.; McElmurry, R.; Chu, S.; Hinderlie, P.; Bendzick, L.; Geller, M.A.; Tolar, J.; Blazar, B.R.; Miller, J.S. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018, 3, e96219. [Google Scholar] [CrossRef]
- Li, Q.; Ye, L.J.; Ren, H.L.; Huyan, T.; Li, J.; Shi, J.L.; Huang, Q.S. Multiple effects of IL-21 on human NK cells in ex vivo expansion. Immunobiology 2015, 220, 876–888. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; He, Z.; Li, L.; Liu, S.; Jiang, M.; Zhao, B.; Deng, M.; Wang, W.; Mi, X. Breakthrough of solid tumor treatment: CAR-NK immunotherapy. Cell Death Discov. 2024, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, A.; Contet, J.; Wong, K.; Chen, C.; Verhoeyen, E.; Slichter, C.K.; Schluns, K.S.; Cursons, J.; Berry, R.; Nikolic, I. Optimisation of a primary human CAR-NK cell manufacturing pipeline. Clin. Transl. Immunol. 2024, 13, e1507. [Google Scholar] [CrossRef]
- Sin, W.-X.; Jagannathan, N.S.; Teo, D.B.L.; Kairi, F.; Fong, S.Y.; Tan, J.H.L.; Sandikin, D.; Cheung, K.-W.; Luah, Y.H.; Wu, X. A high-density microfluidic bioreactor for the automated manufacturing of CAR T cells. Nat. Biomed. Eng. 2024, 8, 1571–1591. [Google Scholar] [CrossRef]
- He, B.; Chen, H.; Deng, S.; Li, C.; Xu, N.; Liu, X.; Zhou, H.; Liu, Q. CD19-specific CAR NK cells coexpressing IL-21 exhibit superior expansion and antitumor activity against CD19-postive lymphoma. Blood 2023, 142, 6824. [Google Scholar] [CrossRef]
- Schmidt, D.; Ebrahimabadi, S.; Gomes, K.R.d.S.; de Moura Aguiar, G.; Cariati Tirapelle, M.; Nacasaki Silvestre, R.; de Azevedo, J.T.C.; Tadeu Covas, D.; Picanço-Castro, V. Engineering CAR-NK cells: How to tune innate killer cells for cancer immunotherapy. Immunother. Adv. 2022, 2, ltac003. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Schussler-Lenz, M.; Bondanza, A.; Buchholz, C.J. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017, 9, 1183–1197. [Google Scholar] [CrossRef]
- Jiang, J.; Ahuja, S. Addressing Patient to Patient Variability for Autologous CAR T Therapies. J. Pharm. Sci. 2021, 110, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, S.; Nunez-Cruz, S.; O’Connor, R.S.; Fraietta, J.A.; Patel, P.R.; Scholler, J.; Barrett, D.M.; Lundh, S.M.; Davis, M.M.; Bedoya, F.; et al. Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells. Cancer Immunol. Res. 2018, 6, 1100–1109. [Google Scholar] [CrossRef]
- Mock, U.; Nickolay, L.; Philip, B.; Cheung, G.W.; Zhan, H.; Johnston, I.C.D.; Kaiser, A.D.; Peggs, K.; Pule, M.; Thrasher, A.J.; et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS prodigy. Cytotherapy 2016, 18, 1002–1011. [Google Scholar] [CrossRef]
- Watanabe, N.; Mo, F.; McKenna, M.K. Impact of Manufacturing Procedures on CAR T Cell Functionality. Front. Immunol. 2022, 13, 876339. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Jensen, M.C.; Lansdorp, P.M.; Gough, M.; Elliott, C.; Riddell, S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Investig. 2008, 118, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.L.; Miskin, J.; Wonnacott, K.; Keir, C. Global Manufacturing of CAR T Cell Therapy. Mol. Ther. Methods Clin. Dev. 2017, 4, 92–101. [Google Scholar] [CrossRef]
- Harrison, R.P.; Zylberberg, E.; Ellison, S.; Levine, B.L. Chimeric antigen receptor-T cell therapy manufacturing: Modelling the effect of offshore production on aggregate cost of goods. Cytotherapy 2019, 21, 224–233. [Google Scholar] [CrossRef]
- Lei, W.; Liu, H.; Deng, W.; Chen, W.; Liang, Y.; Gao, W.; Yuan, X.; Guo, S.; Li, P.; Wang, J.; et al. Safety and feasibility of 4-1BB co-stimulated CD19-specific CAR-NK cell therapy in refractory/relapsed large B cell lymphoma: A phase 1 trial. Nat. Cancer 2025, 6, 786–800. [Google Scholar] [CrossRef]

| General Reagents |
|---|
| HEK293 cells, K562 cells (ATCC, Manassas, VA, USA; CCL-243), phosphate-buffered saline (PBS) without calcium and magnesium, conical centrifuge tubes (15 mL, 50 mL, sterile pipettes, Bovine Serum Albumin (BSA), non-tissue culture coated, and tissue culture coated cell culture plates (24-well or 6-well), RPMI, MACS buffer, penicillin–streptomycin, glutamine, Fetal Bovine Serum, human AB serum, 0.4% Trypan Blue solution, Mr. Frosty, DMSO, cryogenic vials |
| Special Reagents |
| Ficoll–Paque [density: 1.077 g/mL] (Cytiva, Malborough, MA, USA; 17144002) |
| Human peripheral blood or buffy coat (preferably collected within 24 h) |
| RBC lysis buffer (Invitrogen, Carlsbad, CA, USA; 00-4333-57) |
| CD3 microbeads (Miltenyi Biotec, Gaithersburg, MD, USA; 130-097-043) |
| CD56 microbeads (Miltenyi Biotec, Gaithersburg, MD, USA; 130-050-401) |
| Lentiviral vector carrying the desired CAR gene |
| MACS magnetic separator (Miltenyi Biotec, Gaithersburg, MD, USA; 130-042-602) or equivalent |
| Retronectin (TakaraBio, San Jose, CA, USA, T100A) |
| NK MACS (Miltenyi Biotec, Gaithersburg, MD, USA; 130-114-429) |
| Superblock blocking buffer (Thermo Fischer Scientific, Waltham, MA, USA; 37515) |
| Phorbol 12-myristate 13-acetate (PMA) (SelleckChem, Houston, TX, USA; S7791) |
| Ionomycin (SelleckChem, Houston, TX, USA; S7074) |
| Recombinant IL-2 (Miltenyi Biotec, Gaithersburg, MD, USA; 130-095-760) |
| Recombinant IL-15 ((Miltenyi Biotec, Gaithersburg, MD, USA; 130-095-760) |
| Recombinant IL-21 ((Miltenyi Biotec, Gaithersburg, MD, USA; 130-095-767) |
| G-Rex 6-well plate (Wilson Wolf, Saint Paul, MN, USA; P/N 80240M) |
| CryoStor CS10 (STEMCELL Technologies, Cambridge, MA, USA; 100-1061) |
| NK cell expansion media: NKMACs media supplemented with IL-2 (200–500 IU/mL), IL-15 (5 ng/mL), and IL-21(25 ng/mL) |
| Freezing media: 90% FBS + 10% DMSO or CryoStor CS10 |
| Equipment |
| Incubator (37 °C, 5% CO2) |
| Tabletop centrifuge with swinging-bucket rotor |
| Countess 3 FL automated cell counter (Thermo Fischer Scientific, Waltham, MA, USA; AMQAF2000) |
| −80 °C Freezer |
| Liquid nitrogen (LN2) storage |
| Water bath (37 °C) |
| Micropipettes (P10, P20, P100, P200, P1000) |
| Parameter | Day 0–2 | Day 3–7 | Day 8–14 | Expected Outcomes |
|---|---|---|---|---|
| Cell Density | 1 × 106 cells/mL | 0.5–1 × 106 cells/mL | 0.3–0.8 × 106 cells/mL | 15–30-fold expansion |
| IL-15 (ng/mL) | 5 (optional) | 5 | 5–10 | Sustained activation |
| IL-2 (IU/mL) | 200 (optional) | 200–500 | 200–500 | Enhanced proliferation |
| Media Exchange | None | 50% every 48h | 50% every 48–72h | Fresh nutrient supply |
| CAR Expression | N/A | 25–55% | 25–65% | Stable CAR expression |
| Viability | >95% | >90% | >85% | >60% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, S.; Bhattarai, N. A Scalable Protocol for Ex Vivo Production of CAR-Engineered Human NK Cells. Methods Protoc. 2025, 8, 102. https://doi.org/10.3390/mps8050102
Khanal S, Bhattarai N. A Scalable Protocol for Ex Vivo Production of CAR-Engineered Human NK Cells. Methods and Protocols. 2025; 8(5):102. https://doi.org/10.3390/mps8050102
Chicago/Turabian StyleKhanal, Supreet, and Nirjal Bhattarai. 2025. "A Scalable Protocol for Ex Vivo Production of CAR-Engineered Human NK Cells" Methods and Protocols 8, no. 5: 102. https://doi.org/10.3390/mps8050102
APA StyleKhanal, S., & Bhattarai, N. (2025). A Scalable Protocol for Ex Vivo Production of CAR-Engineered Human NK Cells. Methods and Protocols, 8(5), 102. https://doi.org/10.3390/mps8050102








